Abstract
Purpose
Neoadjuvant chemoradiotherapy for rectal cancer is associated with improved local control and may result in complete tumor response. Associations between tumor response and disease control following radical resection should be established before tumor response is used to evaluate treatment strategies. The purpose of this study was to assess and compare oncologic outcomes associated with the degree of pathologic response after chemoradiotherapy.
Patients and Methods
All patients with locally advanced (cT3-4 or cN+ by endorectal ultrasonography, computed tomography, or magnetic resonance imaging) rectal carcinoma diagnosed from 1993 to 2008 at our institution and treated with preoperative chemoradiotherapy and radical resection were identified, and their records were retrospectively reviewed. The median radiation dose was 50.4 Gy with concurrent chemotherapy. Recurrence-free survival (RFS), distant metastasis (DM), and local recurrence (LR) rates were compared among patients with complete (ypT0N0), intermediate (ypT1-2N0), or poor (ypT3-4 or N+) response by using Kaplan-Meier survival analysis and multivariate Cox proportional hazards regression.
Results
In all, 725 patients were classified by tumor response: complete (131; 18.1%), intermediate (210; 29.0%), and poor (384; 53.0%). Age, sex, cN stage, and tumor location were not related to tumor response. Tumor response (complete v intermediate v poor) was associated with 5-year RFS (90.5% v 78.7% v 58.5%; P < .001), 5-year DM rates (7.0% v 10.1% v 26.5%; P < .001), and 5-year LR only rates (0% v 1.4% v 4.4%; P = .002).
Conclusion
Treatment response to neoadjuvant chemoradiotherapy among patients with locally advanced rectal cancer undergoing radical resection is an early surrogate marker and correlate to oncologic outcomes. These data provide guidance with response-stratified oncologic benchmarks for comparisons of novel treatment strategies.
INTRODUCTION
Rectal cancer is one of the most common cancers in the United States, estimated to have affected 39,670 individuals in 2010, accounting for approximately 28% of all colorectal cancers.1 For patients with localized disease, the primary treatment is surgical resection.2 Total mesorectal excision has been associated with improvements in cancer control; however, for patients with locally advanced (stage II to III) cancers, preoperative chemoradiotherapy provides further improvements.3
For most patients, preoperative chemoradiotherapy results in clinically meaningful tumor regression, but the degree of response varies among patients: some show almost no response, and others exhibit a pathologic complete response (CR). CR with absence of viable tumor cells in the surgical specimen occurs in approximately 15% to 20% of patients.4–6 Many, however, will experience an intermediate response with varying residual tumor infiltration. Emerging data suggest that CR is associated with improved local control and survival, and this has led to a growing interest in an organ-preserving “wait-and-see” strategy to avoid the morbidity and functional sequelae of radical resection, which includes the potential need for colostomy or bowel, urinary, and sexual dysfunction. However, before an organ-preserving strategy can be evaluated, the benchmarks of oncologic outcomes, to which new strategies should be compared, must be established.7 Conversely, poor response to chemoradiotherapy may serve as an indicator of adverse tumor biology and the need to consider treatment intensification, and the incremental benefits of such treatments will need to be measured against current outcomes among the same cohort.
Most prior reports examining this issue have required pooling of data from several institutions to provide sufficient power to evaluate the results. Although pooling of data permits additional analyses, interinstitutional biases and variations in treatment practices may affect data interpretation. A large single-institution experience with standardized treatment and pathologic evaluation avoids this limitation. Furthermore, although most prior interest has focused on patients with CR, the influence of various levels of tumor response on recurrence and on survival has been less well characterized.8–10 Although pretreatment staging is the standard for planning the treatment regimen for patients with rectal cancer, pathologic stage may be the more prognostic determinant of cancer-related survival. Therefore, improving the understanding of tumor response on the natural history of rectal cancer on the basis of postchemoradiotherapy pathology will provide practical information for patients and practitioners who are considering prognosis or planning adjuvant treatment.
The aim of this study was to compare the oncologic outcomes of patients with rectal cancer treated by preoperative chemoradiotherapy and radical resection stratified by degree of tumor response to chemoradiotherapy. To improve the generalizability of our findings, we also sought to determine a prognostic tumor response indicator based on ypT and ypN staging.
PATIENTS AND METHODS
Patients
We performed a retrospective consecutive cohort study of patients with biopsy-proven, locally advanced (cT3-4 or cN+ by endorectal ultrasonography, computed tomography, or magnetic resonance imaging) rectal cancer treated with preoperative chemoradiotherapy followed by radical resection at The University of Texas MD Anderson Cancer Center between 1993 and 2008. Patients were identified from our institutional patient colorectal cancer database and tumor registry. Patients with concurrent distant metastasis or concurrent inflammatory bowel disease, hereditary colorectal cancer syndromes, concurrent malignancy, emergent surgery, prior history of radiotherapy to the pelvis, or prior history of malignancy were excluded. The MD Anderson institutional review board approved the study.
Clinical Staging, Treatment, and Pathologic Evaluation
Pretreatment clinical stage was assessed on the basis of EUS, MRI, or CT findings. All pretreatment biopsies were reviewed and diagnoses confirmed by MD Anderson gastrointestinal pathologists. All patients also underwent full colonoscopic evaluation to exclude synchronous tumors, as well as digital rectal examination and proctoscopy to identify the tumor distance from the anal verge. Patients were treated with chemoradiotherapy with a median radiotherapy dose of 50.4 Gy and concurrent fluoropyrimidine-based chemotherapy (mainly single-agent infusional fluorouracil or capecitabine). Surgery generally was performed 6 to 8 weeks following completion of chemoradiotherapy and included low anterior resection, proctectomy with coloanal reconstruction, abdominoperineal resection, or multivisceral rectal resection using total mesorectal excision principles. Adjuvant chemotherapy was recommended for all medically fit patients following resection. The therapy consisted of infusional fluorouracil or capecitabine for a period of 4 to 6 months. Oxaliplatin-containing regimens were introduced in 2003 at the treating physician's discretion. In some cases, protocol-based concurrent chemotherapy included the addition of irinotecan or bevacizumab.
Standard pathologic tumor staging of the resected specimen was performed after resection in accordance with the guidelines of the College of American Pathologists, with histopathologic diagnosis performed by dedicated gastrointestinal cancer pathologists.11 The gross tumor volume was entirely embedded and serially sectioned for hematoxylin and eosin staining and microscopic evaluation. The mesorectum was manually dissected, and lymph nodes were examined with one to three separate sections per node. An independent pathologist blinded to the study results independently confirmed the findings in the pathologic record. CR was defined as absence of viable adenocarcinoma cells in the surgical specimen (ypT0N0). The CR classification included specimens with acellular mucin pools without viable tumor cells. Intermediate response was defined as an improvement in stage to ypT1-2 and ypN0. Patients with ypT3-4 or persistent lymph node metastasis were classified as poor responders. Postoperative follow-up consisted of routine physical examination with carcinoembryonic antigen measurement every 3 to 6 months along with proctoscopy and cross-sectional imaging every 6 to 12 months for 5 to 7 years.
Statistical Analysis
Response outcomes of each of the tumor-related variables as well as the pathologic response rate following chemoradiotherapy were compared by using the Wilcoxon rank sum test or, for multiple predictor and response groups, the Kruskal-Wallis test. Categorical data were summarized by frequency within each cohort, and comparisons were performed by using the χ2 test for proportions. The test for binary correlation was used to assess associations between selected polynomial categorical variables.
For recurrence-free survival (RFS) analysis, patients for whom treatment had failed were identified at the time of disease recurrence or death from any cause (ie, noncancer deaths were not censored). Five-year RFS rates were determined by the Kaplan-Meier method, and univariate comparisons among tumor response groups were performed with the log-rank test. Local recurrence and distant metastasis rates also were compared among groups. Cox proportional hazards regression analysis was performed for multivariate comparisons. P values less than .05 were considered significant.
RESULTS
Patient Population and Tumor Characteristics
In all, 725 patients who were treated for rectal cancer with chemoradiotherapy during the study period were included. The median age was 57 years (interquartile range [IQR], 48 to 66 years). The majority were men (62.6%). Median tumor distance from the anal verge was 5 cm (IQR, 3 to 8 cm). Most tumors were cT3 on preoperative evaluation. The median dose of radiation was 50.4 Gy (IQR, 45 to 52.5 Gy). Concurrent chemotherapy was fluorouracil in 42.1% and capecitabine in 37.9% of the patients. The remaining patients received investigational fluoropyrimidine-based combination regimens (eg, with oxaliplatin or bevacizumab). Surgery was performed at a median of 7 weeks after completion of chemoradiotherapy. All patients underwent total or tumor-specific mesorectal excision depending on the extent of the tumor. Two hundred forty patients (33.1%) underwent low anterior resection, 277 patients (38.2%) underwent proctectomy with coloanal anastomosis, 202 patients (27.9%) underwent abdominoperineal resection, and six patients (0.7%) underwent other procedures. Among them, 122 patients (16.8%) underwent multivisceral resection for adjacent visceral organ involvement. A sphincter-preserving operation was performed for 71.2% of the patients. A total of 24 patients did not have complete pathologic data to evaluate T and N stage and therefore were excluded to yield the final 725-patient cohort.
Pathologic Results and Tumor Characteristics Regarding Response Group
Resections were classified R0 in 706 patients (97.4%). During the earlier period of study, the quantitative radial margin distance was not consistently reported; therefore, the quantitative information was available for the most recent 193 patients. Among these, 19 (9.8%) had a circumferential resection margin ≤ 1 mm.
Among all patients, the median number of examined lymph nodes was 11 (IQR, 6 to 16). Lymphovascular invasion was noted in 104 (14.3%), and perineural invasion in 56 patients (7.7%). Pathologic classification was ypT0N0 in 131 patients (18.1%), ypT1-2N0 in 210 (29.0%), ypT3-4N0 in 164 (22.6%), and any ypT with N+ in 210 (29.0%). Patients with higher ypT categories following chemoradiotherapy were more likely to also have positive ypN status (P < .001). ypN+ tumors accounted for 14 ypT0 tumors (9.7%), 12 ypT1 tumors (17.6%), 42 ypT2 tumors (21.4%), and 152 ypT3-4 tumors (47.9%).
Patients were categorized into three response groups on the basis of their pathologic results: ypT0N0 was classified as CR, ypT1-2N0 was classified as intermediate response (IR), and ypT3-4N0 or ypTanyN+ were classified as poor response (PR). Median age and sex were not significantly different among the three response groups. Presence of lymphovascular and perineural invasion was more frequently observed in the PR group (P < .001). The details of the clinicopathologic features are provided in Table 1.
Table 1.
Patient and Tumor Characteristics and Treatment Factors (N = 725)
| Characteristic | Complete Response (T0N0) |
Intermediate Response (T1-2N0) |
Poor Response (T3-4or N+) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Total No. of patients | 131 | 18.1 | 211 | 29.0 | 384 | 53.0 | |
| Patient and tumor characteristics | |||||||
| Age, years* | .15 | ||||||
| Median | 57 | 58.5 | 56 | ||||
| Range | 48–66 | 49–67 | 48–65 | ||||
| Sex | .64 | ||||||
| Male | 80 | 61.1 | 137 | 65.2 | 237 | 61.7 | |
| Female | 51 | 38.9 | 73 | 34.8 | 147 | 38.3 | |
| Location of tumor, cm from AV | .11† | ||||||
| > 10 | 13 | 10.1 | 4 | 2.0 | 25 | 6.8 | |
| 6-10 | 54 | 42.2 | 84 | 41.0 | 162 | 43.9 | |
| ≤ 5 | 61 | 47.7 | 117 | 57.0 | 182 | 49.3 | |
| Clinical stage | .08 | ||||||
| II | 51 | 38.9 | 95 | 45.2 | 144 | 37.5 | |
| III | 74 | 56.5 | 98 | 46.7 | 195 | 50.8 | |
| Tumor grade | .08 | ||||||
| G1, G2 | 112 | 91.1 | 184 | 91.1 | 321 | 85.6 | |
| G3, G4 | 11 | 8.9 | 18 | 8.9 | 54 | 14.4 | |
| No. of examined lymph nodes* | .22‡ | ||||||
| Median | 11 | 11 | 12 | ||||
| Range | 6–17 | 6–15 | 7–18 | ||||
| Lymphovascular invasion | 0 | 10 | 4.8 | 94 | 24.5 | < .001§ | |
| Perineural invasion | 0 | 1 | 0.5 | 55 | 14.3 | < .001§ | |
| Treatment factors | |||||||
| Radiation dose, Gy* | .10 | ||||||
| Median | 50.4 | 50.4 | 50.4 | ||||
| Range | 45–52.13 | 45–52.5 | 45–52.5 | ||||
| Concurrent chemotherapy | .10 | ||||||
| Fluorouracil | 52 | 39.9 | 101 | 48.6 | 153 | 39.5 | |
| Capecitabine | 47 | 36.2 | 65 | 31.3 | 153 | 39.5 | |
| Other¶ | 14 | 10.8 | 11 | 5.3 | 16 | 4.1 | |
| Interval between completion of chemoradiotherapy and surgery, days* | .14 | ||||||
| Median | 49 | 50 | 52 | ||||
| Range | 46–57 | 44–57 | 45–62 | ||||
| Adjuvant chemotherapy | 109 | 83.2 | 172 | 81.9 | 330 | 86.2 | .36 |
| Fluoropyrimidine only∥ | 85 | 78.0 | 142 | 82.6 | 220 | 66.7 | < .001# |
| Oxaliplatin–based∥ | 19 | 17.4 | 23 | 13.4 | 97 | 29.4 | |
| Others∥ | 2 | 1.8 | 2 | 1.2 | 10 | 3.0 | |
| Follow–up duration, months* | < .001 | ||||||
| Median | 75 | 75 | 59 | ||||
| Range | 39–105 | 43–116 | 36–96 | ||||
Abbreviation: AV, anal verge.
Median value with interquartile range.
Tumor location was analyzed based on distance (cm) from AV.
Compared with complete response group.
Comparison between intermediate and poor response groups.
Targeted agents, oxaliplatin.
Percentage among patients who received adjuvant chemotherapy.
Comparison of fluoropyrimidine only v oxaliplatin-based treatment.
Treatment factors that did not differ among the three groups included dose of radiation, concurrent chemotherapy regimen, interval between treatment and resection, and whether adjuvant chemotherapy was given postoperatively. However, the adjuvant chemotherapy regimen did differ among the response groups. Although single-agent fluoropyrimidine (fluorouracil or capecitabine) was the most common regimen, there was an association between receipt of combination chemotherapy that included oxaliplatin and PR (Table 1). Six hundred eleven patients (84.3%) received postoperative adjuvant chemotherapy. One hundred fourteen patients did not receive adjuvant chemotherapy for the following reasons: postoperative complications or poor performance status in 48 patients (42.1%), patient refusal in 27 (23.7%), comorbidities in 16 (14.0%), intolerance of chemotherapy in six (5.2%), not recommended based on favorable pathologic results in six (5.2%), and unknown in 11 patients (9.6%).
Recurrence and Survival
The median follow-up duration was 65 months (IQR, 38 to 104 months) for the entire cohort and was shorter in the PR group than in other response groups (P < .001), largely because of cancer-related mortality. Overall, recurrences were observed in 153 patients: 20 had local recurrence only, 114 had systemic recurrence only, and 19 had both local and systemic recurrences. Rates of local and systemic recurrences were related to the tumor response group, with a remarkably low recurrence rate associated with CR (Table 2).
Table 2.
Recurrence According to Response to Preoperative Chemoradiotherapy
| Variable | Complete Response |
HR | 95% CI | Intermediate Response |
HR | 95% CI | Poor Response |
HR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||||||
| Total No. of patients | 131 | 210 | 384 | |||||||||
| Local recurrence only | 0 | 3 | 1.4 | 17 | 4.4 | |||||||
| Systemic recurrence only | 8 | 6.21 | 19 | 9.0 | 87 | 22.7 | ||||||
| Both local and systemic recurrence | 1 | 0.8 | 2 | 1 | 16 | 4.2 | ||||||
| Cox regression model for risk of recurrence | ||||||||||||
| Local recurrence | 1 | 3.17 | 0.37 to 27.13 | 12.94 | 1.77 to 94.64 | |||||||
| Systemic recurrence | 1 | 1.48 | 0.68 to 3.23 | 4.29 | 2.17 to 8.48 | |||||||
Abbreviation: HR, hazard ratio.
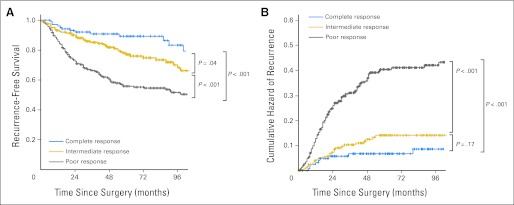
For the entire cohort, overall survival and RFS at 5 years were 82.9% and 70.1%, respectively. The 5-year overall survival rates were 93.4%, 87.0%, and 77.3% for the CR, IR, and PR groups, respectively (P = .002); the 5-year RFS rates were 90.5%, 78.7%, and 58.5% for the CR, IR, and PR groups, respectively (P < .001; Fig 1).
Fig 1.
(A) Recurrence-free survival by response category. (B) Cumulative hazard of relapse by response category.
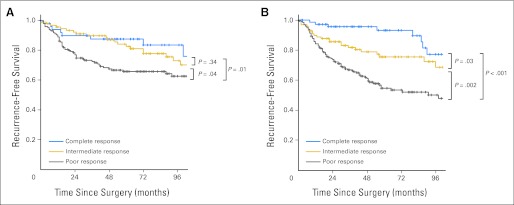
The results of the multivariate Cox regression analysis for RFS are listed in Table 3. In adjusted analysis, RFS was strongly associated with response to chemoradiotherapy but only weakly related to clinical stage (Fig 2). Compared with CR, PR was strongly related to an increased risk of recurrence (hazard ratio, 3.01; 95% CI, 1.75 to 5.16).
Table 3.
Univariate and Multivariate Cox Proportional Hazards Regression Models of Clinical Factors of RFS
| Factor | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Response | ||||
| Complete response | 1 | 1 | ||
| Intermediate response | 1.69 | 0.10 to 2.87 | 1.80 | 1.01 to 3.21 |
| Poor response | 3.21 | 1.99 to 5.17 | 3.01 | 1.75 to 5.16 |
| Clinical T classification | ||||
| cT2 | 1 | 1 | ||
| cT3 | 1.32 | 0.65 to 2.70 | 1.11 | 0.54 to 2.29 |
| cT4 | 2.32 | 1.06 to 5.06 | 1.47 | 0.64 to 3.40 |
| Clinical N classification | ||||
| Negative | 1 | 1 | ||
| Positive | 1.27 | 0.96 to 1.70 | 1.48 | 1.08 to 2.01 |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.96 | 0.73 to 1.26 | 0.93 | 0.69 to 1.25 |
| Age, years | ||||
| ≤ 60 | 1 | 1 | ||
| 61–75 | 1.31 | 0.99 to 1.75 | 1.50 | 1.11 to 2.04 |
| > 75 | 2.08 | 1.34 to 3.23 | 2.24 | 1.36 to 3.68 |
| Lymphovascular invasion | ||||
| None | 1 | 1 | ||
| Present | 2.07 | 1.48 to 2.90 | 1.54 | 1.01 to 2.35 |
| Undetermined | 0.80 | 0.56 to 1.12 | 1.07 | 0.59 to 1.94 |
| Perineural invasion | ||||
| None | 1 | 1 | ||
| Present | 2.52 | 1.69 to 3.75 | 1.46 | 0.90 to 2.36 |
| Undetermined | 0.86 | 0.64 to 1.16 | 0.57 | 0.34 to 0.95 |
| Location of tumor, cm from AV | ||||
| > 10 | 1 | 1 | ||
| 6–10 | 1.02 | 0.77 to 1.35 | 1.15 | 0.85 to 1.55 |
| ≤ 5 | 0.70 | 0.36 to 1.38 | 0.87 | 0.42 to 1.82 |
| Tumor grade | ||||
| G1, G2 | 1 | 1 | ||
| G3, G4 | 1.27 | 0.87 to 1.86 | 1.20 | 0.79 to 1.81 |
| Adjuvant chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 0.63 | 0.46 to 0.86 | 0.56 | 0.38 to 0.82 |
Abbreviations: AV, anal verge; HR, hazard ratio; RFS, recurrence-free survival.
Fig 2.
Recurrence-free survival according to response and pretreatment clinical stage: (A) clinical stage II disease; (B) clinical stage III disease.
DISCUSSION
The results of this study show that oncologic outcomes after preoperative chemoradiotherapy and radical resection for locally advanced rectal cancer are correlated with treatment response. Patients with a CR following radical resection had excellent outcomes without isolated local recurrence and with low rates of distant recurrence. Patients with IR also experienced improved local and distant disease control rates compared with those with PR. Clinical outcomes were strongly associated with the findings at final pathologic evaluation and less related to the clinical staging information. These data indicate that pathologically assessed tumor response to neoadjuvant therapy is a powerful short-term treatment response indicator and surrogate marker of long-term outcomes. Novel response-stratified treatment strategies (eg, watch-and-wait or systemic treatment intensification) should therefore be compared with such benchmarks of response-stratified outcomes.
Recently, there has been great interest in evaluating outcomes among patients with rectal cancer who were treated with neoadjuvant chemoradiotherapy. In a 385-patient subcohort from a randomized trial comparing preoperative with postoperative chemoradiotherapy for rectal cancer, tumor regression was categorized on the basis of a semiquantitative assessment comparing the amount of viable tumor with the amount of fibrosis (tumor regression grade [TRG]), with most patients achieving more than 50% regression.12 But limitations for generalizability of this semiquantitative measure of TRG have resulted in the development of alternative definitions for assessment of tumor regression, which are not yet broadly accepted.11 However, post-treatment T and N classifications are easily identified and are a more practical measure of treatment response than TRG. Furthermore, substratification based on ypT and ypN classifications may be more discriminatory for patients with IR, because fewer patients will have ypT1-2N0 classification than will have an intermediate TRG.
Tumor response to neoadjuvant chemoradiotherapy followed by radical resection, as assessed pathologically, therefore serves as a powerful early response indicator that identifies patients with excellent long-term prognosis, those with a high risk for recurrence who may be candidates for systemic treatment intensification, and those in between. In this study, we observed remarkably low overall rates of recurrence among patients with CR. But when recurrence occurred, the pattern of failure was systemic. Among patients with IR, local recurrences were uncommon, and outcomes were intermediate to those with CR or PR. Although distant disease was the main type of recurrence among patients in our study, it was also infrequent, occurring in only 6.9% of patients with CR and 10.1% of those with IR. Thus the 5-year RFS and overall survival rates progressively increased according to the tumor response from PR to CR. Systemic and local recurrence rates also progressively decreased according to the response group from PR to CR. Oncologic outcomes were less related to clinical stage and showed stratification according to tumor response in patients with clinical stage II or III disease. In multivariate analysis, the tumor response category was the most important predictor of oncologic outcomes after radical resection.
Given the low rates of recurrence among patients with CR following chemoradiotherapy, the feasibility of an organ-preserving strategy is apparent.13–16 However, outcomes of such strategies would need to be compared with those of the current approach. Previous studies of oncologic outcomes associated with resection10,17,18 have been limited by the need to pool data from multiple smaller series or to combine patients with ypT0 tumors with patients with ypT1 or ypT1-2 tumors. This is the largest single-institution study to date of response-stratified outcomes following chemoradiotherapy and radical resection for locally advanced rectal cancer. We have further stratified patients into categories of response because there are uniquely different implications of CRs, IRs, and PRs. These results may be viewed in the context of treatment standardization for chemoradiotherapy, surgical resection, and pathologic evaluation. But our study may be subject to potential limitations associated with a 15-year study period, although the rate of CR during the study period remained generally stable and there were no differences in the radiotherapy dose, type of concurrent chemotherapy, or rate of adjuvant therapy use between the response groups. Because of the inherently favorable outcomes associated with standard treatment in patients with good response, the oncologic outcomes of an organ-preserving strategy for complete responders should be comparable to the exceptionally good outcomes observed with radical resection in the CR cohort. Furthermore, the potential for metastatic nodal disease (9% among ypT0 tumors) within the residual unresected mesorectum cannot be ignored. Because previous comparative studies of organ-preserving approaches versus the radical resection approach have been limited by small sample sizes or the need to compare a highly selected group of patients using the wait-and-see approach to an unselected control group that includes patients with poor as well as good responses, the data from this study provide a benchmark for comparison of outcomes.19–21
Still another issue is the role of adjuvant chemotherapy after chemoradiotherapy and surgery. In this study, 84.3% of the patients received postoperative adjuvant chemotherapy. The most common reasons for not receiving adjuvant chemotherapy were postoperative complications or poor overall performance status. Because of these and other factors affecting patient selection, an evaluation of the impact of adjuvant therapy on survival outcomes is beyond the scope of this study; however, the administration of adjuvant therapy was not dependent on neoadjuvant therapy response. Studies before the era of total mesorectal excision demonstrated improved survival outcomes associated with adjuvant chemotherapy among patients with resected rectal cancer; however, the benefit of adjuvant chemotherapy following neoadjuvant chemoradiotherapy in the modern era is assumed on the basis of the results of trials of adjuvant therapy for colon cancer.22 In the only study23 to formally evaluate the impact of adjuvant fluorouracil-based chemotherapy on survival following neoadjuvant chemoradiotherapy, the magnitude of the effect was much smaller than the differences between response strata observed in this report. Here, the 26.5% 5-year rate of distant recurrence among patients with PR—despite an 85.5% rate of adjuvant therapy administration—indicates that there remains considerable room for improvement. Neoadjuvant treatment response may serve as not only an indicator of prognosis but also an indicator of subsequent response to the same chemotherapeutic agents used for radiosensitization among patients with good responses and of the need for expanded therapeutic options for patients with PR.23,24 Furthermore, CR patients have such favorable outcomes that additional gains from adjuvant chemotherapy are uncertain, and future trials of adjuvant chemotherapy treatment minimization should be considered for this group of patients. Such an early response indicator will also enable us to select patients with poor prognoses for studies of treatment intensification without subjecting patients with good prognoses to potential added toxicities. Thus, the patients most likely to benefit from such treatments can be targeted.
We found that oncologic outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer are correlated with treatment response. Final pathologic stage is an early response indicator for long-term outcomes that provides better prognostication than does the clinical stage. Patients who achieve CR and undergo radical resection have excellent prognosis with low risk for local or distant recurrence. These patients may be eligible for study with organ-preserving strategies; however, the outcomes after such an approach will need to be compared with those achieved with radical resection. Alternatively, patients with PR should be targeted for studies of strategies incorporating treatment intensification. These data provide oncologic reference points to which future novel treatment strategies may be compared.
Supplementary Material
Acknowledgment
We thank Sa Nguyen, MS, for her assistance with data management and Bryan Tutt for his assistance with scientific editing.
Footnotes
Supported in part by a Career Development Award from the American Society of Clinical Oncology Conquer Cancer Foundation (G.J.C.), Grant No. K07-CA133187 from the National Institutes of Health (NIH; G.J.C.), and Grant No. CA016672 from NIH through MD Anderson's Cancer Center Support.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Prajnan Das, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: In Ja Park, John M. Skibber, Miguel A. Rodriguez-Bigas, George J. Chang
Financial support: George J. Chang
Administrative support: George J. Chang
Provision of study materials or patients: John M. Skibber, Miguel A. Rodriguez-Bigas, Cathy Eng, Barry W. Feig, Sunil Krishnan, Christopher H. Crane, George J. Chang
Collection and assembly of data: In Ja Park, Miguel A. Rodriguez-Bigas, Atin Agarwal, John M. Skibber, Miguel A. Rodriguez-Bigas, Cathy Eng, Barry W. Feig, Prajnan Das, Sunil Krishnan, Christopher H. Crane, George J. Chang
Data analysis and interpretation: In Ja Park, Y. Nancy You, Chung-Yuan Hu, George J. Chang
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Atlanta, GA: American Cancer Society; 2011. Cancer Facts & Figures 2011. [Google Scholar]
- 2.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Public-Use, Nov 2005 Sub (1973-2003 varying). National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2006, based on the November 2005 submission.
- 3.O'Connell JB, Maggard MA, Liu JH, et al. Are survival rates different for young and older patients with rectal cancer? Dis Colon Rectum. 2004;47:2064–2069. doi: 10.1007/s10350-004-0738-1. [DOI] [PubMed] [Google Scholar]
- 4.Das P, Skibber JM, Rodriguez-Bigas MA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007;109:1750–1755. doi: 10.1002/cncr.22625. [DOI] [PubMed] [Google Scholar]
- 5.Silberfein EJ, Kattepogu KM, Hu CY, et al. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann Surg Oncol. 2010;17:2863–2869. doi: 10.1245/s10434-010-1119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KD, Tan D, Das P, et al. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg. 2010;251:261–264. doi: 10.1097/SLA.0b013e3181bdfc27. [DOI] [PubMed] [Google Scholar]
- 7.Glynne-Jones R, Wallace M, Livingstone JI, et al. Complete clinical response after preoperative chemoradiation in rectal cancer: Is a “wait and see” policy justified? Dis Colon Rectum. 2008;51:10–19. doi: 10.1007/s10350-007-9080-8. [DOI] [PubMed] [Google Scholar]
- 8.Chang GJ, Rodriguez-Bigas MA, Eng C, et al. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer. 2009;115:5432–5440. doi: 10.1002/cncr.24622. [DOI] [PubMed] [Google Scholar]
- 9.Janjan NA, Crane C, Feig BW, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24:107–112. doi: 10.1097/00000421-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 11.Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539–1551. doi: 10.5858/133.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 13.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg. 2004;240:711–717. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa WT, Rossi BM, de O Ferreira F, et al. Chemoradiation instead of surgery to treat mid and low rectal tumors: Is it safe? Ann Surg Oncol. 2002;9:568–573. doi: 10.1007/BF02573893. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health. ClinicalTrials.gov: Minimal Invasive Strategies for Good and Complete Response to Chemoradiation in Rectal Cancer. http://clinicaltrials.gov/ct2/show/NCT00939666.
- 16.O'Neill BD, Brown G, Heald RJ, et al. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 2007;8:625–633. doi: 10.1016/S1470-2045(07)70202-4. [DOI] [PubMed] [Google Scholar]
- 17.Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99–107. doi: 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Wolthuis AM, Penninckx F, Haustermans K, et al. Outcome standards for an organ preservation strategy in stage II and III rectal adenocarcinoma after neoadjuvant chemoradiation. Ann Surg Oncol. 2011;18:684–690. doi: 10.1245/s10434-010-1324-5. [DOI] [PubMed] [Google Scholar]
- 19.Nair RM, Siegel EM, Chen DT, et al. Long-term results of transanal excision after neoadjuvant chemoradiation for T2 and T3 adenocarcinomas of the rectum. J Gastrointest Surg. 2008;12:1797–1805. doi: 10.1007/s11605-008-0647-z. [DOI] [PubMed] [Google Scholar]
- 20.Habr-Gama A, Perez RO, Nadalin W, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90–99. doi: 10.1016/j.gassur.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Callender GG, Das P, Rodriguez-Bigas MA, et al. Local excision after preoperative chemoradiation results in an equivalent outcome to total mesorectal excision in selected patients with T3 rectal cancer. Ann Surg Oncol. 2010;17:441–447. doi: 10.1245/s10434-009-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: Results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 23.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 24.Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379–4386. doi: 10.1200/JCO.2007.11.9685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.