Abstract
Purpose.
To compare the anterior chamber area/volume (ACA/ACV) and their relationship with the drainage angle between adult Caucasians and Chinese.
Methods.
Study groups were comprised of four age- and sex-matched cohorts: American Caucasians, American Chinese, southern mainland Chinese, and northern mainland Chinese. All subjects were consecutively recruited from general ophthalmology clinics except for southern mainland Chinese participants who were drawn from an ongoing population-based study. Anterior segment optical coherence tomography (ASOCT) images were obtained under dark conditions. Customized software was used to analyze structural indices including ACA/ACV, angle opening distance (AOD), anterior chamber depth (ACD), anterior chamber width (ACW), lens vault (LV), corneal arc depth (CAD), iris thickness (IT), iris curvature (ICurv), and iris area (IArea).
Results.
Data from 121, 124, 121, and 120 participants were obtained of American Caucasians, American Chinese, and southern and northern mainland Chinese, respectively. After multiple linear regression analysis, adjusting for age, sex, pupil diameter (PD), and axial length (AL), ACA/ACV was positively associated with ACD, ACW, CAD, and corneal radius of curvature (CR) but negatively related with ICurv and IArea. Ethnic Chinese had significantly smaller ACA (β = −0.18, P = 0.022) and ACV (β = −3.9, P = 0.001) than Caucasians. ACV contributes the most to AOD variation for both Chinese (standardized regression coefficient [SRC] = 0.47, P < 0.001) and Caucasians (SRC = 0.59, P < 0.001).
Conclusions.
Compared with Caucasians, ethnic Chinese had smaller ACA/ACV independent of ACD, ACW, ICurv, IArea, PD, CR, and AL. ACA/ACV is the most prominent contributor to angle width variation for both Chinese and Caucasians in this study.
ACA/ACV is the most prominent contributor to angle width in both Chinese and Whites. Chinese tended to have smaller ACA/ACV than did whites. Anterior chamber depth, anterior chamber width, and iris curvature are the most important factors associated with ACA/ACV.
Introduction
The well-known higher prevalence of primary angle closure (PAC) in East Asians as compared with Europeans and Africans provides an opportunity to explore the etiology of this disease through comparing possible risk factors for angle closure across racial groups.1 Drainage angle width is the anatomic characteristic most relevant to the risk for primary angle closure glaucoma (PACG). With the emergence of anterior segment optical coherence tomography (ASOCT), a fast, noncontact method for imaging the anterior ocular segment, several innovative anterior segment anatomic parameters such as iris thickness (IT),2 iris curvature (ICurv),3 anterior chamber width (ACW),4 and lens vault (LV)5 have been developed and shown to be useful for angle closure screening due to their close association with angle width. Furthermore, multiethnic studies among subjects free of angle closure diseases indicated that Chinese tend to have thicker peripheral irides6 and smaller ACW7 than that of age- and sex-matched Caucasians even after controlling for refractive status and axial length, suggesting a causative relationship between these anatomic factors and PAC development.
By using a best-predictive model, a population-based study of Singaporean Chinese reported that among all the established and newly identified associated factors of angle closure, anterior chamber area (ACA), anterior chamber volume (ACV), and LV are the three most significant determinants of angle width.8 In this study, our aim was to evaluate the difference in ACA/ACV and their predictors, as measured by ASOCT, between American Caucasians, American Chinese, and mainland Chinese. The contribution of various anterior structural parameters, particularly ACA/ACV, to angle width was also analyzed and compared between racial groups. Results of this study may provide insights into the understanding of mechanisms for narrow angle development from an ethnicity-specific perspective, and therefore give helpful information for ophthalmologists in the clinical prevention and treatment of angle closure-related diseases.
Materials and Methods
Subjects
Institutional Review Board/Ethics Committee approval was obtained from the University of California, San Francisco (UCSF), Zhongshan Ophthalmic Center in Guangzhou, and Peking University Eye Center in Beijing. This study adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). Informed consent was obtained for all individuals who participated in the study.
Four cohorts comprised the study sample. These were American Caucasians and American Chinese residing in San Francisco, southern mainland Chinese residing in Guangzhou, and northern mainland Chinese residing in Beijing. The subject enrollment period was from May 2008 through December 2010. Each cohort was designed to comprise 120 subjects, including 30 people (15 of each sex) in each of the fifth to eighth decades of life. Aside from the Guangzhou site, all subjects were consecutively recruited from general ophthalmology clinics in SF and Beijing. Participants at the Guangzhou site were drawn from an ongoing population-based study. All the participants were recruited with no prior assessment of their angle structures. Inclusion criteria included: (1) age between 40 and 80 years; (2) self-reported Caucasian or Chinese ancestry for both parents (the term “Caucasian” for the purposes of this study included only European-derived white people); and (3) willingness and ability to participate. Exclusion criteria included: (1) bilateral pseudophakia or aphakia or any prior intraocular surgery or laser treatment with the potential to alter natural anterior segment anatomy; (2) corneal or conjunctival abnormalities precluding an adequate view of the anterior chamber on ASOCT imaging; (3) use of any glaucoma medications; (4) active ocular infection in which contact exams might be contraindicated; and (5) high refractive error defined as spherical equivalent (SE) less than −8 or greater than +4.
Image Acquisition
ASOCT imaging was performed in a standard dark room (<1 lux illumination by digital light meter; Easy View model EA30; Extech Instruments, Inc., Waltham, MA). Patients were allowed 5 minutes for dark adaptation before image acquisition. Refractive correction was entered into the program to ensure nonaccommodative status of the tested eye. The fixation angle was adjusted to align the irides of the temporal and nasal quadrants on a horizontal level. The “anterior segment single” mode was used to acquire an image centered over the pupil on the horizontal meridian. Proper eye alignment was indicated by the occurrence of an interference beam along the visual axis. After imaging was complete, a single picture of the best quality judged by the optimal visibility of both scleral spurs (SS) was selected and saved.
Image Analysis
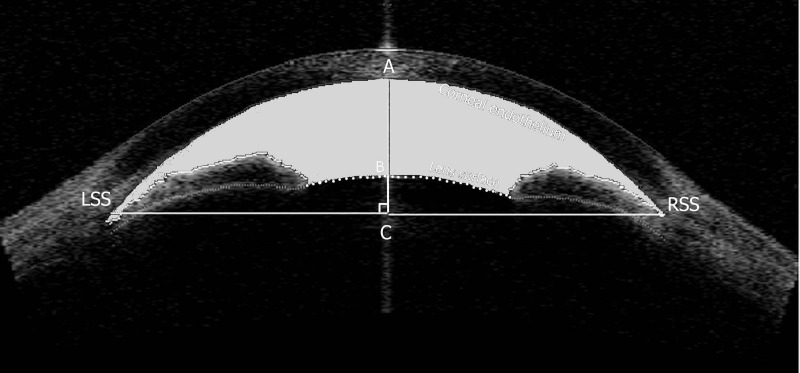
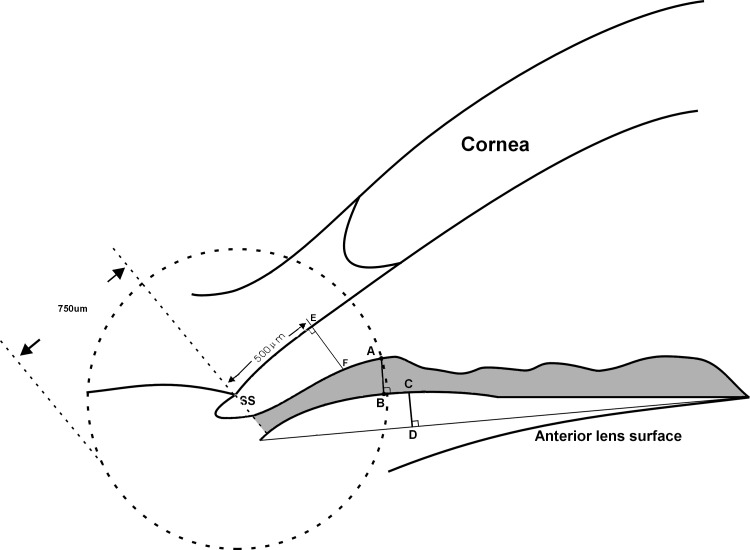
Custom software was used for image analysis.9 After the user manually identifies the left (LSS) and right scleral spurs (RSS) in the image, the algorithm automatically delineates the surfaces of the cornea, irides, and lens. The definitions of anterior segment indices have been described in detail elsewhere.6,7,10 In brief, ACA is defined as the cross-sectional area of the anterior segment bounded by the corneal endothelium, anterior surface of the iris, and anterior surface of the lens (within the pupil) (gray area in Fig. 1). A vertical axis through the midpoint (center) of the ACA was plotted by the program, and ACV was calculated by rotating the ACA 360° around this vertical axis. ACW was calculated as the distance between the LSS and the RSS (Fig. 1). Along the bisector of ACW (AC in Fig. 1), anterior chamber depth (ACD) was calculated as the length of AB. LV was calculated as the length of BC, which indicated the part of the lens above the ACW line. Corneal arc depth (CAD) was calculated as the length of AC, indicating the relative location of the inter-SS line to the apex of the corneal endothelium arc. Pupillary diameter (PD) was calculated as the distance between the pupillary tips of the irides on both sides on the cross-sectional image. The iris thickness was measured at 750 μm anterior to the SS (IT) as the shortest distance between anterior and posterior iris surfaces (AB in Fig. 2). Iris curvature (ICurv) was determined by creating a line from the most peripheral to the pupillary edge of the iris and then measuring the perpendicular distance from this line to the greatest convexity point along the posterior iris surface (CD in Fig. 2). Iris area (IArea) was calculated as the cumulative cross-sectional area of the full length of iris (gray area in Fig. 2). Angle opening distance (AOD500) was defined as the length of a line drawn from the anterior iris to the corneal endothelium perpendicular to a line along the trabecular meshwork at 500 μm from the SS (EF in Fig. 2). An average of the temporal and nasal iris parameters was determined for each eye.
Figure 1. .
Schematic figure illustrating anterior segment biometric parameters measurement. Distance between LSS and RSS was defined as ACW. The perpendicular bisector of the ACW line intersected with corneal endothelium, anterior lens surface, and ACW line at points A, B, and C, respectively. The length of AB represents the anterior chamber depth, BC represents lens vault, and AC represents corneal arc depth. The gray area denotes ACA.
Figure 2. .
Definition of angle- and iris-related parameters. Angle opening distance (AOD500) is measured on a line perpendicular to the plane of the trabecular surface 500 μm anterior to the SS and extended to meet the surface of the iris (length of EF). The iris thickness was measured at 750 μm anterior to the SS (IT750) as the shortest distance between anterior and posterior iris surfaces (length of AB). ICurv was determined by creating a line from the most peripheral to the pupillary edge of the iris and then measuring the perpendicular distance from this line to the greatest convexity point along posterior iris surface (length of CD). IArea is the area within the iris contour bordered by a line through the SS and perpendicular to the meshwork line (gray area).
Images from all study sites were analyzed by a single grader (DW) who was masked with regard to the subjects' demographic or clinical data. As multiple images were acquired according to the study protocol to account for angle status at different time points during dark–light transition, in the rare cases when SS were not completely discernable in the image acquired in the dark, images taken at other time points were referred to aid SS location. Images of 15 subjects randomized from each cohort were collected for intraobserver repeatability testing. All of these images were analyzed once again 2 weeks after the initial measurement by the same observer. The test–retest intraclass correlation coefficients (ICCs) for ACA and ACV were 97% and 95%, respectively.
Slit-lamp (Model BM900; Haag-Streit, Bern, Switzerland) gonioscopy was performed on both eyes under standardized dark room conditions (0–1 lux by light meter; Easy View model EA30, Extech Instruments) using a four-mirror gonioprism (Carl Zeiss, Chester, VA) at American sites and one-mirror Goldmann gonioprism (Ocular Instruments, Inc., Redmond, WA) at Chinese sites at ×25 magnification. An autorefractor (Automatic Refractor/Keratometer, Model 599; Carl Zeiss Meditec, Dublin, CA) was used to measure noncycloplegic refraction and corneal radius of curvature (CR). All raw refractive data were converted to spherical equivalent (SE, sphere + ½ of cylinder) for analysis. Axial length (AL) was measured by A-scan biometry (E-Z Scan A/B 5500+; Sonomed, Inc., Lake Success, NY).
Statistical Analysis
Data from the right eye were used for analysis. The left eye's data were used in the circumstances that only the left eye met the eligibility criteria. For comparisons across cohorts, ANCOVAs were applied for continuous data, and χ2 was used for proportion data. Participants diagnosed with PAC or PACG after the study examinations were excluded from data analysis since peripheral anterior synechiae (PAS) can lead to incorrect assessments of angle- and iris-related parameters. According to the International Society for Geographical and Epidemiological Ophthalmology standards, PAC was defined as eyes with pigmented trabecular meshwork not visible in ≥ three quadrants gonioscopically (occludable eye) with PAS or IOP > 21 mm Hg. PACG was defined as PAC eyes with evidence of glaucomatous optic nerve damage.11 Multiple linear regression models were constructed to assess the independent risk factors of ACA/ACV. Standardized regression coefficients (SRCs) were estimated to convert all independent variables to a standard distribution (mean = 0, SD = 1) and therefore equalize the scale of the independent variables in the multiple regression model. Variance inflation factor (VIF) was calculated to test potential multicollinearity among the independent variables in multiple linear regressions. R2 was calculated to evaluate the adequacy of multiple regression models. To avoid an inflated type I error rate, the Bonferroni-adjusted approach was used for multiple intergroup comparisons and linear regressions in the study. A value of P < 0.05 was considered statistically significant. Data analyses were performed using a commercial package (Stata 10.0; Stata Corp., College Station, TX).
Results
For the American Caucasian, American Chinese, and both southern and northern mainland Chinese cohorts, there were 121, 124, 121, and 120 subjects enrolled, with a mean age of 59.8 ± 11.7, 59.6 ± 12.0, 59.9 ± 11.7, and 58.5 ± 10.7 years (P for ANOVA = 0.782), respectively. None of the participants was found to have PAC or PACG in all four groups. The refractive errors were −0.5 ± 1.8, −0.8 ± 1.7, 0.1 ± 1.4, and 0.2 ± 1.4 diopters (P for ANOVA = 0.516), respectively. Data from the right eyes were collected except for 9 (7.4%), 5 (4.0%), 7 (5.8%), and 6 (5%) participants in each group, respectively, in whom the data were collected in the eligible left eyes because the right eye was ineligible. The age distribution of ACA/ACV in dark conditions for the four groups is summarized in Table 1. Both ACA and ACV were found to decrease with advanced age in each group (P < 0.05). Females tended to have smaller ACA/ACV than that of males in all the Chinese groups, whereas this sex difference was not identified in Caucasians.
Table 1. .
Distribution of Anterior Chamber Area (mm2) and Anterior Chamber Volume (mm3) in Dark Conditions by Age and Sex among American Caucasians, American Chinese, and Southern and Northern Mainland Chinese (mean ± SD)
|
American Caucasians |
American Chinese |
Southern Chinese |
Northern Chinese |
|||||||||
|
Age, y |
n |
ACA |
ACV |
n |
ACA |
ACV |
n |
ACA |
ACV |
n |
ACA |
ACV |
| 40–50 | 30 | 25.6 ± 3.4 | 188.1 ± 33.0 | 30 | 22.6 ± 3.3 | 153.5 ± 27.3 | 30 | 21.3 ± 4.0 | 143.9 ± 34.0 | 30 | 22.4 ± 2.9 | 152.3 ± 27.4 |
| 50–60 | 30 | 25.4 ± 4.3 | 185.0 ± 41.3 | 32 | 20.7 ± 4.6 | 142.1 ± 39.6 | 31 | 19.9 ± 3.7 | 133.3 ± 32.1 | 30 | 20.6 ± 2.7 | 136.1 ± 23.1 |
| 60–70 | 31 | 22.8 ± 5.2 | 162.4 ± 46.0 | 31 | 20.3 ± 3.7 | 135.0 ± 32.7 | 30 | 19.2 ± 2.8 | 125.7 ± 23.6 | 30 | 19.5 ± 3.8 | 128.0 ± 33.8 |
| ≥70 | 30 | 22.1 ± 4.1 | 158.0 ± 35.7 | 31 | 18.7 ± 3.9 | 123.2 ± 33.7 | 30 | 18.6 ± 4.6 | 123.6 ± 38.2 | 30 | 18.7 ± 3.5 | 122.2 ± 28.4 |
| All | 121 | 23.9 ± 4.5 | 172.8 ± 41.3 | 124 | 20.1 ± 4.1 | 136.5 ± 35.1 | 121 | 19.7 ± 4.0 | 132.0 ± 33.6 | 120 | 19.7 ± 3.7 | 129.3 ± 31.3 |
| β (age) | –0.12 | –1.02 | –0.12 | –0.83 | –0.11 | –0.82 | –0.11 | –1.01 | ||||
| β (sex) | −0.59 | −5.84 | –1.81 | –16.2 | –2.35 | –20.5 | –1.80 | –16.0 | ||||
β (age/sex): regression coefficient of age/sex in regression models with angle parameter as dependent variable, and age and sex as independent variables. Numbers in bold and italic style denote the association is statistically significant (P < 0.05). ACA, anterior chamber area; ACV, anterior chamber volume.
When comparing ACA/ACV across the three Chinese cohorts, none of them was found to be different among the three groups (P for ANOVA Bonferroni = 0.274 and 0.147 for ACA and ACV, respectively). Therefore, in subsequent linear regression analyses we pooled the data from the three Chinese groups together to compare with that from Caucasians.
The independent anatomic contributors to ACA/ACV are presented in Table 2. In our study, smaller ACV is significantly related to smaller ACD (SRC = 0.62, P < 0.001), smaller ACW (SRC = 0.24, P < 0.001), greater ICurv (SRC = −0.15, P < 0.001), smaller CAD (SRC = 0.11, P < 0.001), greater IArea (SRC = −0.1, P < 0.001), and smaller CR (SRC = 0.07, P < 0.001). No significant relationship was found between ACA/ACV and axial length. Similar associated factors were identified for ACA as for ACV. After controlling for age, sex, overall eyeball size represented by AL, PD, and the above anatomic features including CAD, ACD, ACW, ICurv, IArea, and CR, Chinese still tended to have smaller ACA (β = −0.18, P = 0.022) and ACV (β = −3.9, P < 0.001) than those of Caucasians.
Table 2. .
Factors Associated with Anterior Chamber Area (mm2) and Anterior Chamber Volume (mm3) in Dark Conditions among the Whole Sample
|
ACA |
ACV |
|||||||
|
β |
SD |
P Value |
SRC |
β |
SD |
P Value |
SRC |
|
| Age | 0.00 | 0.00 | 0.934 | 0.00 | 0.02 | 0.04 | 0.514 | 0.01 |
| Sex | −0.05 | 0.06 | 0.403 | −0.01 | −0.14 | 0.88 | 0.873 | −0.001 |
| Ethnicity | −0.18 | 0.08 | 0.022 | −0.02 | −3.90 | 1.08 | 0.001 | −0.04 |
| CAD (mm) | 1.20 | 0.22 | <0.001 | 0.08 | 19.3 | 3.76 | <0.001 | 0.11 |
| ACD (mm) | 8.27 | 0.13 | <0.001 | 0.74 | 60.93 | 1.82 | <0.001 | 0.62 |
| ACW (mm) | 1.56 | 0.09 | <0.001 | 0.15 | 22.28 | 1.26 | <0.001 | 0.24 |
| ICurv (mm) | −3.04 | 0.28 | <0.001 | −0.11 | −36.56 | 3.92 | <0.001 | −0.15 |
| IArea (mm2) | −1.16 | 0.15 | <0.001 | −0.07 | −14.09 | 2.06 | <0.001 | −0.10 |
| PD (mm) | 0.40 | 0.04 | <0.001 | 0.06 | 1.15 | 0.68 | 0.089 | 0.02 |
| AL (mm) | −0.01 | 0.02 | 0.567 | 0.00 | −0.40 | 0.14 | 0.538 | −0.01 |
| CR (mm) | 0.56 | 0.11 | <0.001 | 0.04 | 7.94 | 1.62 | <0.001 | 0.07 |
| R2 | 0.986 | 0.964 | ||||||
β, regression coefficient; SD, standard deviation of β; SRC, standardized regression coefficients; R2, adequacy of the multiple regression model; ACA, anterior chamber area; ACV, anterior chamber volume; CAD, corneal arc depth; ACD, anterior chamber depth; ACW, anterior chamber width; ICurv, iris curvature; IArea, iris area; PD, pupil diameter; AL, axial length; CR, corneal radius curvature. Numbers in bold and italic style denote significant P values.
We further analyzed the ethnic differences in the anterior chamber parameters associated with ACA/ACV (Table 3). The data suggest that after adjusting for age, sex, refractive status, and AL, Chinese tended to have smaller CAD (β = −0.2, P < 0.001), ACW (β = −0.6, P < 0.001) and ACD (β = −0.21, P < 0.001) than those of Caucasians. However, no ethnic difference was found for LV (β = 0.005, P = 0.871).
Table 3. .
Ethnic Differences (Chinese vs. Caucasian) for Structural Components of Anterior Chamber Area (mm2) and Anterior Chamber Volume (mm3)
|
CAD |
LV |
ACW |
ACD |
|||||
|
β |
P Value |
β |
P Value |
β |
P Value |
β |
P Value |
|
| Age | −0.001 | 0.548 | 0.007 | <0.001 | −0.003 | 0.043 | −0.007 | <0.001 |
| Sex | −0.06 | 0.001 | 0.093 | <0.001 | −0.165 | <0.001 | −0.14 | <0.001 |
| Ethnicity | −0.2 | <0.001 | 0.005 | 0.871 | −0.6 | <0.001 | −0.21 | <0.001 |
| SE | −0.004 | 0.202 | 0.035 | <0.001 | −0.02 | 0.016 | −0.04 | <0.001 |
| AL | 0.002 | 0.820 | −0.012 | 0.193 | 0.038 | 0.01 | 0.02 | 0.19 |
In the multiple linear regressions, the ethnic differences of CAD/LV/ACW/ACD are tested when confounders such as age, sex, SE, and AL are controlled. β, regression coefficient; ACA, anterior chamber area; ACV, anterior chamber volume; CAD, corneal arc depth; ACD, anterior chamber depth; ACW, anterior chamber width; LV, lens vault. Numbers in bold and italic style denote significant P values.
Predictors of angle width (AOD500) were analyzed in Chinese and Caucasians separately in multiple linear regression models (Table 4). After controlling for age, sex, PD, and SE, the independent associated factors with a narrower angle, ranked by their contribution to the variation of angle width were smaller ACV (SRC = 0.59, P < 0.001), more curved iris (SRC = −0.22, P = 0.01), and thicker iris (SRC = −0.15, P = 0.005) for Caucasians. These factors were smaller ACV (SRC = 0.47, P < 0.001), greater LV (SRC = −0.28, P < 0.001), more curved iris (SRC = −0.23, P < 0.001), and thicker iris (SRC = −0.18, P < 0.001) for Chinese. Among all these associated factors, ACV contributes the most to the variation of AOD500 in both ethnicities. No obvious multicollinearity was identified of the independent variables in regression models based on VIF (Table 4).
Table 4. .
Comparison of Potential Factors Associated with Angle Width (AOD500) between Caucasians and Chinese
|
Caucasians |
Chinese |
|||||||||
|
β |
SD |
P Value |
SRC |
VIF |
β |
SD |
P Value |
SRC |
VIF |
|
| Age | <0.001 | 0.001 | 0.839 | 0.01 | 1.35 | 0.001 | 0.000 | 0.111 | 0.06 | 1.28 |
| Sex | 0.02 | 0.021 | 0.45 | 0.04 | 1.19 | 0.01 | 0.010 | 0.221 | 0.04 | 1.12 |
| IT750 (mm) | −0.42 | 0.147 | 0.005 | −0.15 | 1.53 | −0.39 | 0.072 | <0.001 | −0.18 | 1.76 |
| ICurv (mm) | −0.28 | 0.108 | 0.01 | −0.22 | 2.77 | −0.25 | 0.050 | <0.001 | −0.23 | 2.34 |
| IArea (mm2) | −0.05 | 0.06 | 0.357 | −0.07 | 2.21 | −0.03 | 0.03 | 0.371 | −0.04 | 2.38 |
| ACV (mm3) | 0.003 | 0.000 | <0.001 | 0.59 | 2.73 | 0.002 | 0.000 | <0.001 | 0.47 | 2.04 |
| PD (mm) | −0.03 | 0.014 | 0.029 | −0.14 | 2.38 | −0.02 | 0.007 | 0.015 | −0.09 | 2.50 |
| LV (mm) | −0.06 | 0.055 | 0.309 | −0.08 | 2.69 | −0.14 | 0.026 | <0.001 | −0.28 | 2.75 |
| SE (diopter) | −0.01 | 0.004 | 0.063 | −0.12 | 1.42 | 0.00 | 0.002 | 0.771 | −0.01 | 1.23 |
β, regression coefficient; SD, standard deviation of β; SRC, standardized regression coefficients; R2, adequacy of the multiple regression model; VIF, variance inflation factor; ACV, anterior chamber volume; IT, iris thickness; ICurv, iris curvature; IArea, iris area; PD, pupil diameter; LV, lens vault; SE, spherical equivalence; AOD500, angle opening distance at 500 μm anterior to the scleral spur.
ACA was not included in the regression models because ACA and ACV are highly correlated. To replace ACV with ACA in the models reveals the same significant associated factors with angle width. Numbers in bold and italic style denote significant P values.
Discussion
In this prospective multicenter study, American Caucasians and Chinese Americans residing in SF were compared with their age- and sex-matched Chinese counterparts from Guangzhou and Beijing with regard to ASOCT-derived anterior segment parameters, particularly ACA and ACV. Consistent with previous studies,12 we found ACA and ACV decreased with advanced age, in both Chinese and Caucasians. However, the finding that females had smaller ACA/ACV than that of males was detected only in Chinese in this study. This indicated that the higher risk of angle closure among women may be ethnicity specific.
American Caucasians in our study had greater ACA/ACV than that of each Chinese group. A study in Singapore has also suggested that Chinese adults had smaller ACA/ACV than that of Malaysians and Indians.12 This ethnic difference could be mediated by the interethnic variation of certain anterior segment structural features that are associated with ACA/ACV. By multiple linear regressions, we found that the independent and significant factors related to a smaller ACA/ACV included smaller CAD, ACD, ACW, and CR as well as greater ICurv and IArea. According to the standardized regression coefficients, we found that ACD contributes the most to ACA/ACV variation, followed by ACW and ICurv. We further investigated the ethnic differences for the significant contributors of ACA/ACV. After controlling for age, sex, SE, and AL, Chinese in our study had smaller corneal arc depth, smaller ACW, and smaller ACD than that of Caucasians. It also has been reported in our previous study that Chinese had greater iris area than that of Caucasians, even when PD is taken into account.6 Therefore, the smaller CAD, ACD, ACW, and greater iris area found in Chinese are likely to be contributory to the smaller ACA/ACV in Chinese in our study. Meanwhile, we found no ethnic difference for LV in this study. Considering that ACD is CAD minus LV, it could be postulated that a relatively shallower anterior chamber found in Chinese than that in Caucasians is mainly due to the shallower corneal arc in the former group.
Smaller ACA/ACV has been reported to be independently associated with gonioscopically narrow angles in Singaporean Chinese.8,12 In our study, when using AOD500 to represent angle width, we found that a narrower angle is significantly related to a thicker iris, more curved iris, smaller ACV, and larger pupil diameter in both Caucasians and Chinese. Furthermore, our findings are consistent with a previous study that found that angle width is largely dependent on variations in ACV.8 This population-based study among Singaporean Chinese used a predictive model and determined that ACA, ACV, ACW, LV, IT, and IArea explained 85.5% of the variability of the AOD500. Although iris curvature is associated with ACA/ACV, we found that its contribution to angle width variation is independent of that of ACV. Our findings indicated that iris root thickness and curvature were more pertinent to drainage angle width than the overall iris area.
We found that lens vault was significantly associated with angle width, independent of the impact of ACA/ACV. However this phenomenon was identified only in Chinese and not in Caucasians. Furthermore, among Chinese, the contribution of LV to angle width variation was even stronger than that of ICurv and IT. This suggests that although the numerical value of LV did not differ between ethnicities, it plays different roles in Caucasians and Chinese regarding the impact on angle width. Because LV represents the space occupied by the lens under the corneal dome capacity, which is represented in one dimension by corneal arc depth (CAD, perpendicular distance from corneal endothelium dome apex to inter-SS line), the fact that Chinese had smaller CAD than that of Caucasians7 should result in a shallower and more crowded anterior chamber space. It is also possible that for a given LV that is under a smaller CAD, there may be a more curved iris due to the increased pupillary block. This could be an indirect way that LV contributes to narrow angles in Chinese. Future studies focused on the relationship of LV and iris curvature are warranted to answer this issue.
The findings and implications of this study need to be interpreted with caution in light of some limitations. First, subjects enrolled from San Francisco and Beijing were clinic based. Although efforts have been made to rule out patients with ocular abnormalities by comprehensive exclusion criteria, selection bias may still be inevitable with our study design. Second, due to the difficulty in recruiting American-born Chinese, especially those who are elderly, about half of the American Chinese in this study were first-generation immigrants born in China. These immigrants have, of course, lived in the United States for less time than similarly aged U.S.-born Chinese, thus limiting our ability to adequately study the impact of environmental versus genetic factors. Third, ASOCT images of vertical quadrants were not collected due to difficulties in limbal exposure. Regarding the sector variation in anterior chamber anatomy, data from lateral quadrants may not represent the whole circle. However, this may have less impact on the assessment of associated factors. Finally, most eyes in this study were not occludable (115/121 [95.0%] in Whites and 345/365 [94.5%] in Chinese). The results and conclusions should be validated in future studies with more occludable eyes. Although a previous study reported that AOD750 may have better performance in identifying gonioscopic narrow angles in ASOCT images,13 we believe that the use of AOD500 will not alter the analysis of associated factors of angle width, considering this sample consisted of mainly open angle eyes and there is a high association between AOD500 and AOD750.
In summary, ACA/ACV is the most prominent contributor to angle width in both Chinese and Caucasians in this study. Anterior chamber depth, anterior chamber width, and iris curvature are the most important factors associated with ACA/ACV. Due to their smaller ACD and ACW, Chinese in this study tended to have smaller ACA/ACV than that of Caucasians, which is the main contributor to the narrower drainage angle in Chinese.
Footnotes
Supported in part by the George and Rosalie Hearst Foreign Fellowship; That Man May See, Inc.; Research to Prevent Blindness; and National Eye Institute Core Grant EY002161.
Disclosure: D. Wang, None; M. Qi, None; M. He, None; L. Wu, None; S. Lin, None
References
- 1. Yip JL, Foster PJ. Ethnic differences in primary angle-closure glaucoma. Curr Opin Ophthalmol. 2006;17:175–180 [DOI] [PubMed] [Google Scholar]
- 2. Wang BS, Narayanaswamy A, Amerasinghe N, et al. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2011;95:46–50 [DOI] [PubMed] [Google Scholar]
- 3. Cheung CY, Liu S, Weinreb RN, et al. Dynamic analysis of iris configuration with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:4040–4046 [DOI] [PubMed] [Google Scholar]
- 4. Nongpiur ME, Sakata LM, Friedman DS, et al. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology. 2010;117:1967–1973 [DOI] [PubMed] [Google Scholar]
- 5. Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011;118:474–479 [DOI] [PubMed] [Google Scholar]
- 6. Wang D, He M, Wu L, et al. Differences in iris structural measurements among American Caucasians, American Chinese and mainland Chinese. Clin Exp Ophthalmol. 2012;40:162–169 [DOI] [PubMed] [Google Scholar]
- 7. Wang D, Huang G, He M, et al. Comparison of anterior ocular segment biometry features and related factors among American Caucasians, American Chinese and mainland Chinese. Clin Exp Ophthalmol. 2012;40 [DOI] [PubMed] [Google Scholar]
- 8. Foo LL, Nongpiur ME, Allen JC, et al. Determinants of angle width in Chinese Singaporeans. Ophthalmology. 2012;119:278–282 [DOI] [PubMed] [Google Scholar]
- 9. Console JW, Sakata LM, Aung T, et al. Quantitative analysis of anterior segment optical coherence tomography images: the Zhongshan Angle Assessment Program. Br J Ophthalmol. 2008;92:1612–1616 [DOI] [PubMed] [Google Scholar]
- 10. Wang DD, Huang GF, He MG, et al. Inter-ethnic variation of ocular traits-design and methodology of comparison study among American Caucasians, American Chinese and mainland Chinese. Yan Ke Xue Bao. 2011;26:30–34 [DOI] [PubMed] [Google Scholar]
- 11. Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu RY, Nongpiur ME, He MG, et al. Association of narrow angles with anterior chamber area and volume measured with anterior-segment optical coherence tomography. Arch Ophthalmol. 2011;129:569–574 [DOI] [PubMed] [Google Scholar]
- 13. Narayanaswamy A, Sakata LM, He MG, et al. Diagnostic performance of anterior chamber angle measurements for detecting eyes with narrow angles: an anterior segment OCT study. Arch Ophthalmol. 2010;128:1321–1327 [DOI] [PubMed] [Google Scholar]