Abstract
A major challenge in allogeneic hematopoietic cell transplantation is how to transfer T-cell immunity without causing graft-versus-host disease (GVHD). Effector memory T cells (CD62L−) are a cell subset that can potentially address this challenge because they do not induce GVHD. Here, we investigated how CD62L− T cells contributed to phenotypic and functional T-cell reconstitution after transplantation. On transfer into allogeneic recipients, CD62L− T cells were activated and expressed multiple cytokines and cytotoxic molecules. CD62L− T cells were able to deplete host radioresistant T cells and facilitate hematopoietic engraftment, resulting in enhanced de novo T-cell regeneration. Enhanced functional immune reconstitution was demonstrated in CD62L− T-cell recipients using a tumor and an influenza virus challenge model. Even though CD62L− T cells are able to respond to alloantigens and deplete host radioresistant immune cells in GVHD recipients, alloreactive CD62L− T cells lost the reactivity over time and were eventually tolerant to alloantigens as a result of prolonged antigen exposure, suggesting a mechanism by which CD62L− T cells were able to eliminate host resistance without causing GVHD. These data further highlight the unique characteristics of CD62L− T cells and their potential applications in clinical hematopoietic cell transplantation.
Introduction
Hematopoietic cell transplantation begins with a preparatory regimen that destroys the host immune system (especially T cells), thereby allowing the engraftment of donor stem cells.1 The reconstitution of T cells after hematopoietic cell transplantation depends on the mature T cells in the graft and on the de novo regeneration of T cells from hematopoietic stem cells.2 Donor-type mature T cells provide immediate immunity against infectious agents and tumor cells to the host.2 However, donor T cells also cause life-threatening graft-versus-host disease (GVHD).3 Moreover, GVHD and the immunosuppressive treatments used to prevent or control GVHD result in severely impaired thymopoiesis and T-cell deficiency in the graft recipient.4 De novo T-cell regeneration from hematopoietic stem cells is a very slow process, usually taking months and even years.5–8 Under current treatment protocols, the overall T-cell recovery can be very slow after allogeneic hematopoietic cell transplantation, making hematopoietic cell recipients extremely susceptible to a variety of opportunistic infections for a significant period of time.6,9 As a result, infections have remained a major cause of morbidity and mortality after hematopoietic cell transplantation.9
Because of the slow de novo regeneration of stem- and progenitor-derived T cells, a population of T cells that does not cause GVHD would be extremely helpful to protect the recipients from infections in the first few months after transplantation before new T cells can be generated from hematopoietic stem or progenitor cells. We and others have recently observed that allogeneic effector memory T cells (TEM; CD62L−)10 do not cause GVHD and contribute directly to posttransplantation T-cell recovery.11–16 We further demonstrated that CD62L− T cells contribute to after transplantation T-cell reconstitution not only through peripheral expansion but also through thymopoiesis.11 These important observations suggest that CD62L− T cells are capable of protecting hematopoietic cell recipients from infections early after transplantation by providing immediate recall immunity and later by promoting more diverse T-cell regeneration through thymopoiesis. Because depletion of host radioresistant T cells is associated with the enhancement of immune reconstitution,11 it is likely that CD62L− T cells enhance stem/progenitor cell mediated de novo T cell regeneration through facilitating hematopoietic cell engraftment.
Here, we further investigated whether and how CD62L− T cells enhanced functional immune reconstitution after allogeneic stem cell transplantation. CD62L− T cells were able to prolong the survival of T cell–depleted (TCD) bone marrow (BM) recipients after challenge with a tumor cell line or with live influenza viruses. CD62L− T cells facilitated hematopoietic progenitor engraftment, leading to enhanced immune reconstitution after hematopoietic stem cell transplantation. On transfer into irradiated BALB/c recipients, donor CD62L− C57BL/6 T cells were activated, secreted multiple inflammatory cytokines, and expressed many cytotoxic molecules such as perforin and granzyme B. We also investigated why the activation of CD62L− T cells by alloantigens only led to host cell depletion but not GVHD.
Methods
Mice
BALB/c (H2d, CD45.2, Thy1.2, Mls-2a, Mls-3a), C57BL/6, CD45.2, Thy1.2 (H2b, Mls-2b, Mls-3b, termed B6 CD45.2 mice), BALB/c severe immunodeficiency (SCID), NOD.Cg-Prkdcscid (NSG) mice were purchased from The Jackson Laboratory. Rag2−/−γC−/− C57BL/6 mice17 were purchased from Taconic Farms. The breeders of C57BL/Ka, CD45.1, Thy1.1 mice (H2b, Mls-2b, Mls-3b, termed B6 CD45.1 mice) were provided by Dr Jos Domen (Duke University, Durham, NC). All animals were female and were used when they were 8 to 12 weeks old except that some T-cell donors were male and up to 14 months old. Animal were housed in sterile microisolator cages in a specific pathogen-free facility throughout the study. This study was approved by the Duke University Institution Animal Care and Use Committee.
BCL1 cell lines
BCL1 (a kind gift from Drs Defu Zeng and Samuel Strober, Stanford University, Stanford, CA) is a spontaneous B-cell leukemia/lymphoma cell line of BALB/c origin.18 Purified BCL1 cells were obtained from spleen cells from BCL1-bearing mice by positive selection using anti-BCL1 idiotype antibody19 (rat IgG2a, clone 6A5; a generous gift from Dr Ellen S. Vietta, University of Texas, Dallas) followed by using goat anti–rat immunoglobulin G magnetic beads (Miltenyi Biotec).
T-cell separation
CD62L− T cells were isolated based on a published protocol from our laboratory, with modification.20 The only difference was that a mouse pan-T-cell selection kit (Miltenyi Biotec) was used to negatively purify splenic T cells.
Hematopoietic cell transplantation
Recipient BALB/c mice were lethally irradiated (8.5 Gy for regular BALB/c mice; 5 Gy for BALB/c SCID mice; 3.25 Gy for NSG mice; 8 Gy for Rag2−/−γC−/− C57BL/6 mice). Within 4 hours after irradiation, all recipients were transplanted via tail-vein injection. For the rejection model, recipients were transplanted with 5 × 105 TCD BM cells. For the immune reconstitution model, recipients were transplanted with 1 × 107 TCD BM cells or 5000 hematopoietic stem cells (c-Kit+Thy1.1lowLin−/lowSca-1+ or KTLS cells21–23) with or without 1 × 106 T cells. Except where indicated, the experiments were performed in the C57BL/6→BALB/c system.
Peripheral blood cell counts
Blood cells were counted in an automatic hematology analyzer (HEMAVET HV950FS; Drew Scientific).
Intracellular staining
Spleen cells were first stained with surface markers. Cells were then fixed, permeabilized, and intracellularly stained according to the manufacturer's instructions (eBioscience). Except where indicated, all antibodies were purchased from eBioscience. Isotype control was used to define the negative populations.
Tumor model
Lethally irradiated BALB/c or Rag2−/−γC−/− mice were transplanted with 1 × 107 TCD BM cells from B6 CD45.2 mice or total BM cells from Rag2−/−γC−/− C57BL/6 mice with or without CD62L− T cells (1 × 106) from B6 CD45.1 mice via tail-vein injection. BCL1 cells (5 × 105) were injected intravenously to recipients at different times after transplantation. Mortality was recorded daily. Biopsies were taken from spleen, lung, and liver for histologic analyses for evidence of leukemia or lymphoma.
Flu model
Lethally irradiated BALB/c mice were transplanted with TCD BM cells (1 × 107) with or without CD62L− T cells (1 × 106) from C57BL/6 mice via tail-vein injection. At day 7 after transplantation, all recipients were administered with 1.4 × 102 PFU influenza virus (A/PR/8/34 murine influenza; Charles River Laboratories) in 20 μL of PBS by intranasal inoculation. Mock-challenged controls (normal BALB/c mice) were administered 20 μL of PBS in the same way. Weight loss, change in body temperature, and survival were monitored daily. Mice with more than 25% weight loss were humanely killed.
Statistical analysis
Data were presented as mean ± SD or mean + SD. Comparison of continuous data between groups was performed by either unpaired t test (2 groups) or ANOVA (> 2 groups). Survival data were analyzed by log rank test. Statistical analyses were performed using StatView Version 5 (SAS Institute), Excel 2010 (Microsoft), or Prism software Version 5 (GraphPad Software). P values < .05 were considered significant.
Other methods
Other methods used in this study have been described in detail previously.24
Results
CD62L− T cells enhance the ability of TCD BM recipients to prevent tumor growth
Our previously published study11 has demonstrated that CD62L− T cells enhance phenotypic T-cell recovery. To determine whether CD62L− T cells enhance functional immune recovery, lethally irradiated BALB/c recipients were first transplanted with 1 × 107 TCD BM cells with or without 1 × 106 CD62L− T cells from B6 CD45.2 mice. At day 7 after transplantation, the recipient mice were challenged with 5 × 105 BCL1 tumor cells. BCL1 is a host-type leukemia/lymphoma cell line.18 In an allogeneic transplantation setting, T cells play a critical role in preventing BCL1 growth.19
Development of tumor(s) and survival were monitored, and the results are summarized in Figure 1A-B. Although 6 of 8 TCD BM only recipients developed tumors and died within 30 days after transplantation, none of CD62L− T-cell recipients developed tumors, and all recipients survived for more than 100 days after transplantation (P < .001). Consistent with our previously published results,11 none of CD62L− T-cell recipient mice developed GVHD (data not shown).
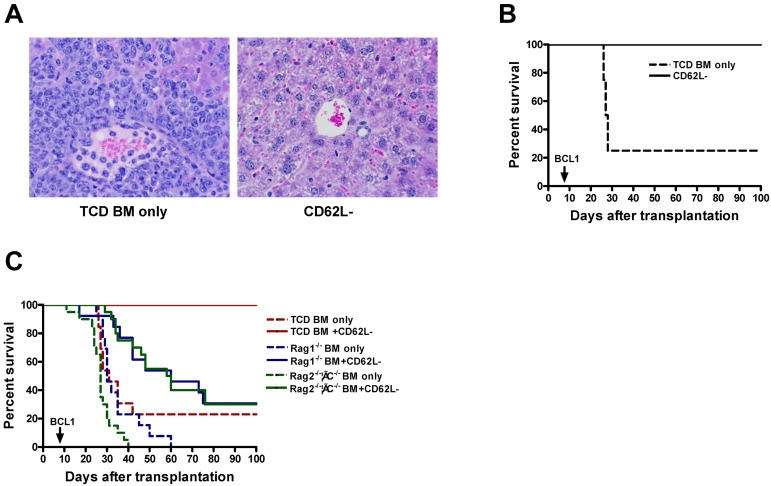
Figure 1.
CD62L− T cells enhance the ability of TCD BM recipients to prevent tumor growth. T cell–depleted bone marrow cells (1 × 107) from B6 CD45.2 (A-C) or Rag1−/− or Rag2−/−γC−/− (C) mice and CD62L− T cells (1 × 106) from unprimed B6 CD45.1 mice were first transplanted into lethally irradiated BALB/c recipients. The recipient mice were then challenged with 5 × 105 host-type BCL1 tumor cells at day 7 after transplantation. The recipient mice were monitored for development of leukemia or lymphoma and survival. Each group contained 8 to 20 mice. The data are pooled from 2 independent experiments. (A) Histologic analysis of liver (H&E stain, ×100). Tissue from TCD BM only group was obtained on day 28; tissue from CD62L− T-cell recipients was obtained on day 166. (B) Survival (P < .001). (C) Survival: TCD BM only versus Rag1−/− BM only or Rag2−/−γC−/− BM only (P < .05); BM only groups versus their corresponding CD62L− groups (P < .01); TCD BM + CD62L− versus Rag1−/− BM + CD62L− or Rag2−/−γC−/− BM + CD62L− (P < .0001).
To determine whether the enhancement of antitumor immunity in CD62L− T-cell recipients is the direct effect of transplanted CD62L− T cells or newly generated immune cells from stem or progenitor cells, we repeated the above-mentioned experiment using bone marrow from T, B, and natural killer (NK) cell–deficient Rag2−/−γC−/− B6 mice. Similar to the data shown in Figure 1A and B, none of CD62L− T-cell recipients who also received TCD BM from wild-type donors (B6 CD45.2) developed tumors, and all recipients survived for more than 100 days after transplantation. By contrast, all CD62L− T-cell recipients transplanted together with Rag2−/−γC−/− bone marrow developed tumors, and 14 of 20 mice died by 100 days (Figure 1C; P < .0001). Similar results were also obtained when Rag1−/− mice (T and B but not NK cell deficient) were used as bone marrow donor (Figure 1C). It is of note that B6 CD45.2 TCD BM only group survived longer than both Rag1−/− and Rag2−/−γC−/− BM only groups (Figure 1C; P < .05), probably because tumor cells were inoculated on day 7 when new T and B cells had already been generated from B6 CD45.2 but not from Rag1−/− or Rag2−/−γC−/− marrow.
CD62L− T cells enhance the ability of TCD BM recipients to counteract the effects of viral infection in vivo
The ability of CD62L− T cells to enhance functional immune recovery in allogeneic hematopoietic cell recipients was further tested using an influenza virus challenge model. Similar to the tumor challenge model (Figure 1A-B), CD62L− T-cell recipients were challenged with 1.4 × 102 PFU influenza virus at day 7 after transplantation. Weight change, body temperature, and survival were monitored. The results were summarized in Figure 2A. On challenge with influenza virus, TCD BM only recipients rapidly developed symptoms of influenza; namely, rapid weight loss and decrease in body temperature, followed by death of all animals within 17 days after challenge. The onset of disease was significantly delayed in allogeneic CD62L− T-cell recipients compared with the TCD BM only recipients. Even though all allogeneic CD62L− T-cell recipients developed flu disease, they survived significant longer than TCD BM control animals (median survival time, 15 vs 30.5 days; P < .0001).
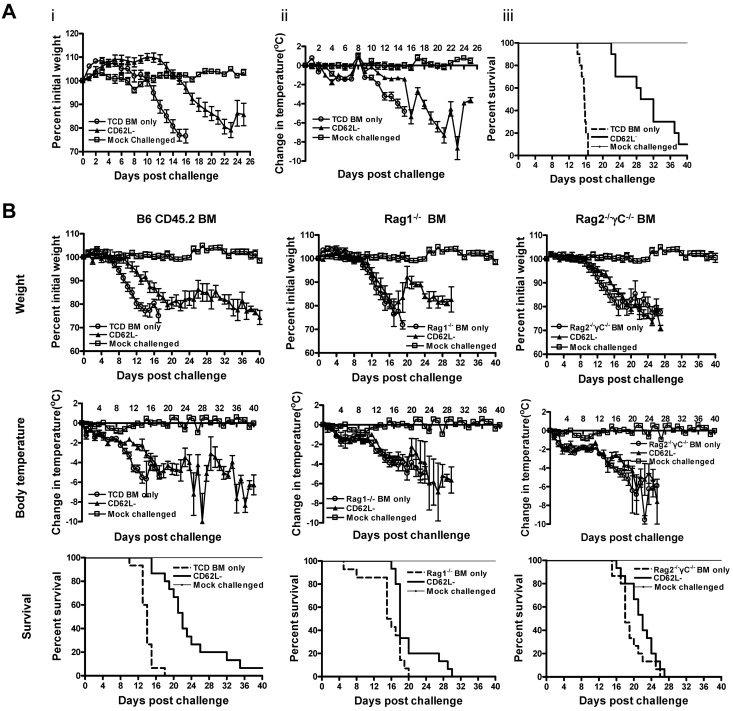
Figure 2.
CD62L− T cells enhance the ability of TCD BM recipients to counteract the effects of viral infection in vivo. Bone marrow cells (1 × 107) from different sources and CD62L− T cells (1 × 106) from unprimed B6 CD45.1 mice were transplanted into lethally irradiated BALB/c recipients. The recipient mice were challenged with influenza virus at day 7 after transplantation. Body weight and body temperature were monitored daily in all recipients. Each group contained 10 (A) or 15 (B) mice. A group of normal BALB/c mice (n = 5) was included as mock-challenged control for each experiment. (A) TCD BM was from B6 CD45.2 mice. CD62L− versus TCD BM only (P < .0001); TCD BM only versus mock-challenged (P < .001). (B) Bone marrow used included TCD BM cells from B6 CD45.2 mice or whole marrow from Rag1−/− B6 or Rag2−/−γC−/− B6 mice. For survival, BM only versus their corresponding CD62L− groups except for Rag2−/−γC−/− (P < .01); TCD BM only verus Rag1−/− BM only or Rag2−/−γC−/− BM only (P < .01); P = NS in all other comparisons.
To understand how CD62L− T cells enhance the ability of TCD BM recipients to fight against influenza virus infection, we performed similar experiments as described in Figure 1C. Although CD62L− T-cell recipients transplanted with B6 CD45.2 TCD BM survived much longer than TCD BM only recipients (Figure 2B; P < .0001), CD62L− T cells did not help Rag2−/−γC−/− bone marrow recipients survive longer (Figure 2B; P = NS). To further define the specific role of NK cells, we also transplanted CD62L− T-cell recipients together with Rag1−/− bone marrow. In contrast to antitumor responses (Figure 1C), CD62L− T-cell recipients transplanted with Rag1−/− bone marrow survived longer than Rag1−/− bone marrow alone recipients (Figure 2B; P < .01). Even though it was not statistically significant, CD62L− T-cell recipients transplanted with B6 CD45.2 bone marrow trended to survive longer than those transplanted together with Rag1−/− bone marrow (median survival time, 22 vs 18 days; P = .06; Figure 2B).
CD62L− T cells enhance hematopoietic cell engraftment after TCD BM transplantation
It was reported previously that CD62L− T cells were able to kill host radioresistant T cells11 and host-type tumor cells,25,26 suggesting that CD62L− T cells might facilitate hematopoietic stem cell engraftment. To determine whether CD62L− T cells could truly facilitate hematopoietic stem cell engraftment, we used a rejection model in which lethally irradiated BALB/c mice were transplanted with a minimum number (5 × 105) of B6 CD45.2 TCD BM cells. As illustrated in Figure 3A, only 40% of TCD BM only recipients survived more than 100 days after transplantation because of engraftment failure. Both total white blood cell and platelet counts were significantly lower in TCD BM only recipients compared with CD62L− T cell recipients at day 30 (Figure 3B; P < .05). All TCD BM only recipients had a mixed chimera phenotype when measured at day 30 (Figure 3C). By contrast, the addition of 1 × 106 donor CD62L− T cells to the TCD BM graft rescued 90% of the recipients (P < .01, compared with TCD BM only group), and all recipients became full donor chimeras by day 30 (P < .05). Similar results on chimerism were obtained at day 70 and day 100 (data not shown), indicating that the engraftment was durable.
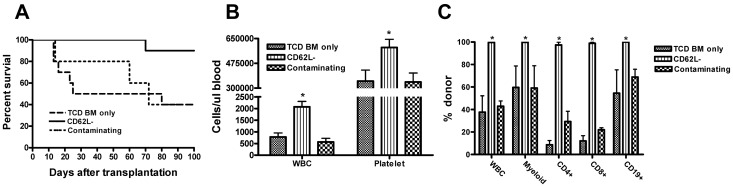
Figure 3.
CD62L− T cells facilitate hematopoietic cell engraftment after allogeneic TCD BM transplantation. TCD BM cells from B6 CD45.2 (5 × 105) and CD62L− T cells (1 × 106) from unprimed B6 CD45.1 mice were transplanted into lethally irradiated BALB/c recipients. Mortality was recorded daily. Each group contained 5 to 10 animals (TCD BM only, 10; CD62L−, 10; and contaminating, 5). The data are the representative of 2 independent experiments with similar data. (A) Survival. CD62L− versus TCD BM only or contaminating (P < .01); TCD BM only versus contaminating (P = .86). (B) Hematologic recovery. The recovery of white blood cells (WBCs) and platelets was analyzed in peripheral blood at day 30 after transplantation. All values represent means + SD (*P < .05; CD62L− vs TCD BM only or contaminating in both WBC and platelets). The mean values of WBCs and platelets in peripheral blood of normal BALB/c mouse (age, 8-12 weeks; female) were 6.1 ± 1.5 K/μL and 716.1 ± 142.4 K/μL, respectively. (C) Chimerism analysis. Chimerism in different cell subsets was analyzed in peripheral blood by flow cytometry on day 30 after transplantation. All values represent means + SD (*P < .05; CD62L− versus TCD BM only or contaminating in all cell subsets except CD62L− vs contaminating in CD19+ cells).
It has been demonstrated in mice27 and humans1 that naive T cells have strong effects on facilitating hematopoietic cell engraftment. Even though the contamination of CD62L+ T cells was very small (always < 0.5%), there was a possibility that the enhancement of hematopoietic cell engraftment was mediated by the contaminating CD62L+ T cells. To exclude this possibility, we included an additional group in which the same number (4 × 103) of CD62L+ T cells contained in the 1 × 106 CD62L− T cell graft was added to the TCD BM graft. As demonstrated in Figure 3A through C, addition of 4 × 103 CD62L+ T cells neither significantly prolonged the survival of TCD BM recipients, accelerated hematopoietic recovery, nor converted mixed chimeras into full donor chimeras.
CD62L− T cells promote purified hematopoietic stem cell–derived T-cell regeneration
Because hematopoietic stem cell dose correlates with the speed of immune reconstitution after stem cell transplantation,24 facilitation of engraftment (Figure 3) by CD62L− T cells may lead to enhanced immune reconstitution. Indeed, our published data11 strongly suggest that allogeneic CD62L− T cells are able to promote stem- or progenitor-derived T-cell generation because TCD BM was used in that study. To directly test whether CD62L− T cells were able to support the growth of stem cell–derived T cells, we performed an experiment using purified stem cells. In this experiment, we transplanted 1 × 106 CD62L− T cells from unprimed B6 CD45.2 mice and 5 × 103 KTLS cells from B6 CD45.1 mice into lethally irradiated BALB/c recipients. CD4+ and CD8+ T-cell counts were monitored in peripheral blood by flow cytometry over time. As illustrated in Figure 4A, the promoting effect of CD62L− T cells on stem cell–mediated T-cell regeneration was maintained in both CD4+ and CD8+ T cells through day 42, directly demonstrating that allogeneic CD62L− T cells were able to enhance T-cell generation from hematopoietic stem cells.
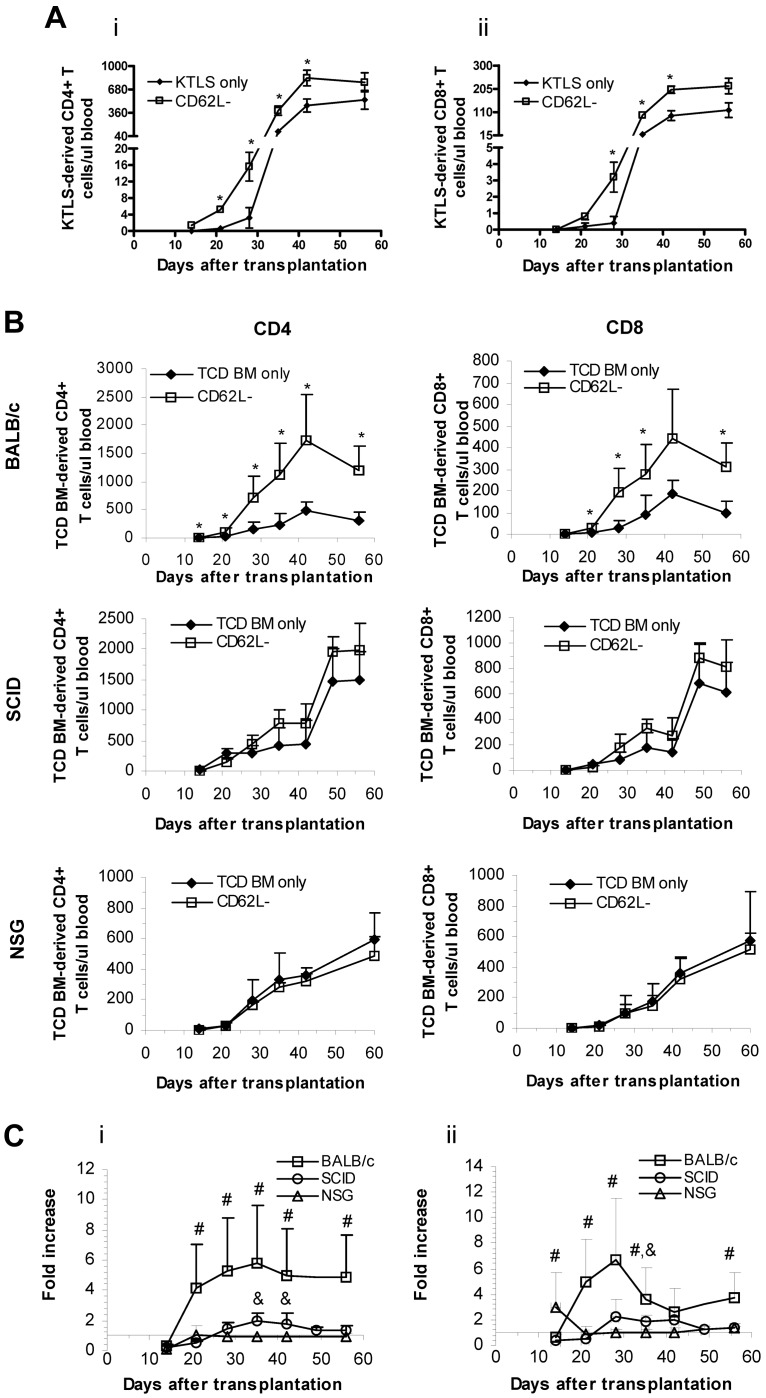
Figure 4.
CD62L− T cells enhance stem cell–mediated T-cell regeneration. (A) Purified hematopoietic stem cells (KTLS, 5 × 103) from B6 CD45.1 mice and CD62L− T cells (1 × 106) from B6 CD45.2 mice were transplanted into lethally irradiated BALB/c recipients. T-cell reconstitution was monitored at different times in peripheral blood by flow cytometry. Data represent absolute counts in peripheral blood (mean ± SD/μL blood). Each group contained 5 mice (*P < .05 between groups). (i) CD4+ T cells. (ii) CD8+ T cells. KTLS indicates c-Kit+Thy1.1lowLin−/lowSca-1+ hematopoietic stem cells. (B-C) TCD BM cells (1 × 107) from B6 CD45.2 mice and CD62L− T cells (1 × 106) from B6 CD45.1 mice were transplanted into irradiated BALB/c or SCID or NSG recipients. T-cell reconstitution was monitored in peripheral blood at different times after transplantation by flow cytometry. Data represent absolute counts in peripheral blood (mean + SD/μL blood). Each group contained 5 to 10 animals. The data were pooled from 2 independent experiments. Fold increase of peripheral T-cell counts in CD62L− T-cell recipients over TCD BM only control is shown in C (*P < .05, TCD BM only vs CD62L−; #P < .05, BALC/c vs SCID or NSG; &P < .05, SCID vs NSG).
CD62L− T cells lose the promoting effects on stem cell–mediated T-cell regeneration in recipients with limited or no host resistance
Our results were consistent with a model in which CD62L− T cells promoted T-cell regeneration from stem cells via depleting host radioresistant T cells that could interfere with T-cell regeneration. Specifically, the finding that host radioresistant T cells were depleted in CD62L− T-cell recipients but not in the TCD BM control mice11 suggested that CD62L− T cells might eliminate radioresistant host cells. To test directly whether CD62L− T cells promote new T-cell generation by overcoming T cell–mediated host resistance, we repeated the immune reconstitution experiments11 using BALB/c SCID mice (lacking both T and B cells but not NK cells) as recipients. We transplanted 1 × 106 CD62L− T cells from unprimed B6 CD45.1 mice and 1 × 107 B6 CD45.2 TCD BM cells into lethally irradiated SCID BALB/c recipients. CD4+ and CD8+ T-cell counts were monitored in peripheral blood by flow cytometry over time. The results presented in Figure 4B and C demonstrated that the promoting effect of CD62L− T cells was dramatically decreased when SCID mice were used as recipients compared with BALB/c mice.
It was observed that there was a trend toward higher T-cell counts in SCID recipients of CD62L− T cells after day 28 even though the differences were not statistically significant (Figure 4B). These potential differences could have reflected the resistance mediated by radioresistant NK cells.28 To investigate whether that was the case, we repeated the experiment using NSG mice (lacking T, B and NK cells and no known host resistance) as recipients. Using NSG mice as recipients, the promoting effect of CD62L− T cells could not be observed any more (Figure 4B-C). These data further indicated that allogeneic CD62L− T cells enhanced immune reconstitution through depletion of host radioresistant immune cells and enhancing hematopoietic stem or progenitor cell engraftment.
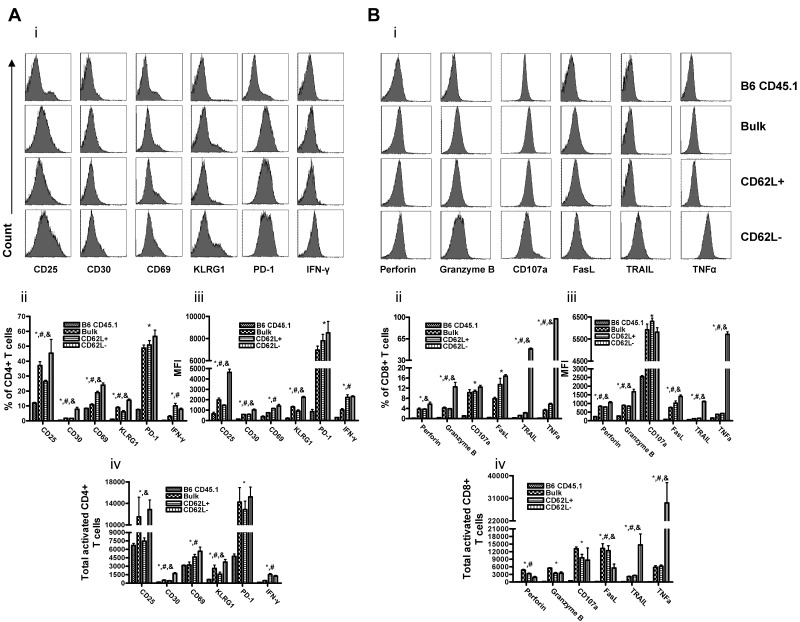
CD62L− T cells are activated, secrete multiple cytokines, and express cytotoxic molecules on transfer into recipients
Even though CD62L− T cells do not induce GVHD,11 the ability of these cells to deplete host-radioresistant T cells11 and kill host-type tumor cells25,26 suggests that CD62L− T cells from unprimed donors may be activated on transfer into allogeneic recipients. To confirm this, we transplanted 1 × 106 CD62L− T cells from unprimed B6 CD45.1 mice and 1 × 107 B6 CD45.2 TCD BM cells into lethally irradiated BALB/c recipients. At day 6, splenocytes were harvested, and the expression of activation molecules, cytokines, and cytotoxic molecules by CD62L− T cells was analyzed directly by flow cytometry without further in vitro stimulation. On transfer into irradiated BALB/c recipients, both CD4+ (Figure 5A) and CD8+ (data not shown) CD62L− T cells expressed many activation markers (eg, CD25, CD30, CD69, and KLRG1) and secreted IFN-γ at similar or even higher levels than not only normal B6 CD45.1 T cells but also transferred bulk and CD62L+ T cells. Moreover, CD8+ CD62L− T cells produced similar or higher amounts of multiple cytotoxic molecules, including perforin, granzyme B, CD107a, FasL, TRAIL, and TNF-α compared with both bulk and CD62L+ CD8+ T cells (Figure 5B). The total numbers of CD62L− T cells expressing most of the activated markers except perforin and FasL were also similar or higher than those of bulk and CD62L+ T cells (Figure 5Aiv,Biv). These results demonstrated that CD62L− T cells could be rapidly and fully activated on transfer into allogeneic recipients, explaining why CD62L− T cells from unprimed donors were able to kill host radioresistant T cells11 and to enhance hematopoietic cell engraftment.
Figure 5.
CD62L− T cells are activated on transfer into allogeneic recipients. Bulk, CD62L+, or CD62L− T cells (1 × 106) from unprimed B6 CD45.1 mice were transplanted into lethally irradiated BALB/c recipients. On day 6, splenocytes were harvested and stained with indicated antibodies for flow cytometry analysis. The data shown including histogram, percentage of positive cells, mean fluorescence intensity (MFI), and total positive cells were gated on CD45.1+ T cells (all transplanted donor T cells). Normal B6 CD45.1 mice were included as a control. Each group contained 5 to 8 mice. The data are the representative of 2 independent experiments. All values represent means + SD (A) CD4+ T cells. (B) CD8+ T cells (*P < .05, CD62L− vs B6 CD45.1; #P < .05, CD62L− vs bulk; &P < .05, CD62L− vs CD62L+).
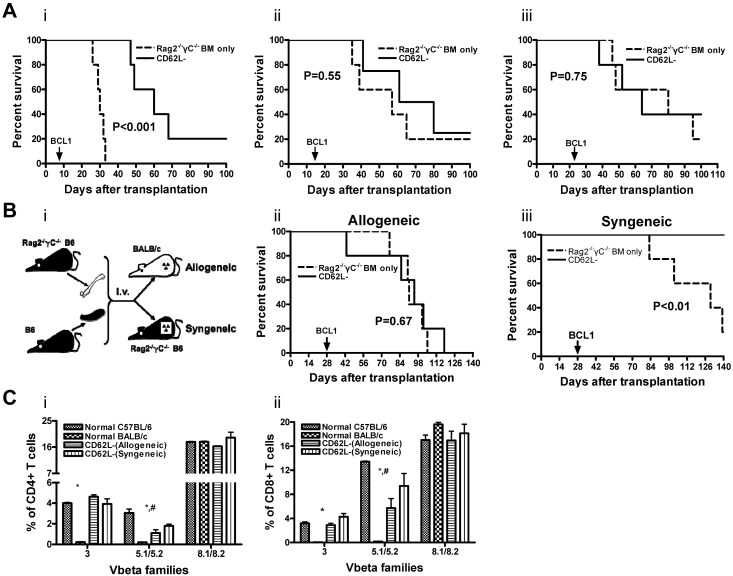
CD62L− T cells lose alloreactivity over time on transfer into allogeneic but not syngeneic recipients
The data presented here have convincingly demonstrated that, on transfer into allogeneic recipients, CD62L− T cells from unprimed donors can be activated (Figure 5) and are able to kill host-radioresistant T cells (Figure 3) and host-type tumor cells (Figure 1C). How can we reconcile these data with the previously published data11,13,14,16 demonstrating that these cells do not induce GVHD? To explain this dichotomy, we hypothesized that donor CD62L− T cells could react to alloantigens initially but the alloresponses could not be sustained on transfer into GVHD recipients based on our previously published in vitro observations.20 To test this hypothesis in vivo, we injected BCL1 cells (5 × 105) into lethally irradiated BALB/c recipients of Rag2−/−γC−/− BM (1 × 107) and B6 CD45.2 CD62L− T cells (1 × 106) at different times after stem cell transplantation (days 7, 14, 21, and 28). Because Rag2−/−γC−/− BM does not have the ability to produce T, B, and NK cells, the direct ability of CD62L− T cells could be tested. When injected at day 7, allogeneic CD62L− T-cell recipients survived significantly longer than Rag2−/−γC−/− BM only controls (Figure 6Ai; P < .001), indicating that CD62L− T cells from unprimed donors had the ability to respond to host-type tumor cells directly. When injected after day 14, the development of tumors and survival were comparable between Rag2−/−γC−/− BM only and allogeneic CD62L− T-cell groups (Figure 6Aii-iii), indicating that CD62L− T cells from unprimed donors lost their ability to react to host antigens in GVHD recipients over time.
Figure 6.
CD62L− T cells lose alloreactivity over time on transfer into allogeneic but not syngeneic recipients. Bone marrow cells (1 × 107) from Rag2−/−γC−/− B6 mice and CD62L− T cells (1 × 106) from unprimed B6 CD45.2 mice were transplanted into lethally irradiated BALB/c (A and Bii, allogeneic) or Rag2−/−γC−/− B6 (Biii, syngeneic) recipients. BALB/c-origin BCL1 cells (5 × 105) were injected into bone marrow recipients at different times after transplantation (Ai, day 7; Aii, day 14; Aii, day 21; B, day 28). The recipient mice were monitored for development of leukemia/lymphoma and survival. For Vβ family experiments (C), peripheral blood was obtained from allogeneic or syngeneic recipients of CD62L− T cells (same as B without BCL1 cell challenge) more than 100 days after transplantation. Percentages of Vβ+ cells were determined by flow cytometry. Each group contained 10 to 12 mice. The data were pooled from 2 independent experiments with similar data (*P < .05, normal BALB/c vs other groups; #P < .05, normal C57BL/6 vs CD62L− [allogeneic]).
Prolonged exposure to antigens has been reported to induce clonal deletion or clonal anergy of antigen-specific T cells in GVHD29 and other settings.30,31 To test whether this mechanism is responsible for the loss of alloreactivity of CD62L− T cells in GVHD recipients, we compared the ability of CD62L− T cells to kill host-type tumor cells in allogeneic and syngeneic recipients 28 days after transplantation. To test only the ability of donor CD62L− T cells to kill tumor cells, Rag2−/−γC−/− B6 mice were used as syngeneic recipients and BM donors (Figure 6Bi). Although CD62L− T cells completely lost the ability to kill BCL1 cells in allogeneic recipients (Figure 6Bii; P = .67; Rag2−/−γC−/− BM only vs CD62L−), they retained the reactivity in syngeneic recipients (Figure 6Biii; P < .01; Rag2−/−γC−/− BM only vs CD62L−). These data demonstrated that the gradual loss of CD62L− T cells' ability to respond to alloantigens on transfer into allogeneic recipients was a result of prolonged exposure to alloantigens.
To further determine whether prolonged exposure of CD62L− T cells to alloantigens leads to clonal anergy or deletion of antigen-specific T cells, we measured the percentages of Vβ3+ and Vβ5.1/5.2+ cells in peripheral blood from allogeneic recipients of CD62L− T cells more than 100 days after transplantation (no BCL1 challenge). Vβ3+ and Vβ5.1/5.2+ cells recognize Mls-2 and Mls-3 superantigens that are present in the recipient BALB/c mice but are absent in the donor C57BL/6 mice. As shown in Figure 6C, CD62L− T-cell allogeneic recipients had similar percentages of Vβ3+ in both CD4+ and CD8+ T-cell subsets compared with normal C57BL/6 mice and CD62L− T-cell syngeneic recipients. Even though the percentages of Vβ5.1/5.2+ among both CD4+ and CD8+ T cells in CD62L− T-cell allogeneic recipients were lower than those in normal C57BL/6 mice (P < .05), the differences between CD62L− T-cell allogeneic and syngeneic recipients were not significant. As a negative control, the percentages of Vβ8.1/8.2+ among both CD4+ and CD8+ T cells were similar in normal C57BL/6 mice, CD62L− T-cell allogeneic and syngeneic recipients.
Discussion
Previously published data from several different groups have independently demonstrated that CD62L− effector TEM from unprimed donors do not induce GVHD.11,13,14,16 Our current work further demonstrates that these T cells are able to facilitate hematopoietic engraftment and T-cell reconstitution. CD62L− T cells contribute to posttransplantation T-cell reconstitution not only directly and but also by promoting de novo T-cell regeneration from hematopoietic stem cells. The ability of CD62L− T cells to enhance stem cell–mediated T-cell reconstitution is of great clinical significance because now CD62L− T cells can not only provide immediate recall immunity11 to the host but also dramatically boost regeneration of new T cells through thymopoiesis. Newly generated T cells would presumably be more potent and have a broader T-cell repertoire capable of protecting the recipients from a wider range of opportunistic infections.2 Indeed, we demonstrate in 2 different models that CD62L− T cells enhance functional immune reconstitution against tumor (Figure 1) and viral (Figure 2) antigens. CD62L− T cells have direct antitumor effects as demonstrated in Figures 1C and 6Ai. In addition, enhanced reconstitution of stem cell–derived adaptive immunity contributes significantly to antitumor effects after transplantation of CD62L− T cells because antitumor activity is dramatically decreased when Rag1−/− BM are used (Figure 1C). In contrast, CD62L− T cells from unprimed donors have very limited direct anti-influenza immunity because CD62L− T cells completely lose their anti-influenza activity when Rag2−/−γC−/− BM is used (Figure 2B). Enhancement of NK cell regeneration is mainly responsible for enhanced anti-influenza activity after CD62L− T-cell transplantation because anti-influenza immunity is preserved even when Rag1−/− BM is used (Figure 2B). Because CD62L− T-cell recipients transplanted with B6 CD45.2 bone marrow seem to survive longer than those transplanted together with Rag1−/− bone marrow (Figure 2B), stem cell–derived adaptive immunity also may contribute to antiviral immunity. Even though CD62L− T cells from unprimed donors have limited direct antiviral activity, it is predicted that the anti-influenza immunity could be enhanced if the donor is immunized against influenza antigens before transplantation.10,12
Previous studies have demonstrated that CD62L− T cells are able to deplete host-radioresistant T cells11 and host-type tumor cells25,26 without causing GVHD, suggesting that allogeneic CD62L− T cells may facilitate hematopoietic engraftment. We further characterized the effect of allogeneic CD62L− T cells on hematopoietic stem or progenitor engraftment using a rejection model, in which a minimum numbers of TCD bone marrow cells were infused. Data from this experiment (Figure 3) demonstrate that CD62L− T cells rescue TCD bone marrow recipients from engraftment failure and significantly prolong survival. The ability of CD62L− T cells to facilitate hematopoietic cell engraftment is further supported by faster hematologic recovery (Figure 3B) and the conversion from mixed to full chimerism (Figure 3C) after addition of CD62L− T cells to the graft. These data are in line with recent data published by Dutt et al.26
Because higher numbers of hematopoietic stem24 and progenitor32,33 cells correlate with faster T-cell reconstitution after transplantation, facilitation of engraftment may lead to accelerated T-cell reconstitution. Indeed, we did observe in the previous study that CD62L− T cells enhance T-cell generation from TCD bone marrow cells.11 However, it is possible that T cells are derived from residual T cell in the TCD bone marrow graft in that study. To further confirm CD62L− T cells indeed promote T-cell regeneration from stem cells, we repeated the experiments using purified stem cells. The data from this experiment (Figure 4A) conclusively demonstrate that the T cells promoted by CD62L− T cells are derived from hematopoietic stem cells. This hypothesis also is supported by the results obtained from the recipients with limited or no host resistance. When BALB/c SCID mice (lacking T and B cells) are used as recipients, the promoting effect of CD62L− T cells is dramatically decreased (Figure 4B-C). Because classic SCID mice contain NK cells34 and NK cells are able to reject stem cell graft,35 host resistance remains in these mice. To further exclude the role of host radioresistant NK cells, we repeated the experiment using NSG mice, in which all T, B, and NK cells are absent. Data from this experiment (Figure 4B-C) demonstrate that the promoting effect of CD62L− T cells is completely lost when host immune barrier is absent. The observation that CD62L− T cells have decreased ability to prevent tumor growth when Rag2−/−γC−/− marrow is used (Figures 1C and 6A) provide functional evidence confirming that CD62L− T cells are able to enhance stem cell–mediated immune recovery. All these data strongly support that facilitation of hematopoietic engraftment by CD62L− T cells lead to enhanced stem cell–mediated T-cell regeneration. It has been reported that CD4+ T cells can stimulate hematopoiesis directly.36 Multiple hematopoietic cytokines that can support hematopoiesis are also produced during alloresponses.37 However, inability of CD62L− T cells to enhance stem cell–mediated T-cell regeneration in allogeneic SCID recipients (Figure 4B-C) argues against these 2 mechanisms.
How do CD62L− T cells deplete host-radioresistant T cells11 and facilitate hematopoietic cell engraftment (Figure 3)? Previously published data20,25 suggest that CD62L− T cells may be activated on transfer into allogeneic recipients. The data from the current work (Figure 5) clearly demonstrate that CD62L− T cells can be activated by alloantigens in vivo. Multiple activation markers including CD25, CD30, CD69, and KLRG1 can be detected in CD62L− T cells. The activated CD62L− T cells are able to produce IFN-γ, a critical cytokine in T-helper 1/cytotoxic T1 immune response.38 Multiple cytotoxicity pathways including perforin, granzyme B, FasL, TRAIL, and TNF-α are activated in CD62L− T cells and likely responsible for the depletion of host-radioresistant T cells. These data are consistent with the data published by Zheng et al,20,25 indicating that cytolytic function is essential for effector memory T cell–mediated GVL effect. Data from the in vivo tumor model (Figures 1C and 6A) demonstrate that CD62L− T cells from unprimed donors have direct effects on host-type tumor cells.
A critical question is why CD62L− T cells are able to kill host radioresistant T cells11 and tumor cells20,25 without inducing GVHD. Previously published in vitro24 and in vivo16,39 observations suggest that CD62L− T cells can react to alloantigens initially but the alloresponses cannot be sustained on transfer into GVHD recipients. Aborted alloresponses may result in decreased overall responses14,20,25 that fall below the threshold required for GVHD induction40,41 and eventually lead to tolerance against host antigens. To test whether this is the case in vivo, we injected host-type tumor cells (BCL1) into lethally irradiated BALB/c recipients of Rag2−/−γC−/− BM and CD62L− T cells from B6 CD45.2 mice at different times (days 7, 14, 21, and 28) after transplantation. Because Rag2−/−γC−/− mice do not contain and cannot generate T, B, and NK cells,17 the alloreactivity of CD62L− T cells could be clearly assessed through their ability to kill host-type tumor cells. The data from this experiment (Figure 6A) clearly demonstrate that CD62L− T cells are able to respond to alloantigens initially but lose alloreactivity over time on transfer into allogeneic recipients. Because previous studies have demonstrated that chronic antigenic stimulation can diminish antigen-specific T-cell responses in vivo,42,43 we speculate the reason why CD62L− T cells lose alloreactivity is because CD62L− T cells become tolerant to host alloantigens because of persistent antigen exposure. Indeed, this is the case because only CD62L− T cells transplanted into allogeneic but not syngeneic recipients lose the ability to kill host-type tumor cells (Figure 6B). Allospecific tolerance is mainly a result of clonal anergy because Vβ3+ and Vβ5.1/5.2+ cells, which recognize Mls-2 and Mls-3 superantigens expressed on host BALB/c cells, are not deleted (Figure 6C; Chen et al11) and do not respond to restimulation against the host H2 as well as Mls antigens.11
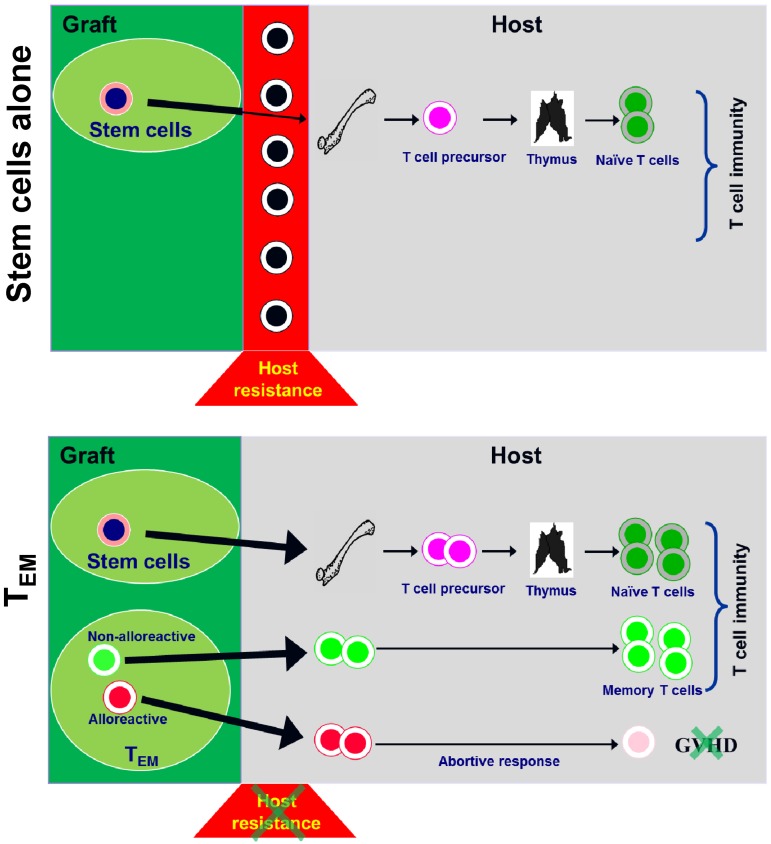
As summarized in Figure 7, besides providing immediate recall immunity to the host,11,13 allogeneic CD62L− T cells from unprimed donors can deplete host-radioresistant T cells and facilitate hematopoietic cell engraftment on transfer into allogeneic recipients. Facilitation of hematopoietic engraftment results in enhanced de novo T-cell regeneration from hematopoietic stem cells. Even though CD62L− T cells are able to respond to alloantigens and deplete host-radioresistant immune cells, CD62L− T cells become tolerant to host antigens as a result of prolonged antigen exposure in GVHD recipients, explaining why CD62L− T cells are able to eliminate host resistance without causing GVHD. It is important to point out that only the alloreactive portion of CD62L− T cell–mediated “abortive” responses. The nonalloreactive portion of CD62L− T cells can survive and function long term.11 The data presented in this article further highlight the unique characteristics of CD62L− T cells and their potential applications in clinical hematopoietic cell transplantation.
Figure 7.
Schema illustrating how CD62L− T cells contribute to T-cell reconstitution after allogeneic hematopoietic cell transplantation.
Acknowledgments
The authors thank Dr Nelson J. Chao for encouragement and support, Drs Defu Zeng and Samuel Strober for BCL1 cells, Dr Ellen S. Vitetta for 6A5 hybridoma, Dr Jos Domen for B6 CD45.1 mice; and Dr Zhiguo Li for advice on statistical analyses.
This study was supported by National Institutes of Health grant P01-CA047741 (B.J.C.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.Z., E.R., and B.J.C. designed research, performed research, analyzed data, and wrote the paper; and B.E.B., W.M., D.D., J.S., and X.C. designed research, performed research, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benny J. Chen, Division of Cellular Therapy/BMT, Department of Medicine, Duke University Medical Center, Box 3289, 247A Carl Bldg, Durham, NC 27710; e-mail: chen0032@mc.duke.edu.
References
- 1.Thomas ED, Blume KG, Forman SJ. Hematopoietic Cell Transplantation. Malden, MA: Blackwell Science; 1999. [Google Scholar]
- 2.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 3.Korngold R, Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. J Exp Med. 1978;148(6):1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dulude G, Roy DC, Perreault C. The effect of graft-versus-host disease on T cell production and homeostasis. J Exp Med. 1999;189(8):1329–1342. doi: 10.1084/jem.189.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 6.Klein AK, Patel DD, Gooding ME, et al. T-cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2001;7(8):454–466. doi: 10.1016/s1083-8791(01)80013-6. [DOI] [PubMed] [Google Scholar]
- 7.Roux E, Helg C, Dumont-Girard F, Chapuis B, Jeannet M, Roosnek E. Analysis of T-cell repopulation after allogeneic bone marrow transplantation: significant differences between recipients of T-cell depleted and unmanipulated grafts. Blood. 1996;87(9):3984–3992. [PubMed] [Google Scholar]
- 8.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97(5):1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 9.Wingard JR. Opportunistic infections after blood and marrow transplantation. Transpl Infect Dis. 1999;1(1):3–20. doi: 10.1034/j.1399-3062.1999.10102.x. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 11.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L− memory T cells without graft-versus-host disease. Blood. 2004;103(4):1534–1541. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 12.Spickett GP, Brandon MR, Mason DW, Williams AF, Woollett GR. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106(3):1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xystrakis E, Bernard I, Dejean AS, Alsaati T, Druet P, Saoudi A. Alloreactive CD4 T lymphocytes responsible for acute and chronic graft-versus-host disease are contained within the CD45RChigh but not the CD45RClow subset. Eur J Immunol. 2004;34(2):408–417. doi: 10.1002/eji.200324528. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Joe G, Zhu J, et al. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103(10):3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 17.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2(3):223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 18.Slavin S, Strober S. Spontaneous murine B-cell leukaemia. Nature. 1978;272(5654):624–626. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- 19.Chen BJ, Cui X, Liu C, Chao NJ. Prevention of graft-versus-host disease while preserving graft-versus-leukemia effect after selective depletion of host-reactive T cells by photodynamic cell purging process. Blood. 2002;99(9):3083–3088. doi: 10.1182/blood.v99.9.3083. [DOI] [PubMed] [Google Scholar]
- 20.Chen BJ, Deoliveira D, Cui X, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109(7):3115–3123. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1(8):661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 22.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 23.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin− Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992;175(1):175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen BJ, Cui X, Sempowski GD, Domen J, Chao NJ. Hematopoietic stem cell dose correlates with the speed of immune reconstitution after stem cell transplantation. Blood. 2004;103(11):4344–4352. doi: 10.1182/blood-2003-07-2534. [DOI] [PubMed] [Google Scholar]
- 25.Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111(4):2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutt S, Baker J, Kohrt HE, et al. CD8+ CD44hi but not CD4+ CD44hi memory T cells mediate potent graft antilymphoma activity without GVHD. Blood. 2011;117(11):3230–3239. doi: 10.1182/blood-2010-10-312751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin PJ. Donor CD8 cells prevent allogeneic marrow graft rejection in mice: potential implications for marrow transplantation in humans. J Exp Med. 1993;178(2):703–712. doi: 10.1084/jem.178.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6(9):938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez M, Quezada SA, Blazar BR, Panoskaltsis-Mortari A, Rudensky AY, Noelle RJ. The balance between donor T cell anergy and suppression versus lethal graft-versus-host disease is determined by host conditioning 2. J Immunol. 2002;169(10):5581–5589. doi: 10.4049/jimmunol.169.10.5581. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Yang Y. The fate of effector CD8 T cells in vivo is controlled by the duration of antigen stimulation. Immunology. 2006;118(3):361–371. doi: 10.1111/j.1365-2567.2006.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362(6422):758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 32.Arber C, BitMansour A, Sparer TE, et al. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102(2):421–428. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 33.Zakrzewski JL, Kochman AA, Lu SX, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12(9):1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 34.Dorshkind K, Keller GM, Phillips RA, et al. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984;132(4):1804–1808. [PubMed] [Google Scholar]
- 35.Gill S, Olson JA, Negrin RS. Natural killer cells in allogeneic transplantation: effect on engraftment, graft- versus-tumor, and graft-versus-host responses. Biol Blood Marrow Transplant. 2009;15(7):765–776. doi: 10.1016/j.bbmt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monteiro JP, Benjamin A, Costa ES, Barcinski MA, Bonomo A. Normal hematopoiesis is maintained by activated bone marrow CD4+ T cells. Blood. 2005;105(4):1484–1491. doi: 10.1182/blood-2004-07-2856. [DOI] [PubMed] [Google Scholar]
- 37.Krenger W, Hill GR, Ferrara JL. Cytokine cascades in acute graft-versus-host disease. Transplantation. 1997;64(4):553–558. doi: 10.1097/00007890-199708270-00001. [DOI] [PubMed] [Google Scholar]
- 38.Pelletier L, Bellon B, Druet P. Cytokines and immune response. Rev Prat. 1993;43(5):536–546. [PubMed] [Google Scholar]
- 39.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8(+) memory stem cells in graft-versus-host disease. Nat Med. 2005;11(12):1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 40.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105(11):4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakraverty R, Cote D, Buchli J, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203(8):2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111(6):837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 43.Kreuwel HT, Aung S, Silao C, Sherman LA. Memory CD8(+) T cells undergo peripheral tolerance. Immunity. 2002;17(1):73–81. doi: 10.1016/s1074-7613(02)00337-0. [DOI] [PubMed] [Google Scholar]