Abstract
A novel stability-indicating gradient RP-UPLC method was developed for the quantitative determination of process related impurities and forced degradation products of fexofenadine HCl in pharmaceutical formulations. The method was developed by using Waters Aquity BEH C18 (100 mm x 2.1 mm) 1.7 μm column with mobile phase containing a gradient mixture of solvent A (0.05% triethyl amine, pH adjusted to 7.0 with ortho-phosphoric acid) and B (10:90 v/v mixture of water and acetonitrile). The flow rate of mobile phase was 0.4 mL/min with column temperature of 30°C and detection wavelength at 220nm. Fexofenadine HCl was subjected to the stress conditions including oxidative, acid, base, hydrolytic, thermal and photolytic degradation. Fexofenadine HCl was found to degrade significantly in oxidative stress conditions, and degradation product was identified and characterized by ESI-MS/MS, 1H and 13C NMR spectroscopic method as the N-oxide 2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]-1-oxido-piperidin-1-yl}butyl)phenyl]-2-methylpropanoic acid. The degradation products were well resolved from fexofenadine and its impurities. The mass balance was found to be satisfactory in all the stress conditions, thus proving the stability-indicating capability of the method. The developed method was validated as per ICH guidelines with respect to specificity, linearity, limit of detection and quantification, accuracy, precision and robustness.
Keywords: Fexofenadine, Validation, Stability-indicating, Forced degradation, Identification, characterization, UPLC
Introduction
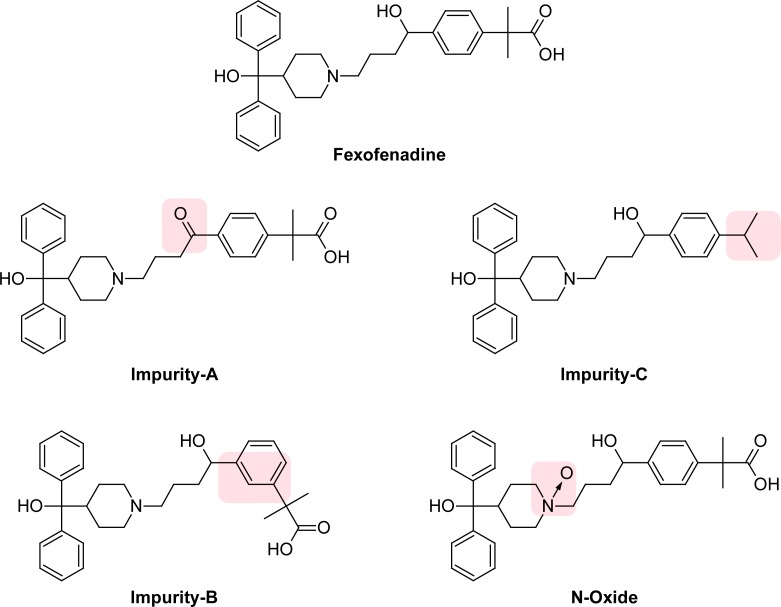
Fexofenadine, 2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]piperidin-1-yl}butyl)phenyl]-2-methylpropanoic acid (Fig. 1), is a highly selective peripheral histamine H1 receptor antagonist used in the treatment of allergic diseases such as allergic rhinitis and chronic urticaria. Fexofenadine is the active derivative of the antihistamine terfenadine, with no anti-chrolinergic or alpha 1-adernergic receptor-blocking effects and without severe cardiac side effects of terfenadine [1, 2].
Fig. 1.
Structures and chemical names of Fexofenadine HCl and its impurities
According to the literature survey, few HPLC assay and dissolution methods have been reported for determination of fexofenadine in pharmaceutical preparation [3–6]. Estimation of fexofenadine in biological fluids using liquid chromatography with mass spectroscopy [7], ionspray tandem mass spectroscopy [8], electronspray tandem mass spectroscopy [9], UV detection [10, 11] and fluorescence detection [12] has been performed. Literature reported the isolation and structure elucidation of photodegradation products of fexofenadine [13] and photodegradation kinetics of fexofenadine HCl using LC method [14]. Methods are reported for the determination of fexofenadine and its two related compounds/degradation compound by HPLC [15, 16]. However, reported methods have not mentioned a new potential oxidative degradent which is N-oxide formation at tertiary amine of feofenadine and eluation of highly non polar related compound imp-c. In this method, we have observed one new potential impurity in our drug product during oxidative forced degradation study. Degradation product formed during oxidative stress was isolated and characterized by ESI-MS/MS,1H and 13C NMR as the N-oxide impurity, 2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]-1-oxidopiperidin-1-yl}butyl)phenyl]-2-methyl-propanoic acid. To our present knowledge, this N-oxide impurity of fexofenadine is not reported in literature and there is no stability-indicating LC method available for the estimation of N-oxide impurity in pharmaceutical formulation. The present work describes the isolation and characterization of N-oxide impurity, as well as the development and validation of a stability-indicating RP-UPLC method for the estimation of degradation and process related impurities of fexofenadine hydrochloride, namely N-oxide, imp-A, imp-B and imp-C [Fig. 1]. The developed LC method was validated with respect to specificity, limit of detection and quantification, linearity, precision, accuracy and robustness. Forced degradation studies were performed on the placebo (all excipient mixture without fexofenadine HCl drug substance) and drug product to show the stability-indicating nature of the method. These studies were performed in accordance with established ICH guidelines [17, 18].
Experimental
Chemicals and reagents
Samples of fexofenadine hydrochloride tablets and its impurities were supplied by Dr. Reddy’s laboratories limited, Hyderabad, India. The HPLC grade acetonitrile, analytical grade triethyl amine and ortho-phosphoric acid were purchased from J.T.Baker, Mumbai, India. High purity water was prepared using Millipore Milli-Q Plus water purification system (Millipore, Milford, MA, USA).
Equipment
Acquity UPLCTM (Water, Milforde, USA) was used which is equipped with a binary solvent manager, a sample manager and a photodiode array (PDA) detector. The output signals were monitored and processed using Empower 2 software. Cintex digital water bath was used for hydrolysis studies. Photo-stability studies were carried out in photo-stability chamber (Sanyo, Leicestershire, UK). Thermal stability studies were performed in a dry air oven (Cintex, Mumbai, India). The pH of the solutions was measured by a pH meter (Mettler-Toledo, Switzerland).
Chromatographic Conditions
The method was developed using Waters Aquity BEH C18 (100 mm x 2.1 mm) 1.7 μm particle size column (Waters, Milforde, USA) with mobile phase containing a gradient mixture of solvent A (0.05% v/v triethyl amine, pH adjusted to 7.0 with ortho-phosphoric acid) and solvent B (mixture of water and acetonitrile in the ratio of 10:90 v/v, respectively). The gradient program (T/%B) was set as 0/25, 10/25, 15/35, 33/60, 35/80, 36/25 and 40/25. The flow rate of the mobile phase was set at 0.4 mL/min. The column temperature was maintained at 30° C and the eluted compounds were monitored at the wavelength of 220 nm. The sample injection volume was 1.5 μl.
Semi-Preparative LC Conditions
The separation and isolation of the degradation products were carried out on a semi-preparative Inertsil ODS 3V (10 mm × 250 mm; particle size 5 μm) LC column using mobile phase containing solvent A (0.01 M ammonium formate buffer) and B (0.01 M ammonium formate buffer and acetonitrile in the ratio of 10:90 v/v) at a flow rate of 4.0 mL/min. The gradient program (Time (min)/%B) was set 0/20, 10/35, 25/50, 35/90, 40/100, 60/100, 65/20 and 75/20 and the detector was maintained at 220 nm.
Liquid Chromatography-Mass Spectroscopy (LC-MS) Conditions
An LC-MS/MS system (Agilent 1100 series liquid chromatograph coupled with Applied Biosystem 4000 Q Trap triple quadrupole mass spectrophotometer with Analyst 1.4 software, MDS SCIEX, USA) was used for the confirmation of atomic mass number of unknown compounds formed during forced degradation studies. The method was developed using Zorbax SB Phenyl, 250 × 4.6 mm, 5μm column as stationary phase with mobile phase containing a gradient mixture of solvent A (0.01 M ammonium formate buffer) and B (0.01 M ammonium formate buffer and acetonitrile in the ratio of 10:90 v/v). Solvent B was used as diluent. The gradient program (Time (min)/%B) was set 0/30, 7/40, 20/50, 25/90, 27/100, 50/100, 52/30 and 60/30. Prior to use mobile phase was mixed thoroughly and degassed. The mobile phase pumped at a flow rate of 1.0 mL/min. The column temperature was maintained at 25°C. The injection volume for sample was 20μL. The analysis was performed in positive electro-spray/ positive ionization mode. The source voltage was 5000 V and source temperature was 450°C. GS1 and GS2 were optimized to 30 and 35 psi, respectively. The curtain gas flow was 20 psi.
NMR Conditions
The 1H and 13C Nuclear magnetic resonance (NMR) spectra were recorded in DMSO-d6 at 500 MHz and 125MHz, respectively, using Varian Unity INOVA 500 MHz spectrometer (Bruker Biospin, Germany). The chemical shift values were reported on δ scale in ppm with respect to TMS (0.00ppm) and DMSO-d6 (δ 39.5 ppm) as internal standard, respectively.
Preparation of system suitability Solution
Mixture of milli-Q water and acetonitrile in the ratio of 50:50 v/v containing 0.1% v/v ortho-phosphoric acid was used as diluent. A system suitability solution of imp-B and fexofenadine HCl with a concentration of 2.4 μg/mL and 1.2 mg/mL, respectively, was prepared by dissolving appropriate amount of drug in the diluent.
Preparation of Standard Solution
Stock solution of fexofenadine HCl was prepared in diluent with a concentration of 960 μg/mL. Working standard solution was prepared from diluting above stock solution of fexofenadine HCl with final concentration of 2.4 μg/mL.
Preparation of sample Solution
Tablet powder equivalent to 120 mg of fexofenadine HCl was dissolved in diluent with sonication for about 25 min to prepare a solution containing 1200 μg/mL drug. This solution was centrifuged at 10000 rpm for about 10 min.
Method validation
The proposed method was validated by determining its performance characteristic regarding specificity, accuracy, precision, limit of detection and quantification, linearity, range and robustness [17, 18].
System Suitability
System suitability was determined before sample analysis. Single injection of system suitability solution and duplicate injections of the standard solution containing 2.4 μg/mL fexofenadine HCl were injected. The acceptance criteria were USP tailing factor not more than 2.0, USP plate count not less than 5000, area similarity ratio between 0.9 to 1.1 for fexofenadine peak (from duplicate injections of standard preparation) and; resolution should be minimum 3.0 between imp-B and fexofenadine peaks (from system suitability solution).
Specificity/stress studies
Specificity is the ability of the method to measure the analyte response in the presence of its potential impurities. The specificity of the developed LC method for fexofenadine HCl was carried out in the presence of its impurities and degradation products. Stress studies were performed at 1200μg/mL concentration of fexofenadine HCl on tablets to provide an indication of the stability-indicating property and specificity of proposed method. The stress condition employed for degradation study included acid hydrolysis (1 N HCl at 60°C for 3.5 hrs), base hydrolysis (2 N NaOH at 60°C for 24 hrs), oxidation (3% H2O2 at 60°C for 5 hrs), hydrolytic (water at 60°C for 24 hrs), thermal (105°C for 24 hrs), humidity (25°C/90% RH for 7 days) and photolytic degradation (drug product exposed to visible light for 240 h resulting in an overall illustration 1.2 million lux h and UV light for 250 h resulting an overall illustration 200 watt h/m2 at 25°C, [19]). Peak purity test was carried out for the fexofenadine peak by using PDA detector in stress samples.
Placebo interference was evaluated by analyzing the placebo prepared as per test method. No peak due to placebo detected at the retention time of fexofenadine and its impurities.
Precision
The precision of method was verified by repeatability and intermediate precision. Repeatability was checked by injecting six individual preparations of fexofenadine HCl tablets spiked with its four impurities; N-oxide and imp-B at 0.10% level, imp-A at 0.2% level and imp-C at 0.15% level (% level of each impurity with respect to 1.2 mg/mL fexofenadine HCl). % RSD of area for each impurity was calculated. The intermediate precision of the method was also evaluated using different analyst and different instrument and performing the analysis on different days.
Limit of Detection (LOD) and Quantification (LOQ)
The LOD and LOQ for fexofenadine HCl impurities were determined at a signal-to-noise ratio of 3:1 and 10:1, respectively, by injecting a series of dilute solutions with known concentrations. Precision study was also carried out at the LOQ level by injecting six individual preparations of fexofenadine HCl impurities and calculating the %RSD of the area.
Linearity
Linearity test solutions were prepared by diluting the stock solutions to the required concentrations. The solutions of each impurity were prepared at six concentration levels from LOQ to 200% of specification level. Calibration curves were plotted between the responses of peak versus analyte concentrations. The coefficient correlation, slope and y-intercept of the calibration curve are reported.
Accuracy
Accuracy of the method for N-oxide, imp-A, imp-B and imp-C was evaluated in triplicate using six concentration levels of LOQ, 50%, 75%, 100%, 125% and 150%. The percentage recoveries for each impurity were calculated.
Robustness
To determine the robustness of the developed method, experimental conditions were deliberately altered and the resolution between imp-B and fexofenadine, and system suitability parameters for fexofenadine HCl standard were recorded. The variables evaluated in the study were pH of the mobile phase buffer (± 0.2), column temperature (± 5°C), flow rate (± 0.04 ml/min) and % organic in the mobile phase (± 10%).
Solution stability and mobile phase stability
The solution stability of fexofenadine HCl and its impurities was determined by keeping test and standard solutions in tightly capped volumetric flasks at room temperature for up to 48 h and measuring the amount of four impurities at every 24 h interval against freshly prepared standard solution. The mobile phase stability was also determined by injecting freshly prepared solutions of fexofenadine HCl and its impurities at 24 h and 48 h. The mobile phase was not changed during the study.
Results and Discussion
Identification of N-oxide impurity
An unknown impurity with relative retention time (RRT) 0.71 with respect to fexofenadine was observed during oxidative degradation study. Fexofenadine (250 mg), dissolved in 20 mL of diluent and 10 mL 30% of hydrogen peroxide, was subjected to oxidative degradation at 80°C temperature for 8 h. About 70% fexofenadine was degraded and degradation product was isolated by semi-preparative HPLC. Fractions from the semi-preparative HPLC separations were collected and evaporated to dryness under vacuum at 40°C. The chromatographic purity of isolated degradation compound was found to be > 97% and used for its identification by LC-MS and NMR studies.
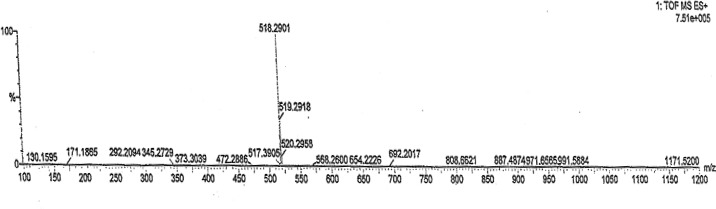
ESI-MS mass spectral analysis (positive mode) (Fig. 2) of degraded compound showed a molecular ion at m/z 518 amu [M+H]+ which was 16 amu more than that of fexofenadine (m/z 502). This data indicated the presence of additional ‘O’ functionality in degradation compound. Use of high resolution mass spectroscopy (HRMS) in this measurement confirms elemental composition C32H39NO5 of the unknown degradant compound.
Fig. 2.
Mass spectrum of N-oxide impurity
To get the structural information, degradation product was further subjected to 1H and 13C NMR study. The number of proton and carbon resonances is the same as that in Fexofenadine. However, the 1H and the 13C chemical shifts of the methylene groups attached to the nitrogen atom in the piperidine ring are deshielded when compared to those of Fexofenadine. Variations were observed in the ppm values (shifted slightly toward downfield) for the hydrogens present on piperidine ring and the aliphatic side chain attached to nitrogen atom. The 13C signals for the carbons of piperidine ring and the aliphatic side chain attached to nitrogen were also shifted slightly toward downfield as well (table 6). This observation lends support to the formation of N-Oxide in the piperidine ring. This is in agreement with the HRMS pattern observed for N-Oxide.
Tab. 6.
NMR assignments of fexofenadine and N-oxide impurity.
| Positiona | 1H | N-Oxide Impurity | Fexofenadine | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| δ (ppm) | J(Hz)b | 13C | δ (ppm) | J(Hz)b | 13C | ||
| 1 | – | – | – | 144.2 | – | – | 144.1 |
| 2,6 | 2H | 7.24–7.57 | – | 125.2 | 7.10–7.30 | – | 125.3 |
| 3,5 | 2H | 7.51 | d, 7.5 | 125.5 | 7.50 | d, 7.4 | 125.7 |
| 4 | – | – | – | 143.8 | – | – | 143.5 |
| 7 | – | – | – | 45.7 | – | – | 45.5 |
| 7′,7″ | 6H | 1.42 | s | 26.6 | 1.45 | s | 26.5 |
| 8 | – | – | – | 178.1 | – | – | 177.6 |
| 9 | 1H | 4.51 | t, 6.5 | 71.4 | 4.51 | t, 6.5 | 71.3 |
| 10 | 2H | 1.28 | m | 35.8 | 1.76 | m | 36.1 |
| 11 | 2H | 1.56 | m | 20.8 | 1.76 | m | 20.0 |
| 12 | 2H | 3.38 | t,12.0 | 68.3 | 2.92 | t, 12.0 | 56.1 |
| 14 | 2H | 3.52 | m | 61.9 | 2.92 | m | 51.6 |
| 15 | 2H | 3.52 | m | 62.0 | 3.44 | m | 51.6 |
| 16,17 | Ha | 1.80–1.92 | m | 22.5 | 1.76 | m | 24.0 |
| He | 2.08 | m | – | m | – | ||
| 18 | 1H | 2.83 | t, 12.0 | 40.4 | 2.92 | t, 12.0 | 40.9 |
| 19 | – | – | – | 78.1 | – | – | 78.3 |
| 20,20′ | – | – | – | 146.6 | – | – | 146.7 |
| 21,21′ | 2H | 7.24–7.57 | – | 125.6 | 7.10–7.30 | – | 125.7 |
| 22,22′ | 2H | 7.24–7.57 | – | 128.0 | 7.10–7.30 | – | 128.0 |
| 23,23′ | 2H | 7.24–7.57 | – | 126.1 | 7.10–7.30 | – | 126.1 |
| 24,24′ | 2H | 7.24–7.57 | – | 127.9 | 7.10–7.30 | – | 127.9 |
| 25,25′ | 2H | 7.24–7.57 | – | 125.7 | 7.10–7.30 | – | 125.7 |
Refer to Fig. 4 the structural formula for numbering;
This column gives the 1H-1H multiplicity and coupling constants; s…Singlet; d…Doublet; t…Triplet; m…Multiplet.
Identification of unknown compound was verified by 1H and 13C NMR study. The assignment of NMR signals were performed for unknown impurity and confirmed as 2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]-1-oxidopiperidin-1-yl}butyl)phenyl]-2-methyl-propanoic acid.
Method Development and Optimization of stability-indicating UPLC method
The main objective of the chromatographic method was to separate critical closely eluting compounds fexofenadine and imp-B and to elute non-polar imp-C with a shorter run time. The blend containing 1.2 mg/ml of fexofenadine and 2.4 μg/ml of each imp-A, imp-B, imp-C and N-oxide impurity was used for separation. A gradient elution method was employed using solvent A (0.05 M sodium dihydrogen orthophosphate and 0.01 M sodium perchlorate, pH 3.0) and acetonitrile as solvent B, Acquity BEH C8 (100 mm X 2.1 mm) 1.7μm column with flow rate of 0.5 mL/min on UPLC equipped with photo diode array detector. Resolution between fexofenadine and imp B was less than 2.0 and baseline disturbance was observed at the retention time of imp-C. To increase the resolution and baseline stabilization an attempt was made with modified solvent A (0.05% triethyl amine, pH adjusted to 7.0 with ortho-phosphoric acid) and solvent B (mixture of water and acetonitrile in the ratio of 10:90 v/v) and Acquity BEH C18 (100 mm X 2.1 mm) 1.7μm column. On the optimization of gradient program, fexofenadine and all four impurity peaks were well resolved from each other and degradation products. Based on these experiments, the final optimized conditions are described below.
Waters Aquity BEH C18 (100 mm × 2.1 mm) 1.7 μm particle size column was used as stationary phase. The mobile phase A consisted of 0.05% triethyl amine, pH adjusted to 7.0 with ortho-phosphoric acid and mobile phase B contained a mixture of water and acetonitrile in the ratio of 10:90 v/v, respectively. The gradient program (T/%B) was set as 0/25, 10/25, 15/35, 33/60, 35/80, 36/25 and 40/25. The flow rate of the mobile phase was set at 0.4 mL/min. The column temperature was maintained at 30° C and the eluted compounds were monitored at the wavelength of 220 nm. The sample injection volume was 1.5 μl.
Method validation
The proposed method was validated as per ICH guidelines [17]. The following validation characteristics were addressed: specificity, accuracy, precision, linearity, range and robustness.
System suitability
System suitability shall be checked for the conformance of suitability and reproducibility of chromatographic system for analysis. The system suitability was evaluated on the basis of USP plate counts, USP tailing factor, peak area ratio for fexofenadine peaks from standard solution and resolution from system suitability solution. All critical parameters tested met the acceptance criteria (Table 1).
Tab. 1.
System suitability test results
| Parameters | Specification |
Observed values
|
|
|---|---|---|---|
| Precision | Intermediate Precision | ||
| Resolutiona | ≥ 3.0 | 7.3 | 6.9 |
| Area ratio | ≥ 0.9 and ≤ 1.1 | 1.0 | 1.0 |
| USP Tailing | ≤ 2.0 | 1.1 | 0.9 |
| USP plate counts | ≥ 5000 | 21047 | 14824 |
Resolution between fexofenadine and Imp-B.
Specificity
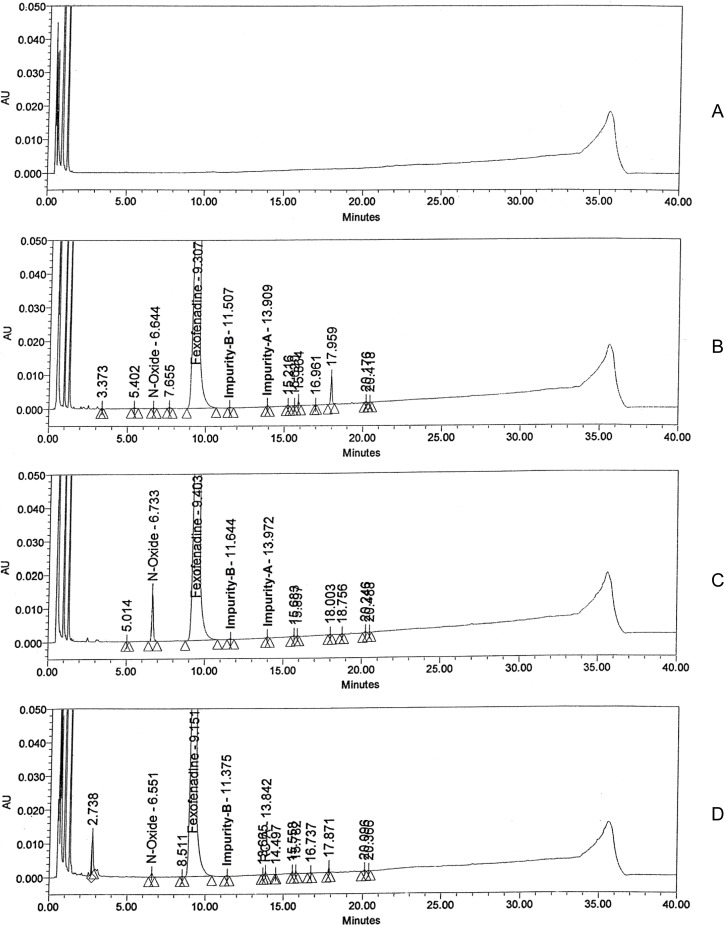
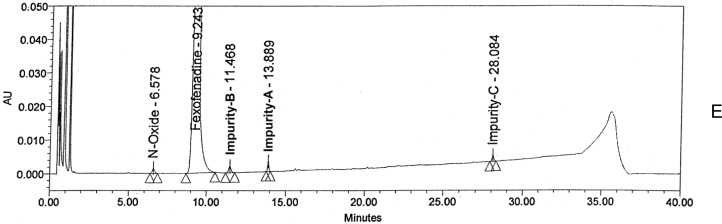
The aim of the specificity study is to assess unequivocally analyte in the presence of components that may be expected to be present. Placebo interference was evaluated by analyzing the placebo prepared as per test method. No peak due to placebo detected at the retention time of fexofenadine and its impurities (Fig. 3A). All force degradation samples were analyzed at 1200 μg/mL concentration of fexofenadine HCl using PDA detector to ensure the homogeneity and purity of fexofenadine peak. Significant degradation was observed in oxidation degradation (3% H2O2 at 60°C for 5 h) and slight degradation was observed in acid hydrolysis (1 N HCl at 60°C for 3.5 h), base hydrolysis (2 N NaOH at 60°C for 24 h) and thermal degradation (105°C for 24 h) (Fig. 3B–D). Oxidation degradation leads to the formation of a major unknown degradation product. Oxidative degradation product was identified and characterized by LC-MS/MS, 1H and 13C NMR as2-[4-(1-hydroxy-4-{4-[hydroxy(diphenyl)methyl]-1-oxidopiperidin-1-yl}butyl)phenyl]-2-methylpropanoic acid. Fexofenadine was found stable under hydrolytic (water at 80°C for 4 h), humidity (25°C/90% RH for 7 days) and photolytic (exposed to 1.2 million lux h visible light and 200 watt h/m2 UV light) degradation conditions. The mass balance (% assay + % sum of all degradants + % sum of all impurities) results were calculated and found to be more than 98.4% (Table 2). The purity of fexofenadine was unaffected by the presence of its impurities and degradation products and thus confirms the stability-indicating power of the developed method.
Fig. 3.
Typical chromatograms of (A) Placebo, (B) Acid degradation sample, (C) Peroxide degradation sample, (D) Thermal degradation sample and (E) Fexofenadine test spiked with its impurities
Tab. 2.
Summary of forced degradation results
| Stress Condition |
% Impurity
|
% Degradation | % Assay of active substance | Mass balance (%) | ||||
|---|---|---|---|---|---|---|---|---|
| N-oxide | Imp-A | Imp-B | Imp-C | MUSI | ||||
| Acid hydrolysis | 0.03 | 0.04 | 0.03 | ND | 0.49 | 0.60 | 97.8 | 98.4 |
| Base hydrolysis | 0.11 | 0.04 | 0.04 | ND | 0.03 | 0.18 | 100.5 | 100.7 |
| Oxidation degradation | 1.72 | 0.05 | ND | ND | 0.05 | 1.98 | 99.0 | 101.0 |
| Thermal Degradation | 0.12 | 0.06 | 0.03 | ND | 0.08 | 0.80 | 102.9 | 103.7 |
| Water Degradation | ND | 0.03 | ND | ND | ND | 0.03 | 101.6 | 101.6 |
| Photolytic degradation | 0.03 | 0.03 | 0.03 | ND | 0.03 | 0.00 | 101.1 | 101.1 |
| Humidity Degradation | ND | 0.02 | ND | ND | ND | 0.02 | 101.0 | 101.0 |
MUSI…Maximum un-specified impurity; ND…Not detected.
Precision
The % RSD for the area of N-oxide, imp-A, imp-B and imp-C in repeatability study was within 3.0% and in intermediate precision study was within 3.6%, which confirms the precision of the method. The %RSD values are presented in Table 3.
Tab. 3.
Linearity and precision data
| Parameter | N-oxide | Imp-A | Imp-B | Imp-C |
|---|---|---|---|---|
| LOD (μg/mL) | 0.196 | 0.166 | 0.190 | 0.159 |
| LOQ (μg/mL) | 0.588 | 0.496 | 0.571 | 0.476 |
| Correlation coefficient | 0.999 | 0.999 | 0.998 | 0.999 |
| Intercept (a) | 361.70 | 13.66 | 249.36 | −478.95 |
| Slope (b) | 5814.98 | 6080.89 | 5118.69 | 6830.94 |
| Bias at 100% response | 2 | 0 | 4 | 4 |
| Precision (%RSD) | 2.9 | 0.4 | 2.6 | 3.0 |
| Intermediate precision (%RSD) | 3.2 | 0.8 | 5.1 | 3.6 |
| Precision at LOQ (%RSD) | 3.3 | 2.4 | 4.8 | 2.8 |
Limits of Detection and Quantification
The limit of detection, limit of quantification and precision at LOQ values for N-oxide, imp-A, imp-B and imp-C are reported in Table 3.
Linearity
The linearity calibration plots for the N-oxide, imp-A, imp-B and imp-C was obtained over the calibration ranges tested, i.e. LOQ to 200% of specification level. The correlation coefficient obtained was greater than 0.998 and % bias at 100% response was within 5% (Table 3). The above result shows that a strong correlation exists between peak area and concentration of N-oxide, imp-A, imp-B and imp-C.
Accuracy
The percentage recovery of N-oxide, imp-A, imp-B and imp-C in fexofenadine samples varied from 92.6 to 108.6%. The LC chromatogram of spiked sample at specification level of all four impurities in fexofenadine hydrochloride tablet sample is shown in Fig. 3E. The recovery values for fexofenadine impurities are presented in Table 4.
Tab. 4.
Recovery data
| Amount spikeda |
% Recoveryb
|
|||
|---|---|---|---|---|
| N-oxide | Imp-A | Imp-B | Imp-C | |
| LOQ | 92.6 ± 2.3 | 104.9 ± 3.5 | 93.1 ± 3.4 | 94.1 ± 1.5 |
| 50% | 107.0 ± 1.3 | 106.6 ± 0.6 | 107.8 ± 1.0 | 104.2 ± 3.5 |
| 75% | 94.8 ± 1.9 | 108.6 ± 2.2 | 95.4 ± 2.8 | 97.2 ± 3.9 |
| 100% | 96.7 ± 0.4 | 104.1 ± 0.6 | 95.1 ± 3.0 | 92.3 ± 2.6 |
| 125% | 98.3 ± 1.2 | 102.3 ± 2.3 | 96.7 ± 1.7 | 94.3 ± 1.7 |
| 150% | 103.0 ± 0.9 | 107.2 ± 1.0 | 107.5 ± 1.8 | 100.2 ± 0.9 |
Amount of four impurities spiked with respect to specification level;
Mean ± %RSD for three determinations.
Robustness
In all the deliberate varied chromatographic conditions (flow rate, column temperature, pH of mobile phase buffer and composition of organic solvent), all analytes were adequately resolved and elution order remained unchanged. The resolution between critical pair, i.e. for imp-B and fexofenadine was greater than 5.4 and tailing factor for fexofenadine peak from standard solution was not more than 1.0, and USP plate count was more than 11434 (Table 5).
Tab. 5.
Robustness results of UPLC method
| Variation in chromatographic condition |
Observed system suitability parameters
|
|||
|---|---|---|---|---|
| Area ratio ≥ 0.9 and ≤ 1.1 | Resolutiona ≥ 3.0 | USP Tailing ≤ 2.0 | USP plate count ≥ 5000 | |
| Column Temperature 25°C | 1.0 | 5.9 | 0.9 | 14654 |
| Column Temperature 35°C | 1.0 | 6.3 | 0.9 | 16417 |
| Flow rate 0.36 mL/min | 1.0 | 6.0 | 1.0 | 16309 |
| Flow rate 0.44 mL/min | 1.0 | 6.0 | 1.0 | 11590 |
| Acetonitrile 90% | 1.0 | 5.5 | 0.9 | 23488 |
| Acetonitrile 110% | 1.0 | 5.9 | 0.9 | 11434 |
| Mobile Phase Buffer pH 6.8 | 1.0 | 6.0 | 1.0 | 12061 |
| Mobile Phase Buffer pH 7.2 | 1.0 | 5.4 | 1.0 | 13461 |
Resolution between fexofenadine and Imp-B.
Stability of Solution and Mobile Phase
The variability in the estimation of all four fexofenadine impurities was within ± 10% during solution stability and mobile phase stability. The results from solution stability and mobile phase stability experiments confirmed that mobile phase was stable up to 48 h and sample solution and standard solutions were stable up to 48 h.
Conclusion
This research paper describes the identification and characterization of a potential oxidative degradant (N-oxide) in fexofenadine hydrochloride in pharmaceutical formulations. The impurity was isolated by semi-preparative liquid chromatography. The isolated impurity was characterized by using spectroscopic techniques. A simple and efficient RP-UPLC method development and validation were discussed. The method was found to be precise, accurate, linear, robust and rugged during validation. The method is stability-indicating and can be used for routine analysis of production samples and to check the stability of the fexofenadine HCl tablets.
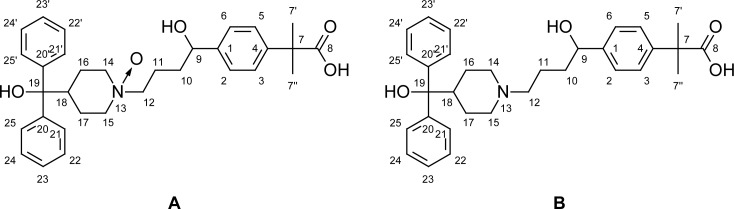
Fig. 4.
Structural formula and numbering of N-Oxide impurity (A) and Fexofenadine (B)
Acknowledgments
The authors are thankful to the management of Dr. Reddy’s Laboratories Ltd., Hyderabad for providing facilities in which to carry out this work.
Dr. Reddy’s internal publication number for this manuscript is PUB00130-11.
Footnotes
This article is available from: http://dx.doi.org/10.3797/scipharm.1111-07
Authors’ Statement
Competing interests
The authors declare no conflict of interest.
References
- [1].The Merck Index, an encyclopedia of chemicals, drugs and biologicals. Merck & Co., Inc; Whitehouse Station, NJ; 2001. [Google Scholar]
- [2].Simpson K, Jarvis B. Fexofenadine: a review of its use in the management of seasonal allergic rhinitis and chronic idiopathic urticaria. Drugs. 2000;59:301–321. doi: 10.2165/00003495-200059020-00020. http://dx.doi.org/10.2165/00003495-200059020-00020. [DOI] [PubMed] [Google Scholar]
- [3].Oliveira DC, Weigch A, Rolim CM. Simple and reliable HPLC analysis of fexofenadine hydrochloride in tablets and its application to dissolution studies. Pharmazie. 2007;62:96–100. http://dx.doi.org/10.1691/ph.2007.2.6120. [PubMed] [Google Scholar]
- [4].Breier AR, Paim CS, Steppe M, Schapoval EES. Development and validation of dissolution test for fexofenadine hydrochloride capsules and coated tablets. J Pharm Pharm Sci. 2005;8:289–298. http://www.ncbi.nlm.nih.gov/pubmed/16124939. [PubMed] [Google Scholar]
- [5].Karakus S, Kucukguzel L, Kucukguzel SG. Development and validation of a RP-HPLC method for the determination of cetirizine or fexofenadine with pseudoephedrine in binary pharmaceutical dosage forms. J Pharm Biomed Anal. 2008;46:295–302. doi: 10.1016/j.jpba.2007.10.018. http://dx.doi.org/10.1016/j.jpba.2007.10.018. [DOI] [PubMed] [Google Scholar]
- [6].Zafar F, Shoaib MH, Yousuf RI. Development of RP-HPLC method for fexofenadine determination in tablet formulations and development of dissolution method. Pak J Pharmacol. 2011;28:43–49. [Google Scholar]
- [7].Hofmann U, Seiler M, Drescher S, Fromm MF. Determination of fexofenadine in human plasma and urine by liquid chromatography-mass spectrometry. J Chromatogr B. 2002;766:227–233. doi: 10.1016/s0378-4347(01)00468-6. http://dx.doi.org/10.1016/S0378-4347(01)00468-6. [DOI] [PubMed] [Google Scholar]
- [8].Flynn CA, Alnouti Y, Reed GA. Quantification of the transporter substrate fexofenadine cell lysates by liquid chromatography/tandem mass spectrometry. Rapid Comm Mass Spectrom. 2011;25:2361–2366. doi: 10.1002/rcm.5111. http://dx.doi.org/10.1002/rcm.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nirogi RV, Kandikere VN, Shukla M, Mudigonda K, Mayurya S, Komarneni P. Simultaneous quantification of fexofenadine and pseudoephedrine in human plasma by liquid chromatography/tandem mass spectrometry with electrospray ionization: method development, validation and application to a clinical study. Rapid Comm Mass Spectrom. 2006;20:3030–3038. doi: 10.1002/rcm.2701. http://dx.doi.org/10.1002/rcm.2701. [DOI] [PubMed] [Google Scholar]
- [10].Miura M, Uno T, Tateishi T, Suzuki T. Determination of fexofenadine enantimers in human plasma with high performance liquid chromatography. J Pharm Biomed Anal. 2007;43:741–745. doi: 10.1016/j.jpba.2006.07.033. http://dx.doi.org/10.1016/j.jpba.2006.07.033. [DOI] [PubMed] [Google Scholar]
- [11].Arayen MS, Sultana N, Shehnaz H, Haider A. RP-HPLC method for the quantitative determination of fexofenadine hydrochloride in coated tablets and human serum. Med Chem Res. 2011;20:55–61. http://dx.doi.org/10.1007/s00044-009-9285-6. [Google Scholar]
- [12].Pathak SM, Kumar AR, Musmade P, Udupa N. A simple and rapid high performance liquid chromatographic method with fluorescence detection for the estimation of fexofenadine in rat plasma-application to preclinical pharmacokinetics. Talanta. 2008;76:338–346. doi: 10.1016/j.talanta.2008.02.047. http://dx.doi.org/10.1016/j.talanta.2008.02.047. [DOI] [PubMed] [Google Scholar]
- [13].Breier AR, Nudelman NS, Steppe M, Schapoval EES. Isolation and structure elucidation of photodegradation products of fexofenadine. J Pharm Biomed Anal. 2008;46:250–257. doi: 10.1016/j.jpba.2007.09.017. http://dx.doi.org/10.1016/j.jpba.2007.09.017. [DOI] [PubMed] [Google Scholar]
- [14].Breier AR, Steppe M, Schapoval EES. Photodegradation Kinetics of Fexofenadine Hydrochloride Using a LC Method Chromatographia. 2006;64:725–729. http://dx.doi.org/10.1365/s10337-006-0096-3. [Google Scholar]
- [15].Radhakrishna T, Reddy GO. Simultaneous determination of fexofenadine and its related compounds by HPLC. J Pharm Biomed Anal. 2002;29:681–690. doi: 10.1016/s0731-7085(02)00181-4. http://dx.doi.org/10.1016/S0731-7085(02)00181-4. [DOI] [PubMed] [Google Scholar]
- [16].Sharaf El-Din MK, Ibrahim F, Eid MI, Wahba MEK. Validated stability-indicating liquid chromatographic method for the determination of fexofenadine hydrochloride in presence of its degradation products. Application to tablets and content uniformity testing. J Pharmacy Res. 2011;4:2377–2380. [Google Scholar]
- [17].International Conference on Harmonisation. ICH Q1A (R2), Stability Testing of new Drug Substances and Products. 2003.
- [18].ICH Q2 (R1), Validation of Analytical Procedures: Text and Methodology. 2005. International Conference on Harmonisation. [Google Scholar]
- [19].International Conference on Harmonisation. ICH Q1B (R2), Photostability testing on new drug substances and products. 1996.