Abstract
Purpose
To investigate changes in bone mineral density (BMD) and fracture risk in men who received intermittent androgen deprivation (IAD) for nonmetastatic, hormone-sensitive prostate cancer.
Patients and Methods
Men with prostate cancer who lacked radiographically detectable metastases were treated in a prospective trial of IAD. After 9 months of treatment with leuprolide and flutamide, androgen deprivation therapy (ADT) was stopped until prostate-specific antigen reached a threshold (1 ng/mL for radical prostatectomy; 4 ng/mL for radiation or primary ADT) for a new cycle. Dual-energy x-ray absorptiometry (DXA) scans were performed before starting ADT and subsequently with each change in therapy. At least two consecutive DXA scans were required for this analysis. Computed tomography, bone scintigraphy, and lumbar spine x-rays were performed at the beginning and end of each treatment period.
Results
Fifty-six of 100 patients met criteria for this analysis. The median age at study entry was 64.5 years (range, 49.8 to 80.9 years). The average percentage change in BMD during the first on-treatment period was −3.4% (P < .001) for the spine and −1.2% (P = .001) for the left hip. During the first off-treatment period (median, 37.4 weeks; range, 13.4 weeks to 8.7+ years), BMD recovery at the spine was significant, with an average percentage change of +1.4% (P = .002). Subsequent periods had heterogeneous changes of BMD without significant average changes. After a median of 5.5 years (range, 1.1 to 13.8+) years on trial, one patient (1.8%) had a compression fracture associated with trauma.
Conclusion
Patients experienced the greatest average change in BMD during early treatment periods of IAD with a smaller average change thereafter. Fractures were rare.
INTRODUCTION
Androgen deprivation therapy (ADT), which is achieved through orchiectomy or gonadotropin-releasing hormone agonists or antagonists, is an established first-line therapy for patients with metastatic disease and is commonly used to treat nonmetastatic, biochemically recurrent prostate cancer.1–4 Men with biochemically recurrent disease alone may live for many years5 and experience long-term exposure to ADT. Prolonged ADT may lead to significant treatment-related toxicities, such as hot flashes, changes in sexual function and libido, hyperlipidemia, increased cardiac risk, and changes in body composition.6–8 ADT also reduces bone mineral density (BMD), which increases risk of developing osteoporosis and skeletal fractures in men with prostate cancer.9–12 In the first year of therapy, men who undergo ADT have a five- to 10-fold higher rate of bone loss, and risk of fracture increases with increasing duration of ADT.10,13 These factors are important contributors to the morbidity associated with ADT.
Because of issues with toxicity, along with cost and efficacy of continuous ADT, multiple randomized studies of intermittent androgen deprivation (IAD) versus continuous ADT have been performed.14,15 There have not been differences in efficacy, but there is evidence of less toxicity in patients treated with IAD. Recently, a large trial of IAD versus continuous ADT for men with biochemically recurrent prostate cancer after radiation revealed an equal overall survival, which suggested that IAD should be the standard of care for patients who are treated with ADT in this setting.14 Although quality-of-life data from this trial has only been partially presented, with IAD leading to improvements in hot flashes and sexual adverse effects,14 IAD has been shown to improve quality-of-life during the off-treatment periods in multiple other studies.16–18 There is, however, limited information on the potential for IAD to attenuate the loss of BMD or fractures. We explored long-term changes in BMD and fracture rates over the course of IAD for men with nonmetastatic, hormone-sensitive prostate cancer.
PATIENTS AND METHODS
Study Design
In 1996, a prospective trial of IAD for biochemically recurrent or locally advanced prostate cancer was initiated with study end points of BMD, lean body mass, weight, body mass index, and cognitive and psychological function.19 Key eligibility requirements included a histologic diagnosis of prostate cancer, previous therapy with radical prostatectomy or definitive radiation with at least two consecutive increases in prostate-specific antigen (PSA) ≥ 2 weeks apart, original American Urological Association stage A2-D1, no detectable metastasis by using a bone scan and computed tomography (CT) scan, Eastern Cooperative Oncology Group performance status 0 or 1 (ie, none or limited effect of disease on daily living abilities), and pretreatment testosterone levels greater than 100 ng/dL. Late enrollment was permitted for patients with biochemical relapse or localized disease who underwent primary ADT as long as the duration of therapy was less than 10 months. Patients could have received previous ADT for the neoadjuvant, adjuvant, or salvage setting as long as the ADT duration was ≤ 3 months and it was completed ≥ 1 year before the patient otherwise met eligibility criteria.
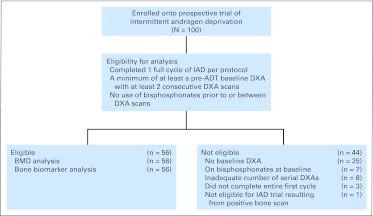
To be eligible for analysis, the patient must have had a baseline pre-ADT DXA and at least two consecutive DXAs at the spine and left hip. The patient also could not have been taking bisphosphonates before or at the time relevant DXA scans were obtained. As a result of late enrollment and other logistic factors, not all 100 patients in the IAD study were eligible for the BMD analysis (Fig 1).
Fig 1.
CONSORT diagram shows the rationale for selection of the 56 patients from the clinical trial for the analysis. ADT, androgen-deprivation therapy; BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; IAD, intermittent androgen deprivation; PSA, prostate-specific antigen.
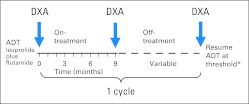
Before starting ADT, formal nutrition and physical therapy consultations were obtained to advise patients about optimal calcium and vitamin D intake, weight-loss strategies, and proper exercises to maintain BMD and prevent weight gain. Bone and CT scans were performed for the determination of eligibility, and a baseline dual-energy x-ray absorptiometry (DXA) scan was obtained when feasible. After all baseline studies and consultations were obtained, flutamide 250 mg three times daily was started approximately 2 weeks before the start of luteinizing hormone-releasing hormone agonist. Flutamide was continued, and patients were treated with a total of 9 months of combined ADT (Fig 2). At the end of the 9 months of therapy, new imaging studies, including bone, CT, and DXA scans were performed, and ADT was stopped provided that the PSA value was ≤ 1 ng/mL and not rising. When the PSA exceeded an arbitrary, prespecified threshold (1 ng/mL for radical prostatectomy; 4 ng/mL for radiation or primary ADT), a new cycle was initiated with another 9 months of ADT, and new imaging studies were repeated. PSA and testosterone levels were measured monthly, and bone biomarkers were measured every 3 months throughout IAD therapy. Each cycle of therapy consisted of a 9-month on-treatment period and a variable off-treatment period. The protocol was amended in 2002 to include lateral lumbar spine films. If a patient had a prolonged off-treatment period ≥ 1 year, DXA, CT, and bone scans were obtained annually. All patients continued cycling on and off therapy until the development of castration resistance, which was defined as at least two serial increases in PSA while undergoing ADT with a castrate level of testosterone less than 50 ng/dL.
Fig 2.
Study schema illustrates one full cycle of intermittent androgen deprivation. Cycles were repeated until the development of castration resistance or death. Dual-energy x-ray absorptiometry (DXA) scan at baseline with at least two consecutive DXA scans were required to be eligible for this analysis. (*) The prostate-specific antigen threshold for radiation therapy or primary androgen-deprivation therapy (ADT) was 4 ng/mL and for radical prostatectomy was 1 ng/mL.
Statistical Analysis Methods
The primary objective of the analysis was to determine the long-term effects of IAD on BMD and fracture risk. The mean change and average percentage change in BMD relative to baseline and the previous DXA measurement were reported at both the spine and left hip. Because DXA was measured by using both the Hologic-4500 (Hologic, Bedford, MA) and Lunar Prodigy (General Electric, Madison, WI) bone densitometers, spine and hip measurements were standardized by using methods that have been deemed suitable for population studies.20,21 Patients who eventually started bisphosphonate therapy were censored from the analysis at that time point.
We calculated averages and 95% CIs for absolute and percentage changes in spine and left-hip BMD across patients within each on- and off-treatment period. Changes in BMD across cycles are illustrated relative to baseline, but the statistical significance of changes in BMD across cycles were tested relative to previous DXA by using t tests with alternative hypotheses negative average change in BMD during on-treatment periods and positive average change in BMD during off-treatment periods.
We estimated the correlation between BMD and other important biomarkers by using multivariate linear mixed models. In each model, the multivariate response contained BMD (spine or left hip) and a biomarker (testosterone, urine N-telopeptide, or alkaline phosphatase) for a total of six models; each model included a patient-specific random intercept, and the cycle number and an indicator of on- or off-treatment were the only covariates. Models were estimated by using the R package MCMCglmm,22 and P values were derived by using a grid search of credible intervals from posterior samples.
RESULTS
Patient Characteristics
Between June 1996 and September 2006, 100 patients were accrued within the IAD trial (Fig 1). Fifty-six patients were eligible for this analysis. Of patients who were ineligible, 25 patients registered late and, therefore, did not have a baseline DXA, seven patients were taking bisphosphonates at baseline, eight patient did not have the required serial DXAs, three patients did not complete a full cycle of ADT (one patient chose to leave the study to pursue alternative therapies, one patient moved/transferred care before the end of the first on-treatment period, and one patient developed progressive disease), and one patient was ineligible as a result of the presence of metastatic disease at baseline.
Baseline patient characteristics of the 56 evaluable patients are listed in Table 1. The median age at study entry was 64.5 years (range, 49.8 to 80.9 years) with a median baseline spine BMD T score of 0.02 (range, −2.48 to 4.64) and a median baseline left-hip BMD T score of −0.14 (range, −2.53 to 2.70). The median number of ADT cycles completed was three (range, one to nine), and the median duration in the trial was 5.5 years (range, 1.1 to 13.8+ years). Fifteen patients were still in the trial with data censored on June 23, 2011. Eight patients were started on bisphosphonates while in the study during IAD (five patients started during the first off-treatment period, two patients started during the second off-treatment period, and one patient started during the fifth on-treatment period). The median time to the start of bisphosphonates for these eight patients was 13 months (range, 9 to 62 months).
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients |
|---|---|
| Age at study start, years | |
| 40-50 | 1 |
| 50-60 | 14 |
| 60-70 | 26 |
| 70-80 | 14 |
| 80-90 | 1 |
| Race | |
| White | 53 |
| African American | 2 |
| Asian | 1 |
| Primary treatment | |
| Radical prostatectomy | 40 |
| Radiation therapy | 13 |
| Primary androgen deprivation therapy | 3 |
| Baseline bone mineral density at spine, T score | |
| > 2.5 | 3 |
| 1.0-2.5 | 18 |
| 0.0-1.0 | 8 |
| −1.0 to 0.0 | 16 |
| −2.5 to −1.0 | 11 |
| Baseline bone mineral density at left hip, T score | |
| > 2.5 | 1 |
| 1.0-2.5 | 5 |
| 0.0-1.0 | 17 |
| −1.0 to 0.0 | 19 |
| −2.5 to −1.0 | 13 |
| < −2.5 | 1 |
| Baseline testosterone, ng/dL* | |
| 100-199 | 3 |
| 200-299 | 15 |
| 300-399 | 14 |
| 400-499 | 7 |
| 500-599 | 1 |
| 600-699 | 2 |
| Previous ADT | |
| None | 51 |
| Neoadjuvant | 2 |
| Adjuvant | 3 |
| Greatest No. of total cycles completed† | |
| 1-2 | 22 |
| 3-4 | 19 |
| 5-6 | 10 |
| 7-8 | 3 |
| 9-10 | 2 |
| Bisphosphonate use | |
| Added later during trial‡ | 8 |
| None | 48 |
| Calcium use | |
| In the study | 44 |
| None | 12 |
| Vitamin D use | |
| In the study | 49 |
| None | 7 |
Abbreviation: ADT, androgen deprivation therapy.
Forty-two of 56 patients had baseline testosterone levels measured.
Fifteen of 56 patients are still in the study.
Initiation of bisphosphonates while on trial was allowed based on treating provider discretion. Of the eight patients who eventually started bisphosphonates on trial, five patients were started for osteopenia, and three patients were started for osteoporosis.
Change in BMD Relative to Last DXA by ADT Cycle
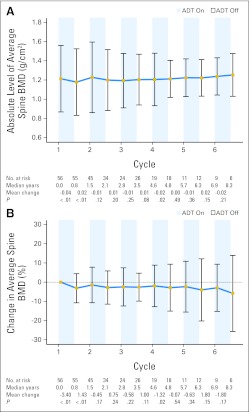
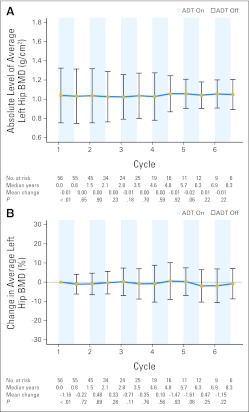
After the first 9 months of ADT, the absolute change in standardized average BMD was −0.04 g/cm2 (P < .001, Fig 3) at the spine and −0.01 g/cm2 (P = .001, Fig 4) at the left hip. The average percentage change in BMD from the first on-treatment period was −3.4% (P < .001) for the spine and −1.2% (P = .001) at the left hip. After the first off-treatment interval, the absolute change in standardized average BMD was +0.02 g/cm2 (P = .001) at the spine and −0.00 g/cm2 (P = .648) at the left hip. The average percentage change in BMD from the first off-treatment interval was +1.4% (P = .002) at the spine and −0.2% (P = .721) at the left hip. Therefore, only BMD recovery at the spine was statistically significant during the first off-treatment period. Other than cycle 4 at the spine (Fig 3), none of the subsequent cycles showed statistically significant changes in BMD at either site.
Fig 3.
Average and 95% CIs of (A) absolute and (B) percentage change in standardized average bone mineral density (BMD) relative to baseline at the spine are represented in the line graph over multiple cycles of intermittent androgen deprivation. Displayed below each graph are the number of patients at risk, median years from initiation of androgen deprivation therapy (ADT), mean change in spine BMD relative to last dual-energy x-ray absorptiometry scan, and P values from one-sided t tests.
Fig 4.
Average and 95% CIs of (A) absolute and (B) percentage change in standardized average bone mineral density (BMD) relative to baseline at the left hip are represented in the line graph over multiple cycles of intermittent androgen deprivation. Displayed below each graph are the number of patients at risk, median years from initiation of androgen deprivation therapy (ADT), mean change in left-hip BMD relative to last dual-energy x-ray absorptiometry scan, and P values from one-sided t tests.
BMD Changes During On- and Off-Treatment Periods
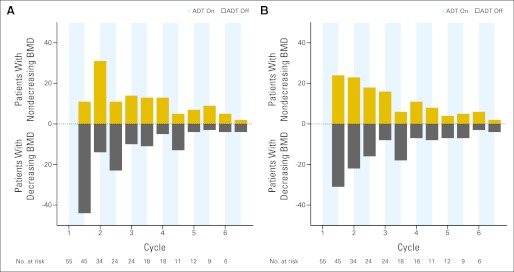
During the first on-treatment period, 44 of 55 patients (80.0%) and 31 of 55 patients (56.4%) experienced a decrease in BMD in the spine and left hip, respectively. During the first off-treatment period, 30 of 45 patients (66.7%) and 21 of 45 patients (46.7%) experienced an increase in BMD at the spine and left hip, respectively. In subsequent cycles, there was more variability in the BMD response to on- or off-treatment periods (Fig 5). The average response to ADT on- and off-treatment relative to baseline for each cycle is also shown in Figures 3 and 4.
Fig 5.
Number of patients with decreasing or nondecreasing (either stable or increasing) bone mineral density (BMD) between consecutive dual-energy x-ray absorptiometry scans at the beginning of on- or off-treatment periods at the (A) spine or (B) left hip demonstrate the heterogeneity of patient BMD response to androgen deprivation therapy (ADT).
Conversion to and From Normal (T score > −1.0), Osteopenia (T score from −1.0 to 2.5), and Osteoporosis (T score < −2.5)
Of the 56 eligible patients, 38 patients (67.9%) had a baseline DXA BMD within a normal range both at the spine and left hip. Of these 38 patients, five patients (13.2%) developed osteopenia during IAD (three patients developed osteopenia at the spine, one patient developed osteopenia at the left hip, and one patient developed osteopenia at both sites). Of these five patients, two patients were eventually administered an oral bisphosphonate 1.1 and 5.2 years from the initiation of ADT. None of these five patients developed frank osteoporosis over a median of 8.0 years (range, 6.1 to 13.7 years) in the study.
Osteopenia was present in 17 of 56 patients (30.4%) at baseline (four patients had osteopenia at the spine, seven patients had osteopenia at the left hip, and six patients had osteopenia at both sites). Interestingly, the BMD of four of 17 patients (23.5%) normalized during IAD (all in the left hip) without bisphosphonates. Of the remaining 13 patients with baseline osteopenia, four patients were eventually started on bisphosphonates after a median of 1.0 years (range, 36.5 weeks to 2.4 years) from the initiation of ADT. One of these four patients developed osteoporosis despite having received oral bisphosphonate therapy 2.4 years from the start of ADT.
Only one of 56 patients (1.8%) had baseline osteoporosis at the left hip with osteopenia at the spine. The patient was started on bisphosphonates while on IAD, with resulting improvement of his left-hip osteoporosis to osteopenia, but osteoporosis developed later at the spine despite antiresorptive therapy.
Fracture Data
All CT scans, bone scans, and lumbar spine x-rays (available after amendment in 2002) were analyzed for radiographic evidence of fracture for all patients while in the study. Twenty-five of 56 patients (44.6%) had at least one lumbar spine x-ray while in the study. After a median duration within the study of 5.5 years (range, 1.1 to 13.8+ years), one patient (1.8%) with normal BMD had a compression fracture of T12 after a fall from a ladder.
Testosterone and Bone Biomarker Analysis
Median baseline levels of testosterone, urine N-telopeptide, and alkaline phosphatase were all within normal limits and were 320 ng/dL (range, 130 to 660 ng/dL), 26 nM bone collagen equivalents/mM (range, 10 to 45 nM bone collagen equivalents/mM), and 66 μg/L (range, 45 to 120 μg/L), respectively. The median time to testosterone recovery to greater than 50 ng/dL was 91 days (range, 0 to 308 days) and to greater than 100 ng/dL was 110 days (range, 49 to 343 days). Six of 45 patients (13.3%) with consistent off-cycle testosterone measurements maintained castrate testosterone levels without recovery during any off cycle, and nine of 45 patients (20.0%) maintained testosterone levels less than 100 ng/dL during any off cycle; thus, most patients resumed testosterone production even after many cycles into IAD. A change in spine BMD was positively correlated with a change in testosterone (correlation, 0.18 [95% CI, 0.04 to 0.27]; P = .009) and negatively correlated with a change in urine N-telopeptide (correlation, −0.31 [95% CI, −0.65 to −0.07]; P = .005) after the cycle number and on- or off-treatment period were controlled for. The change in left hip BMD was similarly positively correlated with a change in testosterone (correlation, 0.16 [95% CI, 0.04 to 0.26]; P = .026) but not with a change in urine N-telopeptide (correlation, 0.007 [95% CI, −0.26 to 0.153]; P = .966). Neither a change in spine nor change in left hip BMD was correlated with a change in alkaline phosphatase.
DISCUSSION
To our knowledge, this study is the first to evaluate the dynamics of BMD on IAD by looking at DXA scans relative to the treatment cycles of IAD. Instead of measuring BMD at baseline and predetermined time points after the initiation of ADT, as was done in the only other report of BMD changes after IAD,23 DXA scans were performed at the beginning of each on- and off-treatment period to more directly assess the impact of testosterone depletion and recovery on BMD changes.
Patients administered IAD have a net improved quality of life with fewer hot flashes and reduced sexual adverse effects.14,16–18 However, there is currently no definitive information on the long-term effects of IAD on BMD, which is a strong predictor of fracture risk.24 On the basis of this trial, patients who were able to remain on IAD for numerous cycles eventually experienced stabilization in BMD with long-term BMD levels that seemed to fall just slightly below baseline. The median period within the study for our patients was 5.5 years (range, 1.1 to 13.8+ years), and during this entire period, there was only one fracture associated with trauma in a patient with normal BMD. Although these trials cannot be directly compared, another study of men without metastatic disease who received continuous ADT reported a fracture rate of 3.9% (in the placebo arm) at 3 years in 673 patients.25 However, most patients were not followed from the initiation of ADT because the median time of ADT before study entry was 20.4 months.
Although we hypothesized that BMD would decline during ADT and increase during testosterone recovery in the off-treatment period, our data showed substantial heterogeneity. This heterogeneity was likely due to a delayed response in DXA scans relative to hormonal changes in either direction. Although the DXA scan shows the actual BMD at that time point, it does not necessarily reflect the actual biology that underlies any changes. Changes in BMD during the off cycles are a function of the time to recovery of testosterone and, hence, estrogen. Thus, changes in BMD, as measured by DXA, may not accurately reflect the real-time biology of the bone at that time point. This is one potential reason why significant changes in BMD in response to on- and off-treatment periods are only observed during cycle 1.
At baseline, all but five patients received consultations with our nutrition and physical therapy services. Patients were counseled on calcium and vitamin D intake on the basis of their individual dietary habits, although compliance logs were not part of the protocol. Despite physical therapy consultations, most patients did not report compliance with resistance and other recommended exercises. It is doubtful, although possible, that differences in patient compliance with these interventions may have contributed to the heterogeneity of BMD changes in response to ADT. It is also important to note that variability of BMD change in response to ADT was present even during cycle 1. Individual biologic and/or environmental factors may have led to this degree of heterogeneity. For instance, the median age of the patients in this trial was 64.5 years (range, 49.8 to 80.9 years); it is possible that an older patient population may have experienced a more uniform loss of BMD.
Other reasons that statistically significant changes in BMD were observed only during cycle 1 may exist. Because patients dropped out of the study as a result of development of castration resistance, fewer patients remained in the study, which decreased the statistical power. It is also possible that patients lose the most bone during early stages of ADT, and changes in BMD over time stabilize regardless of whether patients receive IAD or continuous ADT. This assumption, however, was not supported by Wadwha et al,26 who found that continuous ADT led to persistent annual decreases in BMD over a 7-year period.26 Finally, it is possible that IAD is an intervention that minimizes BMD loss over time.
Concerns over BMD loss and osteoporotic fractures have prompted numerous intervention studies to maintain BMD for men who receive ADT. This maintenance includes the use of bisphosphonates such as alendronate,27,28 pamidronate,29 and zoledronic acid.30,31 Denosumab, which is a receptor activator of nuclear factor kappa-B ligand inhibitor, increases BMD and decreases risk of osteoporotic fractures in patients who receive ADT.25 In September 2011, the US Food and Drug Administration granted approval of denosumab at a dose of 60 mg subcutaneously every 6 months to prevent osteoporotic fracture and loss of BMD for men who receive ADT. Whether denosumab would have a significant impact in men treated with IAD is unknown.
Although the results demonstrated that patients who were treated with IAD experienced a reasonably stable fluctuation in BMD that was slightly below average baseline measurements and did not have an increase in fracture risk, the patient numbers were small. Fracture risk may be better defined in the phase III cooperative group trial of intermittent versus continuous therapy in men treated with biochemically recurrent, nonmetastatic disease after treatment with radiation. In our trial, changes in BMD were unpredictable in the individual patient, and hence, continued monitoring with DXA scans is advised.
Acknowledgment
We thank Charles H. Chesnut III, MD, and Ruth Etzioni, PhD, for their insightful comments and Nigel and Annette Southey for organizing Drive Fore the Cure Northwest.
Footnotes
Supported in part by unrestricted educational grants from Integrated Therapeutics (Schering), TAP Pharmaceuticals, the Pacific Northwest Prostate Cancer SPORE (Grant No. NCI P50 CA097186), and the Prostate Cancer Foundation and a donation from Drive Fore the Cure Northwest.
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, June 4-8, 2010; the 38th Annual Meeting of ASCO, Orlando, FL, May 18-21, 2002; and the 3rd North American Symposium on Skeletal Complications of Malignancy, Bethesda, MD, April 24-26, 2002.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00223665.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Evan Y. Yu, Amgen (C); Celestia S. Higano, Amgen (C) Stock Ownership: None Honoraria: Evan Y. Yu, Amgen; Celestia S. Higano, Amgen Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Evan Y. Yu, Peter Y. Jiang, Celestia S. Higano
Financial support: Evan Y. Yu, Celestia S. Higano
Administrative support: Celestia S. Higano
Provision of study materials or patients: Evan Y. Yu, Peter Y. Jiang, Celestia S. Higano
Collection and assembly of data: Evan Y. Yu, Kevin F. Kuo, Teresa E. Gambol, Suzanne P. Hall, Peggy Pitzel, Celestia S. Higano
Data analysis and interpretation: Evan Y. Yu, Kevin F. Kuo, Roman Gulati, Shu Chen, Celestia S. Higano
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 3.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 4.Shahinian VB, Kuo YF, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M. Adjuvant hormonal treatment with radiotherapy for locally advanced prostate cancer. Eur Urol. 1999;35(suppl 1):23–25. discussion 26. [PubMed] [Google Scholar]
- 6.Haseen F, Murray LJ, Cardwell CR, et al. The effect of androgen deprivation therapy on body composition in men with prostate cancer: Systematic review and meta-analysis. J Cancer Surviv. 2010;4:128–139. doi: 10.1007/s11764-009-0114-1. [DOI] [PubMed] [Google Scholar]
- 7.Schwandt A, Garcia JA. Complications of androgen deprivation therapy in prostate cancer. Curr Opin Urol. 2009;19:322–326. doi: 10.1097/MOU.0b013e32832a082c. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR. Androgen deprivation therapy and risk for diabetes and cardiovascular disease in prostate cancer survivors. Curr Urol Rep. 2008;9:197–202. doi: 10.1007/s11934-008-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69:500–504. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Boyce SP, Moyneur E, et al. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–139. doi: 10.1016/S0022-5347(05)00033-9. discussion 139. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 13.Greenspan SL, Coates P, Sereika SM, et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 14.Crook JM, O'Callaghan CJ, Ding K, et al. A phase III randomized trial of intermittent versus continuous androgen suppression for PSA progression after radical therapy (NCIC CTG PR. 7/SWOG JPR. 7/CTSU JPR. 7/UK Intercontinental Trial CRUKE/01/013) J Clin Oncol. 2011;29(suppl) abstr 4514. [Google Scholar]
- 15.de Leval J, Boca P, Yousef E, et al. Intermittent versus continuous total androgen blockade in the treatment of patients with advanced hormone-naive prostate cancer: Results of a prospective randomized multicenter trial. Clin Prostate Cancer. 2002;1:163–171. doi: 10.3816/cgc.2002.n.018. [DOI] [PubMed] [Google Scholar]
- 16.Bruchovsky N, Klotz L, Crook J, et al. Quality of life, morbidity, and mortality results of a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen relapse after radiation therapy for locally advanced prostate cancer. Clin Genitourin Cancer. 2008;6:46–52. doi: 10.3816/CGC.2008.n.008. [DOI] [PubMed] [Google Scholar]
- 17.Sato N, Akakura K, Isaka S, et al. Intermittent androgen suppression for locally advanced and metastatic prostate cancer: Preliminary report of a prospective multicenter study. Urology. 2004;64:341–345. doi: 10.1016/j.urology.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Spry NA, Kristjanson L, Hooton B, et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur J Cancer. 2006;42:1083–1092. doi: 10.1016/j.ejca.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 19.Yu EY, Gulati R, Telesca D, et al. Duration of first off-treatment interval is prognostic for time to castration resistance and death in men with biochemical relapse of prostate cancer treated on a prospective trial of intermittent androgen deprivation. J Clin Oncol. 2010;28:2668–2673. doi: 10.1200/JCO.2009.25.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui SL, Gao S, Zhou XH, et al. Universal standardization of bone density measurements: A method with optimal properties for calibration among several instruments. J Bone Miner Res. 1997;12:1463–1470. doi: 10.1359/jbmr.1997.12.9.1463. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Fuerst T, Hui S, et al. Standardization of bone mineral density at femoral neck, trochanter and Ward's triangle. Osteoporos Int. 2001;12:438–444. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 22.Hadfield J. MCMC Methods for multi-response generalized linear mixed models: The MCMCglmm R package. J Stat Softw. 2010;33:1–22. [Google Scholar]
- 23.Spry NA, Galvão DA, Davies R, et al. Long-term effects of intermittent androgen suppression on testosterone recovery and bone mineral density: Results of a 33-month observational study. BJU Int. 2009;104:806–812. doi: 10.1111/j.1464-410X.2009.08458.x. [DOI] [PubMed] [Google Scholar]
- 24.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadhwa VK, Weston R, Mistry R, et al. Long-term changes in bone mineral density and predicted fracture risk in patients receiving androgen-deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU Int. 2009;104:800–805. doi: 10.1111/j.1464-410X.2009.08483.x. [DOI] [PubMed] [Google Scholar]
- 27.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: A randomized trial. Ann Intern Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 28.Planas J, Trilla E, Raventós C, et al. Alendronate decreases the fracture risk in patients with prostate cancer on androgen-deprivation therapy and with severe osteopenia or osteoporosis. BJU Int. 2009;104:1637–1640. doi: 10.1111/j.1464-410X.2009.08622.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 30.Israeli RS, Rosenberg SJ, Saltzstein DR, et al. The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer. 2007;5:271–277. doi: 10.3816/CGC.2007.n.003. [DOI] [PubMed] [Google Scholar]
- 31.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]