Abstract
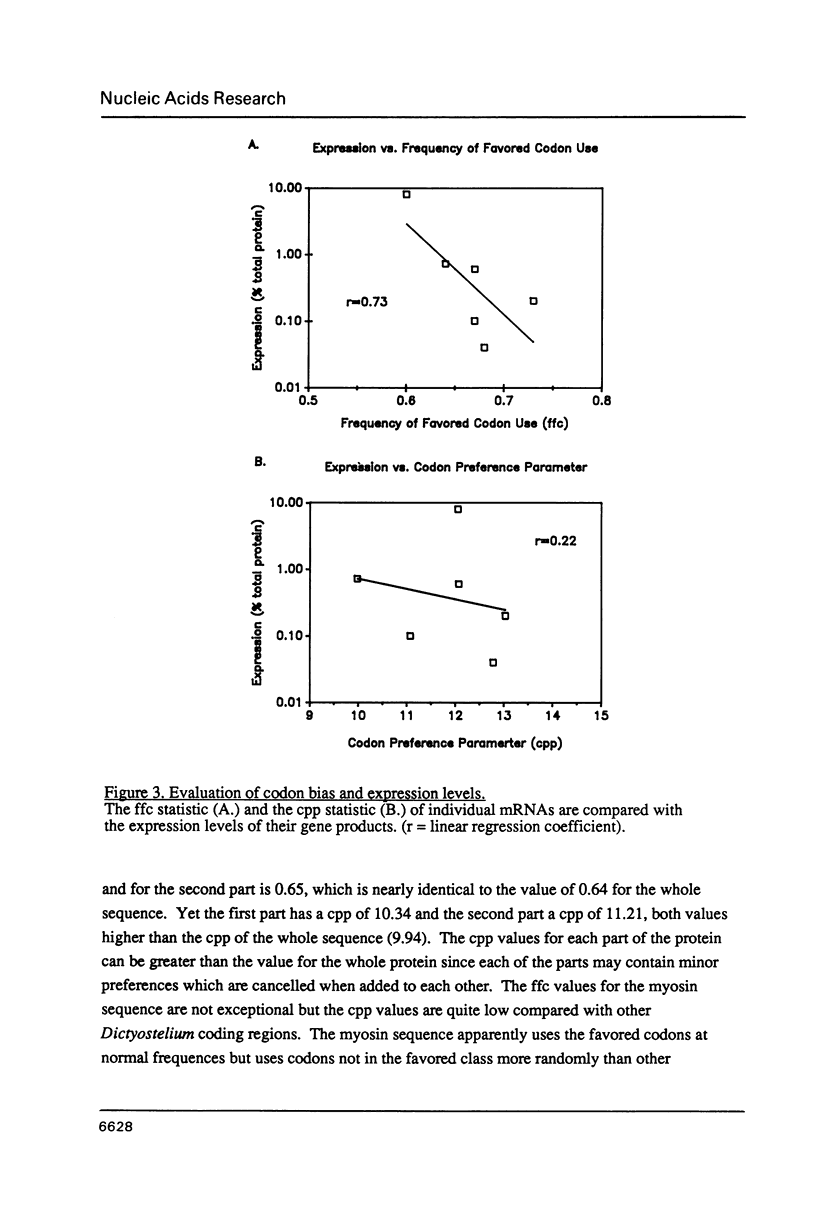
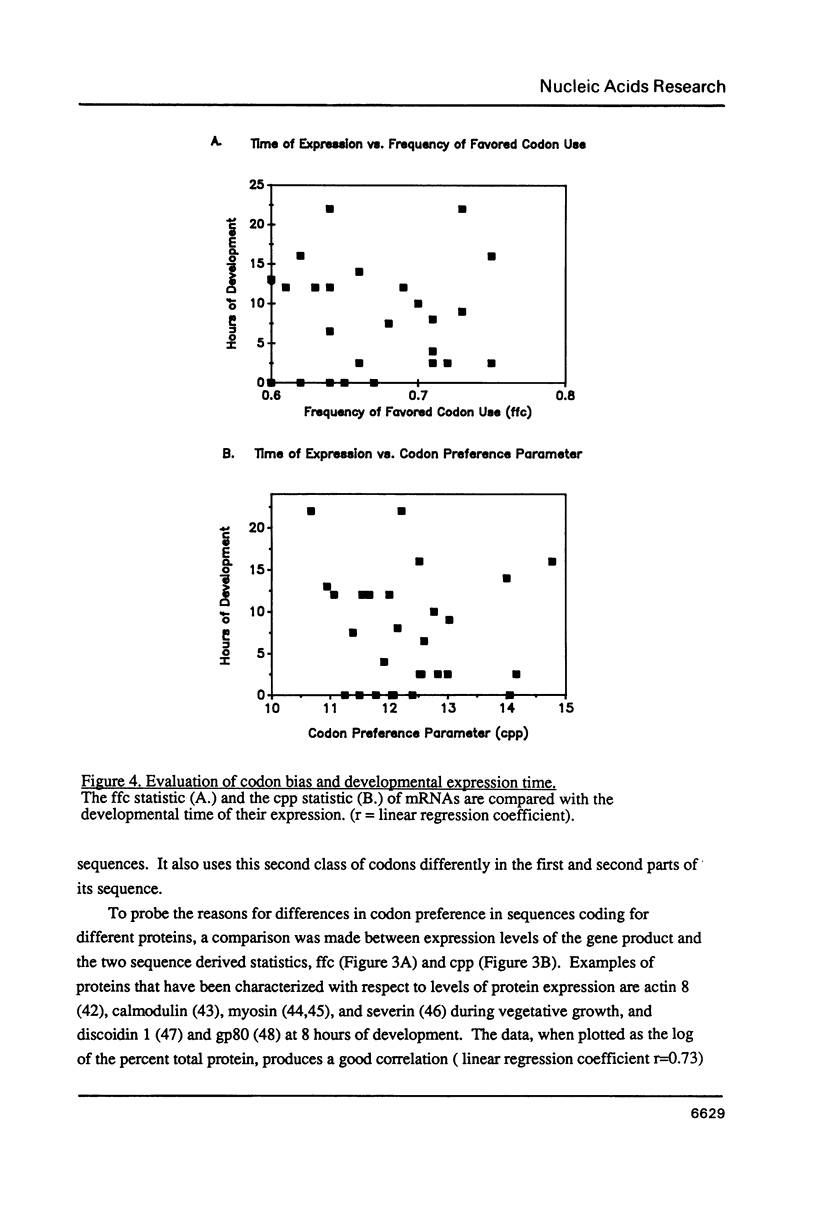
Dictyostelium discoideum is of increasing interest as a model eukaryotic cell because its many attributes have recently been expanded to include improved genetic and biochemical manipulability. The ability to transform Dictyostelium using drug resistance as a selectable marker (1) and to gene target by high frequency homologous integration (2) makes this organism particularly useful for molecular genetic approaches to cell structure and function. Given this background, it becomes important to analyze the codon preference used in this organism. Dictyostelium displays a strong and unique overall codon preference. This preference varies between different coding regions and even varies between coding regions from the same gene family. The degree of codon preference may be correlated with expression levels but not with the developmental time of expression of the gene product. The strong codon preference can be applied to identify coding regions in Dictyostelium DNA and aid in the design of oligonucleotide probes for cloning Dictyostelium genes.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André E., Lottspeich F., Schleicher M., Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988 Jan 15;263(2):722–727. [PubMed] [Google Scholar]
- Barklis E., Lodish H. F. Regulation of dictyostelium discoideum mRNAs specific for prespore or prestalk cells. Cell. 1983 Apr;32(4):1139–1148. doi: 10.1016/0092-8674(83)90297-0. [DOI] [PubMed] [Google Scholar]
- Barklis E., Pontius B., Barfield K., Lodish H. F. Structure of the promoter of the Dictyostelium discoideum prespore EB4 gene. Mol Cell Biol. 1985 Jun;5(6):1465–1472. doi: 10.1128/mcb.5.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barklis E., Pontius B., Lodish H. F. Structure of the Dictyostelium discoideum prestalk D11 gene and protein. Mol Cell Biol. 1985 Jun;5(6):1473–1479. doi: 10.1128/mcb.5.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bilofsky H. S., Burks C., Fickett J. W., Goad W. B., Lewitter F. I., Rindone W. P., Swindell C. D., Tung C. S. The GenBank genetic sequence databank. Nucleic Acids Res. 1986 Jan 10;14(1):1–4. doi: 10.1093/nar/14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy-Marcotte E., Vilaine F., Camonis J., Jacquet M. A DNA sequence from Dictyostelium discoideum complements ura3 and ura5 mutations of Saccharomyces cerevisiae. Mol Gen Genet. 1984;193(3):406–413. doi: 10.1007/BF00382076. [DOI] [PubMed] [Google Scholar]
- Cardelli J. A., Knecht D. A., Wunderlich R., Dimond R. L. Major changes in gene expression occur during at least four stages of development of Dictyostelium discoideum. Dev Biol. 1985 Jul;110(1):147–156. doi: 10.1016/0012-1606(85)90072-7. [DOI] [PubMed] [Google Scholar]
- Chisholm R. L., Rushforth A. M., Pollenz R. S., Kuczmarski E. R., Tafuri S. R. Dictyostelium discoideum myosin: isolation and characterization of cDNAs encoding the essential light chain. Mol Cell Biol. 1988 Feb;8(2):794–801. doi: 10.1128/mcb.8.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M., Bazari W. L., Kayman S. C. Isolation and properties of calmodulin from Dictyostelium discoideum. J Bacteriol. 1980 Jan;141(1):397–400. doi: 10.1128/jb.141.1.397-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. Isolation and characterization of myosin from amoebae of Dictyostelium discoideum. J Mol Biol. 1974 Jun 25;86(2):209–222. doi: 10.1016/0022-2836(74)90013-8. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll D. M., Williams J. G. Two divergently transcribed genes of Dictyostelium discoideum are cyclic AMP-inducible and coregulated during development. Mol Cell Biol. 1987 Dec;7(12):4482–4489. doi: 10.1128/mcb.7.12.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure M., Kalekine M., Boy-Marcotte E., Jacquet M. Developmental control of the expression of the dihydroorotate dehydrogenase and UMP synthase genes in Dictyostelium discoideum. Cell Differ. 1988 Jan;22(2):159–164. doi: 10.1016/0045-6039(88)90028-0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Bonner J. Characterization of the genome of the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1972 May 28;66(3):339–361. doi: 10.1016/0022-2836(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Giorda R., Ennis H. L. Structure of two developmentally regulated Dictyostelium discoideum ubiquitin genes. Mol Cell Biol. 1987 Jun;7(6):2097–2103. doi: 10.1128/mcb.7.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhagen H., Clarke M. Identification of the single gene for calmodulin in Dictyostelium discoideum. Mol Cell Biol. 1986 May;6(5):1851–1854. doi: 10.1128/mcb.6.5.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm G. H., Cameron G. N. The EMBL data library. Nucleic Acids Res. 1986 Jan 10;14(1):5–9. doi: 10.1093/nar/14.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L. Codon usage and gene expression. Nucleic Acids Res. 1986 Apr 11;14(7):3075–3087. doi: 10.1093/nar/14.7.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985 Jan;2(1):13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982 Jul 15;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Guilbaud R., Garreau H. Sequence analysis of the DdPYR5-6 gene coding for UMP synthase in Dictyostelium discoideum and comparison with orotate phosphoribosyl transferases and OMP decarboxylases. Mol Gen Genet. 1988 Mar;211(3):441–445. doi: 10.1007/BF00425698. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kalekine M., Boy-Marcotte E. Sequence analysis of a Dictyostelium discoideum gene coding for an active dihydroorotate dehydrogenase in yeast. Biochimie. 1985 Jun;67(6):583–588. doi: 10.1016/s0300-9084(85)80197-8. [DOI] [PubMed] [Google Scholar]
- Kelly L. J., Kelly R., Ennis H. L. Characterization of cDNA clones specific for sequences developmentally regulated during Dictyostelium discoideum spore germination. Mol Cell Biol. 1983 Nov;3(11):1943–1948. doi: 10.1128/mcb.3.11.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Intervening sequences in a Dictyostelium gene that encodes a low abundance class mRNA. Nucleic Acids Res. 1980 Dec 11;8(23):5599–5610. doi: 10.1093/nar/8.23.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Sequence organization in Dictyostelium: unique structure at the 5'-ends of protein coding genes. Nucleic Acids Res. 1983 Jan 25;11(2):541–552. doi: 10.1093/nar/11.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Sequence organization in Dictyostelium: unique structure at the 5'-ends of protein coding genes. Nucleic Acids Res. 1983 Jan 25;11(2):541–552. doi: 10.1093/nar/11.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Cohen S. M., Loomis W. F., Lodish H. F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986 Nov;6(11):3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli J., Grosjean H. Usage of the three termination codons: compilation and analysis of the known eukaryotic and prokaryotic translation termination sequences. Mol Gen Genet. 1981;182(3):430–439. doi: 10.1007/BF00293932. [DOI] [PubMed] [Google Scholar]
- Lacombe M. L., Podgorski G. J., Franke J., Kessin R. H. Molecular cloning and developmental expression of the cyclic nucleotide phosphodiesterase gene of Dictyostelium discoideum. J Biol Chem. 1986 Dec 25;261(36):16811–16817. [PubMed] [Google Scholar]
- Lam T. Y., Siu C. H. Synthesis of stage-specific glycoproteins in Dictyostelium discoideum during development. Dev Biol. 1981 Apr 15;83(1):127–137. doi: 10.1016/s0012-1606(81)80015-2. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Maniak M., Nellen W. A developmentally regulated membrane protein gene in Dictyostelium discoideum is also induced by heat shock and cold shock. Mol Cell Biol. 1988 Jan;8(1):153–159. doi: 10.1128/mcb.8.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. K., Firtel R. A. Cyclic AMP regulation of early gene expression in Dictyostelium discoideum: mediation via the cell surface cyclic AMP receptor. Mol Cell Biol. 1987 Jan;7(1):458–469. doi: 10.1128/mcb.7.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Gojobori T., Aota S., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1986;14 (Suppl):r151–r197. doi: 10.1093/nar/14.suppl.r151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockrin S. C., Spudich J. A. Calcium control of actin-activated myosin adenosine triphosphatase from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2321–2325. doi: 10.1073/pnas.73.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutzel R., Lacombe M. L., Simon M. N., de Gunzburg J., Veron M. Cloning and cDNA sequence of the regulatory subunit of cAMP-dependent protein kinase from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1987 Jan;84(1):6–10. doi: 10.1073/pnas.84.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Taubenberger A., Westphal M., Jaeger E., Noegel A., Gerisch G. Complete cDNA sequence of a Dictyostelium ubiquitin with a carboxy-terminal tail and identification of the protein using an anti-peptide antibody. FEBS Lett. 1988 Mar 14;229(2):273–278. doi: 10.1016/0014-5793(88)81139-6. [DOI] [PubMed] [Google Scholar]
- Nellen W., Datta S., Reymond C., Sivertsen A., Mann S., Crowley T., Firtel R. A. Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 1987;28:67–100. doi: 10.1016/s0091-679x(08)61637-4. [DOI] [PubMed] [Google Scholar]
- Noegel A., Gerisch G., Stadler J., Westphal M. Complete sequence and transcript regulation of a cell adhesion protein from aggregating Dictyostelium cells. EMBO J. 1986 Jul;5(7):1473–1476. doi: 10.1002/j.1460-2075.1986.tb04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. D., Miller M. J. Elsie 4: quantitative computer analysis of sets of two-dimensional gel electrophoretograms. Anal Biochem. 1988 Feb 15;169(1):49–70. doi: 10.1016/0003-2697(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Katz E. R. Isolation and characterization of transfer RNAs from Dictyostelium discoideum during growth and development. J Biol Chem. 1977 Jan 25;252(2):694–703. [PubMed] [Google Scholar]
- Poole S., Firtel R. A., Lamar E., Rowekamp W. Sequence and expression of the discoidin I gene family in Dictyostelium discoideum. J Mol Biol. 1981 Dec 5;153(2):273–289. doi: 10.1016/0022-2836(81)90278-3. [DOI] [PubMed] [Google Scholar]
- Ragheb J. A., Dottin R. P. Structure and sequence of a UDP glucose pyrophosphorylase gene of Dictyostelium discoideum. Nucleic Acids Res. 1987 May 11;15(9):3891–3906. doi: 10.1093/nar/15.9.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond C. D., Gomer R. H., Mehdy M. C., Firtel R. A. Developmental regulation of a Dictyostelium gene encoding a protein homologous to mammalian ras protein. Cell. 1984 Nov;39(1):141–148. doi: 10.1016/0092-8674(84)90199-5. [DOI] [PubMed] [Google Scholar]
- Robinson M., Lilley R., Little S., Emtage J. S., Yarranton G., Stephens P., Millican A., Eaton M., Humphreys G. Codon usage can affect efficiency of translation of genes in Escherichia coli. Nucleic Acids Res. 1984 Sep 11;12(17):6663–6671. doi: 10.1093/nar/12.17.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romans P., Firtel R. A. Organization of the actin multigene family of Dictyostelium discoideum and analysis of variability in the protein coding regions. J Mol Biol. 1985 Nov 20;186(2):321–335. doi: 10.1016/0022-2836(85)90108-1. [DOI] [PubMed] [Google Scholar]
- Romans P., Firtel R. A., Saxe C. L., 3rd Gene-specific expression of the actin multigene family of Dictyostelium discoideum. J Mol Biol. 1985 Nov 20;186(2):337–355. doi: 10.1016/0022-2836(85)90109-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Tuohy T. M., Mosurski K. R. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986 Jul 11;14(13):5125–5143. doi: 10.1093/nar/14.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu C. H., Lerner R. A., Ma G., Firtel R. A., Loomis W. F. Developmentally regulated proteins of the plasma membrane of Dictyostelium discoideum. The carbohydrate-binding protein. J Mol Biol. 1976 Jan 15;100(2):157–178. doi: 10.1016/s0022-2836(76)80146-5. [DOI] [PubMed] [Google Scholar]
- Staden R., McLachlan A. D. Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):141–156. doi: 10.1093/nar/10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel L. F., Jacobson A. Translational control of ribosomal protein synthesis during early Dictyostelium discoideum development. Mol Cell Biol. 1987 Mar;7(3):965–972. doi: 10.1128/mcb.7.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel L. F., Smyth A., Jacobson A. Nucleotide sequence and characterization of the transcript of a Dictyostelium ribosomal protein gene. Nucleic Acids Res. 1987 Dec 23;15(24):10285–10298. doi: 10.1093/nar/15.24.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- Sussman R., Rayner E. P. Physical characterization of deoxyribonucleic acids in Dictyostelium discoideum. Arch Biochem Biophys. 1971 May;144(1):127–137. doi: 10.1016/0003-9861(71)90462-0. [DOI] [PubMed] [Google Scholar]
- Uyemura D. G., Brown S. S., Spudich J. A. Biochemical and structural characterization of actin from Dictyostelium discoideum. J Biol Chem. 1978 Dec 25;253(24):9088–9096. [PubMed] [Google Scholar]
- Warrick H. M., De Lozanne A., Leinwand L. A., Spudich J. A. Conserved protein domains in a myosin heavy chain gene from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9433–9437. doi: 10.1073/pnas.83.24.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Schleicher M., Lottspeich F., Noegel A. Studies on the transcription, translation, and structure of alpha-actinin in Dictyostelium discoideum. J Cell Biol. 1986 Sep;103(3):969–975. doi: 10.1083/jcb.103.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Pardee J. D., Reidler J., Stryer L., Spudich J. A. Mechanism of interaction of Dictyostelium severin with actin filaments. J Cell Biol. 1982 Dec;95(3):711–719. doi: 10.1083/jcb.95.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]



