Abstract
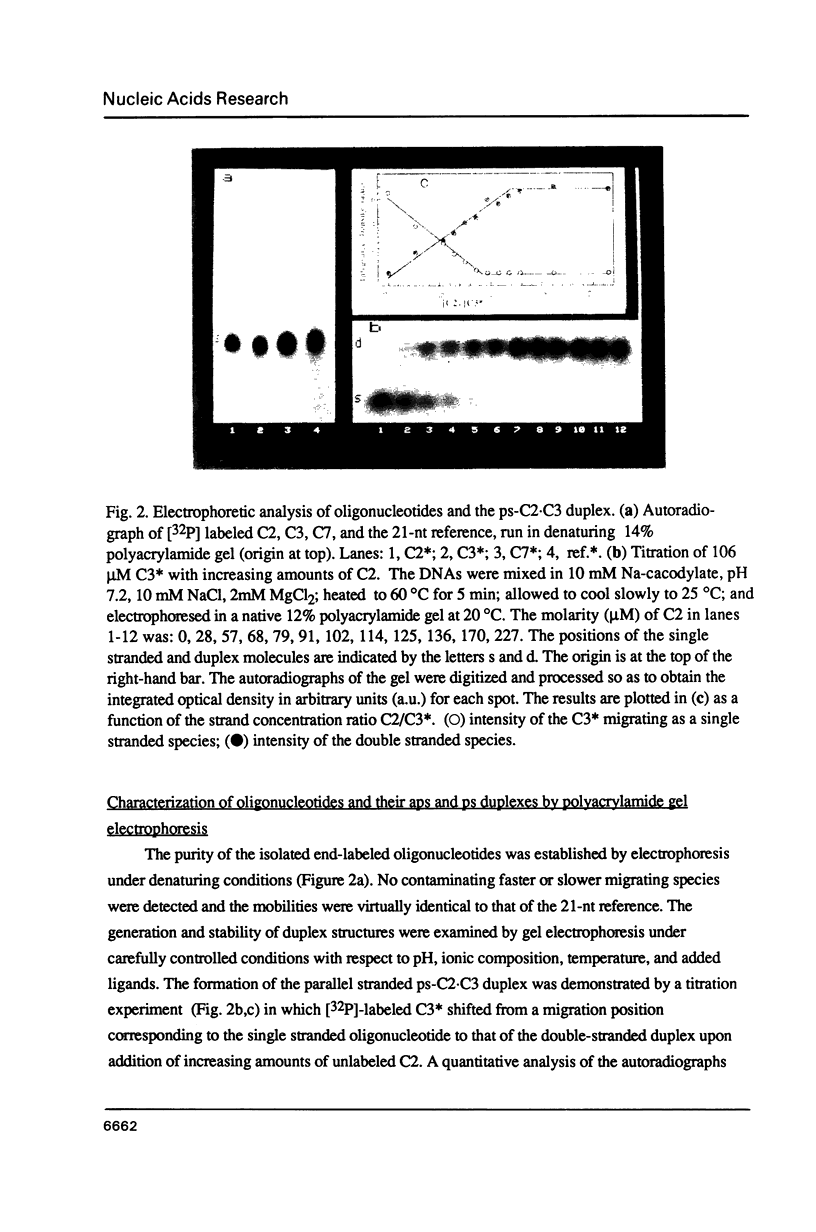
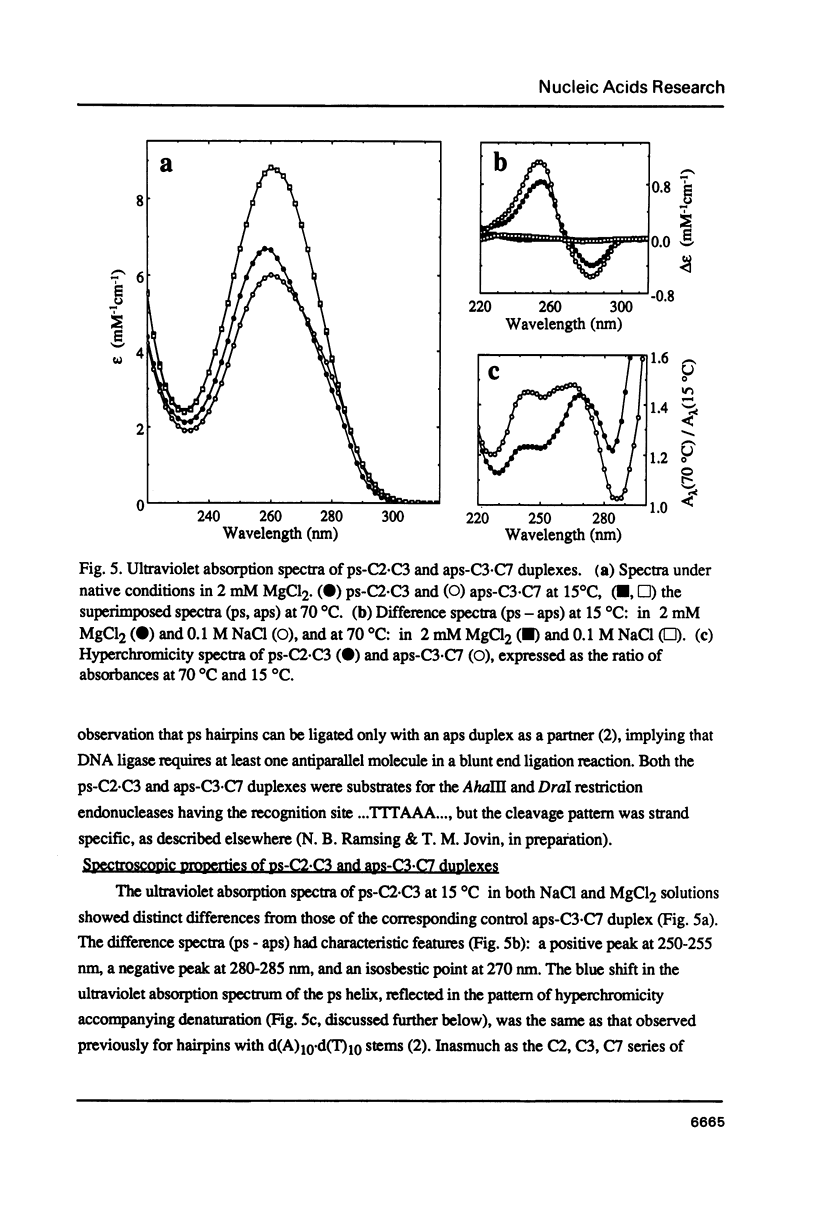
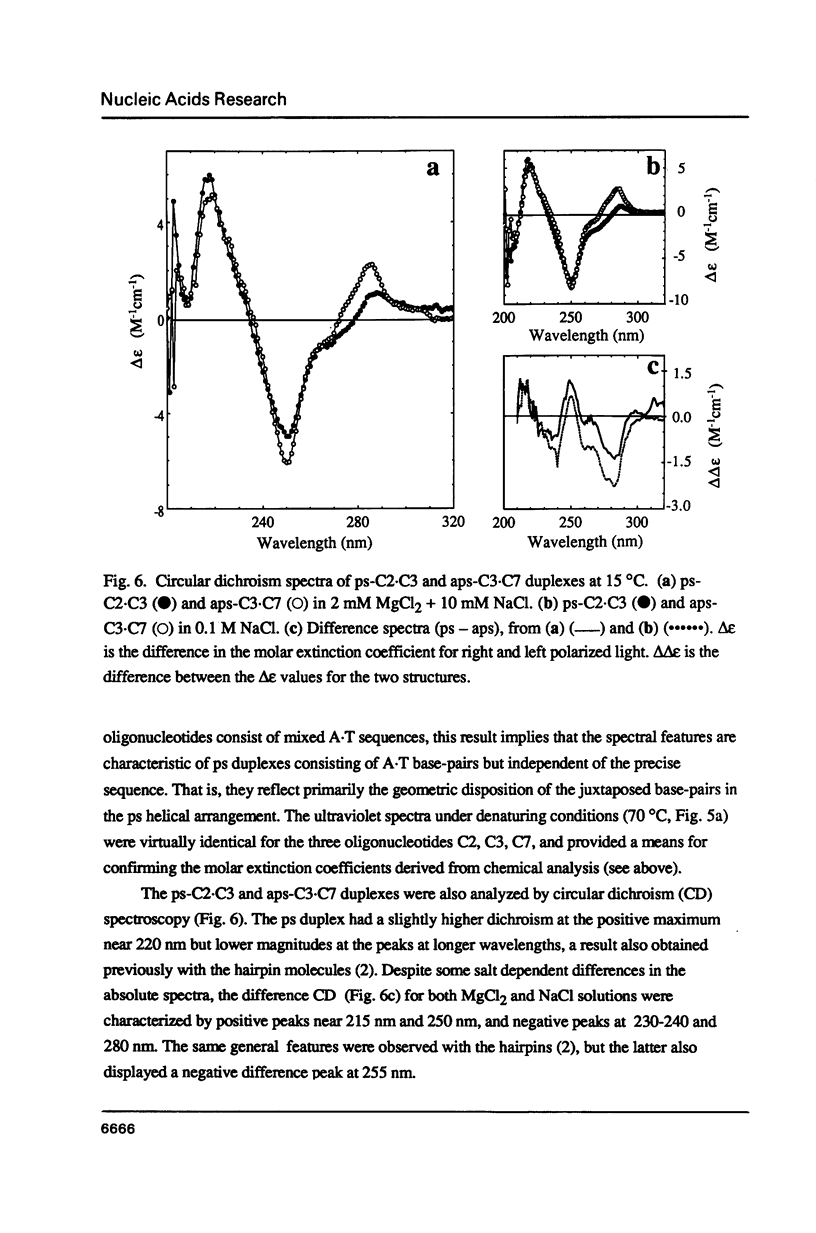
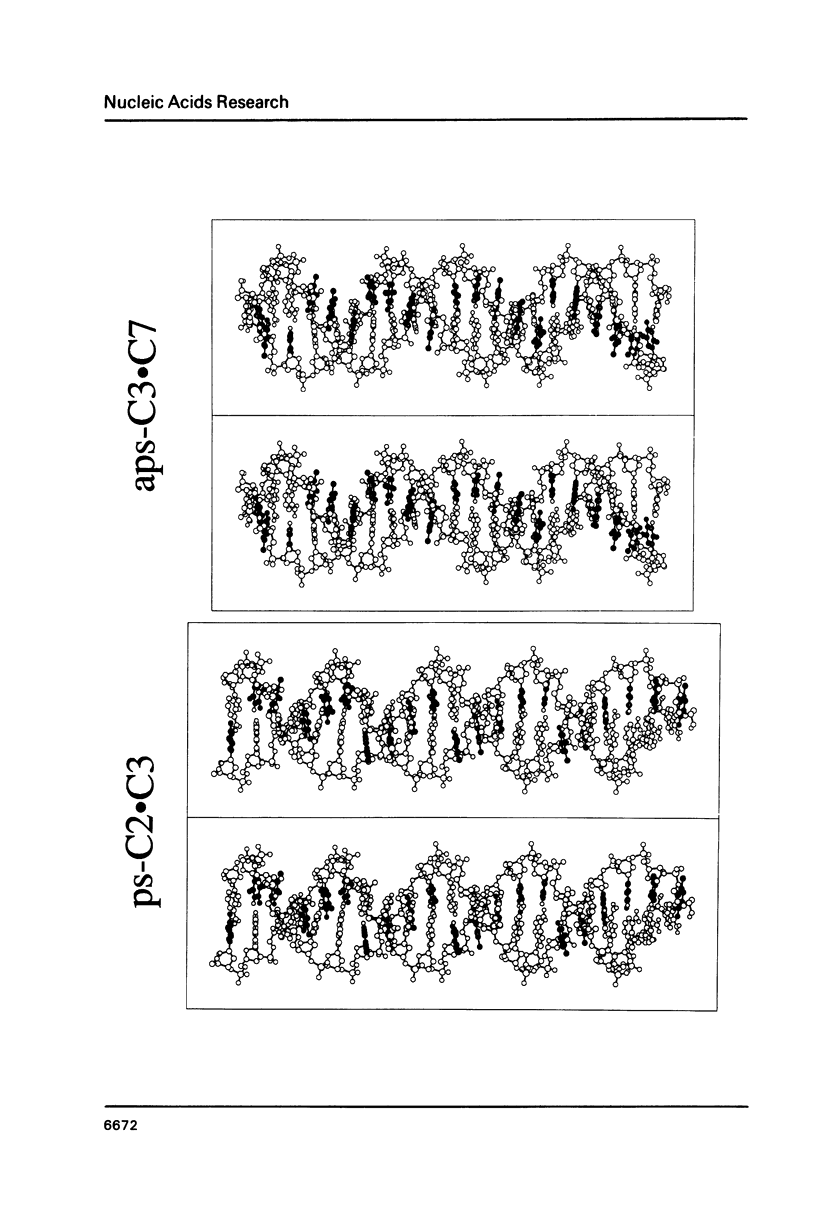
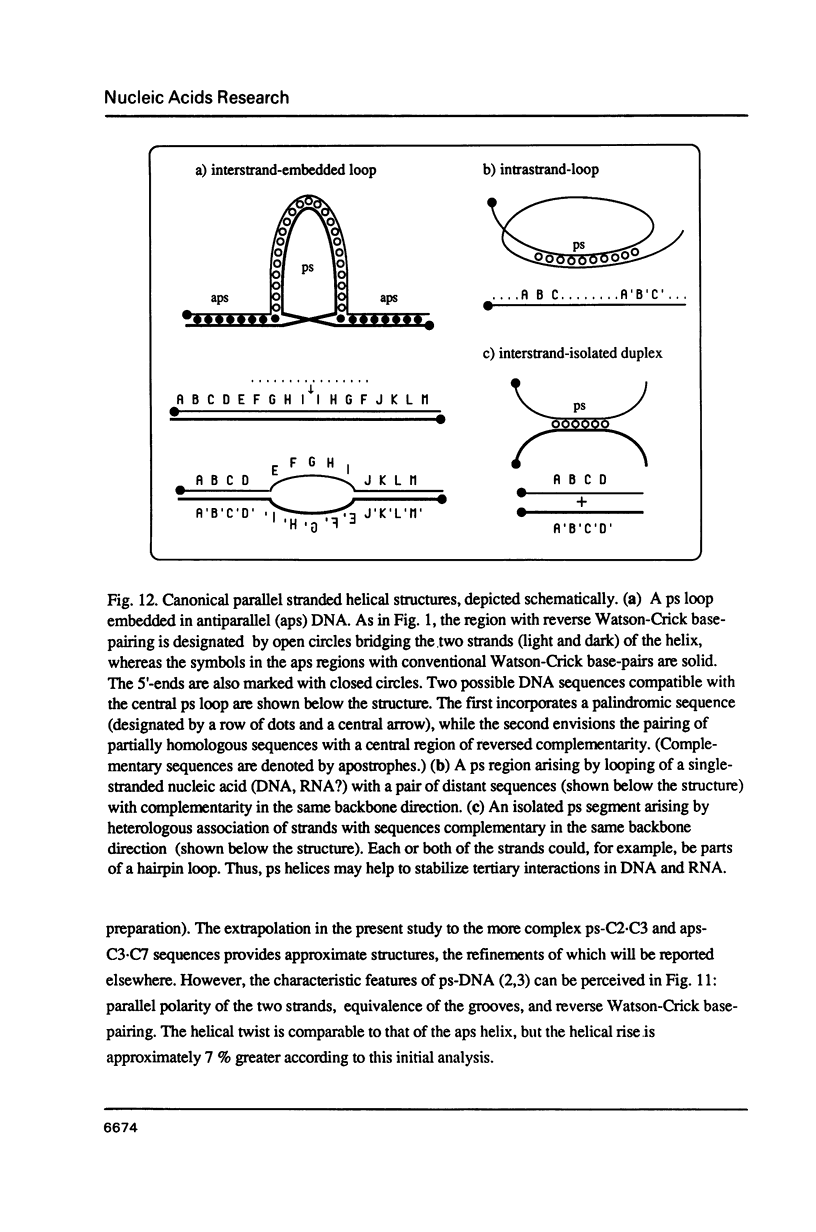
Three linear 21-nt oligonucleotides (C2, C3, C7) have been synthesized with different sequences of A and T residues. One pairwise combination, (C3, C7), hybridizes to form a conventional antiparallel duplex (aps-C3.C7), whereas the pair C2, C3 forms a duplex (ps-C2.C3) in which the two strands are in a parallel orientation and the A.T base-pairs in a reverse Watson-Crick configuration. The existence of the novel ps helical structure was established from the following criteria: (i) The electrophoretic mobilities of the ps and aps duplexes in native and denaturing polyacrylamide gels are similar. (ii) The ps duplex is not a substrate for T4 DNA ligase. (iii) Salt-dependent thermal transitions are observed for the two duplexes, but the melting temperatures of the ps molecules are 15 degrees C lower. (iv) The ultraviolet absorption and circular dichroism spectra of the ps duplex are indicative of a base-paired structure, but differ systematically from that of the aps helix. (v) Based on fluorescent measurements, the bis-benzimidazole drug BBI-258 shows a lower affinity for the ps compared to the aps duplex, whereas the opposite preference holds for the intercalator ethidium bromide. We conclude from the present study that parallel stranded DNA is a stable conformation which can arise by interaction between two conventional strands with appropriate sequence homology.
Full text
PDF




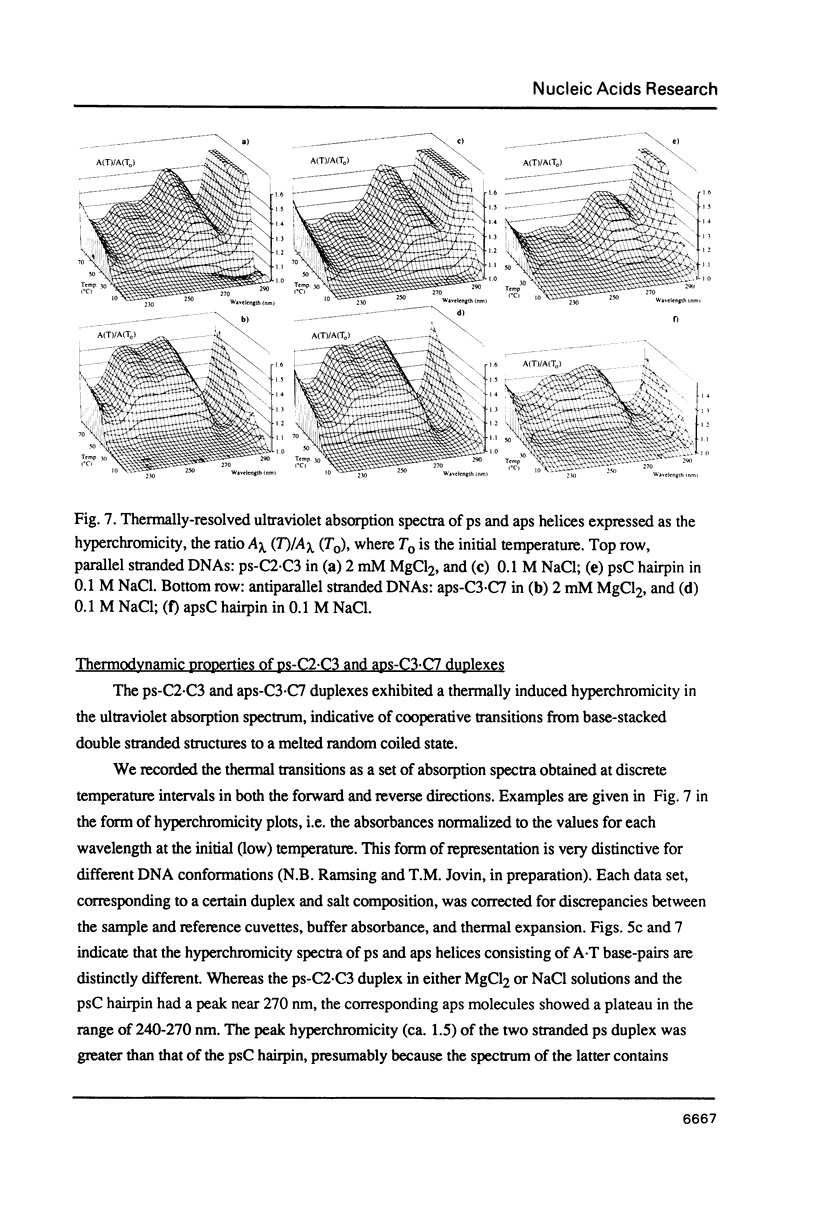

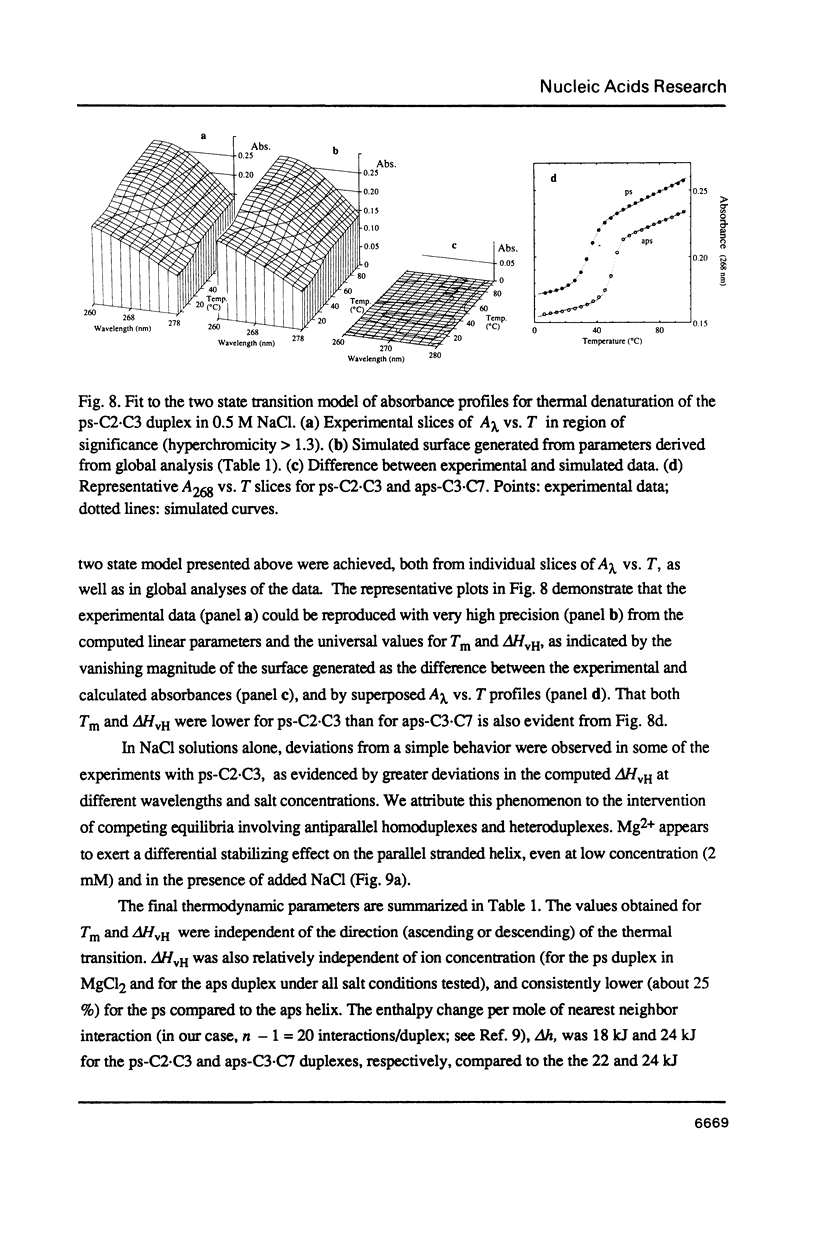
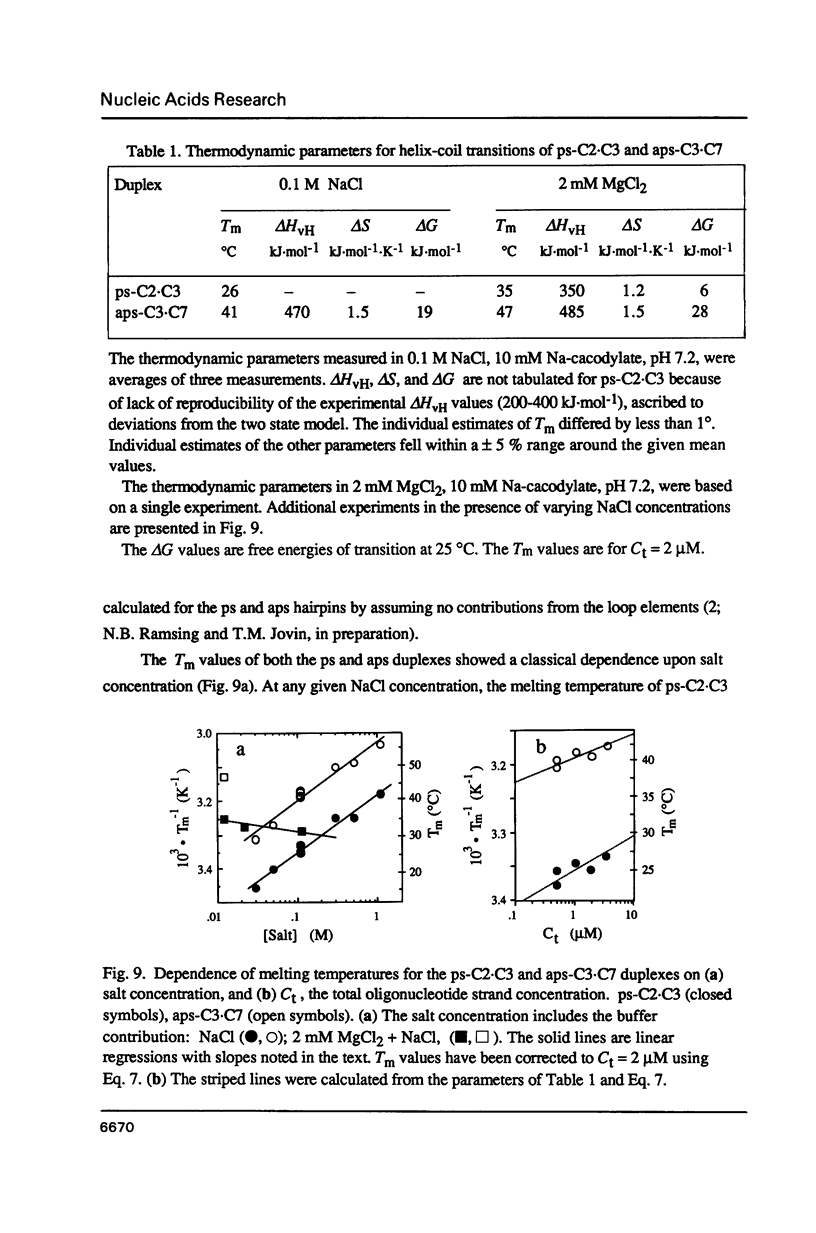
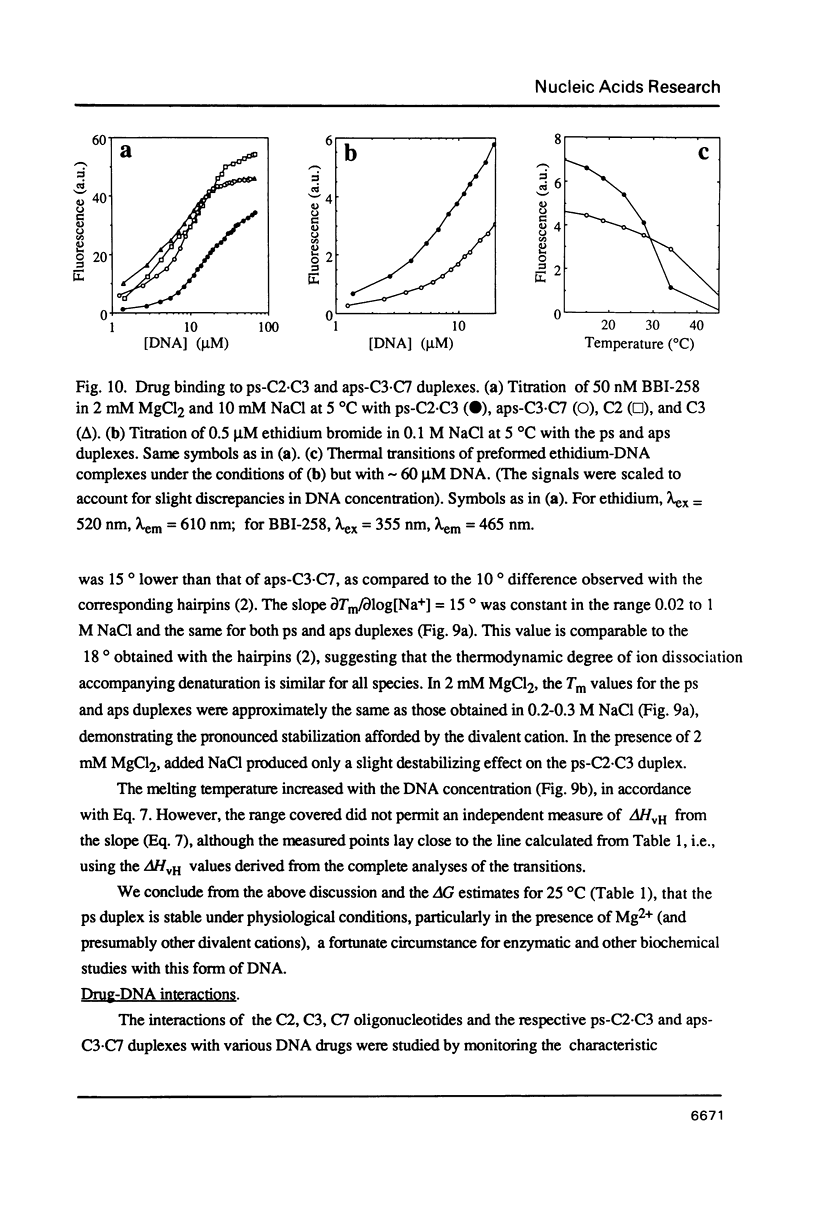



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslauer K. J., Frank R., Blöcker H., Marky L. A. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Harshman K. D., Dervan P. B. Molecular recognition of B-DNA by Hoechst 33258. Nucleic Acids Res. 1985 Jul 11;13(13):4825–4835. doi: 10.1093/nar/13.13.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 1987 Sep;26(9):1601–1620. doi: 10.1002/bip.360260911. [DOI] [PubMed] [Google Scholar]
- Pattabiraman N. Can the double helix be parallel? Biopolymers. 1986 Sep;25(9):1603–1606. doi: 10.1002/bip.360250903. [DOI] [PubMed] [Google Scholar]
- Pjura P. E., Grzeskowiak K., Dickerson R. E. Binding of Hoechst 33258 to the minor groove of B-DNA. J Mol Biol. 1987 Sep 20;197(2):257–271. doi: 10.1016/0022-2836(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Stokke T., Steen H. B. Multiple binding modes for Hoechst 33258 to DNA. J Histochem Cytochem. 1985 Apr;33(4):333–338. doi: 10.1177/33.4.2579998. [DOI] [PubMed] [Google Scholar]
- von Kitzing E., Diekmann S. Molecular mechanics calculations of dA12.dT12 and of the curved molecule d(GCTCGAAAAA)4.d(TTTTTCGAGC)4. Eur Biophys J. 1987;15(1):13–26. doi: 10.1007/BF00255031. [DOI] [PubMed] [Google Scholar]