Abstract
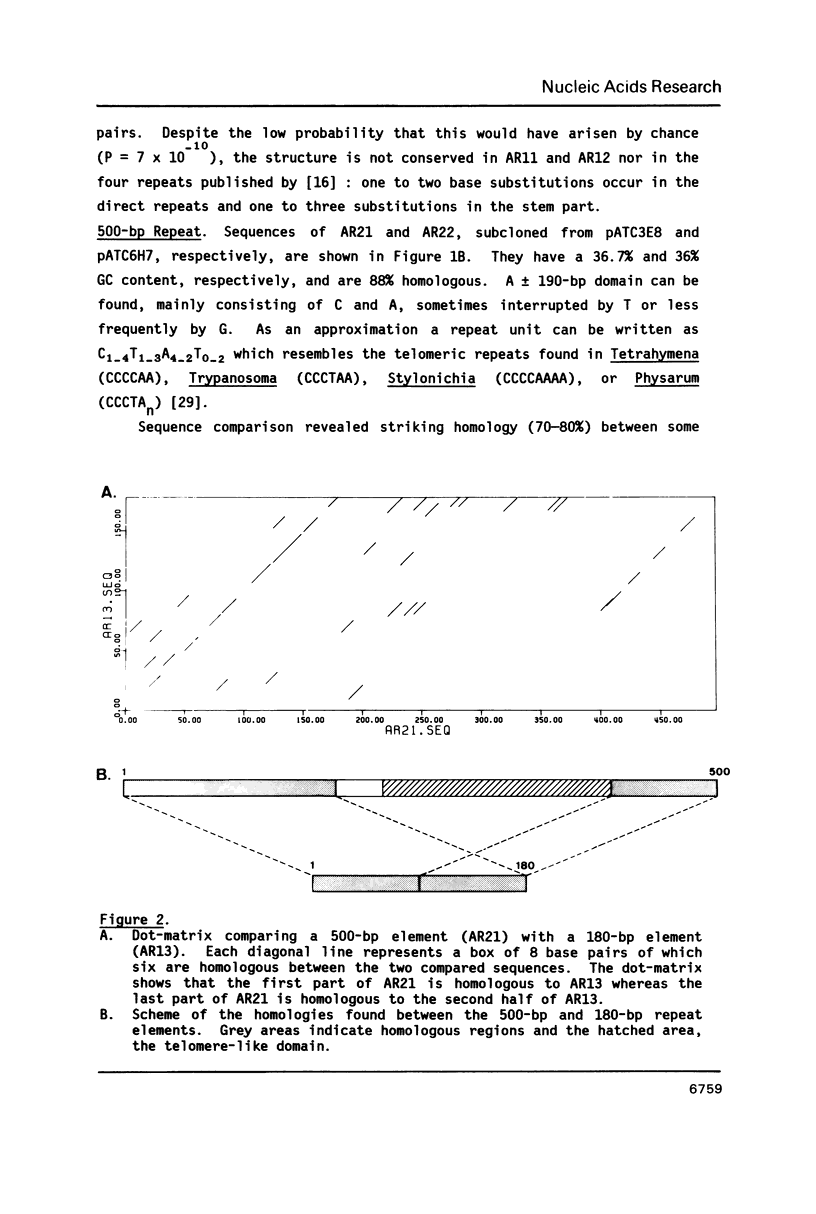
We have analyzed three classes of highly repetitive DNA sequences of Arabidopsis thaliana, composed of tamdemly repeated units of 180 bp, 500 bp, and 160 bp, respectively. The three families comprise approximately 2% of the Arabidopsis genome and are the major component of the highly repetitive DNA. The 500-bp element arose by duplication of one half of a 180-bp ancestor and insertion of a foreign segment between the two duplicated parts followed by amplification. The repeat elements contain occasionally palindromes and other motifs but none are significantly conserved. There is no significant similarity with previously published repetitive elements. Heterogeneity between monomers ranges from 6% to 17%. Monomers derived from different clusters in the genome are more diverged than monomers of the same array.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. D. Nuclear DNA content and minimum generation time in herbaceous plants. Proc R Soc Lond B Biol Sci. 1972 Jun 6;181(1063):109–135. doi: 10.1098/rspb.1972.0042. [DOI] [PubMed] [Google Scholar]
- Bennett M. D., Smith J. B. Nuclear dna amounts in angiosperms. Philos Trans R Soc Lond B Biol Sci. 1976 May 27;274(933):227–274. doi: 10.1098/rstb.1976.0044. [DOI] [PubMed] [Google Scholar]
- Benslimane A. A., Dron M., Hartmann C., Rode A. Small tandemly repeated DNA sequences of higher plants likely originate from a tRNA gene ancestor. Nucleic Acids Res. 1986 Oct 24;14(20):8111–8119. doi: 10.1093/nar/14.20.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Nuclear volume control by nucleoskeletal DNA, selection for cell volume and cell growth rate, and the solution of the DNA C-value paradox. J Cell Sci. 1978 Dec;34:247–278. doi: 10.1242/jcs.34.1.247. [DOI] [PubMed] [Google Scholar]
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980 Apr 17;284(5757):601–603. doi: 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Dover G. Molecular drive: a cohesive mode of species evolution. Nature. 1982 Sep 9;299(5879):111–117. doi: 10.1038/299111a0. [DOI] [PubMed] [Google Scholar]
- Grellet F., Delcasso D., Panabieres F., Delseny M. Organization and evolution of a higher plant alphoid-like satellite DNA sequence. J Mol Biol. 1986 Feb 20;187(4):495–507. doi: 10.1016/0022-2836(86)90329-3. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C. Homocopolymer sequences in the spacer of a sea urchin histone gene repeat are sensitive to S1 nuclease. Nature. 1982 Feb 25;295(5851):714–716. doi: 10.1038/295714a0. [DOI] [PubMed] [Google Scholar]
- John B., Miklos G. L. Functional aspects of satellite DNA and heterochromatin. Int Rev Cytol. 1979;58:1–114. doi: 10.1016/s0074-7696(08)61473-4. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. I. Los Alamos sequence analysis package for nucleic acids and proteins. Nucleic Acids Res. 1982 Jan 11;10(1):183–196. doi: 10.1093/nar/10.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F. T., Hartz J. A., Law M. L., Davidson J. N. Isolation and chromosomal localization of unique DNA sequences from a human genomic library. Proc Natl Acad Sci U S A. 1982 Feb;79(3):865–869. doi: 10.1073/pnas.79.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama R., Matsui H., Okumura K., Oishi M. A group of repetitive human DNA families that is characterized by extrachromosomal oligomers and restriction-fragment length polymorphism. J Mol Biol. 1987 Feb 20;193(4):591–597. doi: 10.1016/0022-2836(87)90342-1. [DOI] [PubMed] [Google Scholar]
- Klee H. J., Muskopf Y. M., Gasser C. S. Cloning of an Arabidopsis thaliana gene encoding 5-enolpyruvylshikimate-3-phosphate synthase: sequence analysis and manipulation to obtain glyphosate-tolerant plants. Mol Gen Genet. 1987 Dec;210(3):437–442. doi: 10.1007/BF00327194. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Pruitt R. E. Arabidopsis thaliana and Plant Molecular Genetics. Science. 1985 Sep 20;229(4719):1214–1218. doi: 10.1126/science.229.4719.1214. [DOI] [PubMed] [Google Scholar]
- Miklos G. L., Gill A. C. The DNA sequences of cloned complex satellite DNAs from Hawaiian Drosophila and their bearing on satellite DNA sequence conservation. Chromosoma. 1981;82(3):409–427. doi: 10.1007/BF00285766. [DOI] [PubMed] [Google Scholar]
- Orgel L. E., Crick F. H. Selfish DNA: the ultimate parasite. Nature. 1980 Apr 17;284(5757):604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Peacock W. J., Dennis E. S., Rhoades M. M., Pryor A. J. Highly repeated DNA sequence limited to knob heterochromatin in maize. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4490–4494. doi: 10.1073/pnas.78.7.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt R. E., Meyerowitz E. M. Characterization of the genome of Arabidopsis thaliana. J Mol Biol. 1986 Jan 20;187(2):169–183. doi: 10.1016/0022-2836(86)90226-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saiga H., Edström J. E. Long tandem arrays of complex repeat units in Chironomus telomeres. EMBO J. 1985 Mar;4(3):799–804. doi: 10.1002/j.1460-2075.1985.tb03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoens C., Alliotte T., Mendel R., Müller A., Schiemann J., Van Lijsebettens M., Schell J., Van Montagu M., Inzé D. A binary vector for transferring genomic libraries to plants. Nucleic Acids Res. 1986 Oct 24;14(20):8073–8090. doi: 10.1093/nar/14.20.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. Highly repeated sequences in mammalian genomes. Int Rev Cytol. 1982;76:67–112. doi: 10.1016/s0074-7696(08)61789-1. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Staden R. A computer program to search for tRNA genes. Nucleic Acids Res. 1980 Feb 25;8(4):817–825. [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Trick M., Dover G. A. Cryptic simplicity in DNA is a major source of genetic variation. Nature. 1986 Aug 14;322(6080):652–656. doi: 10.1038/322652a0. [DOI] [PubMed] [Google Scholar]
- Wu J. C., Manuelidis L. Sequence definition and organization of a human repeated DNA. J Mol Biol. 1980 Sep 25;142(3):363–386. doi: 10.1016/0022-2836(80)90277-6. [DOI] [PubMed] [Google Scholar]
- Wu T. Y., Wu R. A new rice repetitive DNA shows sequence homology to both 5S RNA and tRNA. Nucleic Acids Res. 1987 Aug 11;15(15):5913–5923. doi: 10.1093/nar/15.15.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]