Abstract
Adenosine A1 receptor (A1R)-induced translocation of PKCε to transverse (t) tubular membranes in isolated rat cardiomyocytes is associated with a reduction in β1-adrenergic-stimulated contractile function. The PKCε-mediated activation of protein kinase D (PKD) by endothelin-1 is inhibited by β1-adrenergic stimulated protein kinase A (PKA) suggesting a similar mechanism of A1R signal transduction modulation by adrenergic agonists may exist in the heart. We have investigated the influence of β1-adrenergic stimulation on PKCε translocation elicited by A1R. Immunofluorescence imaging and Western blotting with PKCε and β-COP antibodies were used to quantify the co-localization of PKCε and t-tubular structures in isolated rat cardiomyocytes. The A1R agonist CCPA increased the co-localization of PKCε and t-tubules as detected by imaging. The β1-adrenergic receptor agonist isoproterenol (ISO) inhibited this effect of CCPA. Forskolin, a potent activator of PKA, mimicked, and H89, a pharmacological PKA inhibitor, and PKI, a membrane-permeable PKA peptide PKA inhibitor, attenuated the negative effect of ISO on the A1R-mediated PKCε translocation. Western blotting with isolated intact hearts revealed an increase in PKCε/β-COP co-localization induced by A1R. This increase was attenuated by the A1R antagonist DPCPX and ISO. The ISO-induced attenuation was reversed by H89. It is concluded that adrenergic stimulation inhibits A1R-induced PKCε translocation to the PKCε anchor site RACK2 constituent of a coatomer containing β-COP and associated with the t-tubular structures of the heart. In that this translocation has been previously associated with the antiadrenergic property of A1R, it is apparent that the interactive effects of adenosine and β1-adrenergic agonists on function are complex in the heart.
Keywords: adrenergic, antiadrenergic, heart, RACK2, PKA, PKCε
INTRODUCTION
Adrenergic and adenosinergic receptors play an important role in modulating cardiac function. Stimulation of the β1-adrenoceptor enhances Gs protein cycling (Fenton and Dobson, 2007) resulting in the sequential activation of adenylyl cyclase (Romano and Dobson, 1990) and cyclic AMP-dependent protein kinase A (PKA (Dobson, 1983b). The ensuing phosphorylation of proteins important to the modulation of myocardial metabolism and contractility elicits the manifestations characteristic of β1-adrenergic stimulation (Fenton and Dobson, 1984). The activation of myocardial adenosine A1 receptors (A1R) has been found to attenuate this β1-adrenergic signal cascade by an antiadrenergic action, reducing the β-adrenergic receptor (β1R)-induced elevation of cAMP levels and PKA activity (Dobson, 1983b). This antiadrenergic property reduces the vulnerability of the myocardium to overstimulation by adrenergic neurotransmitters released during ischemia (Rona, 1985; Schomig et al., 1991) and appears to prevent the depression of heart function elicited by catecholamine stimulation during low flow ischemia (Fenton et al., 1995).
Although considerable study has been made of the effect of A1R on the β1-adrenergic-induced signaling cascade, adenosine has also been reported to manifest an activation of protein kinase C (PKC) in the heart (Kudo et al., 2002; Perlini et al., 1998; Sakamoto et al., 1995). Activation of PKC has been shown to be reflected in a translocation, or change of subcellular localization, from the cytosol to a cell membrane where it has been found to bind to an anchor site designaged as a Receptor for Activated C-kinase (RACK) (Csukai et al., 1997; Mochly-Rosen et al., 2000). In the adult cardiomyocyte, PKCε appears to be predominant isoform which is thought to be important in modulating cardiac contractility (Hug and Sarre, 1993; Rybin and Steinberg, 1994). PKCε has been shown to play an important role in the adenosine-induced modulation of heart function, for the overexpression of the PKCε enhances, whereas specific inhibition of the PKCε decreases, the antiadrenergic action of A1R (Miyazaki et al., 2004). Stimulation of A1R elicits the translocation of the PKCε to the vicinity of t-tubules in the cardiomyocytes, and the co-localization of PKCε with β-COP in cardiomyocytes and isolated perfused hearts (Fenton et al., 2009). β-COP is one constituent of the coatomer that also possesses RACK2 (Sheff et al., 1996), the anchor site for activated PKCε (Csukai et al., 1997).
Although it had been found that PKCε is a mediator of the antiadrenergic action of adenosine (Fenton et al., 2009; Miyazaki et al., 2004), this property was only determined by quantifying ventricular function. A subsequent unrelated study investigating the A1R-stimulated phosphorylation of Heat Shock Protein 27 (HSP27) (Fenton et al., 2010) unexpectedly revealed an inhibitory effect of β1-adrenergic stimulation on this effect of A1R. The present study was initiated to investigate directly the interaction of A1R and β1R activation at the level of PKCε activation/translocation.
MATERIALS AND METHODS
Animals used in this study were maintained and used in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council (DHEW Publication, National Institutes of Health no. 85-23, revised 1996). The study was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. Euthanasia resulted from decapitation with subsequent exsanguination prior to removal of the heart.
Isolation of ventricular myocytes
Cardiomyocytes were isolated from 3 - 4 month male Sprague-Dawley rat hearts with the use of collagenase and hyaluronidase as described by us previously (Dobson et al., 2008). Isolated cardiomyocytes were cultured for 2–3 hrs on poly-L-ornithine-coated glass coverslips to allow attachment as described previously (Miyazaki et al., 2004). Myocytes attached to coverslips in this manner were subsequently used in the following imaging experiments.
Immunofluorescence Staining and Image Processing
Immunocytochemistry was performed as described previously (Miyazaki et al., 2004). Briefly, cardiomyocytes were placed in Fixation solution. After extensive washing, cells were made permeable by exposure to 0.1% Triton X-100 in PBS for 10 min. The coverslips with attached myocytes were subsequently washed twice with PBS and incubated for 30 min with 1% BSA in PBS. Immunostaining for PKCε and β-COP was performed using rabbit polyclonal and mouse monoclonal antibodies, respectively, as described previously (Fenton et al., 2009; Miyazaki et al., 2004). Images were observed using a Leica confocal microscope (Leica Microsystems, Germany). Differential interference contrast (DIC) and fluorescence images were viewed using a Leica DM IRB laser scanning confocal microscope controlled by Leica TCS SP II systems (Leica Microsystems, Germany). All images were taken with the same laser output to directly compare the fluorescence signal intensities (Fenton et al., 2009; Komatsu and Ikebe, 2004). For three-dimensional reconstruction, a series of optical sections that were obtained by confocal microscope were collected at 0.5-µm intervals moving progressively across the cell (Komatsu et al., 2002). The images were reconstructed using LCS 3D software of Leica Microsystems and processed using Adobe Photoshop 5.5 software (Adobe Systems).
Biochemical Procedures for Western Blotting
Rat hearts were isolated and perfused at a constant rate as described previously (Fenton and Chung, 2001). After isolation, hearts were allowed to stabilize for 15 min before initiating the experimental protocols. Agents were administered via infusion into the buffer to achieve the desired concentrations. Upon termination of the experimental periods, hearts were frozen with liquid N2-cooled aluminum clamps and stored in liquid N2 until assayed.
Fifty mg samples of frozen hearts were homogenized (PRO200 homogenizer, PRO Scientific, Oxford, CT) in 1 ml of a homogenization buffer containing 20 mM HEPES (pH 7.4), 0.3 mM MgCl2, 0.2 mM EDTA, 0.2 mM N-p-tosyl-l-lysine chloromethyl ketone (TLCK), 0.2 mM tosyl-phenylalanyl chloromethyl ketone (TPCK), 10 mg/ml leupeptin, and 2 mM phenylmethylsulfonyl fluoride (PMSF). After centrifugation for 30 min at 22,000 × g, the pellet was resuspended in a solubilization buffer composed of 30 mM HEPES (pH 7.4), 70 mM NaCl, 50 mM NaF, 1 mM sodium orthovanadate, 0.2 mM TLCK, 0.2 mM TPCK, 10 mg/ml leupeptin, and 2 mM PMSF. These membrane solutions were subjected to Western blot analysis using SDS-PAGE. Immunoblotting was conducted as described previously using nitrocellulose membranes and appropriate antibodies against PKCε (mouse monoclonal) and β-COP (rabbit polyclonal) (Komatsu et al., 2000). The densities of the PKCε bands were normalized to those of β-COP bands. In this manner translocation of PKCε from cytosol to membrane bound anchor sites (β’COP or RACK2) (Csukai et al., 1997) could be quantified since both β-COP and β’-COP are constituents of the coatomer associated with the membrane fraction of the cardiomyocyte (Sheff et al., 1996).
Cardiomyocyte and Isolated Heart Protocols
The following protocols were conducted with immunofluorescence imaging of isolated ventricular cardiomyocytes. The effect of A1R activation on translocation of PKCε to the β-COP containing coatomer was investigated in myocytes that were treated with 1 µM CCPA for 5 min or 10 min (Fenton et al., 2009). In addition, myocytes treated with 1 µM CCPA for 5 min were subsequently exposed to 2 µM isoproterenol (ISO) for 5 min in the continued presence of CCPA. Because cellular PKA activity increases in response to β1-adrenergic stimulation, it was necessary to determine if PKA is integral to the effect of adrenergic stimulation on adenosine actions. PKA activation was induced with 20 µM forskolin and co-localization of PKCε and β-COP in response to this treatment was quantified. Additional experiments were conducted as follows: 1) Cardiomyocytes were pre-treated with 20 mM forskolin for 5 min prior to the administration of 1 µM CCPA for 5 min in the continued presence of forskolin. 2) PKA was inhibited in cardiomyocytes sequentially treated with either 10 µM H89 or 10 µM PKI, a membrane-permeable myristoylated PKA inhibitor amide 14 to 22, for 10 min, 1 µM CCPA for an additional 5 min in the continued presence of H89 or PKI, followed by an administration of 2 µM ISO for 5 min in the continuted presence of CCPA and H89 or PKI. All cardiomyocyte experiments were conducted at room temperature.
The experiments described above utilized immunofluorescence and isolated cardiomyocytes to visualize the co-localization of PKCε and β-COP elicited by agent stimulation. A second approach was conducted to corroborate these results. Activation of PKCε is reflected in the translocation of this kinase from the cytosol to a membrane fraction in the myocyte where the PKCε anchor-site, β’-COP, is localized as quantified by β-COP immunoreactivity (Mochly-Rosen et al., 1990). Western Blotting was utilized to quantify the association of PKCε with the β-COP-containing membrane fraction of the intact myocardium in response to agent stimulation. Isolated hearts were treated to the following agents at the given concentrations: 8-cyclopentyl-1,3-dipropylxanthine (A1R antagonist; DPCPX; 0.1 µM), CCPA at 1.0 µM, H89 at 10 µM, and ISO at 10 nM. All agents were infused into the perfusion solution at 1% of the flow rate to achieve the final concentration. When indicated, DPCPX, H89, and CCPA were administered separately or in combination for 5 min in the absence or presence of a 5 min treatment with ISO.
Materials
8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), forskolin, H89, isoproterenol (ISO), and chlorocyclopentyladenosine (CCPA) were purchased from RBI/Sigma Chemical (St. Louis, MO). PKI, membrane-permeable myristoylated PKA inhibitor peptide was purchased from EMD Chemicals, Inc (Gibbstown, NJ). PKCε rabbit polyclonal antibody and PKCε mouse monoclonal antibody were obtained from Santa Cruz (sc-214; Santa Cruz, CA) and BD Transduction Laboratories, respectively. β-COP rabbit polyclonal antibody and β-COP mouse monoclonal antibody were purchased from Thermo Fisher Scientific and Sigma (St Louis, MO), respectively. All secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA), except for the horseradish peroxidase-conjugated secondary antibodies for Western blotting (Bio-Rad).
Statistical methods
Quantitative imaging analysis to assess the colocalization of the PKCε and β-COP was performed using “Intensity Correlation Analysis” function in ImageJ (http://rsbweb.nih.gov) (Li et al., 2004) as described previously (Fenton et al., 2009). The overlap coefficients generated by Pearson’s correlation coefficient have values between 1 and 0 (1 indicating that 100% of both components of the two images overlap). Quantitative co-localization analysis of the immunofluorescence data was conducted with images obtained from over 15 separate cardiomyocytes. All statistical analyses were performed with a Student’s t-test.
RESULTS
Effect of β1R on the A1R-mediated PKCε translocation to RACK2 in rat cardiomyocytes as determined by co-localization with β-COP
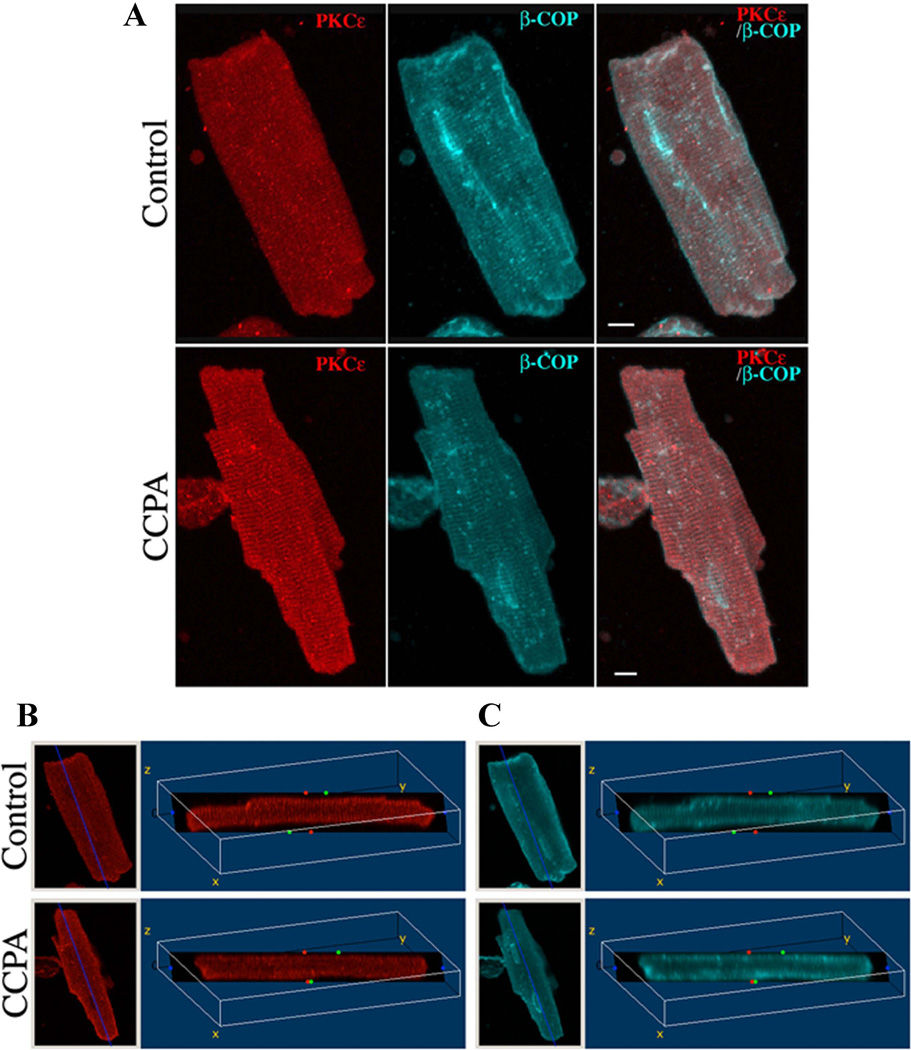
PKCε plays a mediating role in the antiadrenergic effect of A1R in the rat cardiomyocyte (Dobson et al., 2008; Miyazaki et al., 2004). Although A1R activation in the absence of β1R elicited an translocation of PKCε, it remained to be shown what effect β1-adrenergic stimulation would have on this action of A1R. Experiments were conducted to determine this relationship. Consistent with previous our observations, a three-dimensional reconstruction image of PKCε clearly demonstrated that the translocation of PKCε to the vicinity of the β-COP immunoreactive t-tubule membranes occurred after a 10 min of stimulation with CCPA (Fig. 1, CCPA and Movie S1). Conversely, agent-free control cells showed a weak fluorescence intensity of PKCε to the t-tubule membranes and a mostly diffuse cytosolic distribution (Fig 1, CONTROL and Movie S2). Fig 1B depicts a single slice section through the 3D reconstruction image obtained from Fig 1A. The fluorescence signals of PKCε and β-COP were observed within the rat cardiomyocyte. In the CCPA-treated cell, the PKCε was found to localized in a cross-striated pattern, forming the Z-line of the sarcomere. This observation verifies our earlier reports that the cellular distribution of PKCε is modified by the A1R adenosinergic signaling.
Figure 1. Three-dimensional reconstruction images of PKCε in isolated cardiomyocytes.
Isolated rat cardiomyocytes were incubated for 10 min in the absence (Control) or presence (CCPA) of 1 µM CCPA. Cells were fixed and stained as described in “Materials and Methods”. Panel A: A series of optical sections were collected at 0.5-µm intervals from the bottom to the top of the cell and reconstructed into composite images using software from Leica Microsystems. Panels B and C: Distribution of fluorescence in the transverse section of the 3D volume images obtained from Fig 1A as defined by the dark plane. Left image of each panel shows projections of the 3D reconstructions using maximum intensity in the xy (top view) orientation. Right image shows a single slice section image that is rotated about a fixed point (x = 110, z = −70). PKCε (Red), β-COP (Blue) and Merged. Bar = 8 µm.
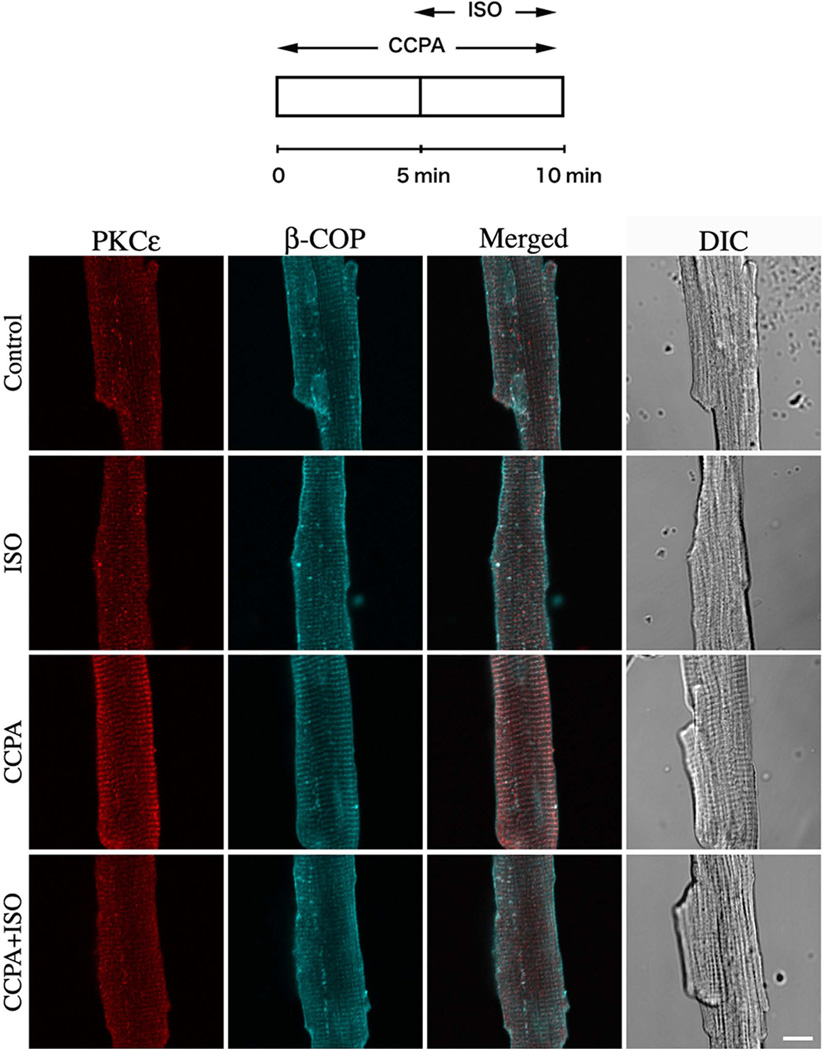
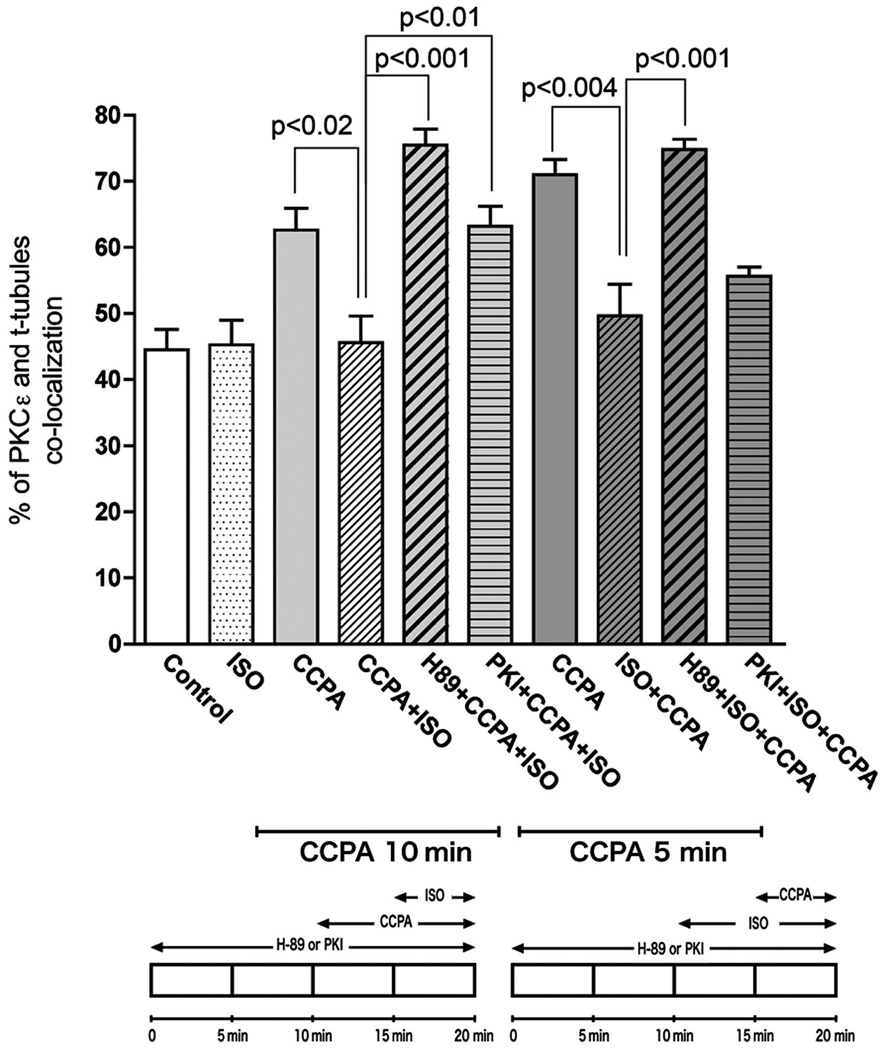
Because activation of PKC isoforms are regulated by the translocation of the inactive form from the cytosol to cellular membranes, the subcellular distribution of PKCε might be coupled to the role of PKCε in the antiadrenergic effect of A1R in the cardiomyocyte. However, little is known about the regulatory mechanism that controls PKCε distribution in the cardiomyocyte. To investigate the effect of β1R activation on the A1R-mediated translocation of PKCε, cardiomyocytes were stimulated with the β1R agonist, isoproterenol (ISO) five min into the administration of CCPA. As shown in Fig. 2, activation of β1R by the agonist ISO attenuated the A1R-mediated translocation of PKCε in the cardiomyocytes. Quantitative co-localization analysis indicated that stimulation with ISO significantly decreased the co-localization of PKCε and β-COP induced by A1R stimulation by CCPA (Fig. 3). Administration of CCPA at 1 µM for 10 min significantly increased the co-localization of PKCε and β-COP by 40%. Subsequent administration of ISO at 2 µM for 5 min inhibited this effect of CCPA. While it is apparent that the order of administering CCPA and ISO together had no effect on the results, i.e., ISO inhibited the effect of CCPA whether administered before or after CCPA, the effect of CCPA alone appeared to wane at longer durations. ISO had an inhibitory effect when administered for 5 min prior to a 5 min CCPA treatment. Furthermore, the effect of the 5 min exposure to CCPA (60% increase) on PKCε co-localization with t-tubules exceeded that of the 10 min exposure (40%). These results suggest that the intracellular distribution of PKCε in the rat cardiomyocyte is regulated by the balance between adenosinergic A1R and adrenergic β1R responses.
Figure 2. Effect of β1 adrenergic agonist, ISO, on the A1R-mediated translocation of PKCε in isolated cardiomyocytes.
Top schematic depicts time course of agent administration. CCPA and ISO were each administered at 2 µM. The focal plane was near the middle of the myocyte viewed by confocal microscope. PKCε (Red), β-COP (Blue) and differential interference contrast (DIC) images. Bar = 10 µm.
Figure 3. Statistical analysis of isolated rat cardiomyocyte images indicating co-localization of PKCε with β-COP, and used as an index of PKCε binding to RACK2 (see text).
Co-localization was determined and statistics conducted as described in “Materials and Methods”. Values are means ± SE for at least 15 cardiomyocytes. Significance is indicated at the respective p value. Bottom schematics depict two protocols for exposure of cardiomyocytes.
Effect of PKA on the A1R-mediated PKCε translocation in rat cardiomyocytes
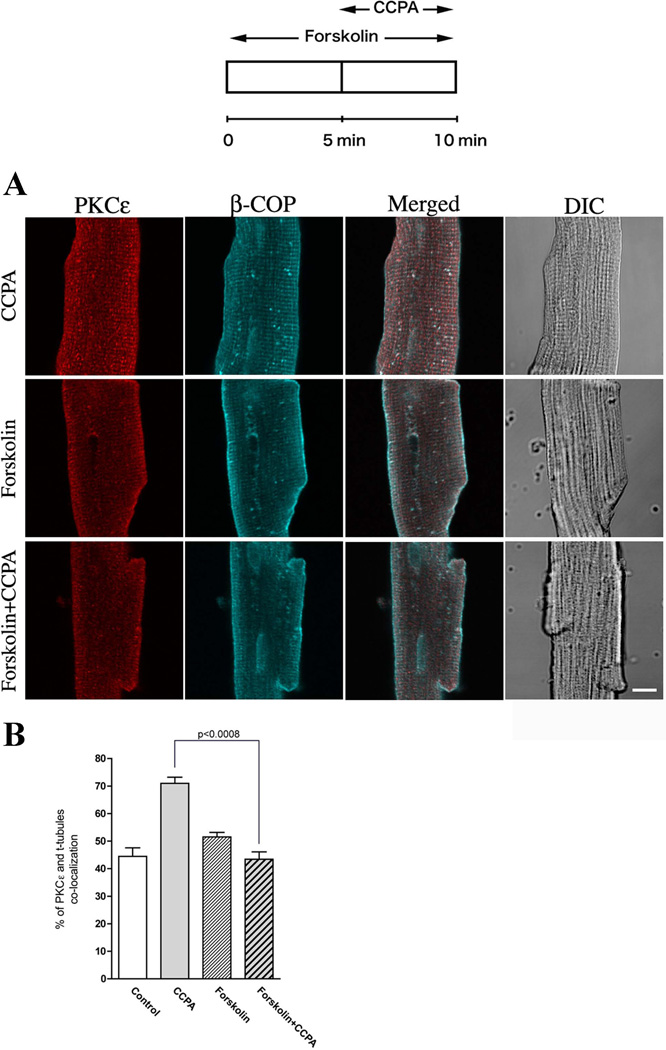
ISO acts on the β1R in the myocardium and subsequently activates adenylyl cyclase thus increasing the cellular levels of cAMP which activate PKA (Dobson, 1978; Dobson, 1983b). It was recently reported that activation of PKA modulates the subcellular distribution of PKCε (Yao et al., 2008). To investigate cAMP and activated PKA as mediators of the ISO attenuation of PKCε translocation, myocytes were treated with forskolin, a highly potent pharmacological PKA activator that is known to directly activate adenylyl cyclase thereby increasing PKA activity. As depicted in Fig. 4, forskolin significantly attenuated the translocation of PKCε elicited by CCPA. A 60% increase in co-localization induced by CCPA was inhibited by the administration of forskolin which alone had no effect on the translocation of PKCε (Fig. 4B). This result suggests that the cAMP is involved in the translocation of PKCε presumably with PKA as an intermediate.
Figure 4. Effect of CCPA on PKCε-induced translocation by forskolin in isolated cardiomyocytes.
(A) Top schematic depicts the time course of the administration of 20 µM forskolin and 1 µM CCPA. The focal plane was near the middle of the myocyte viewed by confocal microscope. PKCε (Red), β-COP (Blue) and DIC images. Bar = 10 µm. (B) Co-localization analysis from the images similar to those presented in (A). Values are means ± SE for at least 15 cardiomyocytes. Significance is indicated at the respective p value.
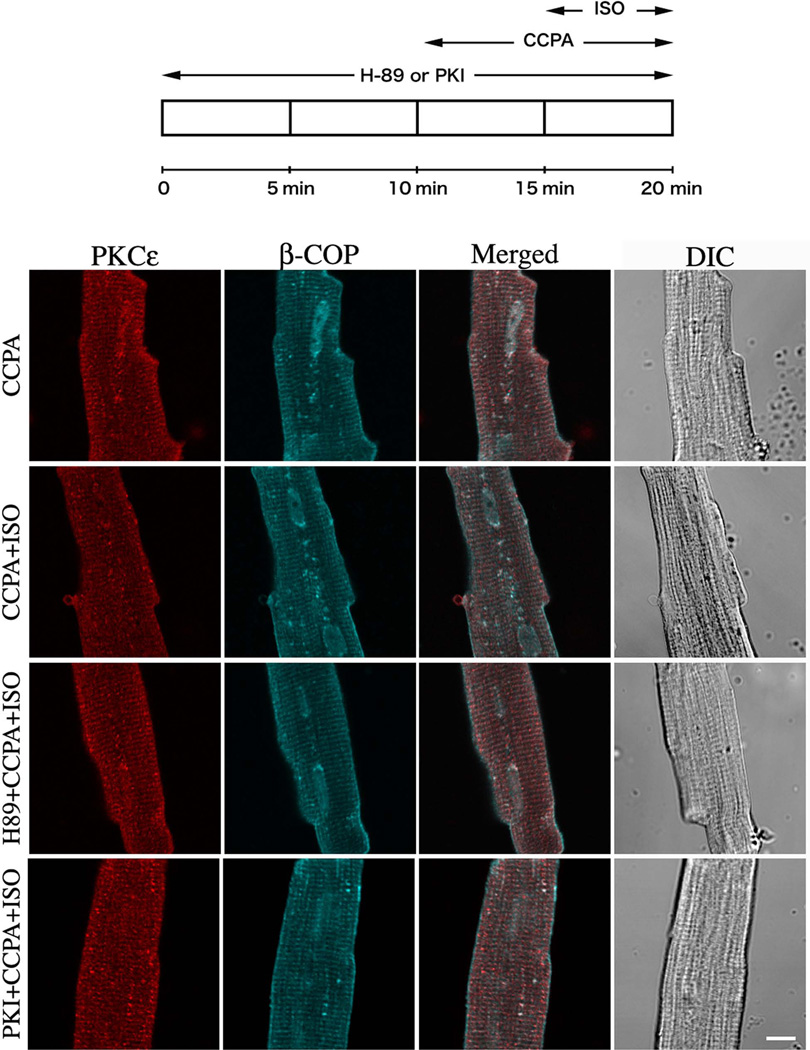
To further investigate the involvement of PKA in the inhibition of A1R-induced PKCε translocation, rat cardiomyocytes were treated with either H89, a pharmacological PKA inhibitor, or PKI, a cell-permeable PKI peptide inhibitor of PKA, prior to the administration of CCPA and ISO (Fig. 5). As visualized, both PKA-specific inhibitors markedly prevented ISO-induced attenuation of the translocation of PKCε induced by CCPA. As shown in Fig. 3, statistical quantitative co-localization analysis indicates that co-localization in the H89 and PKI treated myocytes was elevated as it was in CCPA-treated myocytes. With H89, there was an increase of 70% relative to control. With PKI, co-localization increased 42% relative to control. The administration of H89 reversed the inhibitory action of ISO on CCPA-induced enhancement of co-localization, whereas PKI effectively reduced the ISO-induced attenuation of the translocation of PKCε in Protocol 1, but not that in Protocol 2. Overall, these data obtained from experiments conducted 1) with the activation of PKA by forskolin and 2) the specific inhibition of PKA by H89 and PKI suggest that the redistribution of PKCε from the t-tubules to cytosol in the presence of CCPA is regulated by the ISO-enhanced PKA activity.
Figure 5. Effect of the PKA inhibitor H89 and PKI peptide on the PKCε translocation effects of CCPA and ISO in isolated rat cardiomyocytes.
Top schematic depicts the time course of administration to 10 µM H89 or 10 µM PKI peptide, 1 µM CCPA and 2 µM ISO. The focal plane was near the middle of the myocyte viewed by confocal microscope. PKCε (Red), β-COP (Blue) and DIC images. Bar = 10 µm.
Effect of β1R on the A1R-mediated PKCε translocation in intact rat heart
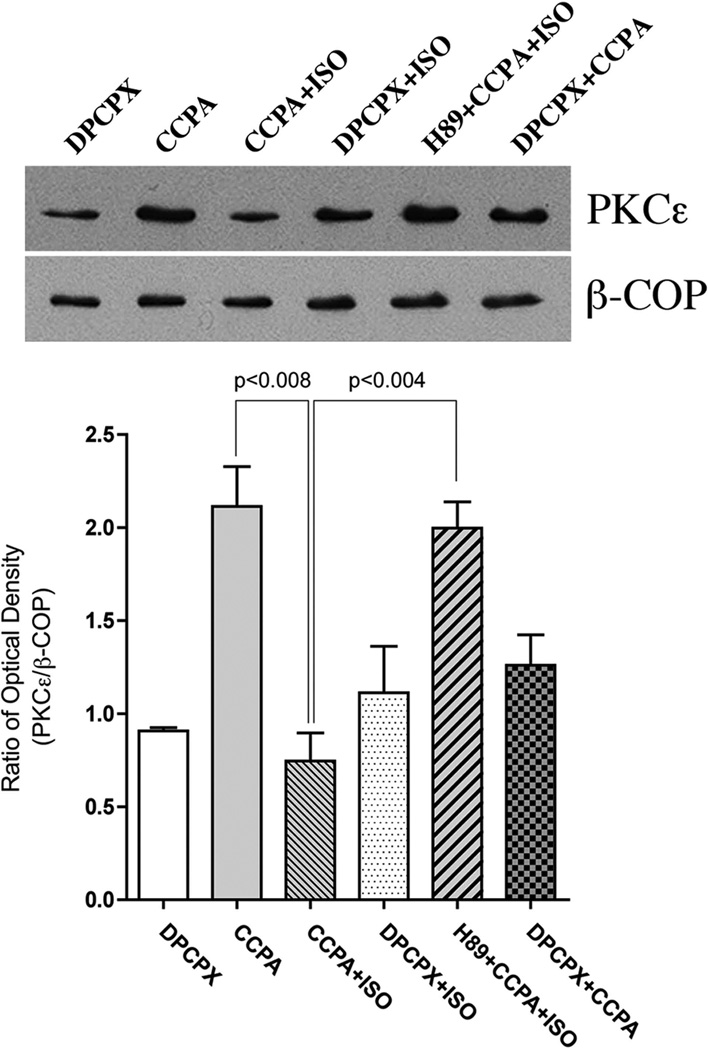
Quantitative co-localization analysis indicates that CCPA induces the translocation of PKCε to the vicinity of t-tubules and that this translocation was inhibited by ISO-activated PKA. An alternative approach was conducted by using Western blotting to examine PKCε and β-COP content in membrane fractions obtained from myocardium treated with the respective agents (Fig. 6). Density values of exposed films for PKCε were normalized for density values obtained for β-COP. Co-localization of PKCε and β-COP in the CCPA-treated hearts was significantly increased 133% compared to the control value. This result is similar to that previously reported (Miyazaki et al., 2004). This increase was effectively inhibited with the administration of ISO at 10 nM to the intact heart. Consistent with the immunostaining observations described earlier, administration of H89 was found to inhibit the effect of ISO. DPCPX, an inhibitor of A1R administered at 1 µM, had no significant effect in ISO treated hearts, but significantly reduced the effect of CCPA alone by 40%. These data indicate that the stimulation of β1R attenuates the A1R-enhanced translocation of PKCε translocation in the intact heart via a mechanism involving PKA.
Figure 6. Effect of various treatments on the translocation of PKCε to the β-COP containing membranes in the perfused rat heart as determined with immunoblotting.
Membrane fractions of treated hearts were subjected to SDS-PAGE, followed by immunoblotting with PKCε Ab and β-COP Ab. Agents were administered as follows: 10 µM H89, 1 µM CCPA, 10 nM ISO and 0.1 µM 8-cyclopentyl-1,3-dipropylxanthine (DPCPX). The amount of PKCε was quantitatively determined by scanning densitometry (NIH image program). The densities of PKCε bands were normalized to those of the β-COP bands. The data are interpreted to reflect the binding of PKCε to its anchor protein β’-COP (RACK2), as described in text. The values shown are means ± SE from three independent hearts. Significance is indicated at the respective p values.
DISCUSSION
The data in this study demonstrate that the translocation of PKCε to the PKCε anchor protein RACK2 induced by A1R stimulation of the isolated ventricular myocyte is effectively inhibited by concurrent β-adrenergic stimulation. Furthermore, it is suggested that the inhibitory signal cascade involves PKA activation. This interaction between adrenergic and adenosinergic stimulation is particularly interesting in that the adenosine-elicited translocation PKCε has been associated with the functional anti-adrenergic property of this nucleoside. In effect, adrenergic stimulation inhibits the anti-adrenergic action of adenosine.
Since 1977, adenosine has been known to be a modulator of the positive inotropic effects resulting from adrenergic stimulation (Schrader et al., 1977). Since then, the signal transduction cascade by which this action is manifest has been found to include the adenosine A1R-elicited reduction in the β1-adrenergic-stimulated Gs protein cycling (Fenton and Dobson, 2007), adenylyl cyclase activity (Romano et al., 1989), cAMP formation (Dobson, 1983b) and activation of PKA (Dobson, 1983b). The subsequent reduction in the β1-adrenergic-enhanced phosphorylation states of various contractile and metabolic proteins (Fenton and Dobson, 1984) results in decreased cellular Ca2+ levels (Fenton et al., 1991) and the β1-adrenergic ventricular contractile function response (Dobson, 1983a). It is thought that the antiadrenergic action of adenosine serves as a mechanism of cardioprotection (Fenton et al., 1995) against cardiotoxicity (Rona, 1985) which may accompany excessive adrenergic stimulation. In effect, adenosine released in response to both ischemia (Fenton and Dobson, 1987) and β1-adrenergic stimulation (Fenton et al., 1990) serves as a negative feedback reducing the responsiveness of the myocardium to norepinephrine released in the ischemic myocardium (Schomig, 1990). This effect of endogenous adenosine has been found to improve the functional recovery of low-flow ischemic myocardium stimulated by ISO (Fenton et al., 1995).
More recently, adenosine has been found to exert an antiadrenergic action in isolated cardiomyocytes via the activation of PKCε (Miyazaki et al., 2004). This is of particular interest with respect to the increasingly recognized role that PKC plays in heart pathology (Ferreira et al., 2011; Palaniyandi et al., 2009). When this isoform of PKC was overexpressed in ventricular myocytes, attenuation by A1R of the contractile response to β1R stimulation was significantly enhanced compared to cells that had not been transfected (Miyazaki et al., 2004). The translocation of the PKCε to t-tubular structures was also observed. This has been interpreted to indicate the activation of the PKC isozyme (Disatnik et al., 1994). Subsequent studies indicated that A1R initiated the signaling cascade by activating phospholipase C (PLC) (Fenton et al., 2010) resulting in the co-localization of the PKCε with β-COP located at the Z-line of the sarcomere. Others have reported a similar structural localization (Robia et al., 2005). β-COP is a coatomer constituent together with β’-COP (Sheff et al., 1996), the latter thought to exist as the anchor binding site, RACK2, for PKCε (Csukai et al., 1997). Thus co-localization of PKCε with β-COP is tantamount to describing the binding of PKCε to β’-COP or RACK2. It is interesting to note that in experiments with primary cultures of neonatal ventricular myocytes, β’-COP, but not β-COP, was localized to cross-striated structures suggesting that an incomplete coatomer complex is localized at the Z-lines (Csukai et al., 1997). It is suggested that these results may have reflected the de-differentiation of cardiomyocytes in culture and that the freshly isolated adult cardiomyocytes as used in the present study correctly reflect the presence of β-COP at the Z-lines.
In the present study, activation of β1R by ISO inhibited the CCPA-induced co-localization of PKCε and β-COP regardless of the order in which these agents were introduced to the myocytes. This effect was apparently mediated by PKA as indicated from data utilizing H89 to inhibit PKA and forskolin to elevate cellular levels of cAMP independent of β1-adrenergic stimulation. The conclusion obtained with H89 was extended with the use of a membrane permeable PKI peptide found to be highly selective as a PKA inhibitor. Interestingly, the percentages of co-localization in the cardiomyocytes treated with H89 to block the effects of ISO were similar regardless of whether cells were treated with CCPA for 5 min or 10 min (Fig. 3). This would suggest a maximum response to CCPA. Recently, we demonstrated that the translocation of PKCε increased within one min after the initiation of CCPA stimulation, reaching a maximum approx. 3 min after stimulation (Fenton et al., 2009). Reversal occurred within 5 min after removal of the adenosine agonist. In the present study, a lower co-localization with 10 min as compared to 5 min of CCPA alone would suggest a waning of the CCPA response with time. However, at present it is not understood why the rate of co-localization with 10 min of CCPA was restored to maximum level by treatment with H89.
Our previous investigations of HSP27 as a mediator of the PKCε-sensitive antiadrenergic action of adenosine indirectly suggested an adrenergic-mediated inhibition of A1R-induced activation of PKCε (Fenton et al., 2010). A similar conclusion was reached in a study by Haworth et. al. (Haworth et al., 2007), where an endothelin-1-induced activation of PKCε was reported to induce the phosphorylation of PKD. This phosphorylation was inhibited by adrenergic stimulation via PKA activation. One can postulate that the action of catecholamines in the heart as described in the present study would prevent an excessive negative feedback of β1-adrenergic stimulation by adenosine that is released in response to the adrenergic stimulation. In any case, as alluded to above, it is interesting that an adrenergic stimulation itself would inhibit the PKCε-initiated antiadrenergic action of adenosine if this nucleoside has importance as a cardioprotective agent in hearts overstimulated by catecholamines. It may be hypothesized that at low concentrations of adenosine, adrenergic responses are more fully expressed as a result of the PKA-effected inhibition of the antiadrenergic action of adenosine mediated through PKCε. However, at higher concentrations of adenosine such as occur with ischemia, A1R stimulation by endogenously produced adenosine reduces PKA activity by inhibiting adenylyl cyclase. This results in a reduced adrenergic-induced inhibition of the PKCε activation by A1R. In this manner, the overall antiadrenergic action of adenosine would be amplified, thus providing greater protection to the heart experiencing adrenergic stimulation during a period of reduced oxygenation. This hypothesis requires further study.
In conclusion, data has been presented showing that the stimulation of isolated cardiomyocytes with the A1R agonist CCPA results in the translocation of PKCε toward the Z-line of the sarcomeres. Immunofluorescence imaging and immunoblotting indicates that this translocation is targeted to the anchor binding protein RACK2. Concomitant stimulation of the myocytes with the agonist ISO inhibits the effect of A1R activation on the co-localization of PKCε and RACK2 by a mechanism involving PKA. A complex interaction between signaling cascades initiated by A1R and β1R is suggested that provides a homeostatic balance between adrenergic stimulation and the antiadrenergic action of adenosine.
ACKNOWLEDGEMENTS
This study was made possible by National Heart, Lung and Blood Institute Grant HL-84160 (JGD). The contents of this study are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
REFERENCES
- Csukai M, Chen C-H, DeMatteis MA, Mochly-Rosen D. The coatomer protein β'COP: a selective binding protein (RACK) for epsilon protein kinase C. J Biol Chem. 1997;272:29200–29206. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- Disatnik M-H, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210:287–297. doi: 10.1006/excr.1994.1041. [DOI] [PubMed] [Google Scholar]
- Dobson JG., Jr Reduction by adenosine of the isoproterenol-induced increase in cyclic adenosine 3',5'-monophosphate formation and glycogen phosphorylase activity in rat heart muscle. Circ Res. 1978;43:785–792. doi: 10.1161/01.res.43.5.785. [DOI] [PubMed] [Google Scholar]
- Dobson JG., Jr Adenosine reduces catecholamine contractile responses in oxygenated and hypoxic atria. Am J Physiol. 1983a;245:H468–H474. doi: 10.1152/ajpheart.1983.245.3.H468. [DOI] [PubMed] [Google Scholar]
- Dobson JG., Jr Mechanism of adenosine inhibition of catecholamine-induced elicited responses in heart. Circ Res. 1983b;52:151–160. doi: 10.1161/01.res.52.2.151. [DOI] [PubMed] [Google Scholar]
- Dobson JG, Jr, Shea LG, Fenton RA. Adenosine A2A and β-adrenergic calcium transient and contractile responses in rat ventricular myocytes. Am J Physiol. 2008;295:H2364–H2372. doi: 10.1152/ajpheart.00927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton RA, Chung ES. Chronic ethanol enhances adenosine antiadrenergic actions in the isolated rat heart. Alcoholism: Clin Exp Res. 2001;25(7):968–975. [PubMed] [Google Scholar]
- Fenton RA, Dobson JG., Jr Adenosine and calcium alter adrenergic-induced intact heart protein phosphorylation. Am J Physiol. 1984;246:H559–H565. doi: 10.1152/ajpheart.1984.246.4.H559. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Dobson JG., Jr Measurement by fluorescence of interstitial adenosine levels in normoxic, hypoxic and ischemic perfused rat hearts. Circ Res. 1987;60:177–184. doi: 10.1161/01.res.60.2.177. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Dobson JG., Jr Adenosine A1 and A2A receptor effects on G-protein cycling in β-adrenergic stimulated ventricular membranes. J Cell Physiol. 2007;213:785–792. doi: 10.1002/jcp.21149. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Galeckas KJ, Dobson JG., Jr Endogenous adenosine reduces depression of cardiac function induced by β-adrenergic stimulation during low flow perfusion. J Mol Cell Cardiol. 1995;27:2373–2383. doi: 10.1016/s0022-2828(95)92055-2. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Komatsu S, Ikebe M, Shea LG, Dobson JG., Jr Adenoprotection of the heart involves phospholipase C-induced activation and translocation of PKCε to RACK2 in adult rat and mouse. Am J Physiol. 2009;297:H718–H725. doi: 10.1152/ajpheart.00247.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton RA, Moore EDW, Fay FS, Dobson JG., Jr Adenosine reduces the Ca2+ transients of isoproterenol-stimulated rat ventricular myocytes. Am J Physiol. 1991;261:C1107–C1114. doi: 10.1152/ajpcell.1991.261.6.C1107. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Shea LG, Doddi C, Dobson JG., Jr Myocardial adenosine A1-receptor-mediated adenoprotection involves phospholipase C, PKC-ε, and p38 MAPK, but not HSP27. Am J Physiol. 2010;298:H1671–H1678. doi: 10.1152/ajpheart.01028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton RA, Tsimikas S, Dobson JG., Jr Influence of β-adrenergic stimulation and contraction frequency on heart interstitial adenosine. Circ Res. 1990;66:457–468. doi: 10.1161/01.res.66.2.457. [DOI] [PubMed] [Google Scholar]
- Ferreira JC, Brum PC, Mochly-Rosen D. βIIPKC and εPKC isozymes as potential pharmacological targets in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:479–481. doi: 10.1016/j.yjmcc.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth RS, Roberts NA, Cuello F, Avkiran M. Regulation of protein kinase D activity in adult myocardium: Novel counter-regulatory roles for protein kinase Cε and protein kinase A. J Mol Cell Cardiol. 2007;43:686–695. doi: 10.1016/j.yjmcc.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Ikebe M. ZIP kinase is responsible for the phosphorylation of myosin II and necessary for cell motility in mammalian fibroblasts. J Cell Biol. 2004;165:243–254. doi: 10.1083/jcb.200309056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Miyazaki K, Tuft RA, Ikebe M. Translocation of telokin by cGMP signaling in smooth muscle cells. Am J Physiol. 2002;283:C752–C761. doi: 10.1152/ajpcell.00501.2001. [DOI] [PubMed] [Google Scholar]
- Komatsu S, Yano T, Shibata M, Tuft RA, Ikebe M. Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J Biol Chem. 2000;275:34512–34520. doi: 10.1074/jbc.M003019200. [DOI] [PubMed] [Google Scholar]
- Kudo M, Wang Y, Xu M, Ayub A, Ashraf M. Adenosine A1 receptor mediates late preconditioning via activation of PKC-δ signaling pathway. Am J Physiol. 2002;283:H296–H301. doi: 10.1152/ajpheart.01087.2001. [DOI] [PubMed] [Google Scholar]
- Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1 Gαo N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Komatsu S, Ikebe M, Fenton RA, Dobson JG., Jr Protein kinase C epsilon and the antiadrenergic action of adenosine in rat ventricular myocytes. Am J Physiol. 2004;287:H1721–H1729. doi: 10.1152/ajpheart.00224.2004. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Henrich CJ, Cheever L, Khaner H, Simpson PC. A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regulation. 1990;1:693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Wu G, Hahn H, Osinska H, Liron T, Lorenz JN, Yatani A, Robbins J, Dorn GW., II Cardiotrophic effects of protein kinase Cε. Analysis by In Vivo modulation of PKCε translocation. Circ Res. 2000;86:1173–1179. doi: 10.1161/01.res.86.11.1173. [DOI] [PubMed] [Google Scholar]
- Palaniyandi SS, Sun L, Ferreira JCB, Mochly-Rosen D. Protein kinase C i heart failure: a therapeutic target? Cardiovasc Res. 2009;82:229–239. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlini S, Khoury E, Norton GR, Chung ES, Fenton RA, Dobson JG, Jr, Meyer TE. Adenosine mediates sustained antiadrenergic depression via activation of protein kinase C in the rat heart. Circ Res. 1998;83:761–771. doi: 10.1161/01.res.83.7.761. [DOI] [PubMed] [Google Scholar]
- Robia SL, Kang M, Walker JW. Novel determinant of PKC-ε anchoring at cardiac Z-lines. Am J Physiol. 2005;289:H1941–H1950. doi: 10.1152/ajpheart.01111.2004. [DOI] [PubMed] [Google Scholar]
- Romano FD, Dobson JG., Jr Adenosine modulates β-adrenergic signal transduction in guinea pig heart ventricular membranes. J Mol Cell Cardiol. 1990;22:1359–1370. doi: 10.1016/0022-2828(90)90981-7. [DOI] [PubMed] [Google Scholar]
- Romano FD, MacDonald SG, Dobson JG., Jr Adenosine receptor coupling to adenylate cyclase of rat ventricular myocyte membranes. Am J Physiol. 1989;257:H1088–H1095. doi: 10.1152/ajpheart.1989.257.4.H1088. [DOI] [PubMed] [Google Scholar]
- Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985;17:291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994;74:299–309. doi: 10.1161/01.res.74.2.299. [DOI] [PubMed] [Google Scholar]
- Sakamoto J, Miura T, Goto M, Iimura O. Limitation of mocardial infarct size by adenosine A1 receptor activation is abolished by protein kinase C inhibitors in the rabbit. Cardiovasc Res. 1995;29:682–688. [PubMed] [Google Scholar]
- Schomig A. Catecholamines in myocardial ischemia: Systemic and cardiac release. Circulation. 1990;82(suppl II):II-13–II-22. [PubMed] [Google Scholar]
- Schomig A, Haass M, Richardt G. Catecholamine release and arrhythmias in acute myocardial ischemia. Eur Heart J. 1991;124:F38–F47. doi: 10.1093/eurheartj/12.suppl_f.38. [DOI] [PubMed] [Google Scholar]
- Schrader J, Baumann G, Gerlach E. Adenosine as inhibitor of myocardial effects of catecholamines. Pflugers Arch. 1977;372:29–35. doi: 10.1007/BF00582203. [DOI] [PubMed] [Google Scholar]
- Sheff D, Lowe M, Kreis TE, Mellman I. Biochemical Heterogeneity and Phosphorylation of Coatomer Subunits. J Biol Chem. 1996;271:7230–7236. doi: 10.1074/jbc.271.12.7230. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Gordon A, Mochly-Rosen D, Diamond I. Dopamine and ethanol cause translocation of εPKC associated with εRACK: cross-talk between cAMP-dependent protein kinase A and protein kinase C signaling pathways. Mol Pharmacol. 2008;73:1105–1112. doi: 10.1124/mol.107.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]