Abstract
The molecular pathways regulating cell lineage determination and regeneration in epithelial tissues are poorly understood. The secretory epithelium of the lung is required for production of mucus to help protect the lung against environmental insults, including pathogens and pollution, that can lead to debilitating diseases such as asthma and chronic obstructive pulmonary disease. We show that the transcription factors Foxp1 and Foxp4 act cooperatively to regulate lung secretory epithelial cell fate and regeneration by directly restricting the goblet cell lineage program. Loss of Foxp1/4 in the developing lung and in postnatal secretory epithelium leads to ectopic activation of the goblet cell fate program, in part, through de-repression of the protein disulfide isomerase anterior gradient 2 (Agr2). Forced expression of Agr2 is sufficient to promote the goblet cell fate in the developing airway epithelium. Finally, in a model of lung secretory cell injury and regeneration, we show that loss of Foxp1/4 leads to catastrophic loss of airway epithelial regeneration due to default differentiation of secretory cells into the goblet cell lineage. These data demonstrate the importance of Foxp1/4 in restricting cell fate choices during development and regeneration, thereby providing the proper balance of functional epithelial lineages in the lung.
Keywords: Foxp1, Foxp4, Endoderm, Goblet cell, Lung, Regeneration, Mouse
INTRODUCTION
The tracheal and bronchiolar airways of mammals are essential for providing defense against inhaled pathogens and external environmental insults, such as cigarette smoke and pollution. The pseudostratified epithelium lining the trachea and proximal bronchiolar airways is composed of multiple cell lineages, including secretory and ciliated epithelium as well as basal cells, which act as progenitors for these lineages (reviewed by Morrisey and Hogan, 2010). The molecular pathways controlling cell fate and the precise allocation of epithelial lineages during both lung development and lung regeneration are poorly understood. Such insight is crucial for developing better therapeutic options for lung diseases such as asthma and chronic obstructive pulmonary disease (COPD).
The proximal airways of the respiratory system are lined with several differentiated cell lineages, including secretory Clara cells, ciliated epithelial cells, neuroendocrine cells and, in large mammals such as humans, goblet cells. Goblet cells are large morphologically distinct cells containing large vacuoles needed for the secretion of mucus in response to airway irritation or injury. Goblet cells are thought to differentiate from other secretory lineages in the proximal airways such as Clara secretory cells (Park et al., 2007; Chen et al., 2009). In normal proximal airways of mammals, only a small number of goblet cells are found and in mice this lineage is absent unless an injury or disease model is provoked. However, in diseases such as asthma and COPD, an increased numbers of goblet cells generate large amounts of mucus secretions in response to airway sensitization and to the chronic injury/repair cycle indicative of these diseases (reviewed by Fahy, 2001; Rogers, 2003; Tagaya and Tamaoki, 2007). The molecular pathways that regulate the cell fate of proximal airway epithelium, which generates the proper composition of the proximal airways, are poorly understood. However, previous studies have demonstrated important roles for the ETS-related transcription factor Spdef and the Notch signaling pathway (Park et al., 2007; Chen et al., 2009; Guseh et al., 2009; Tsao et al., 2009; Rock et al., 2011). Despite these findings, little is understood about how cell fate decisions are regulated in the developing and regenerating airway epithelium and whether there are factors that actively restrict cell lineage differentiation versus those that promote specific cell fates, such as Spdef (Park et al., 2007; Chen et al., 2009).
Foxp1, Foxp2 and Foxp4 transcription factors are conserved transcriptional repressors expressed in overlapping patterns in the epithelium and mesenchymal derivatives of the developing foregut (Shu et al., 2001; Lu et al., 2002; Shu et al., 2007). Foxp1/2/4 are each expressed at high levels during lung development as well as in the adult lung with Foxp1/4 being the predominant paralogs expressed in proximal bronchiolar and tracheal epithelium (Lu et al., 2002). Previous studies have shown that Foxp1/2 cooperatively regulate distal lung epithelial development as well as esophageal development (Shu et al., 2007). Foxp2−/−:Foxp1+/− mutants express lower levels of the key transcription factors N-myc (Mycn – Mouse Genome Informatics) and Hopx, leading to perinatal demise (Shu et al., 2007). However, the role of Foxp1/2/4 in development and homeostasis of the epithelium in the proximal airways of the respiratory system was unknown.
In this article, we show that conditional deletion of Foxp1/4 in developing lung epithelium leads to decreased secretory cell differentiation, in particular loss of Clara cells, but ectopic activation of the goblet cell program, thus indicating a key role for these factors in controlling secretory cell fate. Using ChIP-seq and microarray, we show that Foxp1/4 repress key factors in the goblet cell differentiation program, including the protein disulfide isomerase anterior gradient 2 (Agr2). Overexpression of Agr2 leads to ectopic activation of the goblet cell gene expression program, indicating that this gene is sufficient to drive many aspects of goblet cell differentiation. Foxp1/4 are also required to restrict the goblet cell differentiation program during lung secretory cell homeostasis and regeneration after postnatal injury, as shown by the dramatic inhibition of airway epithelial regeneration in mutants lacking Foxp1/4 expression. Together, these data point to a crucial role for Foxp1/4 in restricting secretory cell fate determination during both lung development and epithelial regeneration, indicating a pivotal role for these transcription factors in defining epithelial cell fates within developing and regenerating foregut derivatives.
MATERIALS AND METHODS
Animals
Shh-cre and Foxp1flox/flox mice were previously generated and genotyped as described (Harfe et al., 2004; Feng et al., 2010). Foxp4flox/+ mice were generated by introducing two loxP sites that flank exons 12 and13 coding the forkhead DNA-binding domain (supplementary material Fig. S1) using standard recombination techniques. The targeting vector was linearized and electroporated into R1 embryonic stem (ES) cells. ES cell clones were isolated and DNA was extracted and Southern blot analysis was performed using both 5′ and 3′ probes to verify correct integration of both loxP sites. Two correctly targeted ES clones were injected into blastocysts to generate chimeric mice, which were further bred to C57BL/6 mice to generate germline transmission of the Foxp4flox/+ allele. The neomycin cassette was removed using Flpe mice. The Scgb1a1-cre mouse was generated by homologous recombination in ES cells to insert the iCre cDNA with SV40 polyadenylation sequence at the Scgb1a1 start codon. Recombination in Scgb1a1-cre/R26R animals shows activity in the majority of airway epithelial cells including the trachea, the bronchi and bronchioles. Shh-cre and Scgb1a1-cre animals were used as controls in the relative experiments. Genotypes of all mutant lines were determined by PCR amplification using primers listed in supplementary material Table S1.
To generate SFTPC-Agr2 mice, the full mouse Agr2 coding region was cloned downstream of the human 3.7 kb SFTPC promoter and upstream of a SV40 polyadenylation sequence as previously described (Tian et al., 2011). The transgenic cassette was excised from the resulting plasmid, purified and injected into FVBN fertilized oocytes. Transgenic positive embryos were collected at E18.5 and genotypes using PCR primers listed in supplementary material Table S1. Three SFTPC-Agr2 genotype-positive animals were examined in these studies and the data were consistent amongst all three F0 founders. Genotype-negative littermates were used as controls for these experiments. All animal procedures were performed in accordance with the Institute for Animal Care and Use Committee at the University of Pennsylvania.
Naphthalene injury
Eight- to ten-week-old mice were injected interperitoneally with 200 mg/kg body weight of naphthalene dissolved in corn oil; control mice were injected with the same volume of corn oil. At days 3, 10 and 20 after injection, right lung lobe was collected for RNA extraction and left lobes were inflation fixed at 25 cm H2O pressure with 10% formalin followed by an additional 24 hours of fixation in 10% formalin. Five animals were used for each genotype and time point tested.
Histology
Tissues were fixed in 10% formalin, embedded in paraffin wax and sectioned at a thickness of 5 μm. Hematoxylin and Eosin (H&E) staining was performed using standard procedures. Immunohistochemistry was performed using the following antibodies: mouse anti-phospho-histone 3 (Cell Signaling Technology, 1:200), goat anti-Scgb1a1 (Santa Cruz, 1:20), rabbit anti-SP-C (Chemicon, 1:500), rabbit anti-Nkx2.1 (Santa Cruz, 1:50), rabbit anti-Sox9 (Santa Cruz, 1:100), rabbit anti-Sox2 (Seven Hills Bioreagents, 1:500), mouse anti-β-tubulin IV (BioGenex, 1:20), rabbit anti-e-cadherin (Cell Signaling, 1:100) and mouse anti-mucin5ac (Abcam, 1:100). The Foxp1 (1:200) and Foxp4 (1:200) polyclonal antibodies have been previously described (Lu et al., 2002). Slides were mounted with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA). In situ hybridization was performed as previously described (Morrisey et al., 1996).
Microarray studies
RNA was isolated from E14.5 lungs from Foxp1/4ShhcreDKO and Shh-cre control embryos and used to generate a biotinylated cRNA probe for Affymatrix Mouse Gene 1.0ST array. Data analysis and heatmap generation were performed as previously described (Zhang et al., 2008). Genes with 1.5-fold or greater changes over that of the experimental mean with a value of P<0.01 (ANOVA) were considered to be statistically significant. Microarray data have been deposited in Gene Expression Omnibus under accession number GSE34584.
Quantitative PCR
Total RNA was isolated from lung tissue at the indicated time points using Trizol reagent, reverse transcribed using SuperScript First Strand Synthesis System (Invitrogen), and used in quantitative real-time PCR analysis with the primers listed in supplementary material Table S1.
ChIP and ChIP-seq analysis
For ChIP-seq analysis, each biological sample was generated from 20-30 embryonic day (E) 12.5 lungs. The ChIP and ChIP-seq analysis was performed as previously described (Zhang et al., 2010). Briefly, chromatin was prepared using a ChIP Assay Kit (Millipore), except protein-G agarose beads were used to pre-clear chromatin. The previously characterized Foxp1 polyclonal antibody as well as control rabbit IgG were used to immunoprecipitate protein–DNA complexes from this chromatin. The purified immunoprecipitated DNA was end-repaired, ligated to sequencing adapters, and enriched by PCR following the manufacturer's protocol (Illumina). The resulting DNA was sequenced on the Illumina Genome Analyzer II. Sequences were aligned to the mouse genome [University of California Santa Cruz Genome Browser on Mouse, July 2007 Assembly (mm9)] following pipeline processing. Peaks within a region covering 10 kb upstream and downstream from the transcriptional start site unique to the Foxp1 antibody and not found in the normal rabbit IgG were used as criteria for potential targets of Foxp1. ChIP-seq binding data for Agr2 was confirmed by directed ChIP in chromatin samples from E12.5 and E18.5 lung using primers listed in supplementary material Table S1.
Cell culture transfection assays
The proximal 600 bp covering forkhead-binding sites within the Foxp1 ChIP-seq peak region (~8 kb upstream mouse Agr2 transcriptional start site) was cloned into the pGL3 lucifersase reporter plasmid to generate pGL3Agr2.luc. pGL3Agr2.luc was co-transfected into NIH 3T3 cells using Fugene 6 reagent with Renilla luciferase control expression plasmid and the indicated amount of Foxp1 expression vector. Forty-eight hours following transfection, cell extracts were assayed for luciferase expression using a commercially available kit (Promega). Relative reporter activities are expressed as luminescence units normalized for Renilla expression in the cell extracts. Data represent the average of three assays ± s.e.m.
RESULTS
Loss of Foxp1/4 expression inhibits early secretory cell differentiation
Foxp1/4 are expressed in proximal airway epithelium in both the developing and adult lung (Lu et al., 2002). To assess the role of Foxp1/4 in lung epithelial differentiation, we generated a floxed allele of Foxp4 (Foxp4flox/flox) and, along with our previously described Foxp1flox/flox allele (Feng et al., 2010), we deleted both genes using the Shh-cre line which is active throughout the developing lung epithelium from the earliest stages of lung development (Fig. 1A-E) (Goss et al., 2009). Shh-cre-mediated loss of either Foxp1 or Foxp4 using Shh-cre did not result in any prenatal or postnatal lethality (data not shown). However, all Shh-cre:Foxp1flox/flox:Foxp4flox/flox mutants, from now on referred to as Foxp1/4ShhcreDKO, die within minutes after birth of apparent respiratory distress. Foxp1/4ShhcreDKO mutants expressed normal levels of the important lung epithelial transcription factor Nkx2.1 and the alveolar epithelial type 2 cells marker surfactant protein C (Sftpc), suggesting that lung epithelial specification was not dramatically altered by Foxp1/4 deficiency (Fig. 1F-J). Although overall lung morphology of Foxp1/4ShhcreDKO mutants appeared to be relatively normal, a reduction in Clara secretory cells was observed in the proximal airways of E18.5 Foxp1/4ShhcreDKO mutants as determined by immunostaining and quantitative PCR (Q-PCR) for the Clara secretory cell marker gene Scgb1a1 (Fig. 1K-O). This was associated with an increase in ciliated epithelial cell differentiation, as revealed by increased β-tubulin IV immunostaining and Q-PCR for the ciliated epithelial specific transcription factor Foxj1 (Fig. 1K-N,P). These data indicate that secretory epithelial cell differentiation is defective in Foxp1/4ShhcreDKO mutants with a decrease in Clara secretory cells and an increase in ciliated cells in the proximal airway epithelium.
Fig. 1.
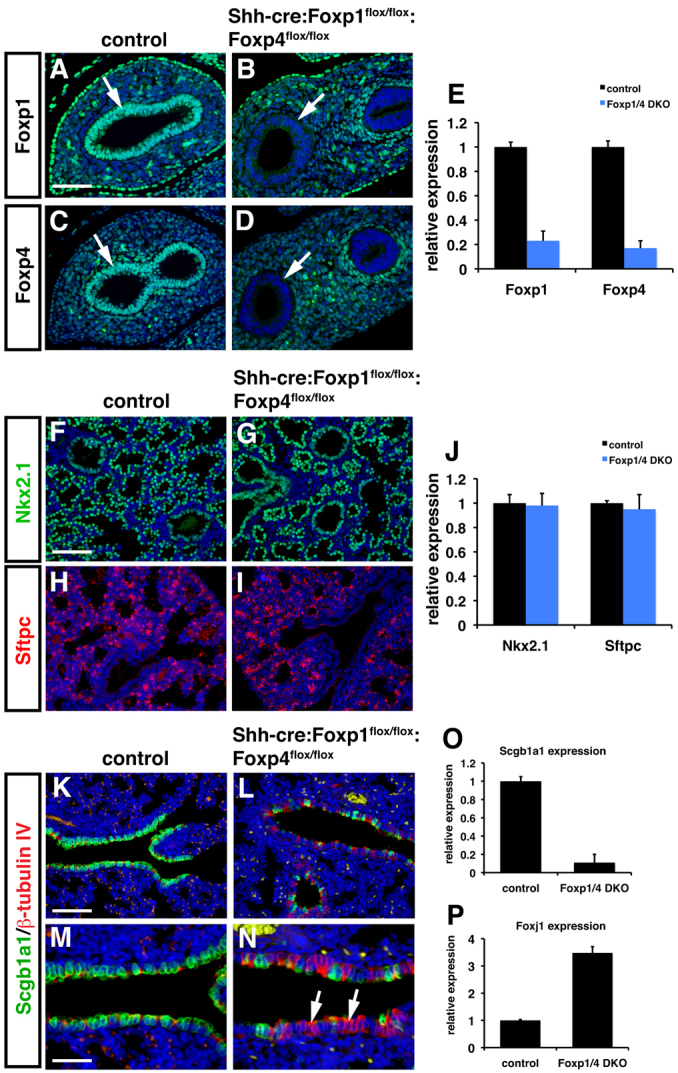
Loss of Foxp1/4 leads to defective proximal airway epithelial development in mouse. (A-E) Foxp1 and Foxp4 were efficiently deleted using the Shh-cre line as noted by loss of expression in the developing airway epithelium using immunostaining (A-D, arrows) and Q-PCR (E) at E12.5. (F-J) Immunostaining for Nkx2.1 (F,G) and Sftpc (H,I) expression indicate normal lung endoderm specification and distal alveolar epithelial differentiation in Foxp1/4ShhcreDKO mutants at E18.5. Q-PCR also indicates normal expression of both Nkx2.1 and Sftpc at E18.5 (J). (K-N) Expression of the proximal secretory cell differentiation marker Scgb1a1 was decreased in Foxp1/4ShhcreDKO mutants as revealed by immunostaining whereas immunostaining for the ciliated epithelial marker β-tubulin IV was increased at E18.5 (K-N, arrows). Panels M and N represent higher magnification pictures of K and L to illustrate better the decrease in Scgb1a1-positive secretory cells (green) and an increase in β-tubulin IV-positive ciliated epithelial cells (red). (O,P) Q-PCR shows decreased expression of the secretory epithelial cell marker Scgb1a1 (O) and an increase in the ciliated epithelial marker Foxj1 (P) in Foxp1/4ShhcreDKO mutants at E18.5. Shh-cre embryos were used as controls for all of these experiments. Error bars represent s.e.m. Scale bars: 100 μm for A-D,M,N; 200 μm for F-I,K,L.
Proximal airway epithelial differentiation defects in Foxp1/4ShhcreDKO mutants
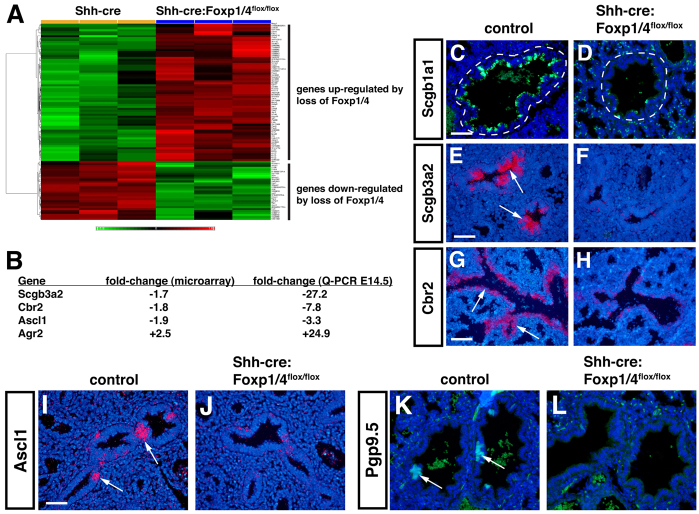
To understand better the genome-wide consequences of Foxp1/4 deficiency on secretory epithelial differentiation in the lung, we performed microarray analysis of Shh-cre control and Foxp1/4ShhcreDKO mutant lungs at E14.5. Greater than 1.5-fold differences were observed for 49 upregulated genes and for 28 downregulated genes (Fig. 2A; supplementary material Table S2). Microarray, Q-PCR and in situ hybridization analyses indicate that the early secretory cell marker genes Scgb3a2 and Cbr2 were significantly downregulated in a fashion similar to Scgb1a1, further suggestive of early defects in the cell fate of the proximal airway epithelium of Foxp1/4ShhcreDKO mutants (Fig. 2A-H). Interestingly, expression of Agr2, a protein disulfide isomerase expressed in goblet cells in the lung (Chen et al., 2009; Zhao et al., 2010), was upregulated in Foxp1/4ShhcreDKO mutants using both microarray and Q-PCR assays (Fig. 2A,B).
Fig. 2.
Decreased secretory and neuroendocrine cell fates and an increase in goblet cell lineage markers in Foxp1/4ShhcreDKO mutants. (A,B) Microarray and Q-PCR analyses show that several genes expressed in the Clara secretory cell (Scgb3a2, Cbr2) and neuroendocrine lineages (Ascl1) are decreased in Foxp1/4ShhcreDKO mutants at E14.5. By contrast, the goblet cell marker gene Agr2 is increased in both microarray and Q-PCR analyses. Red and green indicate up- and downregulated genes, respectively, versus Shh-cre controls. (C-H) Decreased expression of the secretory cell markers Scgb1a1 (C,D), Scgb3a2 (E,F) and Cbr2 (G,H) are observed in Foxp1/4ShhcreDKO mutants at E18.5 using immunostaining (C,D) and in situ hybridization (E-H). Airways are encircled by a dashed line. (I-L) The neuroendocrine markers Ascl1 (I,J) and Pgp9.5 (K,L) are both decreased in Foxp1/4ShhcreDKO mutants at E18.5 as revealed by in situ hybridization and immunostaining, respectively. Arrows indicate probe hybridization or immunostaining. Shh-cre embryos were used as controls for all of these experiments. Scale bars: 200 μm for C-H,K,L; 150 μm for I,J.
In addition to loss of secretory cell marker genes, expression of the neuroendocrine (NE) cell marker Ascl1 was shown to be decreased by microarray and Q-PCR analyses at E14.5 (Fig. 2B). NE cells are found in the large proximal airways of the lung and are thought to derive from a Sox2+ proximal airway precursor (Tompkins et al., 2009). In situ hybridization for Ascl1 as well as immunostaining for Pgp9.5 (also known as Uch1l), an additional marker of the NE lineage, indicated that differentiation of the NE lineage is inhibited in Foxp1/4ShhcreDKO mutants (Fig. 2I-K). Thus, loss of Foxp1/4 in the developing lung epithelium leads to a defect in cell fate choice in the proximal airway epithelium with decreased differentiation of both secretory and NE lineages but increased expression of the goblet cell gene expression program.
Lung endoderm progenitor allocation and Notch signaling are unaffected in Foxp1/4ShhcreDKO mutants
Sox2+ endoderm cells are thought to act as precursors for most, if not all, of the proximal airway epithelial cell lineages, whereas expression of Sox9 is thought to mark an early distal endoderm progenitor cell lineage (Okubo et al., 2005; Lu et al., 2007). As defects in secretory and NE epithelial differentiation could arise from altered development of Sox2+ endoderm progenitors or from altered expression of Sox2 itself, we assessed expression of Sox2 as well as the distal epithelial progenitor marker Sox9 in Foxp1/4ShhcreDKO mutants. Immunohistochemistry as well as Q-PCR showed that expression of Sox2 and Sox9 were unchanged in Foxp1/4ShhcreDKO mutants (Fig. 3A-F). Moreover, the pattern of expression of these progenitor markers was not altered in Foxp1/4ShhcreDKO mutants, with expression of Sox2 confined to the proximal lung endoderm and Sox9 expression confined to the distal lung endoderm (Fig. 3A-D). These data indicate that the defects in secretory epithelial cell fate and differentiation observed in Foxp1/4ShhcreDKO mutants were not due to impaired allocation of Sox2+ or Sox9+ progenitors or from de-regulated expression of the Sox2 or Sox9 genes.
Fig. 3.
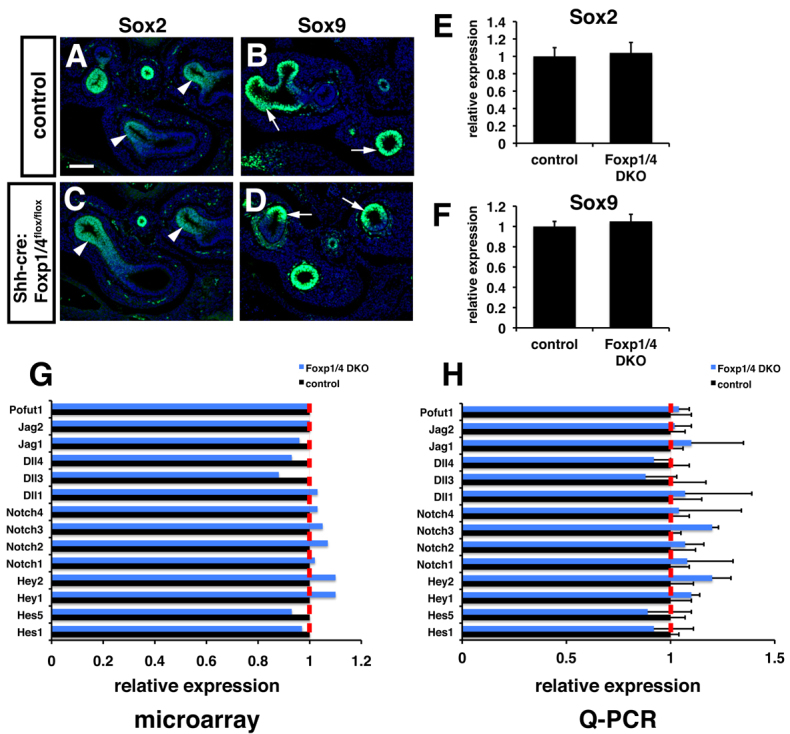
Normal expression of early endoderm progenitor markers and Notch signaling components upon loss of Foxp1/4 expression in lung endoderm. (A-D) The pattern of Sox2 (A,C; arrowheads) and Sox9 (B,D; arrows) expression appears normal by immunostaining in Foxp1/4ShhcreDKO mutants at E12.5. (E,F) Q-PCR reveals normal expression of Sox2 and Sox9 in Foxp1/4ShhcreDKO mutants at E12.5. (G,H) Microarray (G) and Q-PCR (H) analyses show normal expression of Notch signaling components, including the important Notch signaling activity readout genes Hes1 and Hes5. Shh-cre embryos were used as controls for these experiments. Error bars represent s.e.m. Scale bar: 150 μm.
Previous studies have demonstrated that Notch signaling plays a key role in directing the cell fate and differentiation of proximal airway progenitors into the secretory cell lineage (Guseh et al., 2009; Tsao et al., 2009; Morimoto et al., 2010; Rock et al., 2011). Loss of Notch signaling leads to a complete loss of the secretory cell lineage in the developing lung whereas increased Notch signaling promotes secretory cell differentiation (Guseh et al., 2009; Tsao et al., 2009; Morimoto et al., 2010; Rock et al., 2011). Because the phenotype in Foxp1/4ShhcreDKO mutants was one of decreased secretory cell differentiation, we sought to determine whether Notch signaling was de-regulated in these mutants. Multiple Notch ligands, receptors, and transcriptional downstream effectors, such as Hes1, are expressed in the lung (Post et al., 2000; Tsao et al., 2009). Microarray and Q-PCR analyses detected no significant alterations in the expression of all Notch components tested in Foxp1/4ShhcreDKO mutants (Fig. 3G,H). These data suggest that Foxp1/4 act either downstream or independently of Notch signaling to regulate secretory epithelial cell fate in the lung.
Ectopic activation of the goblet cell program in Foxp1/4ShhcreDKO mutants
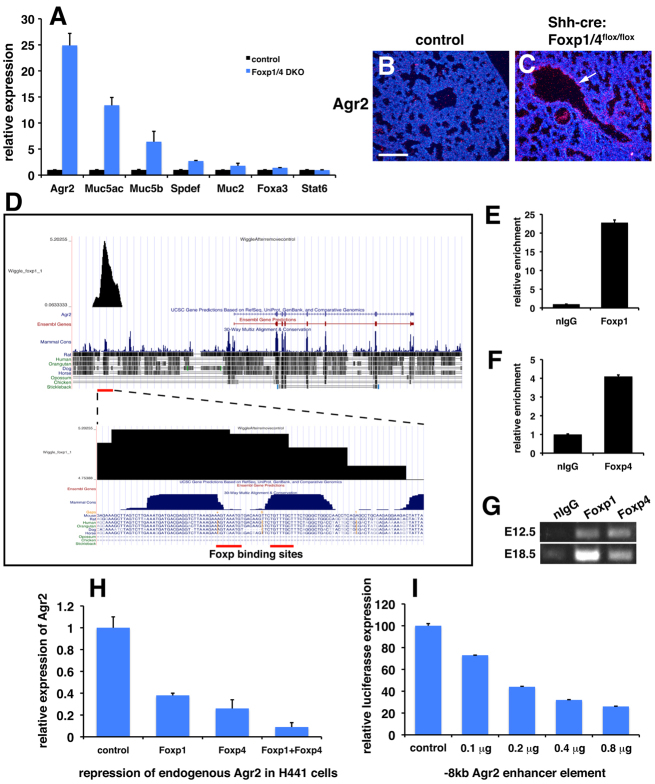
Increased expression of Agr2 along with decreased expression of genes associated with the Clara secretory cell lineage suggested that Foxp1/4 normally restricts the goblet cell lineage within the developing airway epithelium. Goblet cells do not form in mouse airways except during injury or in disease models such as those mimicking human asthma (Rogers, 2003; Sumi and Hamid, 2007). Moreover, goblet cells dramatically increase in number in the human lung after injury or in diseases such as asthma and COPD (Rogers, 2003; Sumi and Hamid, 2007). To assess the extent of activation of the goblet cell lineage program upon loss of Foxp1/4 expression, we characterized E18.5 Foxp1/4ShhcreDKO mutants for expression of additional goblet cell genes and proteins. Expression of Muc5ac, Muc5b, Spdef and Foxa3 was upregulated although Spdef and Foxa3 expression was increased less dramatically than the other genes (Fig. 4A). However, Stat6, which has been shown to regulate Spdef expression (Park et al., 2007; Chen et al., 2009), was not upregulated in Foxp1/4ShhcreDKO mutants (Fig. 4A). In situ hybridization shows increased expression of Agr2 in the proximal airways of Foxp1/4ShhcreDKO mutants (Fig. 4B,C). These data suggested that Foxp1/4 might act downstream of Stat6 but upstream of Agr2 to restrict the goblet cell lineage during lung development.
Fig. 4.
Ectopic activation of the goblet cell lineage program in Foxp1/4ShhcreDKO mutants and identification of Agr2 as a direct target of Foxp1/4. (A) Q-PCR reveals increased expression of goblet cell related genes, including Agr2, in Foxp1/4ShhcreDKO mutants at E18.5. (B,C) In situ hybridization showing increased Agr2 expression in the proximal airways of Foxp1/4ShhcreDKO mutants at E18.5 (arrow). (D) Foxp1 ChIP-seq data reveals a single strong peak 8 kb upstream of the Agr2 transcriptional start site that contains two evolutionarily conserved Foxp DNA-binding sites. (E-G) Directed ChIP for Foxp1 and Foxp4 on the Agr2 −8 kb enhancer element using Q-PCR at E18.5 (E,F) and PCR agarose gel analysis at E12.5 and E18.5 (G). (H) Foxp1 and Foxp4 can cooperatively repress endogenous AGR2 expression in H441 human lung epithelial cells. (I) The −8 kb Agr2 enhancer element can be repressed by Foxp1 in a dose-dependent manner when cloned into a luciferase reporter. Error bars represent s.e.m. Scale bar: 200 μm.
As noted above, previous models of goblet cell development have identified a hierarchical set of factors with Notch and Stat6 residing at the top. These activate Spdef, which, in turn, activates additional goblet cell genes, including Agr2 and the mucin-related proteins Muc5ac and Muc5b (Park et al., 2007; Chen et al., 2009). Of these, Agr2 expression was increased the most and its expression was increased as early as E14.5 (Fig. 2). This is prior to full development of the secretory lineage in the proximal airways of the lung and before the increase of other goblet cell lineage genes, as noted by our microarray results (Fig. 2). Agr2 is a protein disulfide isomerase that has been implicated in trafficking mucin proteins in goblet cells of the intestine (Zhao et al., 2010). Moreover, Agr2 expression is essential for goblet cell development as Agr2 null mice lack goblet cells in the intestine (Zhao et al., 2010). To determine whether Agr2 is a direct transcriptional target of Foxp1/4 in the lung, we performed ChIP-seq for Foxp1 on chromatin from E12.5 mouse lungs (Zhang et al., 2010). The Foxp1 ChIP-seq data reveal that Foxp1 binds to an evolutionarily conserved region of the Agr2 locus (located ~8 kb upstream of the predicted Agr2 transcriptional start site) that contains two conserved forkhead DNA-binding sites (Fig. 4D). Directed ChIP experiments confirmed that both Foxp1 and Foxp4 bind to these evolutionarily conserved forkhead-binding sites in chromatin generated from both E12.5 and E18.5 lungs (Fig. 4E-G).
To assess whether Foxp1 and Foxp4 can directly repress Agr2 gene expression, we overexpressed each transcription factor individually or in combination in the human lung epithelial cell line H441, which has characteristics of Clara secretory epithelial cells (Sawaya et al., 1993). These studies show that Foxp1 and Foxp4 can cooperatively repress endogenous AGR2 expression in lung secretory epithelium (Fig. 4H). Foxp1 can also repress this −8 kb Agr2 enhancer element in a dose-dependent manner when cloned into a luciferase reporter construct (Fig. 4I). These data demonstrate that Foxp1 and Foxp4 can directly target and repress expression of Agr2 in the secretory epithelium of the lung.
Overexpression of Agr2 can elicit the goblet cell fate in the developing lung epithelium
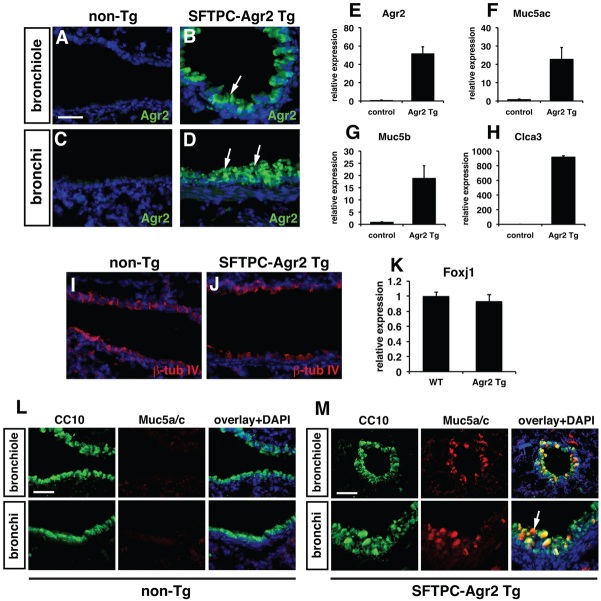
Agr2 is required for intestinal goblet cell development and its expression is associated with metastasis and increased cellular stress responses (Zweitzig et al., 2007; Barraclough et al., 2009; Zhao et al., 2010). Because goblet cells numbers are increased upon epithelial stress in injury and disease in both mice and humans, the increased expression of Agr2 might lead directly to increased expression of mucin genes along with other markers of the goblet cell program. Therefore, we generated transgenic mice using the human surfactant protein C (SFTPC) promoter to drive widespread ectopic Agr2 expression in the early lung epithelium in order to determine whether increased Agr2 expression could activate the goblet cell lineage gene expression program. Examination of transgenic F0 founders at E18.5 showed that three of the five total F0 genotype-positive founders expressed increased levels of Agr2 in the proximal airway epithelium including the bronchi and smaller bronchiols (Fig. 5A-D). These SFTPC-Agr2 transgenic animals expressed increased levels of Muc5ac, Muc2 and Clca3, which are all expressed in the goblet cell lineage in the lung (Fig. 5E-H). Expression of the ciliated epithelial markers β-tubulin IV and Foxj1 was unchanged in SFTPC-Agr2 transgenic lungs (Fig. 5I-K). The increase in Clca3, a calcium-activated chloride channel expressed in goblet cells (Zhou et al., 2002; Thai et al., 2005), suggests that ectopic expression of Agr2 promotes a broader goblet cell gene expression program than induction of mucin genes alone. Co-immunostaining for the secretory protein Scgb1a1 and Muc5ac shows that both proteins are expressed in the secretory epithelium upon overexpression of Agr2 (Fig. 5L,M). This is consistent with previous studies showing that goblet cells express both Sgb1a1 and Muc5ac (Boers et al., 1999; Evans et al., 2004). These data demonstrate that increased Agr2 is sufficient to promote many aspects of the goblet cell fate and that its de-repression in Foxp1/4ShhcreDKO mutants is responsible, at least in part, for the ectopic activation of the goblet cell lineage program.
Fig. 5.
Overexpression of Agr2 can ectopically activate the goblet cell lineage gene expression program. (A-D) SFTPC-Agr2 transgenic lungs express elevated levels of Agr2 protein in both small bronchiolar and larger bronchi at E18.5. (E-H) Q-PCR analysis of Agr2 (E), Muc5ac (F), Muc5b (G) and Clca3 (H) expression in SFTPC-Agr2 transgenic lungs at E18.5. (I,J) Immunostaining for β-tubulin IV for non-transgenic (I) and SFTPC-Agr2 transgenic (J) lungs. (K) Q-PCR for Foxj1 expression in non-transgenic and SFTPC-Agr2 transgenic lungs. (L,M) Non-transgenic littermates do not express Muc5ac in proximal airway epithelium (L) whereas Muc5ac and Scgb1a1 are co-expressed in epithelial cells of SFTPC-Agr2 transgenic lungs at E18.5 (M, arrow). The Q-PCR data in E-H and K are the average ± s.e.m. of the three different F0 founders at E18.5. Non-transgenic littermates were used as controls for these experiments. Scale bars: 150 μm for A-D; 200 μm for L,M.
Foxp1/4 are required for regeneration of secretory cells in lung airways by restricting the goblet cell fate
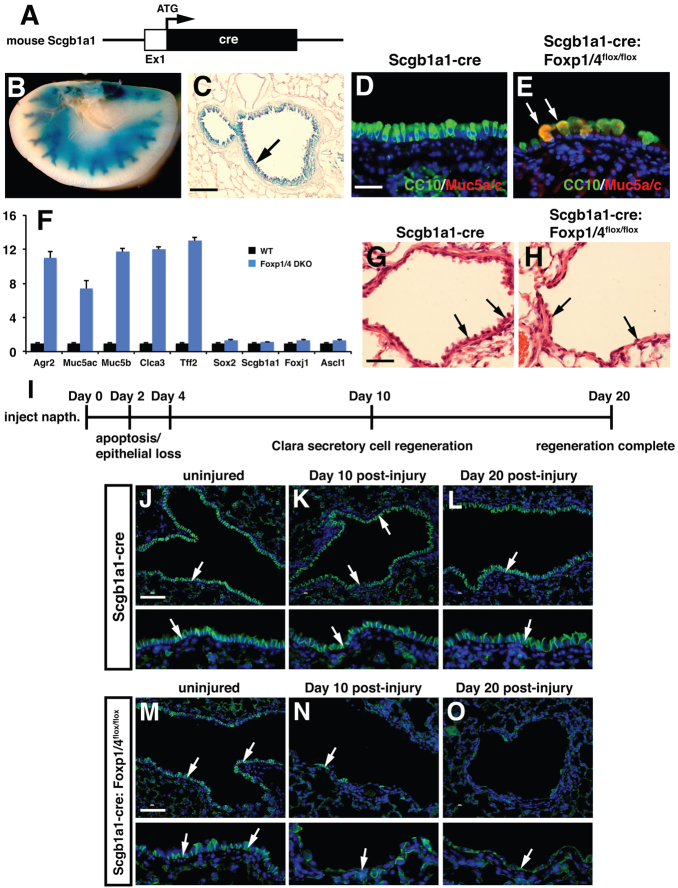
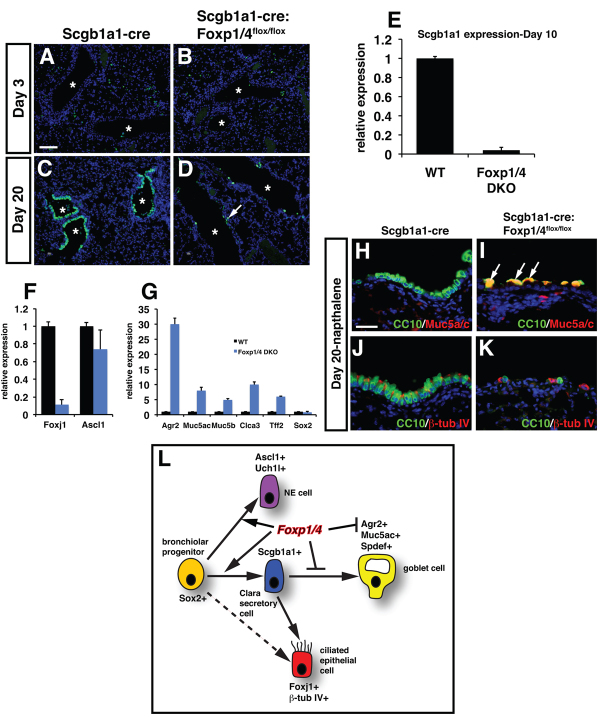
Because Foxp1/4 are necessary for development of the early Clara secretory cell lineage while restricting the goblet cell lineage during lung development, we assessed whether Foxp1/4 were required to maintain the Clara cell secretory lineage and restrict the goblet lineage in the postnatal mouse. We employed a newly generated Clara secretory cell-specific cre line in which cre recombinase was inserted into the endogenous Scgb1a1 locus (Scgb1a1-cre; Fig. 6A) to generate Foxp1/4Scgb1a1creDKO mutants. The Scgb1a1-cre line is active by E18.5 and its activity is restricted to the proximal airway epithelium of the lung (Fig. 6B,C; data not shown). Foxp1/4Scgb1a1creDKO mutants were viable and no significant lethality was observed for up to one year of age (data not shown). Histological analysis showed that although the bronchiolar epithelium of Foxp1/4Scgb1a1creDKO mutants expressed Scgb1a1, many of these cells also expressed Muc5ac as noted by co-immunostaining (Fig. 6D,E; supplementary material Fig. S2). The large bulbous shape of these cells as well as their increased expression of mucins indicative of a goblet cell phenotype was noted by Alcian Blue staining (supplementary material Fig. S3). Other markers of the goblet cell lineage were significantly upregulated in adult Foxp1/4Scgb1a1creDKO mutants, including Agr2, whereas markers of Clara (Scgb1a1, Sox2), ciliated (Foxj1) and NE (Ascl1) cells were unchanged (Fig. 6F). These data suggested that Foxp1/4 are required to restrict the goblet cell lineage during secretory cell homeostasis in the lung.
Fig. 6.
Loss of Foxp1/4 expression in the mature secretory epithelial cell lineage leads to ectopic activation of the goblet cell lineage program and inhibits epithelial regeneration. (A) Diagram of cre insertion into the Scgb1a1 locus. (B,C) Activity of the Scgb1a1-cre: R26RlacZ is confined to the proximal airway epithelium at two months as shown by whole-mount β-galactosidase histochemical staining (B) and histological sections from these lungs (C, arrow). (D,E) Loss of Foxp1/4 leads to ectopic activation of Muc5ac expression in Scgb1a1-positive cells of the proximal airways of Foxp1/4Scgb1a1creDKO adult mutants. (F) Q-PCR for markers of the goblet cell lineage program, including Agr2 as well as the secretory, ciliated and neuroendocrine lineages in Foxp1/4Scgb1a1creDKO adult lungs. Error bars represent s.e.m. (G,H) Naphthalene-induced secretory epithelial injury and regeneration leads to decreased numbers of airway epithelial cells 20 days after injury (arrows). (I) Schematic of the temporal nature of the naphthalene injury repair and regeneration model. (J-O) Immunostaining for E-cadherin shows normal regeneration of airway epithelium in Scgb1a1-cre control animals but reveals a dramatic loss of epithelial cells lining the injured airways of Foxp1/4Scgb1a1creDKO mutants at both 10 and 20 days post-injury (arrows). Lower panels in J-O are higher magnification images to illustrate better the presence or absence of E-cadherin-positive epithelium in the airways. Scgb1a1-cre animals were used as controls for these experiments. Scale bars: 200 μm for C,J-O; 100 μm for D,E; 150 μm for G,H.
We determined next whether Foxp1/4 were required for proper secretory cell repair and regeneration after naphthalene-induced injury. Naphthalene injury of the lung involves a severe and rapid loss of the majority of Clara secretory epithelium from the bronchiolar airways. A small population of Clara secretory cells that lack expression of the cytochrome Cyp2f2 are resistant to naphthalene injury and are thought to act as facultative progenitors during regeneration of the bronchiolar epithelium (Reynolds et al., 2000b; Giangreco et al., 2002). Loss of the secretory epithelium is complete after four days whereas regeneration and re-establishment of the airway epithelium is usually complete after two to three weeks. Naphthalene injury was induced in both Scgb1a1-cre control and Foxp1/4Scgb1a1creDKO mutants at 8 weeks of age. Both control and Foxp1/4Scgb1a1creDKO mutants survived the naphthalene treatment and displayed extensive airway apoptosis and loss of secretory cells three days after naphthalene treatment (Fig. 7A,B; data not shown). In Scgb1a1-cre control animals, extensive regeneration of the airway epithelium was observed at both 10 and 20 days post-injury as judged by E-cadherin (cadherin 1) immunostaining (Fig. 6G,J-L). However, in Foxp1/4Scgb1a1creDKO mutants, only a few airway epithelial cells were observed in the bronchiolar airways, indicating defective epithelial regeneration in the absence of Foxp1/4 expression (Fig. 6H,M-O).
Fig. 7.
Foxp1/4 are required for inhibition of the goblet cell lineage during secretory epithelial regeneration in the lung. (A-D) Scgb1a1-positive secretory cells are depleted at equal levels in Scgb1a1-cre and Foxp1/4Scgb1a1creDKO mutants at 3 days post-injury (A,B). However, Foxp1/4Scgb1a1creDKO mutants do not effectively regenerate Clara cell secretory epithelium after naphthalene injury as noted by Scgb1a1 immunostaining at 20 days post-injury (C,D). Asterisks denote airways. Arrow indicates the small number of Scgb1a1-positive cells in the Foxp1/4Scgb1a1creDKO mutants. (E) Q-PCR for Scgb1a1 expression in control and Foxp1/4Scgb1a1creDKO mutants at 10 days post-injury. (F) Q-PCR for Foxj1 and Ascl1 in control and Foxp1/4Scgb1a1creDKO mutants at 10 days post-injury. (G) Goblet cell markers are expressed at high levels in Foxp1/4Scgb1a1creDKO mutants 10 days post-naphthalene-induced injury, including high levels of Agr2. (H-K) The few epithelial cells in the proximal airways of Foxp1/4Scgb1a1creDKO mutants are strongly positive for both Scgb1a1 and Muc5ac expression (H,I). There are only a few β-tubulin IV-positive ciliated cells in Foxp1/4Scgb1a1creDKO mutants 10 days post-naphthalene-induced injury. Scgb1a1-cre animals were used as controls for these experiments. (L) Model of how Foxp1/4 act to restrict secretory cell lineage fate in the lung. Loss of Foxp1/4 leads initially to decreased secretory cell differentiation followed by ectopic activation of the goblet cell lineage program, which inhibits normal airway epithelial development and airway epithelial regeneration in the postnatal lung. Scale bars: 200 μm for A-D; 100 μm for H-K.
To assess the lineage of the surviving and/or regenerated cells in the naphthalene-treated Foxp1/4Scgb1a1creDKO mutants, Scgb1a1 expression was measured by immunostaining and Q-PCR at 3 and 10 days after injury. At 3 days post-injury, very few Scgb1a1 cells were present in either control or Foxp1/4Scgb1a1creDKO mutants owing to naphthalene-induced depletion (Fig. 7A,B). By 20 days post-injury, Scgb1a1 cells had readily regenerated in Scgb1a1-cre control animals whereas very few Scgb1a1-positive cells were observed in Foxp1/4Scgb1a1creDKO mutants (Fig. 7C,D). This lack of regeneration of Scgb1a1-positive cells is supported by a dramatic decrease in Scgb1a1 gene expression 10 days after injury in Foxp1/4Scgb1a1creDKO mutants (Fig. 7E). There was also a decrease in the ciliated epithelial markers Foxj1 and β-tubulin IV but no significant decrease in the neuroendocrine marker Ascl1 (Fig. 7F,J,K). Conversely, Agr2 expression was significantly increased relative to other goblet cell markers 10 days following naphthalene injury in Foxp1/4Scgb1a1creDKO mutants (Fig. 7G). Immunostaining shows that the majority of the cells remaining after naphthalene airway injury in Foxp1/4Scgb1a1creDKO mutants express Muc5ac, consistent with the acquisition of a goblet cell phenotype (Fig. 7G,H; supplementary material Fig. S2). This loss of secretory epithelial regeneration is not associated with changes in Cyp2f2 expression, absence of expression of which denotes a putative secretory epithelial progenitor capable of airway regeneration after naphthalene injury (supplementary material Fig. S3) (Reynolds et al., 2000a; Li et al., 2011). Thus, loss of Foxp1/4 in postnatal Clara secretory cells inhibits airway epithelial regeneration by ectopically activating the goblet cell fate. Together, these data indicate that Foxp1/4 are required for both development and maintenance of the secretory cell lineage but are also required to restrict the mature goblet cell lineage, in part, through direct repression of Agr2 (Fig. 7L).
DISCUSSION
Lung diseases that involve dysfunctional development and regeneration of the pseudostratified proximal airway epithelium are a leading cause of morbidity and mortality. A hallmark of the response of the proximal airways to both injury and disease is an increase in the goblet cell lineage with a concomitant increase in mucin production and altered morphology of the pseudostratified epithelium. We show that Foxp1/4 play a central role in restricting secretory cell fate in the proximal airways of the lung by regulation of a novel pathway involving direct repression of the protein disulfide isomerase Agr2. Ectopic expression of Agr2 alone can promote the goblet cell fate in the secretory epithelium of the lung. This result, along with previous findings that Agr2 is essential for intestinal goblet cell development (Zhao et al., 2010), suggests that Agr2 is a central player in defining goblet cell fate and function in the epithelium of multiple tissues including the lung and intestine. Given that increased goblet cell formation is associated with severe pulmonary diseases, such as asthma and COPD, our studies provide crucial insight into how secretory cell fate is established, maintained and regenerated in the lung, which may prove beneficial in developing future therapies for lung disease.
The mechanisms that control epithelial lineage fate, differentiation and regeneration are poorly understood. Within the proximal portion of the developing respiratory system, Sox2 is thought to act as an essential regulator of most, if not all, of the epithelial lineages. However, less is understood about the factors and pathways that direct specific epithelial lineage fate choices downstream of Sox2. Notch signaling has been shown to be essential for the fate choice between the secretory lineage and the ciliated lineage, both of which derive from Sox2+ progenitors (Que et al., 2007; Guseh et al., 2009; Tompkins et al., 2009; Tsao et al., 2009; Morimoto et al., 2010; Rock et al., 2011). The ETS transcription factor Spdef is sufficient and necessary for goblet cell differentiation in both gain- and loss-of-function experiments (Park et al., 2007; Chen et al., 2009). Whether other factors or pathways could regulate the balance of epithelial lineages by restricting certain cell fates was unclear. Our studies show that Foxp1/4 restrict the goblet cell fate through a novel pathway that appears to act either independently or does not appear to involve either disruption in Notch signaling or a dramatic increase in Spdef. Foxp1/4 regulate the protein disulfide isomerase Agr2, which, when ectopically expressed, can promote the goblet cell fate.
Other forkhead factors have been implicated in regulating goblet cell formation. Loss of Foxa2 in the developing lung epithelia leads to goblet cell hyperplasia (Wan et al., 2004). Conversely, loss of Foxa1 and Foxa2 leads to decreased goblet cell formation in the developing intestine (Ye and Kaestner, 2009). The mechanism by which Foxa1/2 regulate goblet cell formation is likely to be very different to that of Foxp1/4, as Foxa1/2 are classic pioneer transcription factors responsible for opening compacted chromatin to allow for increased gene transcription (Cirillo et al., 2002). By contrast, Foxp1/4 are transcriptional repressors that interact with the NuRD chromatin-remodeling complexes and repress gene transcription through the action of histone deactylases (Chokas et al., 2010). Thus, these two different subfamilies of forkhead transcription factors are not likely to act redundantly in the regulation of lung endoderm development or regeneration. In addition to defective airway epithelial development, Foxp1/4ShhcreDKO mutants are likely to have additional defects in lung development, possibly in alveolar epithelial differentiation as they die prenatally owing to respiratory distress (data not shown). Therefore, further analysis of Foxp1/4ShhcreDKO mutants is likely to uncover additional roles for Foxp1/4 in alveolar epithelial development.
We observed that de-repression of Agr2 expression closely correlated with loss of Foxp1/4 expression, both developmentally and in the postnatal Clara secretory cell lineage. Accordingly, ectopic expression of Agr2 can induce many characteristics of the goblet cell lineage, including increased expression of multiple mucin genes as well as Clca3, a chloride channel expressed in goblet cells (Zhou et al., 2002). However, the mechanisms by which increased levels of Agr2 lead to promotion of the goblet cell fate in the lung is unclear. Agr2 has been shown to bind mucin proteins directly and by managing their conformation as they traffick through the endoplasmic reticulum (ER), Agr2 might increase their stability and expression (Zhao et al., 2010; Higa et al., 2011; Niederreiter and Kaser, 2011). Expression of Agr2 is also induced by ER stress and is highly enhanced in multiple carcinomas, leading to stimulation of proliferation (Liu et al., 2005; Zweitzig et al., 2007; Ramachandran et al., 2008; Barraclough et al., 2009; Edgell et al., 2010; Maresh et al., 2010; Bu et al., 2011). More relevant in light of our results are previous findings showing that Agr2 can alter cell fates by inducing ectopic cement gland differentiation and anterior neural fate in Xenopus laevis (Aberger et al., 1998). Additional studies will be required to understand better how Agr2 controls cell fate decisions in lung epithelial cells.
Several human lung diseases exhibit increased levels of goblet cell differentiation. However, goblet cells are not apparent in the developing mouse lung and only develop in adult animals upon subjection to stressors, such as airway injury or asthma disease models induced with allergens (Fahy, 2001; Rogers, 2003; Izuhara et al., 2009). As previous studies have demonstrated that Foxp1/4 act as transcriptional repressors, our finding that loss of Foxp1/4 lead to ectopic activation of the goblet cell program and that this can be recapitulated by ectopic expression of Agr2 reveals that the developing secretory epithelium is under restrictive constraints to regulate cell fate decisions. This function of Foxp1/4 is independent of Notch signaling, which is known to regulate the secretory cell fate in the lung. Notch signaling also plays important roles in the developing and regenerating airway epithelium. In the developing lung, Notch signaling is essential for development of the secretory cell lineage (Guseh et al., 2009; Tsao et al., 2009; Morimoto et al., 2010). In the postnatal lung, loss of Notch signaling leads to decreased Clara cell differentiation and increased goblet cell differentiation (Rock et al., 2011). Our findings show that Foxp1/4 promote initial development of the secretory epithelial cell fate and restrain the goblet cell fate in both the developing and postnatal secretory epithelium.
The secretory epithelium is the site of several debilitating human diseases including asthma and COPD. In these diseases, goblet cell formation is increased, which leads to excess mucus production and further degradation of airway function. The lack of airway epithelial regeneration in Foxp1/4Scgb1a1creDKO mutants demonstrates that the ectopic activation of the goblet cell fate leads to a failure of these cells to regenerate in response to airway injury. This would lead to decreased airway repair, exposure of the underlying basal lamina and promotion of an inflammatory response commonly observed in airway diseases such as asthma. Thus, Foxp1/4 are essential for generating the proper balance of epithelial lineages in the proximal airways, which is required for the response to injury and inhibition of diseases of the airway.
Supplementary Material
Footnotes
Funding
These studies were supported by funding from the National Institutes of Health [to E.E.M. (R01 HL071589 and R01 HL087825 to E.E.M.; and P.W.T. (R01 CA31534). Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.079699/-/DC1
References
- Aberger F., Weidinger G., Grunz H., Richter K. (1998). Anterior specification of embryonic ectoderm: the role of the Xenopus cement gland-specific gene XAG-2. Mech. Dev. 72, 115-130 [DOI] [PubMed] [Google Scholar]
- Barraclough D. L., Platt-Higgins A., de Silva Rudland S., Barraclough R., Winstanley J., West C. R., Rudland P. S. (2009). The metastasis-associated anterior gradient 2 protein is correlated with poor survival of breast cancer patients. Am. J. Pathol. 175, 1848-1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers J. E., Ambergen A. W., Thunnissen F. B. (1999). Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 159, 1585-1591 [DOI] [PubMed] [Google Scholar]
- Bu H., Bormann S., Schafer G., Horninger W., Massoner P., Neeb A., Lakshmanan V. K., Maddalo D., Nestl A., Sultmann H., et al. (2011). The anterior gradient 2 (AGR2) gene is overexpressed in prostate cancer and may be useful as a urine sediment marker for prostate cancer detection. Prostate 71, 575-587 [DOI] [PubMed] [Google Scholar]
- Chen G., Korfhagen T. R., Xu Y., Kitzmiller J., Wert S. E., Maeda Y., Gregorieff A., Clevers H., Whitsett J. A. (2009). SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 119, 2914-2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokas A. L., Trivedi C. M., Lu M. M., Tucker P. W., Li S., Epstein J. A., Morrisey E. E. (2010). Foxp1/2/4-NuRD interactions regulate gene expression and epithelial injury response in the lung via regulation of interleukin-6. J. Biol. Chem. 285, 13304-13313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo L. A., Lin F. R., Cuesta I., Friedman D., Jarnik M., Zaret K. S. (2002). Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9, 279-289 [DOI] [PubMed] [Google Scholar]
- Edgell T. A., Barraclough D. L., Rajic A., Dhulia J., Lewis K. J., Armes J. E., Barraclough R., Rudland P. S., Rice G. E., Autelitano D. J. (2010). Increased plasma concentrations of anterior gradient 2 protein are positively associated with ovarian cancer. Clin. Sci. (Lond.) 118, 717-725 [DOI] [PubMed] [Google Scholar]
- Evans C. M., Williams O. W., Tuvim M. J., Nigam R., Mixides G. P., Blackburn M. R., DeMayo F. J., Burns A. R., Smith C., Reynolds S. D., et al. (2004). Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am. J. Respir. Cell Mol. Biol. 31, 382-394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy J. V. (2001). Remodeling of the airway epithelium in asthma. Am. J. Respir. Crit. Care Med. 164, S46-S51 [DOI] [PubMed] [Google Scholar]
- Feng X., Ippolito G. C., Tian L., Wiehagen K., Oh S., Sambandam A., Willen J., Bunte R. M., Maika S. D., Harriss J. V., et al. (2010). Foxp1 is anessential transcriptional regulator for the generation of quiescent naive T cells during thymocyte development. Blood 115, 510-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A., Reynolds S. D., Stripp B. R. (2002). Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am. J. Pathol. 161, 173-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss A. M., Tian Y., Tsukiyama T., Cohen E. D., Zhou D., Lu M. M., Yamaguchi T. P., Morrisey E. E. (2009). Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev. Cell 17, 290-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseh J. S., Bores S. A., Stanger B. Z., Zhou Q., Anderson W. J., Melton D. A., Rajagopal J. (2009). Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136, 1751-1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe B. D., Scherz P. J., Nissim S., Tian H., McMahon A. P., Tabin C. J. (2004). Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 118, 517-528 [DOI] [PubMed] [Google Scholar]
- Higa A., Mulot A., Delom F., Bouchecareilh M., Nguyen D. T., Boismenu D., Wise M. J., Chevet E. (2011). Role of the pro-oncogenic protein disulfide isomerase (PDI)-family member anterior gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis. J. Biol. Chem. 286, 44855-44865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuhara K., Ohta S., Shiraishi H., Suzuki S., Taniguchi K., Toda S., Tanabe T., Yasuo M., Kubo K., Hoshino T., et al. (2009). The mechanism of mucus production in bronchial asthma. Curr. Med. Chem. 16, 2867-2875 [DOI] [PubMed] [Google Scholar]
- Li L., Wei Y., Van Winkle L., Zhang Q. Y., Zhou X., Hu J., Xie F., Kluetzman K., Ding X. (2011). Generation and characterization of a Cyp2f2-null mouse and studies on the role of CYP2F2 in naphthalene-induced toxicity in the lung and nasal olfactory mucosa. J. Pharmacol. Exp. Ther. 339, 62-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Rudland P. S., Sibson D. R., Platt-Higgins A., Barraclough R. (2005). Human homologue of cement gland protein, a novel metastasis inducer associated with breast carcinomas. Cancer Res. 65, 3796-3805 [DOI] [PubMed] [Google Scholar]
- Lu M. M., Li S., Yang H., Morrisey E. E. (2002). Foxp4: a novel member of the Foxp subfamily of winged-helix genes co-expressed with Foxp1 and Foxp2 in pulmonary and gut tissues. Mech. Dev. 119 Suppl. 1, S197-S202 [DOI] [PubMed] [Google Scholar]
- Lu Y., Thomson J. M., Wong H. Y., Hammond S. M., Hogan B. L. (2007). Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev. Biol. 310, 442-453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresh E. L., Mah V., Alavi M., Horvath S., Bagryanova L., Liebeskind E. S., Knutzen L. A., Zhou Y., Chia D., Liu A. Y., et al. (2010). Differential expression of anterior gradient gene AGR2 in prostate cancer. BMC Cancer 10, 680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M., Liu Z., Cheng H. T., Winters N., Bader D., Kopan R. (2010). Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J. Cell Sci. 123, 213-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey E. E., Hogan B. L. (2010). Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18, 8-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey E. E., Ip H. S., Lu M. M., Parmacek M. S. (1996). GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev. Biol. 177, 309-322 [DOI] [PubMed] [Google Scholar]
- Niederreiter L., Kaser A. (2011). Endoplasmic reticulum stress and inflammatory bowel disease. Acta Gastroenterol. Belg. 74, 330-333 [PubMed] [Google Scholar]
- Okubo T., Knoepfler P. S., Eisenman R. N., Hogan B. L. (2005). Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development 132, 1363-1374 [DOI] [PubMed] [Google Scholar]
- Park K. S., Korfhagen T. R., Bruno M. D., Kitzmiller J. A., Wan H., Wert S. E., Khurana Hershey G. K., Chen G., Whitsett J. A. (2007). SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 117, 978-988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. C., Ternet M., Hogan B. L. (2000). Notch/Delta expression in the developing mouse lung. Mech. Dev. 98, 95-98 [DOI] [PubMed] [Google Scholar]
- Que J., Okubo T., Goldenring J. R., Nam K. T., Kurotani R., Morrisey E. E., Taranova O., Pevny L. H., Hogan B. L. (2007). Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development 134, 2521-2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V., Arumugam T., Wang H., Logsdon C. D. (2008). Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer Res. 68, 7811-7818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S. D., Giangreco A., Power J. H., Stripp B. R. (2000a). Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am. J. Pathol. 156, 269-278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S. D., Hong K. U., Giangreco A., Mango G. W., Guron C., Morimoto Y., Stripp B. R. (2000b). Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am. J. Physiol. Lung Cell Mol. Physiol. 278, L1256-L1263 [DOI] [PubMed] [Google Scholar]
- Rock J. R., Gao X., Xue Y., Randell S. H., Kong Y. Y., Hogan B. L. (2011). Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 8, 639-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. F. (2003). The airway goblet cell. Int. J. Biochem. Cell Biol. 35, 1-6 [DOI] [PubMed] [Google Scholar]
- Sawaya P. L., Stripp B. R., Whitsett J. A., Luse D. S. (1993). The lung-specific CC10 gene is regulated by transcription factors from the AP-1, octamer, and hepatocyte nuclear factor 3 families. Mol. Cell. Biol. 13, 3860-3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W., Yang H., Zhang L., Lu M. M., Morrisey E. E. (2001). Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J. Biol. Chem. 276, 27488-27497 [DOI] [PubMed] [Google Scholar]
- Shu W., Lu M. M., Zhang Y., Tucker P. W., Zhou D., Morrisey E. E. (2007). Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development 134, 1991-2000 [DOI] [PubMed] [Google Scholar]
- Sumi Y., Hamid Q. (2007). Airway remodeling in asthma. Allergol. Int. 56, 341-348 [DOI] [PubMed] [Google Scholar]
- Tagaya E., Tamaoki J. (2007). Mechanisms of airway remodeling in asthma. Allergol. Int. 56, 331-340 [DOI] [PubMed] [Google Scholar]
- Thai P., Chen Y., Dolganov G., Wu R. (2005). Differential regulation of MUC5AC/Muc5ac and hCLCA-1/mGob-5 expression in airway epithelium. Am. J. Respir. Cell Mol. Biol. 33, 523-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Zhang Y., Hurd L., Hannenhalli S., Liu F., Lu M. M., Morrisey E. E. (2011). Regulation of lung endoderm progenitor cell behavior by miR302/367. Development 138, 1235-1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins D. H., Besnard V., Lange A. W., Wert S. E., Keiser A. R., Smith A. N., Lang R., Whitsett J. A. (2009). Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS ONE 4, e8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao P. N., Vasconcelos M., Izvolsky K. I., Qian J., Lu J., Cardoso W. V. (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297-2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Kaestner K. H., Ang S. L., Ikegami M., Finkelman F. D., Stahlman M. T., Fulkerson P. C., Rothenberg M. E., Whitsett J. A. (2004). Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 131, 953-964 [DOI] [PubMed] [Google Scholar]
- Ye D. Z., Kaestner K. H. (2009). Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology 137, 2052-2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Goss A. M., Cohen E. D., Kadzik R., Lepore J. J., Muthukumaraswamy K., Yang J., DeMayo F. J., Whitsett J. A., Parmacek M. S., et al. (2008). A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat. Genet. 40, 862-870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li S., Yuan L., Tian Y., Weidenfeld J., Yang J., Liu F., Chokas A. L., Morrisey E. E. (2010). Foxp1 coordinates cardiomyocyte proliferation through both cell-autonomous and nonautonomous mechanisms. Genes Dev. 24, 1746-1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Edwards R., Dizon D., Afrasiabi K., Mastroianni J. R., Geyfman M., Ouellette A. J., Andersen B., Lipkin S. M. (2010). Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev. Biol. 338, 270-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Shapiro M., Dong Q., Louahed J., Weiss C., Wan S., Chen Q., Dragwa C., Savio D., Huang M., et al. (2002). A calcium-activated chloride channel blocker inhibits goblet cell metaplasia and mucus overproduction. Novartis Found. Symp. 248, 150-165; discussion 165-170, 277-282 [PubMed] [Google Scholar]
- Zweitzig D. R., Smirnov D. A., Connelly M. C., Terstappen L. W., O'Hara S. M., Moran E. (2007). Physiological stress induces the metastasis marker AGR2 in breast cancer cells. Mol. Cell. Biochem. 306, 255-260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.