Abstract
Purpose.
Uncorrected presbyopia is a significant cause of visual disability globally. Greater comprehension of the etiology of presbyopia and its contributing factors among medical and vision care providers could lead to changes in correction methods and account for sex differences in near-vision requirements.
Methods.
A meta-analysis was performed using nine cross-sectional studies that provided sufficient data to compare the prevalence and magnitude of presbyopia among men and women. This analysis was further subdivided into measurement methods to determine what differences in presbyopia might exist between men and women.
Results.
Studies of presbyopia including sex as a contributing factor were highly heterogenic (P = 0.01) but overall found female sex to be statistically significant in predicting earlier onset for presbyopia with an adjusted confidence interval (CI) using the Shore method of 95% CI [1.02, 1.45]. When limited to studies only measuring accommodative amplitude, female sex was not associated with presbyopia in a fixed effects model with a 95% CI [0.49, 1.07].
Conclusions.
While an association between female sex and presbyopia for subjective measurements (near spectacle prescriptions and add powers) was indicated, measurements of accommodative amplitude show a weak tendency toward the opposite conclusion. This suggests that increased association of presbyopia for women is not due to a physiologic difference in accommodation but rather due to other sex differences, such as tasks performed and viewing distances. Age-based correction nomograms for presbyopia should therefore consider these sex differences when prescribing add powers for near tasks.
While sex differences in presbyopia exist, they are likely due to other factors besides focusing ability, such as arm length, preferred reading distance, or uncorrected hyperopia.
Introduction
It has been estimated that worldwide more than a billion adults are now affected by presbyopia, the age-related loss of accommodation that blurs near vision due to decreased focusing ability. According to worldwide census data, one third of the population is older than 40, an age when the effects of presbyopia become symptomatic enough that individuals begin using near-vision spectacles. As the global population ages, the prevalence of presbyopia will increase. By 2030, the global population older than 40 is expected to rise to 41%.1 Although estimates show uncorrected presbyopia as one of the leading causes of disability and worthy of attention as a significant contributor to the global burden of disease,2 it is commonly overlooked as a major source of disability due to the ease of acquiring spectacle readers in wealthy countries.
With age, presbyopia eventually affects everyone but is generally measured and diagnosed only when an individual becomes symptomatic and presents to an eye care provider with need for near-vision correction. Due to the need for trained vision care providers, the burden of presbyopia is greatest among vulnerable populations, with sex, race, ethnicity, climate, rurality, and geographic locations considered to be contributing factors.2–5 While presbyopia often manifests as a difficulty in reading small text, an inability to see near objects clearly can have a substantial impact on the quality of life regardless of literacy or profession.3
In 1623, Benito Daza de Valdes noted that “women with blurred vision [presbyopia] cannot follow the same guidelines as men—they require eyeglasses possessing more degrees because they do more delicate work and because they have weaker vision.”6 Recent studies confirm that women are indeed still being prescribed with higher near corrections than men of the same age.7–12 The reason for this sex disparity is not immediately clear. Daza de Valdes made two different claims: women perform “more delicate work” and women have naturally “weaker vision.” These claims would imply that there are biological, societal, and environmental components to the need for higher-powered near prescriptions in women. Despite findings supporting the conclusion that women are given higher reading prescriptions than men of equal age, there is often no differentiation made among the biological, social, psychological, and cultural factors that could explain this difference.
As illustrated in Figure 1, the onset of presbyopia is primarily influenced by three factors: focusing ability, habitual reading distance (or the preferred distance for near tasks), and depth of focus (the tolerance of an optical system such as the eye to defocus). Secondary factors that can influence the onset of presbyopia include occupation, refractive error and other ocular aberrations, arm length, pupil size, and possible differences in lens optical density. Other tertiary factors that could lead to differences in onset time of presbyopia, and therefore underlying sex differences, could include solar radiation, complexity of near tasks, indoor light levels, and/or other task-specific conditions that could have a sex bias.
Figure 1. .
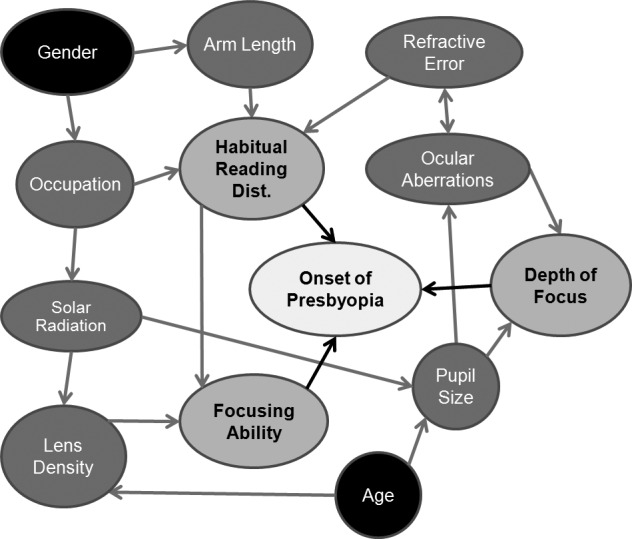
Directed acyclic graph of the causes of presbyopia. Onset of presbyopia is primarily determined by habitual reading distance, depth of focus, and focusing ability. Sex could be associated with the onset of presbyopia through a variety of different pathways.
Loss of focusing ability, an underlying cause of presbyopia, occurs due to a loss of elasticity of the crystalline lens, which makes it less effective at increasing optical power with attempts at accommodation. As the ciliary muscle contracts during accommodation, tension on the zonules decreases, but a larger, stiffer presbyopic lens fails to increase in optical power to the same magnitude as a younger, more pliable crystalline lens.13–20 While the onset, progression, and endpoint of the physiological focusing ability have been studied extensively,19,21,22 the relationship between focusing ability and the subjective need for reading correction is less well understood. Measurements of focusing ability, such as accommodative amplitude, often reveal that women have greater focusing ability than men of the same age, although these findings are often mixed.23–28
In addition to focusing ability, habitual reading distance and depth of focus are primary factors that influence the onset of presbyopia. There is evidence that adult women have a shorter measured habitual reading distance than adult men.29 This could be one cause for the greater need for near correction in women. If women had a smaller depth of focus than men, they would be more affected by near blur than men would be. There are no studies, however, that indicate a difference between men and women in terms of measured depth of focus. Pupil size and higher-order aberrations can affect depth of focus, but neither of these has been demonstrated to be different in women and men.23,30–33
There is evidence that refractive error, or the spectacle prescription needed to bring distant targets into focus, is different among men and women older than 40.34 Women older than 40 have higher rates of hyperopia than men older than 40. While hyperopia and presbyopia have different etiologies, low amounts of undiagnosed hyperopia would manifest as an earlier need for near-vision correction with the onset of presbyopia. This classification has been termed “functional presbyopia,” which is defined as the need for a significant optical correction added to a “presenting” distance refraction correction to achieve a near visual acuity criterion.2 By this definition, a 10-year-old +3.00 hyperope presenting with no distance correction could be described as a “functional presbyope,” due to the need for near-vision correction. This is separate from the standard “objective presbyopia,” which is defined as needing a significant optical correction added to the best distance optical correction to achieve a near-vision criterion. Since functional presbyopia combines hyperopes and presbyopes into one group, it might be useful in describing the need for near-vision correction but could add to the confusion due to grouping of vision problems with very different etiologies. Hyperopia is caused by either insufficient refractive power of the ocular structures or by reduced eye length, whereas presbyopia is caused by loss of accommodative ability. It should be noted that any solution or correction that benefits “objective presbyopia” could also benefit “functional presbyopia,” although a full distance refractive correction would probably be the ideal solution.
In clinical practice, presbyopia is diagnosed by measurements of accommodative amplitude, near subjective refraction, and/or patient's reported symptoms. Studies reporting presbyopia are therefore varied in their methods of diagnosis; but, for purposes of providing a more complete evaluation of the relationship between sex and presbyopia, this meta-analysis will consider the various methods to be equally valid.
Methods
A literature search was performed for studies that were published prior to 2012 that report data regarding presbyopia and sex. Studies were excluded for which the data reported could not be interpreted to provide an odds ratio (OR) of the association between sex and presbyopia when controlling for age. OR was selected because it was the most commonly reported measure of association in the literature search. Studies that reported measures of presbyopia other than measures of prevalence were converted into an OR (using methods described later) in order to be included in the statistical analysis. A meta-analysis was then performed on included studies using the OR. No attempt was made to weight the studies based on the quality of the measurements taken or on the manner used to determine the status of presbyopia since there was no objective way for determining such a method of weighting and a subjective method would not be defensible.
Each OR was weighted (W) based on the inverse square of its standard error (SE) (W = 1/SE2). SEs were calculated by dividing the natural log of the ratio of the upper and lower 95% confidence intervals (CIs) by 3.92 (SE = ln(CIup/CIlow)/3.92). For each study, the weight was then multiplied by the natural log of the OR to calculate a summary measure. A pooled summary was determined by dividing the sum of the summary measures by the sum of the weights. A summary OR was produced by taking the exponent of the pooled summary.
Heterogeneity among studies was assessed using χ2 for heterogeneity.35 When evidence of heterogeneity was present, the 95% CI of the fixed effects summary OR was adjusted using the Shore method.36 For a summary OR in which the χ2 test statistic was greater than the number of degrees of freedom, the variance of the log of the pooled relative risk was multiplied by the ratio of the heterogeneity χ2 statistic to its degrees of freedom. This adjusted variance was then used to adjust the 95% CI.
A total of 15 studies were found that report presbyopia data with the sex of the participants. Of these studies, six were excluded for reasons that follow. Burke et al. (2006) and Patel et al. (2006) utilize the same data set of 1709 individuals in Tanzania, so only the first was included.9,11 Duarte et al. (2003) report a 22% increased risk for women to develop presbyopia but do not include CIs to allow inclusion into a meta-analysis.12 Kragha et al. (1986) report that women had 0.54 diopters (D) greater accommodative amplitude than age-matched men but do not provide sufficient population demographics information (age and sex of participants) to convert this finding into a risk value for presbyopia based on age.24 Carnevali et al. (2005) report no significant differences for sex in accommodative amplitudes but do not provide the data used to arrive at this conclusion.28 Millodot et al. (1989) find women to have greater accommodative amplitudes than men but find that for the overall study this value is not statistically significant.29 The study does not provide standard deviation values for age groups that would allow inclusion into the meta-analysis.
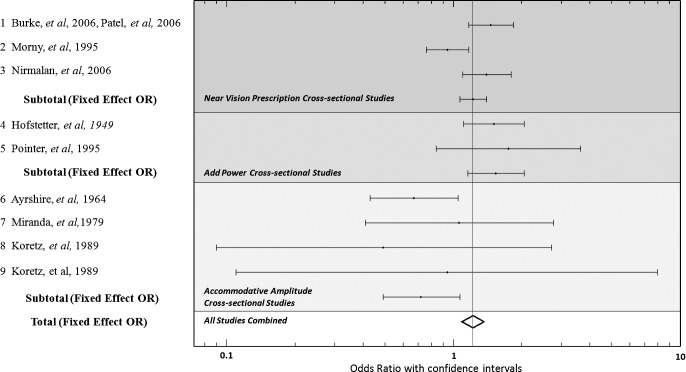
Nine cross-sectional studies were found to meet inclusion criteria (see Fig. 2). From these, three studies (Burke et al. [2006], Nirmalan et al. [2006], and Morny et al. [1995]) report the OR of women being diagnosed with presbyopia compared to men when adjusted for age.9,10,37 Two studies (Hofstetter [1949] and Pointer et al. [1995]) report the values of prescribed near add powers for men and women of various ages.7,8 The Hoffstetter study was converted into an OR of women being diagnosed with presbyopia compared to men by using the need for a near add as a diagnosis of presbyopia. Because Pointer (1995) does not include data for patients who were found to have no need for a near-vision add, a cutoff value of 1.00 D near add was used as the minimum value for a diagnosis of presbyopia. Ayrshire (1964), Miranda et al. (1979), and two studies by Koretz et al. (1989) were included.23,25,27,38 For studies that measure subjective accommodative amplitudes, a cut-off value of 3.75 D was chosen for the diagnosis of presbyopia (an amount reported in the study by Miranda et al. [1979]). For the Koretz study, which measures objective accommodative amplitudes, a cutoff value of 2.5 D was chosen. This amount correlates with subjective values, and it can be inferred that an individual with more than 2.5 D of measured objective focusing ability would not require a near correction for the standard reading distance of 40 cm. When individual data was not provided, a normal distribution of the metric value being measured was assumed to occur across a given age category.
Figure 2. .
Forrest plot of studies used in the meta-analysis of sex and presbyopia.
The initial meta-analysis was performed combining all nine cross-sectional studies that met inclusion criteria. Subsequently, smaller groups were analyzed based on three categories of methods in which the data was gathered: near-vision spectacles prescribed, near add power measured, and accommodative amplitudes (see Table 1).
Table 1. .
Results of Meta-Analysis of Sex and Presbyopia
|
Author, Year of Publication |
OR |
CIlow |
CIup |
n |
Location |
%Wall |
%Wsubgroup |
|
| Near vision prescription cross-sectional studies | ||||||||
| 1 | Burke et al., 2006 | 1.46 | 1.17 | 1.84 | 1709 | Tanzania | 25.5 | 33.9 |
| Patel et al., 2006 | … | … | … | … | … | … | … | |
| 2 | Morny et al., 1995 | 0.94 | 0.76 | 1.17 | 1884 | Ghana | 21.5 | 28.7 |
| 3 | Nirmalan et al., 2006 | 1.40 | 1.10 | 1.80 | 5587 | South India | 28.2 | 37.4 |
| Subtotal (fixed effect OR) | 1.22 | 1.07 | 1.4 | 75.2 | 100.0 | |||
| Add power cross-sectional studies | ||||||||
| 4 | Hofstetter et al., 1949 | 1.51 | 1.11 | 2.05 | 3917 | United States | 13.8 | 85.1 |
| 5 | Pointer et al., 1995 | 1.75 | 0.84 | 3.64 | 600 | UK | 2.4 | 14.9 |
| Subtotal (fixed effect OR) | 1.54 | 1.16 | 2.05 | 16.2 | 100.0 | |||
| Accommodative amplitude cross-sectional studies | ||||||||
| 6 | Ayrshire et al., 1964 | 0.67 | 0.43 | 1.06 | 1307 | United States | 6.4 | 74.9 |
| 7 | Miranda et al., 1979 | 1.06 | 0.40 | 2.77 | 1000 | Puerto Rico | 1.4 | 16.5 |
| 8 | Koretz et al., 1989 | 0.49 | 0.09 | 2.71 | 100 | United States | 0.5 | 5.3 |
| 9 | Koretz et al., 1989 | 0.94 | 0.11 | 7.95 | 100 | United States | 0.3 | 3.3 |
| Subtotal (fixed effect OR) | 0.72 | 0.49 | 1.07 | 8.5 | 100.0 | |||
| Total (fixed effect OR) | 1.21 | 1.08 | 1.36 | 100.0 | ||||
Burke et al. (2006) and Patel et al. (2006) utilize the same data set of 1709 individuals in Tanzania, so only the first was included. CIlow, lower bound of 95% confidence interval; CIup, upper bound of 95% confidence interval; n = number of participants in study; %Wall, percent weight of this study among all studies based on the inverse square of its standard error; %Wsubgroup, percent weight of this study among the subgroup of studies based on the inverse square of its SE.
Results
Using a funnel plot, no evidence of publication bias was found in the included studies.
As seen in Table 2, with all nine studies included, sex was found to be a statistically significant predictor of presbyopia onset in the fixed effects model, with females more likely than males to meet the study criterion for being diagnosed with presbyopia when controlling for age; fixed effect OR of 1.21 (95% CI [1.08, 1.36]). The studies were found to be highly heterogenic with a probability value of 0.01. When controlling for heterogeneity across studies using a random effects model, a reduced OR of 1.19 (95% CI [0.95, 1.48]) was found.39 When adjusting the CI using the Shore method (Table 3), statistical significance was again achieved (95% CI [1.02, 1.45]). Two of the excluded studies (Carnevali et al. 2005, Millodot et al. 1989) report no statistically significant difference between men and women for accommodative amplitudes. One excluded study reports an increased risk of presbyopia for women based on a self-assessment questionnaire (Duarte et al. 2003), and another excluded study reports that women had a greater accommodative amplitude than men (Kragha et al. 1985). These mixed results for the excluded studies would further increase the heterogeneity of the studies and could weaken the statistical significance of the meta-analysis reported here.
Table 2. .
Results of Meta-Analysis of Sex and Presbyopia
|
Category |
n |
Fixed Effects |
Random Effects |
Heterogeneity |
|||||
|
OR |
CIlow |
CIup |
OR |
CIlow |
CIup |
χ2 |
P |
||
| All studies | 9 | 1.21 | 1.08 | 1.36 | 1.19 | 0.95 | 1.48 | 19.74 | 0.01 |
| Near vision prescription studies | 3 | 1.22 | 1.07 | 1.40 | 1.24 | 0.93 | 1.64 | 9.12 | 0.01 |
| Add power studies | 2 | 1.54 | 1.16 | 2.05 | 0.13 | 0.72 | |||
| Accommodative amplitude studies | 4 | 0.72 | 0.49 | 1.07 | 0.95 | 0.81 | |||
CIlow, lower bound of 95% confidence interval; CIup, upper bound of 95% confidence interval; n = number of studies. Random effects model only performed when χ2 > df, where df = number of studies minus one.
Table 3. .
Results of Meta-Analysis of Sex and Presbyopia
|
Category |
n |
Shore Adjusted CI |
||
|
OR |
CIlow |
CIup |
||
| All studies | 9 | 1.19 | 1.02 | 1.45 |
| Near vision prescription studies | 3 | 1.24 | 0.92 | 1.62 |
CIlow, lower bound of 95% confidence interval; CIup, upper bound of 95% confidence interval; n = number of studies. Random effects model only performed when χ2 > df, where df = number of studies minus one.
When evaluating the studies that measure presbyopia by using data for individuals who were given near-vision spectacles, the fixed effect model showed that females were more likely than males to need a near-vision correction when controlling for age (fixed effect OR of 1.22 (95% CI [1.07, 1.40]), though the studies remained heterogenous with a P value of 0.01. Controlling for heterogeneity through a random effects model resulted in a slightly increased OR of 1.24 (95% CI [0.93, 1.64]) that no longer achieved statistical significance even when adjusted using the Shore method (95% CI [0.92, 1.62]).
Hofstetter (1949) and Pointer (1995) compare females and males based on the add powers that were prescribed by their eye doctors. They revealed a greater prevalence of presbyopia among females with a fixed effect OR of 1.54 (95% CI [1.16, 2.05]). Heterogeneity among these studies was much lower and did not reach statistical significance (P = 0.72).
Koretz (1989), Ayshire (1964), and Miranda (1979) find significantly greater subjective accommodative amplitudes for women than men of the same age; but, when the data from these measures were converted into a predictive metric for presbyopia, the data predicted lower levels of presbyopia among women, although not to a level of statistical significance. The fixed effect OR was calculated at 0.72 (95% CI [0.49, 1.07]).
Discussion
Although presbyopia is commonly defined as the loss of focusing ability with age, the detection of presbyopia and need for near-vision correction is dependent on not just the loss of focusing ability but also on the habitual reading distance and the depth of focus. Based on the findings of this analysis, there is no significant sex difference in accommodative amplitudes, a direct measure of focusing ability.
The tendency toward exclusion of studies that report no statistically significant difference between men and women, because those studies fail to report sufficient data, could be problematic in the meta-analysis. While such a selective process would, of course, not bias results toward a specific sex, it would tend to decrease the likelihood of a meta-analysis revealing that there was no significant difference in presbyopia between sexes.
While the overall meta-analysis did provide some evidence that females might have a greater risk for presbyopia in broad terms than males of equivalent age, the smaller group analysis of near add powers for presbyopic prescriptions showed that women have a need for higher-power near adds than do men of an equivalent age. This finding is particularly important when combined with evidence that women in developing countries might often be underserved in receiving near-vision spectacles.4 This sex bias in receiving presbyopia correction would lead to an even greater disparity among men and women in terms of uncorrected presbyopia versus corrected presbyopia. Women, who have a greater need for presbyopia correction than men of equivalent age, may find themselves less likely to receive that aid. A 5-year update of their 2007 study by Ramke et al. shows that improvements were being made in sex disparities in presbyopia correction through a National Spectacle Program, as reported in 2012.5
The summary finding of a meta-analysis that combines the results of studies using differing methods for diagnosing presbyopia might be questionable since the weights of the various methods of measurement were not equal (a result of the number of participants in the studies not being equal). There were far more individuals in the cross-sectional studies that reported presbyopia based on the need for a near-vision prescription by age and sex than in the studies that measured accommodative amplitude by age and sex. While both accommodative amplitude and the need for spectacle prescriptions are used clinically for determining the onset of presbyopia, they were not equally predictive of the differences between men and women. By using different measurements of presbyopia, the various studies also implicitly subscribe to slightly different definitions of the term “presbyopia” itself. Such an internal discrepancy could be rightly viewed as a limitation to the results of a meta-analysis that combined such a varied array of studies.
Due to the wide variety of primary, secondary, and tertiary factors that can be attributable to the onset of presbyopia, it would be impossible to compare all the possible reasons for sex differences through a meta-analysis of previous research. The evidence presented supports the conclusion that sex differences are not due to differences in focusing ability but rather to sex differences related to preferred reading distances, such as arm length, occupation, indoor light levels, and specific conditions related to desired tasks.
Following submission of this meta-analysis for review, a study performed by Hashemi et al. (2012) was accepted for publication.40 This study collected data from 5019 participants in Iran; the results agree with the other cross-sectional studies already included in this meta-analysis, concluding that females require higher add powers than men of similar age. If included in the meta-analysis, this study would strengthen the conclusions already determined.
Conclusions
Although there are no significant sex differences in presbyopia due to focusing ability when measured by accommodative amplitudes, there are significant sex differences in the add requirements for near-vision spectacles for men and women of the same age. These differences are likely due to differences in preferred viewing distances (as a result of arm length or preferred near tasks) or due to uncorrected hyperopia. Measurements of accommodative amplitudes are therefore not sufficient to diagnose presbyopia without considerations of an individual's specific needs.
Presbyopia is a global challenge. More than a billion individuals require near-vision aids to perform basic tasks of daily living. This number will continue to increase as the number of individuals older than 40 increases. Global health policies that seek to overcome the disability caused by visual impairment should consider the specific needs that women have for near vision and adjust policies to meet these needs to provide equitable care for all individuals. In the future, more carefully performed studies should be executed to better isolate and measure the various factors that contribute to the development of presbyopia while controlling for age and sex. Metrics used to determine presbyopia must be carefully chosen. Future studies should also consider depth of focus as a factor in the development of presbyopia and should consider the potential for differences in depth of focus between men and women by looking at potential causes such as higher-order aberrations and pupil size. Longitudinal studies that consider the interaction between the preferred reading distance and the change in accommodative amplitude across time for males and females could help determine to what extent biological factors or environmental factors play a role in the loss of focusing ability with increasing age.
Footnotes
Supported by National Institutes of Health Grant K12 EY017269.
Disclosure: A. Hickenbotham, None; A. Roorda, None; C. Steinmaus, None; A. Glasser, None
References
- 1. Census data and global changes in demographics. Available at: http://www.census.gov/cgi-bin/broker. Accessed March 15, 2012
- 2. Holden BA, Fricke TR, Ho SM, et al. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol. 2008;126:1731–1739 [DOI] [PubMed] [Google Scholar]
- 3. Patel I, West S. Presbyopia: prevalence, impact, and interventions. Community Eye Health J. 2007;20:40–41 [PMC free article] [PubMed] [Google Scholar]
- 4. Ramke J, du Toit R, Palagyi A, Brian G, Naduvilath T. Correction of refractive error and presbyopia in Timor-Leste. Br J Ophthalmol. 2007;91:860–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramke J, Brian G, Naduvilath T. Refractive error and presbyopia in Timor-Leste: the impact of 5 years of a national spectacle program. Invest Ophthalmol Vis Sci. 2012;53:434–439 [DOI] [PubMed] [Google Scholar]
- 6. Daza de Valdés B. (1623) The use of eyeglasses Runge PE. and trans. Sarasota, FL: Wayenborgh Publishing;. 2004:118
- 7. Hofstetter HW. A survey of practices in prescribing presbyopic adds. Am J Optom Arch Am Acad Optom. 1949;26:144–160 [DOI] [PubMed] [Google Scholar]
- 8. Pointer JS. The presbyopic add, II: age-related trend and a gender difference. Ophthalmic Physiol Opt. 1995;15:241–248 [PubMed] [Google Scholar]
- 9. Burke AG, Patel I, Munoz B, et al. Population-based study of presbyopia in rural Tanzania. Ophthalmology. 2006;113:723–727 [DOI] [PubMed] [Google Scholar]
- 10. Nirmalan PK, Krishnaiah S, Shamanna BR, Rao GN, Thomas R. A population based assessment of presbyopia in the state of Andhra Pradesh, south India: the Andhra Pradesh Eye Disease Study. Invest Ophthalmol Vis Sci. 2006;47:2324–2328 [DOI] [PubMed] [Google Scholar]
- 11. Patel I, Munoz B, Burke AG, et al. Impact of presbyopia on quality of life in a rural African setting. Ophthalmology. 2006;113:728–734 [DOI] [PubMed] [Google Scholar]
- 12. Duarte WR, Barros AJ, Dias-da-Costa JS, Cattan JM. Prevalence of near vision deficiency and related factors: a population-based study [in Portuguese]. Cad Saude Publica. 2003;19:551–559 [DOI] [PubMed] [Google Scholar]
- 13. von Helmholtz HH. (1909). Handbuch der Physiologischen Optik. Helmholtz's treatise on physiological optics Southall JPC. Mechanisms of Accommodation. Vol. 1, section 12. New York: Dover;. 1962:143–172
- 14. Gullstrand A. (1909). Appendix to Helmholtz's treatise on physiological optics Southall JPC. Mechanisms of Accommodation. New York: Dover;. 1962:143–172
- 15. Fisher RF. Elastic constants of the human lens capsule. J Physiol. 1969;201:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher RF. The elastic constants of the human lens. J Physiol. 1971;212:147–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher RF. Presbyopia and the changes with age in the human crystalline lens. J Physiol. 1973;228:765–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fisher RF. The force of contraction of the human ciliary muscle during accommodation. J Physiol. 1977;270:51–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glasser A, Campbell MCW. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229 [DOI] [PubMed] [Google Scholar]
- 20. Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999;39:1991–2015 [DOI] [PubMed] [Google Scholar]
- 21. Hamasaki D, Ong J, Marg E. The amplitude of accommodation in presbyopia. Am J Optom Arch Am Acad Optom. 1956;33:3–14 [DOI] [PubMed] [Google Scholar]
- 22. Charman WN. The path to presbyopia: straight or crooked?. Ophthalmic Physiol Opt. 1989;9:424–430 [PubMed] [Google Scholar]
- 23. Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye—aging of the anterior segment. Vision Res. 1989;29:1685–1692 [DOI] [PubMed] [Google Scholar]
- 24. Kragha IK. Amplitude of accommodation: population and methodological differences. Ophthalmic Physiol Opt. 1986;6:75–80 [PubMed] [Google Scholar]
- 25. Ayrshire Study Circle An investigation into accommodation. Br J Physiol Opt. 1964;21:31–35 [PubMed] [Google Scholar]
- 26. Fitch RC. Procedural effects on the manifest human amplitude of accommodation. Am J Optom Arch Am Acad Optom. 1971;48:918–926 [DOI] [PubMed] [Google Scholar]
- 27. Miranda MN. An amplitude of accommodation curve for Puerto Rico. Bol Assoc Med PR. 1979;71:291–297 [PubMed] [Google Scholar]
- 28. Carnevali T, Southaphanh P. A retrospective study on presbyopia onset and progression in a Hispanic population. Optometry. 2005;76:37–46 [DOI] [PubMed] [Google Scholar]
- 29. Millodot M, Millodot S. Presbyopia correction and the accommodation in reserve. Ophthalmic Physiol Opt. 1989;9:126–132 [DOI] [PubMed] [Google Scholar]
- 30. García Serrano JL, López Raya R. Mylonopoulos Caripidis T. Variables related to the first presbyopia correction [in Spanish]. Arch Soc Esp Oftalmol. 2002;77:597–604 [PubMed] [Google Scholar]
- 31. Jones R. Do women and myopes have larger pupils?. Invest Ophthalmol Vis Sci. 1990;31:1413–1415 [PubMed] [Google Scholar]
- 32. Birren JE, Casperson RC, Botwinick J. Age changes in pupil size. J Gerontol. 1955;5:216–225 [DOI] [PubMed] [Google Scholar]
- 33. Applegate RA, Donnelly WJ, III , Marsack JD, Koenig DE, Pesudovs K. Three-dimensional relationship between high-order root-mean-square wavefront error, pupil diameter, and aging. J Opt Soc Am A Opt Image Sci Vis. 2007; March 24:578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kempen JH, Mitchell P, Lee KE, et al. Eye Diseases Prevalence Research Group. The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch Ophthalmol. 2004;122:495–505 [DOI] [PubMed] [Google Scholar]
- 35. Petitti D. Meta-analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine New York: : Oxford University Press; 1994. [Google Scholar]
- 36. Shore R, Gardner M, Pannett B. Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. Br J Ind Med. 1993;50:971–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morny FK. Correlation between presbyopia, age and number of births of mothers in the Kumasi area of Ghana. Ophthalmic Physiol Opt. 1995;15:463–466 [PubMed] [Google Scholar]
- 38. Koretz JF, Cook CA, Kaufman PL. Accommodation and presbyopia in the human eye: changes in the anterior segment and crystalline lens with focus. Invest Ophthalmol Vis Sci. 1997;38:569–578 [PubMed] [Google Scholar]
- 39. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 40. Hashemi H, Khabazkhoob M, Jafarzadehpur E, et al. Population-based study of presbyopia. Clin Exp Ophthalmol. 2012; In Press; [DOI] [PubMed] [Google Scholar]