Abstract
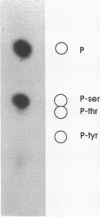
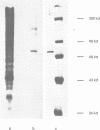
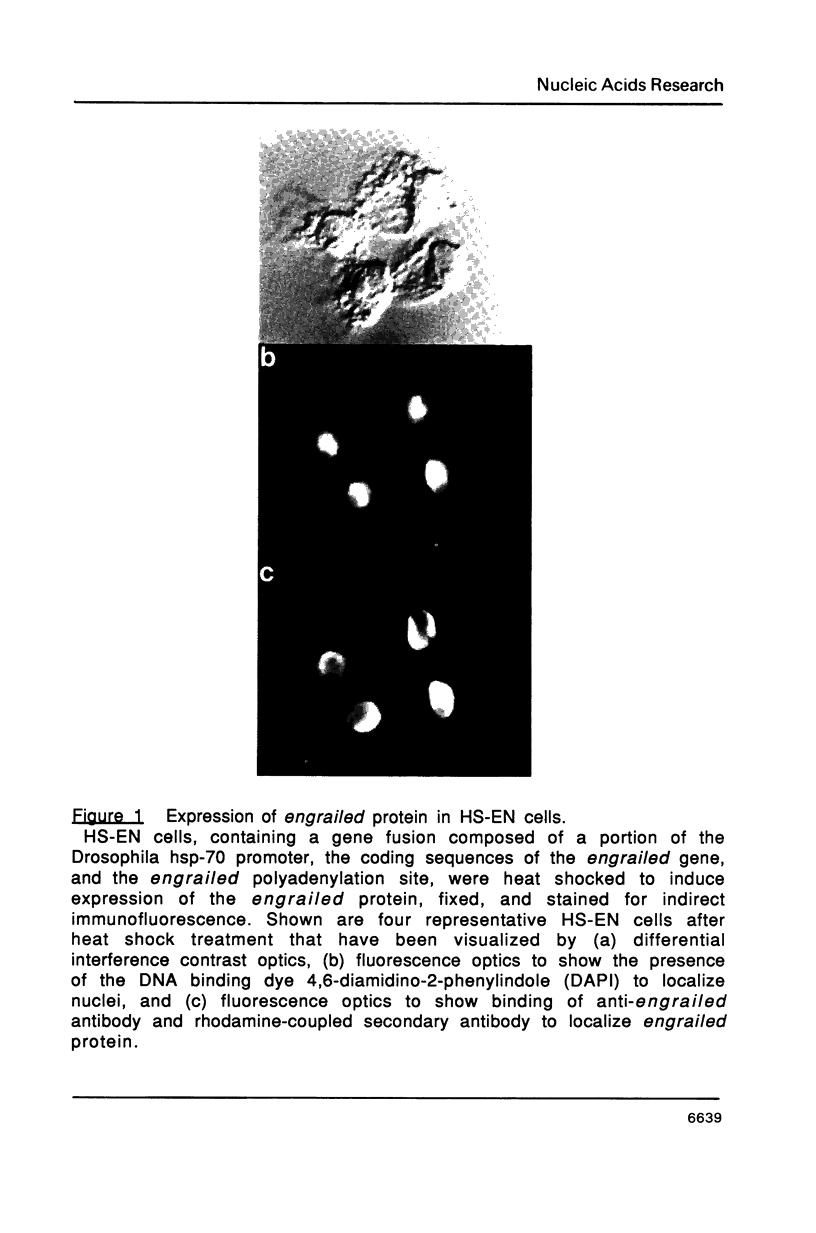
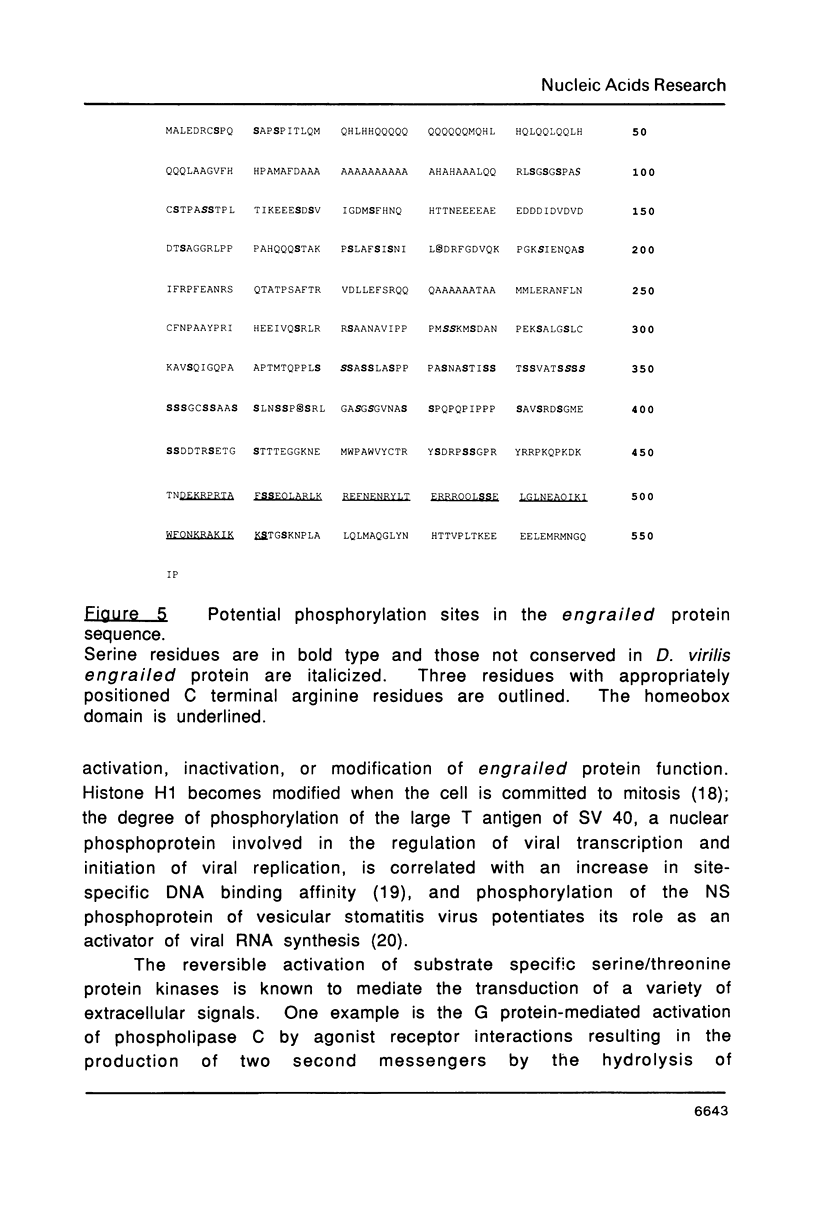
The engrailed gene is required during embryogenesis of Drosophila melanogaster for normal segmental development and for differentiation of posterior compartments. The protein encoded by the engrailed gene contains a homeodomain, has sequence specific DNA binding activity, and has been proposed as a transcriptional regulator. We show here that the engrailed protein, isolated from both cultured cells and embryos, has been modified by a serine-specific protein kinase. This is the first report that homeobox proteins are post-translationally modified. Phosphorylation of the engrailed protein may directly or allosterically modify its function, and offers the possibility that the engrailed protein becomes phosphorylated in response to extracellular, mitogenic or positional stimuli.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Coleman K. G., Poole S. J., Weir M. P., Soeller W. C., Kornberg T. The invected gene of Drosophila: sequence analysis and expression studies reveal a close kinship to the engrailed gene. Genes Dev. 1987 Mar;1(1):19–28. doi: 10.1101/gad.1.1.19. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature. 1985 Dec 19;318(6047):630–635. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Kuner J. M., Theis J., O'Farrell P. H. Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell. 1985 Nov;43(1):59–69. doi: 10.1016/0092-8674(85)90012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. L., Anderson W. B. Interleukin-2 stimulates association of protein kinase C with plasma membrane. Nature. 1985 May 16;315(6016):233–235. doi: 10.1038/315233a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Santamaria P. Developmental analysis of the wing disc in the mutant engrailed of Drosophila melanogaster. Genetics. 1972 Sep;72(1):87–104. doi: 10.1093/genetics/72.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. P., Awgulewitsch A., Fainsod A., McGinnis W., Ruddle F. H. Homeo box gene complex on mouse chromosome 11: molecular cloning, expression in embryogenesis, and homology to a human homeo box locus. Cell. 1985 Nov;43(1):9–18. doi: 10.1016/0092-8674(85)90007-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B. Methodology for the study of the effect of drugs on development and DNA replication in Drosophila melanogaster embryonic tissue. Biochim Biophys Acta. 1984 Dec 14;783(3):205–215. doi: 10.1016/0167-4781(84)90030-7. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Martin G. R. En-1 and En-2, two mouse genes with sequence homology to the Drosophila engrailed gene: expression during embryogenesis. Genes Dev. 1987 Mar;1(1):29–38. doi: 10.1101/gad.1.1.29. [DOI] [PubMed] [Google Scholar]
- Karr T. L., Ali Z., Drees B., Kornberg T. The engrailed locus of D. melanogaster provides an essential zygotic function in precellular embryos. Cell. 1985 Dec;43(3 Pt 2):591–601. doi: 10.1016/0092-8674(85)90231-4. [DOI] [PubMed] [Google Scholar]
- Kassis J. A., Poole S. J., Wright D. K., O'Farrell P. H. Sequence conservation in the protein coding and intron regions of the engrailed transcription unit. EMBO J. 1986 Dec 20;5(13):3583–3589. doi: 10.1002/j.1460-2075.1986.tb04686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T. Engrailed: a gene controlling compartment and segment formation in Drosophila. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1095–1099. doi: 10.1073/pnas.78.2.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg T., Sidén I., O'Farrell P., Simon M. The engrailed locus of Drosophila: in situ localization of transcripts reveals compartment-specific expression. Cell. 1985 Jan;40(1):45–53. doi: 10.1016/0092-8674(85)90307-1. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Morata G. Compartments in the wing of Drosophila: a study of the engrailed gene. Dev Biol. 1976 Jun;50(2):321–337. doi: 10.1016/0012-1606(76)90155-x. [DOI] [PubMed] [Google Scholar]
- Montenarh M., Henning R. Simian virus 40 T-antigen phosphorylation is variable. FEBS Lett. 1980 May 19;114(1):107–110. doi: 10.1016/0014-5793(80)80870-2. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Rio D. C., Rubin G. M. Transformation of cultured Drosophila melanogaster cells with a dominant selectable marker. Mol Cell Biol. 1985 Aug;5(8):1833–1838. doi: 10.1128/mcb.5.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Rhee L., Yadegari R., Paro R., Ullrich A., Goeddel D. V. Structure and nucleotide sequence of a Drosophila melanogaster protein kinase C gene. EMBO J. 1987 Feb;6(2):433–441. doi: 10.1002/j.1460-2075.1987.tb04773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]