Abstract
Background
In clinical practice, clinicians often need to switch antipsychotic medications in patients with schizophrenia to optimize treatment outcomes. Here, we describe the safety and tolerability of switching existing antipsychotic treatments to asenapine or olanzapine monotherapy using various switching regimens.
Methods
Data were pooled from 949 patients in two 26-week randomized double-blind studies. Patients with persistent negative symptoms of schizophrenia, stable for at least 5 months prior to screening and 1 additional month before randomization, were randomized to and treated with either asenapine (n = 485) or olanzapine (n = 464), and were tapered off existing antipsychotic(s) at variable rates within 28 days.
Results
Prior to randomization, most patients were treated with second-generation antipsychotics (SGAs) (asenapine: 79.6%; olanzapine: 78.2%) and first-generation antipsychotics (FGAs) (31.1%; 29.7%), while depot formulations were used by 12.4% and 11.4%, respectively. Median time to taper off previous antipsychotics was 7 days, with approximately 40% of patients abruptly discontinuing their previous medication. Similar percentages of patients in each group reported at least one adverse event (AE) (asenapine: 76.9%; olanzapine: 75.2%). The majority of AEs occurred within the first 28 days. The most frequently reported AEs were somnolence, insomnia, and headache. The incidence of AEs in patients switching from SGAs, FGAs, or depot medications was similar between asenapine and olanzapine (77.5% vs 74.6%, 75.5% vs 79.7%, 85.0% vs 86.8%, respectively). AEs were more frequent in subjects previously treated with two antipsychotics (asenapine: 79.4%; olanzapine: 83.9%) versus one antipsychotic (asenapine: 76.3%; olanzapine: 72.2%) in the switch period.
Conclusion
The presented data from post hoc pooled analyses may provide practical guidance for physicians switching partially stabilized patients with schizophrenia and persistent negative symptoms to asenapine or olanzapine.
Keywords: antipsychotics, asenapine, monotherapy, olanzapine, schizophrenia, switch
Introduction
A substantial proportion of patients treated with antipsychotics either fail to respond adequately or do not tolerate drug treatment well, which may result in treatment discontinuation.1–6 Because combining medications may increase the potential for adverse drug reactions and may also make adherence problematic, clinicians often seek to switch these patients to an alternative drug, hoping for greater efficacy and/or better tolerability. Patients may be switched between multiple antipsychotics until an effective and acceptably tolerated treatment has been identified. The decision of whether, when, and how to switch is usually made by weighing the potential risks before, during, and following the transition from one antipsychotic to another.5,7 Certain strategies may be more appropriate than others in individual cases. In clinical situations, in which a patient has discontinued treatment and an exacerbation of symptoms has occurred, a change to an alternative medication can often be quickly implemented. The situation is more complex, and clinicians may be more hesitant to change treatment, if a certain level of symptom control has already been achieved with the existing drug, yet the efficacy is not satisfactory and/or tolerability issues make a longer-term treatment problematic. In such situations, the clinician will often have to make a decision on the most appropriate switching regimen for a given patient. The clinician will seek to answer questions such as: how quickly can I safely switch from the previous medication to the new medication? Is a cross-tapering period required? If “yes,” how long should the cross-tapering period be? Does it matter whether I switch a patient from a previous first-generation antipsychotic (FGA) or second-generation (SGA)? Can I safely switch the patient from a previous depot medication?
These questions are of particular clinical importance if a new treatment becomes available to the clinician. To inform the clinician facing the need for a switch to such a new treatment option, clinical data from studies in which switching has been performed are particularly informative, especially when they come from substantial numbers of patients.
Asenapine is an antipsychotic with a unique pharmacological profile that is approved for adults in the USA for the treatment of schizophrenia and the acute treatment – as monotherapy or adjunctive therapy to lithium or valproate – of manic or mixed episodes of bipolar I disorder. In the EU, it is approved for the treatment of moderate to severe manic episodes associated with bipolar I disorder. This report was written to provide empirical data on switching regimens using pooled data from a large sample of patients previously stabilized on various treatments and then switched. Two large, randomized, double-blind, multicenter clinical trials with identical design were retrospectively analyzed for this purpose. Both studies included patients with persistent negative symptoms in schizophrenia, whose positive symptoms were stabilized with one or more antipsychotics and who were switched to the study medications asenapine or olanzapine as monotherapies for 26 weeks following a 4-week period during which the previous medication was tapered off. These two studies offer a good opportunity for a comprehensive post hoc evaluation of the safety and tolerability of switching to asenapine, as well as to olanzapine, using different switch regimes controlled by the clinician. The aim of this study is to provide guidance for physicians who seek to switch partially stabilized patients with schizophrenia from their existing antipsychotic(s) to either asenapine or olanzapine.
Methods
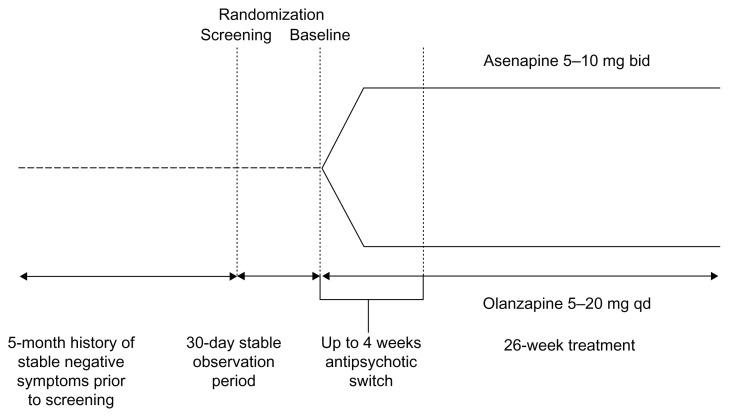
This analysis incorporates results from two studies with identical design (Figure 1). A total of 949 patients received at least one dose of double-blind trial medication.
Figure 1.
Trial design.
Abbreviations: bid, twice daily; qd, once daily.
Study A7501013 (November 2004 to December 2008) included 468 patients, randomized and treated to receive either asenapine (n = 244) or olanzapine (n = 224).8
Study P25543 (March 2005 to July 2006) included 481 patients randomized and treated to receive either asenapine (n = 241) or olanzapine (n = 240).9
These studies were randomized, double-blind, multicenter trials conducted in patients with persistent negative symptoms of schizophrenia. The main results of these studies have been reported previously.10 Psychiatric diagnosis was corroborated with the Mini International Neuropsychiatric Interview; patients were clinically stable on their positive symptoms for at least 5 months at the time of screening and were subsequently followed by a prospective 30-day observation period before baseline. Continued stability had to be demonstrated, as subjects continued taking their pretrial antipsychotic medication. “Stability” was defined as no significant changes in symptoms, no periods of hospitalization/imprisonment, or no increase in the level of psychiatric care due to worsening symptoms. This 6-month period of stability before baseline provided unambiguous data regarding pretrial treatment stability and provided the basis for adequate characterization of a patient’s clinical progression during the switch period. Clinical evaluations included the Negative Symptoms Assessment 16 scale and the Positive and Negative Syndrome Scale (PANSS) as well as safety and tolerability assessments. Patients were randomized 1:1 at baseline to receive either asenapine or olanzapine, administered over 26 weeks. The first 4 weeks (up to 28 days) constituted a switch period, during which the pre-switch antipsychotic medication was discontinued. The first dose of asenapine or olanzapine was coadministered with the existing medication(s). Subjects were then tapered off their existing antipsychotic(s) at variable rates and based on the investigator’s clinical judgment. The maximum tapering period was scheduled 28 days’ post-randomization. This was followed by monotherapy with the study drug for the remainder of the study duration. Safety, efficacy, and tolerability data were analyzed over the duration of the study.
Study drug and other treatments
The packaging and labeling of the clinical trial medication maintained a double-blind, double-dummy design. Fast-dissolving asenapine tablets and oral film-coated olanzapine tablets were made to appear identical to their matched placebo controls. Sublingual asenapine was administered at 5 mg twice daily (bid) for the first week of therapy and then at 5 or 10 mg bid at the discretion of the investigator. Olanzapine was administered at 10 mg once daily (qd) for the first week and then 5–20 mg qd at the discretion of the investigator.
Subjects could continue to receive concomitant propranolol or anticholinergics for extrapyramidal syndrome (EPS) during the 30-day prospective observation period. Following randomization to study treatment, subjects were permitted to continue receiving concomitant medication for the treatment of EPS for the first week of double-blind therapy. After 1 week, an attempt was made to gradually decrease concomitant medication for the treatment of EPS, with complete discontinuation by day 28 unless a requirement for continued treatment was documented. If EPS reappeared, the medications were reinstituted and additional EPS assessments were performed.
Benzodiazepines (<4 mg/day lorazepam or equivalent dose) could be used for the management of agitation or anxiety. Partial benzodiazepine agonists, including zolpidem 2.5–10 mg/day, zaleplon 5–20 mg/day, or zopiclone 7.5–15 mg/day, could be used on a daily basis for insomnia or sleep disturbances. Any equivalent short half-life nonbenzodiazepine hypnotic could be substituted if zolpidem, zaleplon, or zopiclone were not available. It was recommended that no more than half of the recommended maximum suggested hypnotic dose be used for patients >65 years of age. These drugs were not used 12 hours prior to assessment.
Statistical evaluations
Switching strategy was determined by the investigator according to the individual patient, with no specific switching strategy offered by the protocol. The mean and median number of days to finish switching, as well as the interquartile range and its Kaplan–Meier plot, were measured for both treatment groups. The distribution difference of the time to event between groups was tested by a log-rank test. The percentage of patients reporting at least one adverse event (AE) was summarized for each treatment group. AEs were summarized by the antipsychotic drug class with which patients were treated during the switch period (FGA, SGA, or depot), by the number of antipsychotics used in-treatment, and by the individual antipsychotic drug. Sensitivity analyses were also performed; these excluded subjects who switched from olanzapine, as olanzapine was the active study control and this may have led to confounding results.
Results
Patient baseline demographics and characteristics were similar between the asenapine and olanzapine treatment groups in both studies (Table 1).
Table 1.
Patient demographic and baseline characteristics
| Characteristic | Asenapine (n = 244) | Olanzapine (n = 224) | All patients (N = 468) |
|---|---|---|---|
| Study A75010138 | |||
| Sex, male, n (%) | 176 (72.1) | 170 (75.9) | 346 (73.9) |
| Race, n (%) | |||
| White | 110 (45.1) | 95 (42.4) | 205 (43.8) |
| Black | 98 (40.2) | 95 (42.4) | 193 (41.2) |
| Asian | 5 (2) | 2 (0.9) | 7 (1.5) |
| Other | 31 (12.7) | 32 (14.3) | 63 (13.5) |
| Age (years) | |||
| 18–64, n (%) | 239 (98) | 217 (96.9) | 456 (97.4) |
| ≥65, n (%) | 5 (2) | 7 (3.1) | 12 (2.6) |
| Mean (SD) | 43.1 (11.43) | 42.8 (11.27) | 42.9 (11.34) |
| Median | 45 | 45 | 45 |
| Weight (kg) | |||
| Mean (SD) | 84 (19.01) | 85.7 (18.71) | 84.8 (18.86) |
| Median (min, max) | 82.1 (46, 188) | 83.9 (42, 150) | 82.5 (42, 188) |
| BMI kg/m2 | |||
| Mean, n (SD) | 28.3 (5.72) | 28.8 (5.81) | 28.5 (5.76) |
| Median (min, max) | 27 (18, 55) | 28.3 (17, 51) | 28 (17, 55) |
| Total PANSS score (LOCF) | |||
| n | 228 | 217 | |
| Mean (SD) | 75.9 (11.45) | 75.4 (11.94) | |
| Median (min, max) | 75.0 (48, 118) | 74.0 (51, 124) | |
| PANSS positive subscale score (LOCF) | |||
| n | 234 | 218 | |
| Mean (SD) | 18.2 (4.08) | 18.2 (4.21) | |
| Median (min, max) | 18.0 (9, 29) | 18.0 (9, 28) | |
| PANSS Marder negative score (LOCF) | |||
| n | 234 | 218 | |
| Mean (SD) | 26.9 (4.76) | 27.0 (4.50) | |
| Median (min, max) | 26.0 (9, 48) | 26.0 (20, 47) | |
| NSA total score (LOCF) | |||
| n | 234 | 218 | |
| Mean (SD) | 60.1 (10.17) | 61.1 (10.79) | |
| Median (min, max) | 60.0 (29, 88) | 62.0 (23, 87) | |
| Asenapine (n = 241) | Olanzapine (n = 240) | All patients (N = 481) | |
|
| |||
| Study P255439 | |||
| Sex, male n (%) | 164 (68.0) | 164 (68.3) | 328 (68.2) |
| Race, n (%) | |||
| White | 216 (89.6) | 214 (89.2) | 430 (89.4) |
| Black | 16 (6.6) | 17 (7.1) | 33 (6.9) |
| Asian | 9 (0.0) | 1 (0.4) | 1 (0.2) |
| Other | 9 (3.7) | 8 (3.3) | 17 (3.5) |
| Age (years) | |||
| 18–64, n (%) | 231 (95.9) | 235 (97.9) | 466 (96.9) |
| ≥65, n (%) | 10 (4.1) | 5 (2.1) | 15 (3.1) |
| Mean (SD) | 40.7 (12.73) | 40.3 (11.65) | 40.5 (12.19) |
| Median | 40 | 40 | 40 |
| Weight (kg) | |||
| Mean (SD) | 79.3 (15.89) | 80.3 (16.07) | 79.8 (15.97) |
| Median (min, max) | 79 (44.0, 122.2) | 79 (43.0, 140.0) | 79 (43.0, 140.0) |
| BMI kg/m2 | |||
| Mean, n (SD) | 26.6 (4.53) | 26.9 (4.83) | 26.7 (4.68) |
| Median (min, max) | 26 (16.4, 41.0) | 26 (17.5, 44.4) | 26 (16.4, 44.4) |
| Total PANSS score (LOCF) | |||
| n | 216 | 217 | |
| Mean (SD) | 74.6 (11.3) | 73.7 (12.0) | |
| Median (min, max) | 74.0 (51, 108) | 72.0 (48, 124) | |
| PANSS positive subscale score (LOCF) | |||
| n | 216 | 217 | |
| Mean (SD) | 11.4 (2. 99) | 11.2 (3.05) | |
| Median (min, max) | 11.0 (7, 20) | 11.0 (7, 20) | |
| PANSS Marder negative score (LOCF) | |||
| n | 216 | 217 | |
| Mean (SD) | 27.0 (3.87) | 26.7 (4.07) | |
| Median (min, max) | 27.0 (20, 38) | 26.0 (20, 44) | |
| NSA total score (LOCF) | |||
| n | 216 | 217 | |
| Mean (SD) | 60.3 (9.03) | 60.3 (8.62) | |
| Median (min, max) | 60.0 (38, 81) | 60.0 (28, 81) | |
Abbreviations: BMI, body mass index; PANSS, Positive and Negative Syndrome Scale; LOCF, last observation carried forward; NSA, Negative Symptom Assessment; SD, standard deviation.
Approximately two-thirds of the patients in this study switched to asenapine or olanzapine from previous therapy with one antipsychotic and approximately one-quarter from two or more (Table 2). SGA antipsychotics comprised the majority (almost 80%) of the used pre-switch antipsychotics. The percentage of patients previously treated with an FGA or SGA was similar between the asenapine and olanzapine treatment groups.
Table 2.
Summary of pretreatment antipsychotic drug use during cross-titration period
| Asenapine (n = 485) | Olanzapine (n = 464) | |
|---|---|---|
| Antipsychotics, n (%) | ||
| Total | 467 (96.3) | 452 (97.4) |
| 1 | 329 (67.8) | 335 (72.2) |
| 2 | 107 (22.1) | 93 (20.0) |
| 3 or more | 31 (6.4) | 24 (5.2) |
| Antipsychotic class, n (%) | ||
| FGA | 151 (31.1) | 138 (29.7) |
| SGA | 386 (79.6) | 363 (78.2) |
| Depot | 60 (12.4) | 53 (11.4) |
| Antipsychotic, n (%) | ||
| SGA | ||
| Olanzapine | 132 (27.2) | 109 (23.5) |
| Risperidone | 163 (33.6) | 151 (32.5) |
| Quetiapine | 63 (13.0) | 57 (12.3) |
| Ziprasidone | 16 (3.3) | 22 (4.7) |
| Aripiprazole | 28 (5.8) | 41 (8.8) |
| Amisulpride | 17 (3.5) | 14 (3.0) |
| Clozapine | 14 (2.9) | 8 (1.7) |
| Clotiapine | 4 (0.8) | 0 (0.0) |
| Paliperidone | 2 (0.4) | 3 (0.6) |
| Ziprasidone mesilate | 2 (0.4) | 2 (0.4) |
| Zotepine | 0 (0.0) | 2 (0.4) |
| Melperone hydrochloride | 0 (0.0) | 1 (0.2) |
| Sertindole | 0 (0.0) | 1 (0.2) |
| FGA | ||
| Haloperidol | 53 (10.9) | 47 (10.1) |
| Chlorpromazine | 12 (2.5) | 23 (5.0) |
| Levomepromazine | 14 (2.9) | 9 (1.9) |
| Fluphenazine | 11 (2.3) | 9 (1.9) |
| Trifluoperazine | 9 (1.9) | 11 (2.4) |
| Promethazine | 10 (2.1) | 7 (1.5) |
| Sulpiride | 8 (1.6) | 5 (1.1) |
| Thioridazine | 5 (1.0) | 3 (0.6) |
| Chlorprothixene | 4 (0.8) | 11 (2.4) |
| Loxapine | 4 (0.8) | 2 (0.4) |
| Cyamemazine | 4 (0.8) | 2 (0.4) |
| Tiapride | 2 (0.4) | 0 (0.0) |
| Pipotiazine | 1 (0.2) | 1 (0.2) |
| Benperidol | 1 (0.2) | 0 (0.0) |
| Pimozide | 1 (0.2) | 0 (0.0) |
| Thioproperazine | 1 (0.2) | 0 (0.0) |
| Zuclopenthixol | 1 (0.2) | 1 (0.2) |
| Depot | ||
| Fluphenazine decanoate | 11 (2.3) | 13 (2.8) |
| Flupentixol | 19 (3.9) | 21 (4.5) |
| Zuclopenthixol | 15 (3.1) | 6 (1.3) |
| Flupentixol decanoate | 0 (0.0) | 2 (0.4) |
| Haloperidol decanoate | 8 (1.6) | 8 (1.7) |
| Perphenazine | 8 (1.6) | 3 (0.6) |
| Perphenazine enantate | 0 (0.0) | 2 (0.4) |
| Zuclopenthixol decanoate | 1 (0.2) | 1 (0.2) |
| Perphenazine decanoate | 1 (0.2) | 0 (0.0) |
Abbreviations: FGA, first-generation antipsychotic; SGA, second-generation antipsychotic.
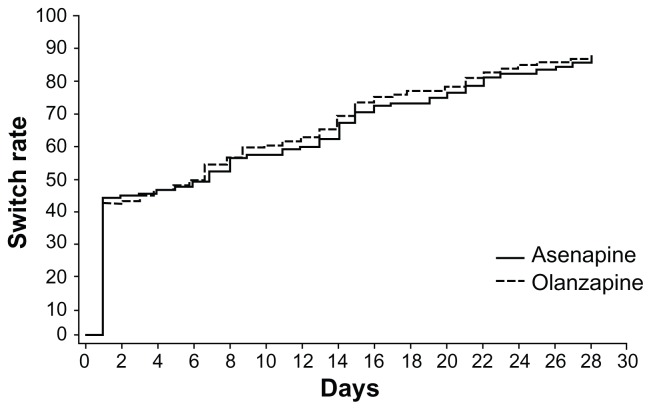
There were no major differences between treatment groups in the tapering strategies. Over 40% of patients in both treatment groups were discontinued abruptly (defined as ≤3 days of switch period). Figure 2 shows a Kaplan–Meier curve of time to cessation of antipsychotic therapy during the switch period (P = 0.4374, log-rank test). Overall, the timing of the switch or duration of the tapering period was similar between the two treatment groups. The median (interquartile range) was 7.0 (1.0, 20.0) days for asenapine and 7.0 (1.0, 17.0) days for olanzapine.
Figure 2.
Kaplan–Meier curve of time to discontinuation of antipsychotics.
In the asenapine group on the day that the switch occurred, the mean treatment doses of previous therapies were: olanzapine 14.1 mg/day, risperidone 6.2 mg/day, quetiapine 378.5 mg/day, ziprasidone 97.5 mg/day, and aripiprazole 18.9 mg/day. For olanzapine, the mean treatment doses of previous therapies were: olanzapine 13.2 mg/day, risperidone 6.8 mg/day, quetiapine 453.6 mg/day, ziprasidone 93.6 mg/day, and aripiprazole 21.0 mg/day.
A slight decrease from baseline was observed on day 28 for PANSS positive subscale and PANSS Marder-factor positive scores (mean change [standard deviation (SD)]) both with asenapine (−0.5 [2.34] and −1.1 [2.86], respectively) and olanzapine (−0.6 [2.12] and −1.2 [2.70], respectively).
Discontinuations
Overall discontinuation rates were numerically higher in the asenapine group (171/467; 36.6%) over the full treatment period than with olanzapine (111/452; 24.6%). Difference in discontinuation rates was compared for the abrupt-switch versus slow-tapering protocols. The discontinuation rate of patients in the asenapine group (N = 467) switched abruptly was 38.8% (81/209) and 36.5% (62/170) in patients switched by slow tapering. The overall discontinuation rate in patients switched to olanzapine (n = 452) was 29.7% (58/195) in patients switched abruptly and 21.3% (33/155) in patients switched by slow tapering.
Over the full treatment period, AEs and serious adverse effects (SAEs) contributed to a numerically higher rate of discontinuations in the asenapine group (74/467; 15.8%) versus the olanzapine group (48/452; 10.6%). A large proportion of the AE/SAE-related discontinuations occurred as a result of the patients’ underlying disease. Similar rates of patients discontinuing treatment as a result of lack of efficacy were seen between the asenapine and olanzapine groups (3.0% and 2.6%, respectively). While investigators were requested to make a distinction, it is possible that some AEs relating to worsening of schizophrenia were reported as a result of a lack of efficacy.
Adverse events
In patients receiving at least one antipsychotic medication during the switch period, 76.9% receiving asenapine and 75.2% receiving olanzapine experienced at least one treatment-emergent AE over the full 26-week treatment period. The majority of these events were reported in the initial 28-day switching period (asenapine, 53.1%; olanzapine, 49.3%). Over the first 28 days, the most frequently reported AEs were somnolence (asenapine, 9.9%; olanzapine, 13.3%), insomnia (asenapine, 9.9%; olanzapine 5.3%), and headache (asenapine, 7.5%; olanzapine 5.5%). Over the full treatment period, the incidence of somnolence was 13.5% with asenapine and 15.3% with olanzapine, insomnia was 16.9% with asenapine and 11.5% with olanzapine, and headache was 13.5% with asenapine and 10% with olanzapine.
The majority of AEs in these patients were of mild or moderate intensity. A total of 11.6% of patients in the asenapine group and 6.0% of patients in the olanzapine group experienced at least one SAE. The most common SAEs appeared to be disease rather than treatment related.
Of those patients switched to asenapine, 209 were switched abruptly (≤3 days) and 170 were switched slowly (≥14 days). Of those patients switched to olanzapine, 195 were switched abruptly and 155 were switched slowly. Over the first 28 days, slightly more treatment-emergent AEs were reported when switched to asenapine with a slow tapered switch (52.4%) than with a rapid switch (48.8%). Patients switched to olanzapine reported numerically more treatment-emergent AEs when switched abruptly (50.3%) than when a slow tapered switch (44.5%) was made. Over the full treatment period, numerically more patients experienced treatment-emergent AEs with a slow tapered switch than with a rapid switch to asenapine (82.9%, 71.8%) or olanzapine (80.6%, 70.3%).
Three deaths were reported during the study. Two deaths were in the asenapine treatment group (one suicide, judged by the investigator as possibly related to the study drug, and one lung cancer death unrelated to the study drug). The one death (suicide) occurring in the olanzapine study group was considered unrelated to the study drug.
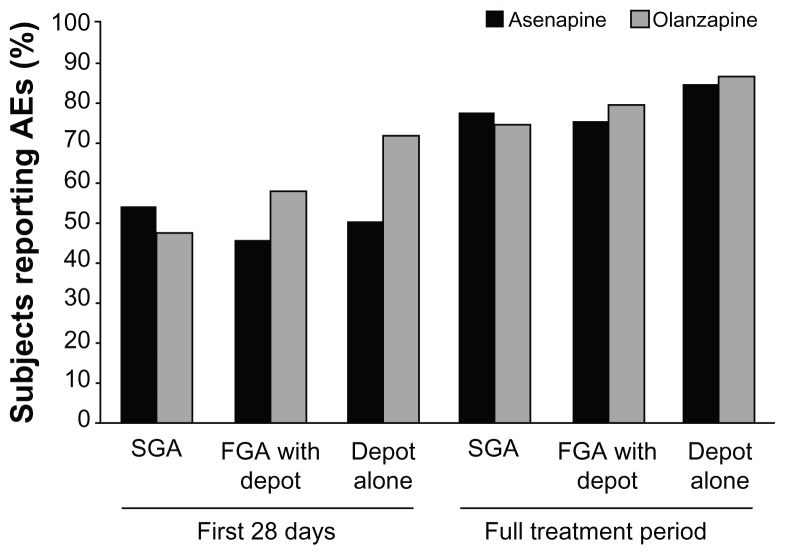
To analyze whether the type or number of previous medications influenced the safety and tolerability of switching to asenapine or olanzapine, the AE profile of patients in each treatment group was summarized by drug class (SGAs, FGAs, or depot medications) and by the number of antipsychotics that patients were taking during the switch period. Over the full time period, similar overall rates of AEs were reported when switching to either asenapine or olanzapine from SGAs, FGAs, or depot medications (Figure 3).
Figure 3.
Subjects reporting adverse events (AEs) by antipsychotic agent type during the switch period.
Abbreviations: FGA, first-generation antipsychotic; SGA, second-generation antipsychotic.
Previous treatment: SGAs
Of patients treated with an SGA before randomization and during the switch period, 386 were switched to asenapine and 363 to olanzapine. Details of the incidence of AEs in patients switched from an SGA to asenapine or olanzapine over the first 28 days and during the entire treatment period are reported in Table 3. The AE with the observed highest incidence in patients switched to asenapine during the first 28 days was insomnia, whereas the AE with the highest incidence in patients switched to olanzapine was somnolence.
Table 3.
Adverse events (AEs) in patients treated with second-generation antipsychotics during the switch period
| Preferred term, n (%) | Switch period (first 28 days) | Full treatment period (26 weeks) | ||
|---|---|---|---|---|
|
|
|
|||
| Asenapine (n = 386) | Olanzapine (n = 363) | Asenapine (n = 386) | Olanzapine (n = 363) | |
| At least one AE | 209 (54.1) | 173 (47.7) | 299 (77.5) | 272 (74.9) |
| Insomnia | 43 (11.1) | 22 (6.1) | 73 (18.9) | 47 (12.9) |
| Somnolence | 38 (9.8) | 46 (12.7) | 53 (13.7) | 52 (14.3) |
| Headache | 33 (8.5) | 23 (6.3) | 56 (14.5) | 37 (10.2) |
| Nausea | 18 (4.7) | 9 (2.5) | 27 (7.0) | 16 (4.4) |
| Agitation | 11 (2.8) | 5 (1.4) | 19 (4.9) | 7 (1.9) |
| Anxiety | 17 (4.4) | 11 (3.0) | 42 (10.9) | 31 (8.5) |
| Dizziness | 17 (4.4) | 12 (3.3) | 21 (5.4) | 20 (5.5) |
| Akathisia | 14 (3.6) | 7 (1.9) | 24 (6.2) | 15 (4.1) |
| Sedation | 14 (3.6) | 15 (4.1) | 20 (5.2) | 21 (5.8) |
| Hypoesthesia oral | 12 (3.1) | 0 (0.0) | 12 (3.1) | 0 (0) |
| Dysgeusia | 10 (2.6) | 1 (0.3) | 10 (2.6) | 2 (0.6) |
| Fatigue | 9 (2.3) | 12 (3.3) | 18 (4.7) | 20 (5.5) |
| Nasopharyngitis | 9 (2.3) | 5 (1.4) | 16 (4.1) | 16 (4.4) |
| Vomiting | 8 (2.1) | 4 (1.1) | 14 (3.6) | 5 (1.4) |
| Weight increase | 7 (1.8) | 8 (2.2) | 28 (7.3) | 65 (17.9) |
| Weight decrease | 2 (0.5) | 0 (0) | 17 (4.4) | 3 (0.8) |
| Increased appetite | 6 (1.6) | 10 (2.8) | 8 (2.1) | 13 (3.6) |
| Dry mouth | 3 (0.8) | 14 (3.9) | 9 (2.3) | 18 (5.0) |
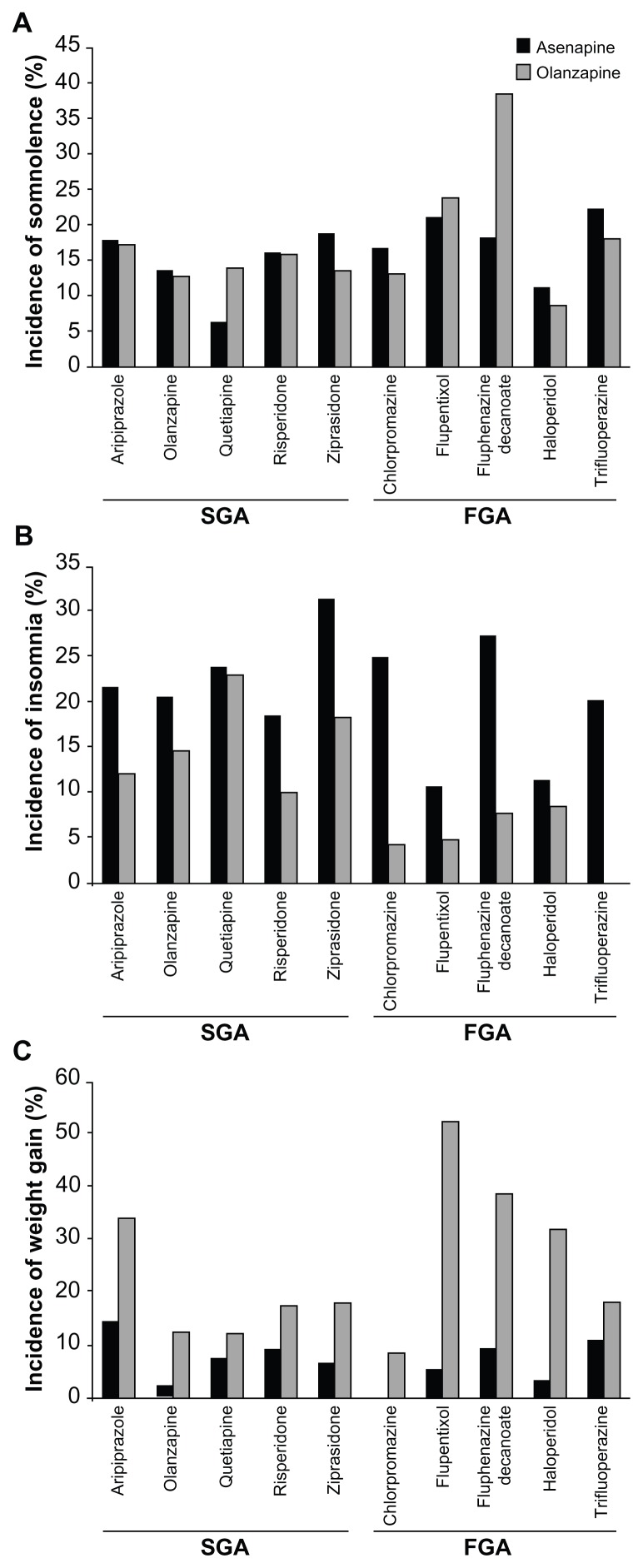
The rates of reported somnolence when switching from an SGA to either asenapine or olanzapine are given in Figure 4A. Overall, the rates were comparable, with possible differences for quetiapine (numerically higher rate for olanzapine) or ziprasidone (numerically higher rate for asenapine). With the exception of the switch from aripiprazole to asenapine, the majority of the cases of somnolence reported occurred in the initial 28 days following switching from an SGA to either asenapine or olanzapine.
Figure 4.
Rate of (A) somnolence, (B) insomnia, and (C) incidence of weight gain by antipsychotic taken during the switch period.
Abbreviations: FGA, first-generation antipsychotic (most frequently used); SGA, second-generation antipsychotic.
The respective rates for insomnia when switching from an SGA to either asenapine or olanzapine (plotted in Figure 4B) show numerically higher rates of insomnia when switching from an SGA to asenapine than to olanzapine (the possible exception here being quetiapine, where insomnia was more frequent in the first 28 days when switching to asenapine, but the rates for asenapine and olanzapine were similar over the complete treatment period).
The respective rates for reported weight gain were numerically lower in patients switched from SGAs to asenapine than to olanzapine over the first 28 days and the entire treatment period (Table 3). When switching from an SGA to asenapine or olanzapine, weight gain rates were generally numerically higher in patients switched to olanzapine (Figure 4C).
Previous treatment: FGAs
Of patients treated with an FGA (including depot medications) prior to randomization and during the switch period, 151 were randomized to asenapine and 138 were randomized to olanzapine. Details of the incidence of AEs in patients switched from an FGA to asenapine or olanzapine over the first 28 days and during the entire treatment period are reported in Table 4.
Table 4.
Adverse events (AEs) in patients treated with first-generation antipsychotics (including depot) during the switch period
| Preferred term, n (%) | Switch period (first 28 days) | Full treatment period (26 weeks) | ||
|---|---|---|---|---|
|
|
|
|||
| Asenapine (n = 151) | Olanzapine (n = 138) | Asenapine (n = 151) | Olanzapine (n = 138) | |
| At least one AE | 69 (45.7) | 80 (58.0) | 114 (75.5) | 110 (79.7) |
| Somnolence | 13 (8.6) | 21 (15.2) | 20 (13.2) | 23 (16.7) |
| Nausea | 7 (4.6) | 3 (2.2) | 8 (5.3) | 7 (5.1) |
| Insomnia | 7 (4.6) | 5 (3.6) | 17 (11.3) | 13 (9.4) |
| Dizziness | 5 (3.3) | 5 (3.6) | 8 (5.3) | 7 (5.1) |
| Headache | 5 (3.3) | 5 (3.6) | 12 (7.9) | 12 (8.7) |
| Dyspepsia | 5 (3.3) | 2 (1.4) | 6 (4.0) | 3 (2.2) |
| Anxiety | 5 (3.3) | 1 (0.7) | 12 (7.9) | 11 (8.0) |
| Agitation | 4 (2.6) | 3 (2.2) | 9 (6.0) | 4 (2.9) |
| Dysgeusia | 4 (2.6) | 0 (0.0) | 5 (3.3) | 0 (0.0) |
| Sedation | 4 (2.6) | 4 (2.9) | 5 (3.3) | 5 (3.6) |
| Weight increase | 2 (1.3) | 17 (12.3) | 9 (6.0) | 43 (31.2) |
| Weight decrease | 0 (0.0) | 1 (0.7) | 4 (2.6) | 2 (1.4) |
| Back pain | 1 (0.7) | 4 (2.9) | 1 (0.7) | 7 (5.1) |
| Increased appetite | 1 (0.7) | 6 (4.3) | 2 (1.3) | 7 (5.1) |
| Paresthesia | 1 (0.7) | 3 (2.2) | 1 (0.7) | 3 (2.2) |
| Fatigue | 0 (0.0) | 3 (2.2) | 3 (2.0) | 3 (2.2) |
| Myalgia | 0 (0.0) | 3 (2.2) | 0 (0.0) | 3 (2.2) |
For patients switched from an FGA pretreatment to either asenapine or olanzapine, the respective rates of reported AEs over the entire study period can be found for somnolence in Figure 4A, for insomnia in Figure 4B and for weight gain in Figure 4C. The plots show comparable rates of somnolence for the different FGAs used. The majority of reports occurred in the first 28 days.
Previous treatment: depot formulations only
Of the patients who received a depot medication prior to randomization (last injection of a depot was given no later than one dosing cycle prior to randomization), 60 switched to asenapine and 53 switched to olanzapine. Details of the incidence of AEs in patients switched from a depot medication to asenapine or olanzapine over the first 28 days and during the entire treatment period are reported in Table 5.
Table 5.
Adverse events (AEs) in patients treated with depot medications during the switch period
| Preferred term, n (%) | Switch period (first 28 days) | Full treatment period (26 weeks) | ||
|---|---|---|---|---|
|
|
|
|||
| Asenapine (n = 60) | Olanzapine (n = 53) | Asenapine (n = 60) | Olanzapine (n = 53) | |
| At least one AE | 30 (50.0) | 38 (71.7) | 51 (85.0) | 46 (86.8) |
| Somnolence | 7 (11.7) | 14 (26.4) | 9 (15.0) | 14 (26.4) |
| Nausea | 1 (1.7) | 1 (1.9) | 1 (1.7) | 3 (5.7) |
| Insomnia | 6 (10.0) | 2 (3.8) | 9 (15.0) | 3 (5.7) |
| Dizziness | 1 (1.7) | 2 (3.8) | 2 (3.3) | 3 (5.7) |
| Headache | 2 (3.3) | 3 (5.7) | 7 (11.7) | 7 (13.2) |
| Dyspepsia | 2 (3.3) | 1 (1.9) | 3 (5.0) | 2 (3.8) |
| Anxiety | 1 (1.7) | 0 (0.0) | 5 (8.3) | 3 (5.7) |
| Agitation | 4 (6.7) | 2 (3.8) | 6 (10.0) | 2 (3.8) |
| Dysgeusia | 1 (1.7) | 0 (0.0) | 1 (1.7) | 0 (0.0) |
| Sedation | 0 (0.0) | 1 (1.9) | 0 (0.0) | 2 (3.8) |
| Weight increase | 1 (1.7) | 13 (24.5) | 5 (8.3) | 25 (47.2) |
| Weight decrease | 0 (0.0) | 0 (0.0) | 3 (5.0) | 0 (0.0) |
| Back pain | 1 (1.7) | 2 (3.8) | 1 (1.7) | 3 (5.7) |
| Increased appetite | 1 (1.7) | 4 (7.5) | 1 (1.7) | 0 (0.0) |
| Paresthesia | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (1.9) |
| Fatigue | 0 (0.0) | 1 (1.9) | 2 (3.3) | 1 (1.9) |
Few patients switched from a depot medication to asenapine reported weight gain throughout the duration of the studies compared with nearly half of the patients switched to olanzapine. Those switched from flupentixol reported notably increased rates of weight gain as detailed earlier.
Effect of the number of antipsychotics in pretreatment
The incidences of reported AEs grouped by the number of antipsychotics taken as pretreatments by patients during the switch period are listed in Table 6. Over the full treatment period, the number of antipsychotics during the switch appeared to have no major influence on somnolence rate when switching to asenapine, while the respective numbers varied for olanzapine.
Table 6.
Effect of number of pre-switch antipsychotics used during switch on incidence of adverse events over the full treatment period (26 weeks)
| Asenapine (5–10 mg twice daily) | Olanzapine (5–20 mg once daily) | |||||
|---|---|---|---|---|---|---|
| Patients (n) | 329 | 107 | 23 | 335 | 93 | 19 |
| Pretreatment antipsychotics (n) | 1 | 2 | 3 | 1 | 2 | 3 |
| Total (%) | 76.3 | 79.4 | 73.9 | 72.2 | 83.9 | 84.2 |
| Somnolence (%) | 14.0 | 14.0 | 8.7 | 14.6 | 21.5 | 0 |
| Sedation (%) | 5.5 | 2.8 | 4.3 | 6.9 | 3.2 | 0 |
| Insomnia (%) | 14.9 | 24.3 | 8.7 | 9.9 | 15.1 | 15.8 |
| Weight increase (%) | 7.6 | 5.6 | 0 | 20.9 | 26.9 | 5.3 |
| Weight decrease (%) | 3.6 | 5.6 | 8.7 | 0.6 | 1.1 | 5.3 |
| Headache (%) | 14.9 | 11.2 | 4.3 | 11.0 | 8.6 | 0 |
| Nausea (%) | 7.0 | 8.4 | 0 | 3.0 | 7.5 | 0 |
| Anxiety (%) | 8.5 | 15.0 | 8.7 | 7.2 | 7.5 | 15.8 |
| Agitation (%) | 4.3 | 6.5 | 4.3 | 0 | 6.5 | 0 |
Patients switched to olanzapine from olanzapine
A subpopulation of patients randomized to switch to olanzapine was taking olanzapine prior to randomization and during the switch period. This previous treatment with the comparator drug was not exclusionary unless insufficient clinical response, despite adequate dosing, was documented at screening. These patients did not effectively undergo switch therapy and their inclusion in the AE analysis may have decreased the sensitivity of AE detection in olanzapine switch patients. To eliminate this possible bias, AE data have also been analyzed to exclude all patients who were taking olanzapine as pretrial medication before the switch phase (132 patients were excluded from the group randomized to asenapine and 109 from the group randomized to olanzapine). After these subjects were excluded from analysis, 77.3% of the asenapine group and 73.4% of the olanzapine group reported at least one AE over the full treatment period. This was comparable to the 76.9% and 75.2% of patients reporting at least one AE from the full treatment groups.
When data from patients using olanzapine prior to the switch period were excluded from the analysis, the overall discontinuation rates increased in both treatment groups (45.5% of patients in the asenapine group [n = 132] and 24.8% in the olanzapine group [n = 109] discontinued treatment). In this subpopulation of patients, lack of efficacy as a cause of discontinuation in those switched to asenapine was numerically greater (9.8%) than in the full patient population (3.0%) and numerically decreased in those patients switched to olanzapine (1.8%). AEs or SAEs as a cause of discontinuation increased in both the asenapine group (20.5%) and the olanzapine group (13.8%).
Discussion
The results from these post hoc analyses can be a useful source of information for clinicians faced with the clinical need to switch medication in patients partially stabilized with an antipsychotic and who consider switching to the relatively new treatment option asenapine or the well-established alternative olanzapine. Though switching treatments was not the primary objective of the studies, the analyses are informative in this regard because they were performed in a large pooled dataset of patients previously treated with various antipsychotic agents who had achieved partial symptom control and stabilization for at least 6 months (both historically and prospectively documented) before either asenapine or olanzapine were added in a double-blind fashion and variable regimens were used according to the treating clinician’s judgment to taper off the previous treatment.
The overall results from our analyses suggest the following clinical conclusions:
When clinicians applied various tapering regimens of pretreatment antipsychotics, including abrupt discontinuation up to 3 days after the addition of either asenapine or olanzapine at adequate doses, the switch appeared to be generally safe and well tolerated. Discontinuation rates were numerically higher for asenapine than with olanzapine, however, the discontinuation rates were within the range seen in other studies conducted with compounds deemed to be well tolerated in comparable populations.
The three most frequently reported AEs within 28 days for asenapine were insomnia, somnolence, and headache, and for olanzapine the three most frequently reported AEs were somnolence, headache, and insomnia (all AEs occurring in approximately ≤10% patients).
For asenapine, the overall rate of reported AEs was similar irrespective of whether patients were pretreated with an SGA, an FGA, or a depot formulation. For olanzapine, a larger variability depending on the pretreatment was observed.
Upon the switch, minimal variation was observed in mean changes of positive symptoms. This indicates that for a majority of patients the previously achieved symptom control over positive symptoms continued after the switch. While individual reactions may have differed from patient to patient, this result is encouraging, as there may be a good chance to maintain symptom control after a switch to the used compounds. The changes in negative symptoms over time, the primary objectives of these trials, have been reported and discussed elsewhere.10
When clinicians select a candidate drug to switch to, the pharmacological profile of an antipsychotic drug, its propensity to induce weight gain and metabolic changes, as well as its overall side-effect profile will be taken into account, particularly if longer-term treatment is necessary. Treatment with olanzapine is reported to be associated with a high risk of substantial weight gain.11–13 In this study, we also found that olanzapine induced weight gain in a sizable subset of patients.
In 2011, the US Food and Drug Administration issued a warning that serious allergic reactions had been reported with the use of asenapine.14 These included 52 cases of Type I hypersensitivity reactions (allergic reactions). In the pooled data from the two studies reported here, one case of hypersensitivity was observed. The subject was switched from olanzapine abruptly to asenapine and reported mild nasal allergies on day 126 of treatment.
Limitations
The presented analyses are retrospective rather than prospective and some subanalyses are based on a limited number of patients. The presented data are exploratory in nature; they had not been designed and powered for the different analyses performed in the pooled dataset. The absence of a control group of patients who did not switch therapy may also limit the generalizability of the results. Also, the fact that the data are derived from a set of patients with schizophrenia partially stabilized on positive symptoms may not allow the conclusions to be generalized to patients who are acutely ill, treatment resistant, or who have been diagnosed with bipolar disorder. Given the mentioned limitations, the clinical conclusions should be regarded as tentative. Further, it should be kept in mind that a large variability of patient reactions can be expected in the clinical setting and patients may react differently than expected based on the presented data. This may be relevant for the comparability of the safety of the switching methods of abrupt discontinuation versus slow taper. The physician must weigh the risks associated with both methods: an abrupt discontinuation’s association with a higher risk of recurrence versus a slow taper’s association with drug–drug interaction on a case-by-case basis. Nevertheless, the study does provide relevant data for clinicians and patients, if switching is considered clinically necessary.
Acknowledgments
This study was sponsored by Merck. Medical writing assistance in the preparation of this manuscript was provided by Karen Pemberton, PhD, of PAREXEL.
Footnotes
Disclosure
All authors were employees of Merck at the time these studies were performed. The authors declare no other conflicts of interest in this work.
References
- 1.Lee CT, Conde BJ, Mazlan M, et al. Switching to olanzapine from previous antipsychotics: a regional collaborative multicenter trial assessing 2 switching techniques in Asia Pacific. J Clin Psychiatry. 2002;63(7):569–576. doi: 10.4088/jcp.v63n0706. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA, Stroup TS, McEvoy JP, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 3.Masand PS, Berry SL. Switching antipsychotic therapies. Ann Pharmacother. 2000;34(2):200–207. doi: 10.1345/aph.18458. [DOI] [PubMed] [Google Scholar]
- 4.Nyhuis AW, Faries DE, Ascher-Svanum H, Stauffer VL, Kinon BJ. Predictors of switching antipsychotic medications in the treatment of schizophrenia. BMC Psychiatry. 2010;10:75. doi: 10.1186/1471-244X-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiden PJ, Young AH, Buckley PF. The art and science of switching of antipsychotic medications, part 1. J Clin Psychiatry. 2006;67(11):e15. doi: 10.4088/jcp.1106e15. [DOI] [PubMed] [Google Scholar]
- 6.Weiden PJ. Discontinuing and switching antipsychotic medications: understanding the CATIE schizophrenia trial. J Clin Psychiatry. 2007;68(Suppl 1):12–19. [PubMed] [Google Scholar]
- 7.Golebiewski K. Antipsychotic switching: when, how, why? Graylands Hospital Drug Bulletin. 2006;14(1):1–4. [Google Scholar]
- 8.Schering-Plough. ClinicalTrials.gov. Bethseda, MD: US National Library of Medicine; 2010. [Accessed April 19, 2012]. Efficacy and safety of asenapine compared with olanzapine in patients with persistent negative symptoms of schizophrenia (A7501013) (COMPLETED) (P05771) [updated April 8, 2010]. Available from: http://clinicaltrials.gov/ct2/show/NCT00145496. NLM identifier: NCT00145496. [Google Scholar]
- 9.Schering-Plough. ClinicalTrials.gov. Bethseda, MD: US National Library of Medicine; 2010. [Accessed April 19, 2012]. Efficacy and safety of asenapine compared with olanzapine in patients with persistent negative symptoms of schizophrenia 25543) (COMPLETED) (P05817) [updated October 2, 2009]. Available from: http://clinicaltrials.gov/ct2/show/NCT00212836. NLM identifier: NCT00212836. [Google Scholar]
- 10.Buchanan R, Panagides J, Kouassi A, Szegedi A. Asenapine versus olanzapine in patients with predominent persistent negative symptoms of schizophrenia. Schizophrenia Research. 2008;102(2):252. [Google Scholar]
- 11.Hester EK, Thrower MR. Current options in the management of olanzapine- associated weight gain. Ann Pharmacother. 2005;39(2):302–310. doi: 10.1345/aph.1D423. [DOI] [PubMed] [Google Scholar]
- 12.Mukundan A, Faulkner G, Cohn T, Remington G. Antipsychotic switching for people with schizophrenia who have neuroleptic-induced weight or metabolic problems. Cochrane Database Syst Rev. 2010;12:CD006629. doi: 10.1002/14651858.CD006629.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(Suppl 4):8–13. [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. FDA drug safety communication: serious allergic reactions reported with the use of Saphris (asenapine maleate) Rockville, MD: US Food and Drug Administration; 2011. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm270243. [Google Scholar]