Abstract
Axonal growth from both intact and severed fibers is limited after adult mammalian CNS injury. Myelin proteins contribute to inhibition of axonal growth. Semaphorin6A protein inhibits the extension of developing axons and is highly expressed in adult oligodendrocytes. This expression pattern suggests that a developmental axon guidance cue contributes to the restriction of adult CNS growth. Here, we assessed the role of a Sema6A receptor, PlexinA2, in recovery from adult trauma. Adult sensory neuron inhibition by Sema6A requires PlexinA2, with complete protection in PlexinA2-/- cultures. Mice lacking another myelin inhibitor receptor, NgR1, are known to exhibit greater axonal sprouting and functional recovery after lesions of the corticospinal tract at the medullary pyramid, so we investigated PlexinA2 in this lesion. Without injury, the corticofugal projection into the cervical spinal cord is normal in adult PlexinA2 null mice. After unilateral pyramidotomy, unlesioned PlexinA2-/- corticospinal fibers sprout across the midline to innervate the contralateral gray matter of the spinal cord to a significantly greater extent than do fibers in wild type mice. Sprouted fibers display frequent synaptophysin-positive synaptic puncta. The increased axonal growth in PlexinA2-/- mice after injury is accompanied by improved behavioral recovery in a pellet retrieval task using the impaired forelimb, and in a tape removal task. Thus, PlexinA2, as a receptor for oligodendrocyte-derived Sema6A and for secreted class 3 Semaphorins, plays a role in limiting adult axon growth and recovery after trauma.
INTRODUCTION
Developmental axonal repulsion plays a key role in sculpting adult neural connectivity by inhibiting growth to inappropriate targets (Tessier-Lavigne and Goodman, 1996). In the adult, axonal growth is strongly inhibited after injury. Although the cellular phenomenon of axon growth inhibition is shared by developing and mature CNS, most developmental axonal guidance cues are down regulated in the adult (Harel and Strittmatter, 2006). Adult-specific inhibitory factors include molecules expressed by myelinating oligodendrocytes, such as Nogo, MAG and OMgp, and by reactive astroctyes, such as CSPGs (Liu et al., 2006). The most widely studied myelin inhibitors, Nogo, MAG and OMgp, function via NgR1, NgR2 and/or PirB (Atwal et al., 2008; Fournier et al., 2001; Huebner et al., 2011; Kim et al., 2004; Venkatesh et al., 2005; Zheng et al., 2005). CSPGs can signal through PTPsigma (Coles et al., 2011; Shen et al., 2009). The current data suggest separate and distinct molecular pathways for axon regeneration versus developmental guidance, despite the reliance of both phenomenons on axon growth inhibition.
Semaphorins are prominent axonal guidance cues with secreted and transmembrane forms (Tran et al., 2007), but levels in the adult CNS with or without injury is low. Sema3A can be expressed by invading fibroblasts at SCI sites but without dural interruption there is not limited evidence for Sema expression related to SCI (Harel and Strittmatter, 2006). Sema6A is a transmembrane protein with axon repulsion activity (Suto et al., 2005; Xu et al., 2000), and a role in patterning thalamocortical, hippocampal and corticospinal projections as well as cerebellar granule and retinal cell migration (Kerjan et al., 2005; Leighton et al., 2001; Matsuoka et al., 2011; Runker et al., 2008; Suto et al., 2007). It has been reported that Sema6A expression is strong and widespread in adult oligodendrocytes (Cohen et al., 2003; Goldberg et al., 2004; Runker et al., 2008), but its oligodendrocyte function is unknown.
The Plexins are transmembrane receptors for Semaphorins (Winberg et al., 1998). For secreted Sema3s, Neuropilins function as accessory subunits linking Sema3s to Plexin signal transduction (Rohm et al., 2000; Takahashi et al., 1999; Tamagnone et al., 1999). In development, PlexinA2 and PlexinA4 serve as receptors for Sema6A and related Sema6s (Kerjan et al., 2005; Matsuoka et al., 2011; Runker et al., 2008; Suto et al., 2007). Structural studies reveal that PlexinA2 oligomerization contributes to ligand activation (Janssen et al., 2010; Nogi et al., 2010). For the corticospinal tract, PlexinA4, but not PlexinA2, participates in Sema6A-dependent developmental patterning (Runker et al., 2008). In adult brain, neuronal PlexinA2 expression is widespread while PlexinA4 is down regulated.
Here, we investigated the role of PlexinA2 in recovery from adult CNS axotomy based on the hypothesis that Sema6A is a myelin-associated inhibitor of axon growth. PlexinA2-/- mice are amenable to injury studies because their health and corticospinal tracts are wild type (Runker et al., 2008; Suto et al., 2007). We find that outgrowth from adult PlexinA2-/- neurons is not inhibited by Sema6A in vitro. Furthermore, after a pyramidotomy lesion, the PlexinA2-/- corticospinal tract sprouts more than wild type corticospinal fibers, and PlexinA2-/- mice recover greater skilled forelimb function than do wild types. Thus, PlexinA2 contributes to restriction of adult axon growth in the mature CNS.
MATERIALS AND METHODS
Mice
PlexinA2-/- mice have been previously described (Runker et al., 2008; Suto et al., 2007).
Immunoblotting
Cortex was freshly dissected from wild type and PlexinA2-/- mice immediately homogenized in lysis buffer (10 mM Tris/HCl, 1% NP-40, 150 mM NaCl, 0.1% SDS, 1% deoxycholate, supplemented with protease inhibitors), sonicated and supernatant collected after centrifugation. Protein (50 μg) was separated by SDS-PAGE and blotted onto PVDF. Membranes were probed with antibodies to detect PlexinA2 (1:500, C-18, Santa Cruz) and ßIII tubulin (1:5,000, Promega). Immunoreactivity was visualized with anti-rabbit and anti-mouse IRDye 680 or 800 (1:10,000, Rockland) – conjugated secondary antibodies. Protein bands were detected with the Li-Cor Odyssey system (Li-Cor Biosciences).
Neurite outgrowth
Adult wild type dorsal root ganglia (DRG) neurons from wild type and PlexinA2-/- mice of either sex were dissociated as previously described (Cafferty et al., 2010). DRG cells were cultured in poly-D-lysine pre-coated 96-well plates, which had been incubated with vehicle or Sema6A (R&D systems), washed and coated with 10 μg/ml laminin prior to cell addition. Cells were cultured for 18 hours at 37°C and 5% CO2, after which time they fixed with 8% paraformaldehyde in 20% sucrose for 1 hour then washed with PBS. Cells were stained with antibodies to ßIII tubulin (1:5,000, Promega) and visualized with Alexa-Fluor 488 conjugated secondary antibodies. Analysis of neurite outgrowth was completed using ImageExpress (Molecular Devices). Each preparation was exposed to wild type and PlexinA2-/- DRG cells from 4 different mice on 4 separate days with each preparation run in quadruplicate. Therefore, data are presented as average neurite outgrowth ±SEM from n = 4 independent experiments.
Surgery
Unilateral pyramidotomy was completed as previously described (Cafferty and Strittmatter, 2006). Animals of either sex were anesthetized deeply with ketamine (100 mg/kg)/xylazine (15 mg/kg) [n= 18 wild type (15 PyX, 3 sham), n = 13 PlexinA2-/- (10 PyX, 3 sham)] and placed in a supine position; an incision was made left of the trachea. Blunt dissection was performed to expose the skull base, and a craniotomy in the occipital bone allowed for access to the medullary pyramids. The dura mater overlaying the pyramids was pierced with a 32-gauge hypodermic needle, and the left pyramid was cut with fine iridectomy scissors medially up to the basilar artery. The wound was closed in layers with 5.0 Vicryl. Four weeks later the intact CST was traced with biotinylated dextran amine (BDA; 1.5 μl total) infused into the sensorimotor cortex at four sites (coordinates from bregma: 1.0 mm anterior/1.0 mm lateral, 1.0 mm anterior/0.5 mm lateral, 1.0 mm posterior/1.0 mm lateral, and 1.0 mm posterior/0.5 mm lateral, all at a depth of 0.8 mm into cortex). Animals received postoperative ampicillin twice a day for 3 d. The surgeon was blind to mouse genotype at surgery. All surgical procedures and postoperative care were performed in accordance with guidelines of the Yale Animal Care and Use Committee.
Behavior
Pellet retrieval. Fine motor skill was assessed in animals that underwent unilateral pyramidotomy by using a modified food pellet retrieval task (Cafferty and Strittmatter, 2006; Thallmair et al., 1998). Animals were trained daily for 2 weeks before pyramidotomy to retrieve and consume 10 food pellets from a smooth surface through a small window displaced to one side of a transparent plastic box. Pellet retrieval was judged as successful if the animal clearly extended its forelimb through the window, grasped the pellet after paw pronation, retrieved the pellet after paw supination, and consumed it. Pellets swept into the testing box were not counted. The biased location of the aperture in the box resulted in animals’ preferentially and consistently using one forelimb to retrieve food pellets. Wild type and PlexinA2-/- mice were trained to retrieve food pellets with their right forelimbs and subsequently underwent ablation of the left pyramidal tract. Their time taken and success (% of successful reaches) for retrieving 10 food pellets was recorded once prior to lesion and then on days 7 and 28 post lesion.Tape removal. Animals had a square of adhesive tape placed on the dorsal surface of the right forepaw, and the time taken to identify the tape (the “sense” component) and the time taken for the mice to remove the tape after sensing it (the “motor” component) was recorded once before lesion and on days 2 and 26 post lesion.
Histology
Animals were perfused transcardially with PBS, followed by 4% paraformaldehyde/PBS. Spinal cords were dissected, post-fixed overnight at 4°C, and gelatin embedded for vibratome sectioning. Transverse sections of cervical cord (25–30 μm) were processed for BDA with streptavidin secondary antibodies (Invitrogen, Eugene, OR) and tyramide signal amplification (PerkinElmer Life Sciences, Emeryville, CA). Immunofluorescence of spinal cord sections used primary antibodies directed against protein kinase Cγ (PKCγ; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), Sema6A (1:1000; R&D Systems), MBP (Myelin Basic Protein, 1:1000, Sigma), Synaptophysin (1:300, Santa Cruz), SV2 (1:100, the synapsin monoclonal antibody SV2, developed by K. M. Buckley was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242), PSD-95 (1:200, Sigma), and DRG sections with CGRP (1:5 000, Sigma) and IB4 (5 μg/ml, Sigma) with Alexa Fluor 568 and 488 secondary antibodies (1:500; Invitrogen). Myelin was visualized with FluoroMyelin green fluorescent myelin stain (1:300, Invitrogen). Where specified, specific in situ hybridization studies were completed by the Allen Institute for Brain Science and can be found at <mouse.brain-map.org>.
Corticospinal tract analysis
The extent of CST fiber innervation was determined by using NIH ImageJ, version 1.62 (National Institutes of Health, Bethesda, MD). Labeled fibers were selected by thresholding, and fiber length within gray matter from each side of each section (five sections/mouse) was measured after the skeletonize function was used.
RESULTS
Sema6A and PlexinA2 are expressed in the adult brain
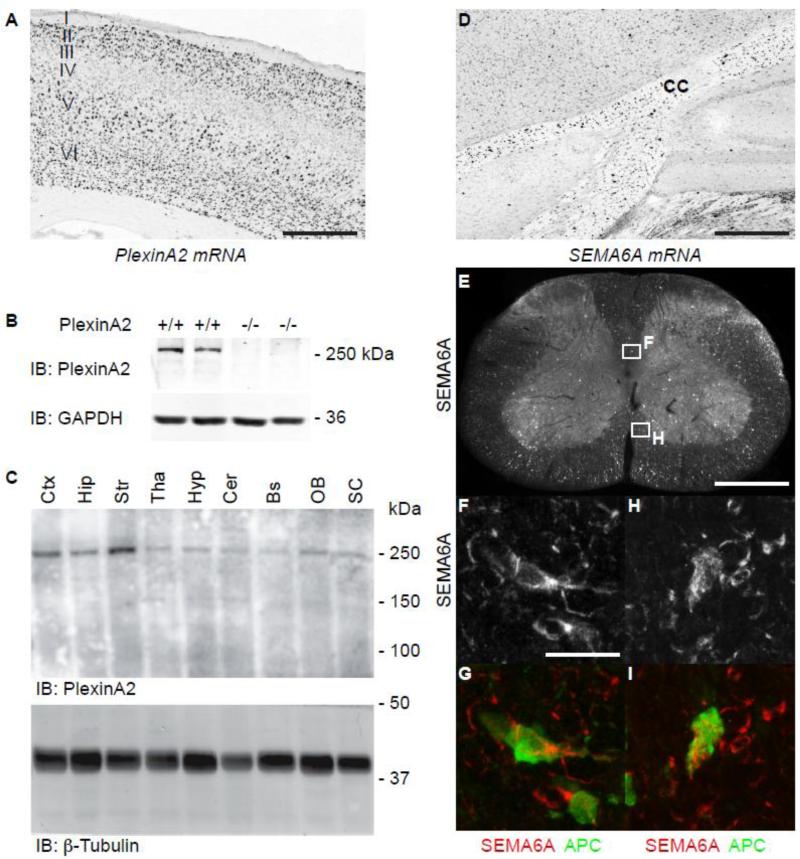
Semaphorin 6A and its cognate receptor PlexinA2 are highly expressed in the mature mouse CNS (Figs. 1A -1G). Images of in situ hybridization from the Allen Brain Atlas (<mouse.brain-map.org>) illustrate that PlexinA2 mRNA is enriched in cortical neurons particularly those in layer V in the adult (Fig. 1A) consistent with a function in corticospinal projection neurons. At the protein level, PlexinA2 is expressed strongly in the brain of adult wild type mice (Fig. 1B), is absent from PlexinA2-/- mice (Fig. 1B) and is enriched in the cortex and striatum (Fig. 1C). The mRNA for Sema6A, a high affinity ligand for PlexinA2, is expressed in sub-cortical white matter postnatally (Fig. 1D, CC = corpus callosum). To assess the identity of Sema6A-expressing cells, we double stained sections of adult spinal cord of wild type mice with antibodies against Sema6A (Figs. 1E-I) and the oligodendrocyte marker, APC (Adenomatus polyposis coli protein, clone CC1). Low power photomicrographs of spinal cord illustrate Sema6A immunoreactivity in dorsal, lateral and ventral white matter tracts at the circumference of the spinal cord (Fig. 1E). Higher power photomicrographs show Sema6A expression in cells morphologically consistent with oligodendrocytes in the dorsal corticospinal tract, (Fig. 1F, G) and ventral funiculus (Fig.1H, I) (Fig. 1F), co-labeled with APC (Fig. 1G, 1I, APC in green, Sema6A in red). Thus, Sema6A ligand is positioned in adult white matter and PlexinA2 in adult cortical projection neurons, such that their interaction might inhibit axonal regrowth after CNS injuries.
Figure 1. Expression of PlexinA2 and Sema6A in Adult CNS.
Photomicrograph of in situ hybridization demonstrates PlexinA2 mRNA expression is enriched in layer V primary motor and somatosensory cortical neurons in adult wild type mice (A). PlexinA2 expression can also be detected at the protein level in adult wild type but not PlexinA2-/- mice via immunoblot (B). PlexinA2 protein expression is enriched in cortex and striatum, but can be detected throughout the brain and spinal cord (C). Sema6A mRNA is detected in subcortical white matter regions in adult wild type mice, including the corpus callosum (CC) (D). Photomicrographs of transverse cervical spinal sections from adult wild type mice show Sema6A expression in dorsal, lateral and ventral white matter (E). Higher power photomicrographs of dorsal and ventral white matter (insets, F and H), show Sema6A expression in cells morphologically consistent with oligodendrocytes (F, H) identified by co-localization with the mature oligodendroctye marker, APC (G, I). (C, Ctx = cortex, Hip = hippocampus, Str = Striatum, Tha = Thalamus, Hyp = Hypothalamus, Cer = Cerebellum, Bs = brainstem, OB = Olfactory Bulb, SC = spinal cord). Scale bar A, D and E = 500 μm, F = 10 μm.
PlexinA2 deletion alleviates neurite outgrowth inhibition by Sema6A
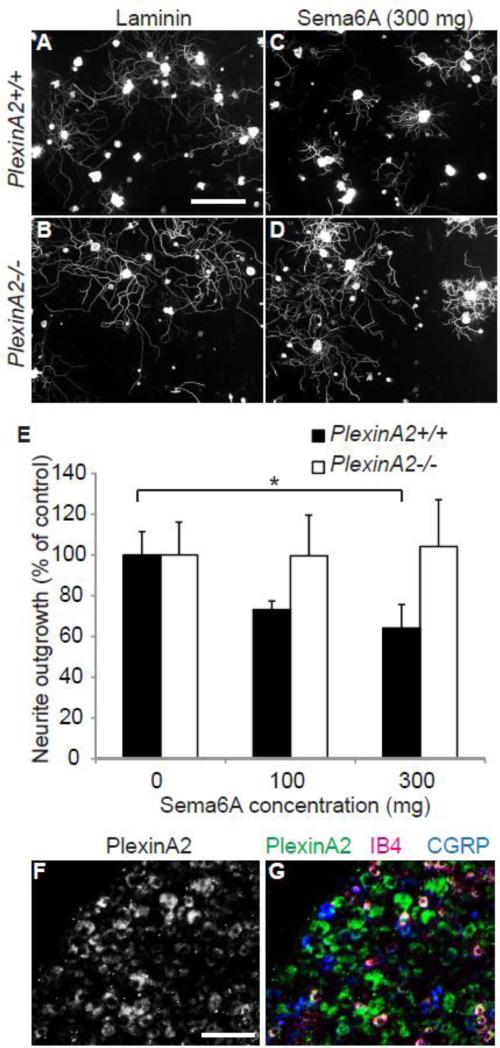
In order to test whether PlexinA2 transduces Sema6A-mediated axonal growth inhibition in adult neurons, we assessed neurite outgrowth from dissociated DRG cells from adult wild type and PlexinA2-/- mice plated on substrata coated with Sema6A. DRG neurons were tested because they can be cultured with high efficiency from mature mice. There was no significant difference in the extent of neurite outgrowth from wild type (353 ± 39 μm) and PlexinA2-/- (307 ± 26 μm) DRGs plated on laminin (Fig. 2A, B, E). Increasing concentrations of substrate-bound Sema6A significantly inhibit neurite outgrowth from wild type DRGs (Figs. 2C, 2E, P<0.05, ANOVA). In contrast, there is no significant difference in neurite outgrowth from PlexinA2-/- DRGs in the presence or absence of Sema6A (Figs. 2D, 2E). Sections of DRG from adult wild type mice demonstrate the expression of PlexinA2 in a subset of large and small diameter (co-labeled with the neuropeptide CGRP, calcitonin gene related peptide and the plant lectin IB4, Griffonia simplicifolia I-B4), sensory neurons (Fig. 1F, 1G), consistent with their sensitivity to Sema6A. Thus, fully adult mouse neurons have an active Sema6A/PlexinA2 signaling system that might play a role after injury.
Figure 2. Adult PlexinA2-/- DRG cells are insensitive to neurite outgrowth inhibition by Sema6A.
Photomicrographs of dissociated adult wild type (A, C) and PlexinA2-/- (B,D) DRG cells immunostained for ßIII-tubulin after being cultured for 18 hours on substrates coated without (A, B) or with 300 μg Sema6A (C, D). Neurite outgrowth was quantitated for wild type and PlexinA2-/- DRGs on subtrates coated with the indicated amounts of Sema6A per well (E). Increasing the concentration of Sema6A significantly attenuated neurite outgrowth from wild type DRG cells, while PlexinA2-/- DRG cells were insensitive to Sema6A mediated inhibition (mean sem from n = 4 independent mouse cultures; * P<0.05, ANOVA). Photomicrograph of a 15 μm section of wild type DRG from an adult mouse demonstrates PlexinA2 expression by a majority of large and small diameter sensory neurons (F, G). CGRP, Calcitonin gene related peptide; IB4, Griffonia Simplicifolia I-B4. Scale bar A = 100 μm, F = 50 μm.
Pyramidotomy-induced sprouting of intact corticospinal tract fibers in PlexinA2-/- mice
To test the potential role of PlexinA2 in mediating axonal growth inhibition in vivo, we utilized a unilateral corticospinal tract (CST) lesion model (pyramidotomy, PyX) in PlexinA2-/- mice. Pyramidotomy allows for the functional assessment of the growth of intact CST axons into CST-denervated spinal cord in the absence of astroglial scarring which typify more severe lesion models. Consequently, growth of intact CST axons is modulated by inhibitors present in myelin (Cafferty and Strittmatter, 2006). Using the PyX model, we examined injury induced axon growth in wild type and PlexinA2-/- mice. Sprouting of intact CST axons was assessed via cortical tracing with BDA injection into intact cortex 4 weeks after injury.
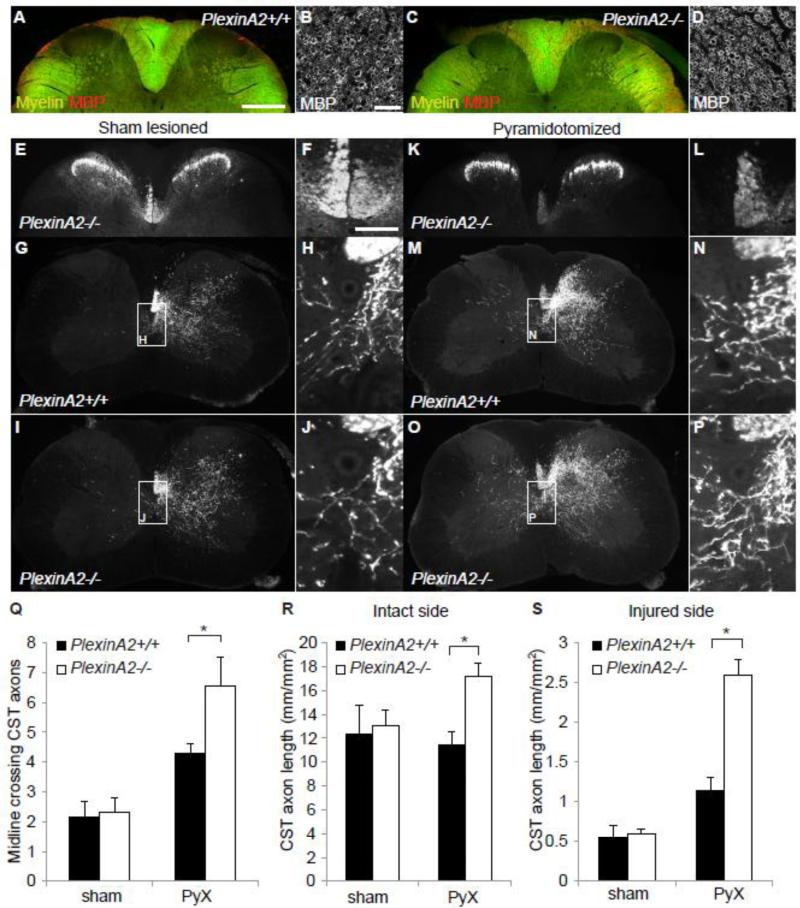
Prior to examining injury responses, we assessed adult anatomy in PlexinA2-/- mice. Intact sham-lesioned wild type (Fig. 3A, 3G) and PlexinA2-/- (Fig. 3C, 3I) mice show normal myelination patterns and normal CST wiring. In particular, MBP-immunoreactive myelinated profiles in the spinal cord dorsal columns are indistinguishable between genetoypes for adult mice (Fig. 3A-D). With regard to CST projections, a tightly fasciculated bundle of BDA+ fibers is observed to descend into the spinal cord dorsal columns of both genotypes (Fig. 3G, 3I). Collaterals project ventrally from the dorsal columns at all spinal levels to branch unilaterally and terminate in all lamina of grey matter. Few CST axons cross the midline in either genotype without injury (Fig. 3Q).
Figure 3. PyX-induced sprouting of intact CST fibers in PlexinA2-/- mice.
Myelination appears normal in adult PlexinA2-/- mice. Photomicrographs of transverse cervical sections from intact adult wild type (A, B) and PlexinA2-/- mice (C, D) demonstrate myelin staining (myelin stain in green, MBP in red) to be indistinguishable between genotypes (compare A and C). High power photomicrographs show a normal pattern of MBP staining around axon profiles of lateral column white matter of PlexinA2-/- mice (D), identical to that of wild type mice (B). Photomicrographs of transverse sections of cervical spinal cord from adult wild type (G, H) and PlexinA2-/- mice (E, F, I, J). PKCγ-immunoreactivity reveals a normal faciculated dorsal CST (E, F) in intact PlexinA2-/- mice. BDA+ CST axons can be seen in the right ventrodorsal column, from which CST collaterals project unilaterally into both dorsal and ventral horns in sham lesioned wild type (G, H) and PlexinA2-/- mice (I, J). A few BDA+ CST axons cross the midline in both wild type and PlexinA2-/- mice without injury (Q). Unilateral PyX is confirmed in all experimental mice by the complete absence of PKCγ-immunoreactivity in the contralateral ventrodorsal column (K, L). PyX PlexinA2-/- mice illustrate robust sprouting of intact BDA+ CST axons into the deafferented side of the spinal cord (O, P). The average number of BDA+ CST axons crossing the midline is significantly greater in PlexinA2-/- compared to wild type controls (compare O and P to M and N, Q, * P<0.01, ANOVA). As another measure of CST innervation, fiber length per cross sectional area was measured throughout the spinal cord grey matter (R, S). Fiber length was significantly greater on the intact side after PyX in PlexinA2-/- mice compared to fiber length on the intact side in sham lesioned PlexinA2-/-, wild type and PyX wild type mice (R, * P<0.01, ANOVA). CST fiber length was also significantly greater on the injured side of PlexinA2-/- mice in comparison to fiber length on the injured side of sham lesioned PlexinA2-/-, wild type and PyX wild type mice (S, * P<0.01 ANOVA). Scale bars in A = 250 μm; B = 25 μm; F = 50 μm. All data are mean ± sem from separate mice.
Pyramidotomized wild type mice demonstrate a lesion-induced increase in the number of CST fibers crossing the midline of the spinal cord (Figs. 3M, 3N, 3Q). The injury-induced midline crossing is significantly greater in PlexinA2-/- mice (Figs. 3O, 3P, 3Q). PyX was confirmed by the absence of PKC-gamma-IR in the contralateral ventral dorsal column of the lesioned mice (compare intact Fig. 3E to PyX 3K). The overall length of CST fibers is significantly greater after PyX in both the intact (Figs. 3R, P<0.01 ANOVA) and denervated sides (Fig. 3S, P<0.01 ANOVA) of the spinal cord for PlexinA2-/- mice compared to wild type mice. Thus, there is greater injury-induced CST axon growth in mice lacking PlexinA2.
Sprouted CST fibers form synaptic specializations
To assess the potential functional impact of the newly sprouted CST fibers, sections of transverse cervical spinal cord were co-stained for BDA and the presynaptic marker synaptophysin (Fig. 4). In wild type mice, few BDA+ fibers are detected on the denervated side after PyX (Fig. 4A-D), but these fibers show colocalization of synaptophysin-immunoreactive (synaptophysin-IR) puncta with BDA+ fibers. This indicates that midline-crossing fibers of wild type mice have the anatomical potential to mediate functional synaptic connections. For PlexinA2-/- mice, significantly greater numbers of BDA+ fibres sprout into the denervated side after PyX (Fig. 3). This increased density of sprouted fibers is densely co-stained for synaptophysin puncta (Fig. 4E-L). ). Furthermore, presumptive synaptic specializations observed on BDA+ fibers in PlexinA2-/- mice were found to co-label with both the presynaptic vesicle marker SV2 (Fig. 4M, O, P) and the post-synaptic marker PSD-95 (Fig. 4N, O, P). This provides anatomical evidence that the enhanced injury-induced CST sprouting released by deletion of PlexinA2 has the anatomical potential for synaptic function.
Figure 4. Synaptic specialization on BDA+ fibers.
Photomicrographs of transverse sections of deafferented cervical spinal cord from PyX wild type (A - D) and PlexinA2-/- (E - L). BDA+ CST fibers can be seen arborizing into deafferented spinal grey matter at low (A, E and I) and high power (B, F and J). Synaptophysin-immunoreactive puncta can be seen colocalizing with BDA+ CST axons in both wild type (C and D) and PlexinA2-/- (G and H, K and L) mice. BDA+ CST axons in PlexinA2-/- mice demonstrate puncta of colocalization with pre- and post-synaptic specializations (white arrows O and P) detected with the SV2 (M, O and P) and PSD-95 (N, O and P), respectively. Scale bar A = 50 μm, B and M = 10 μm.
Improved recovery of function after pyramidotomy in PlexinA2-/- mice
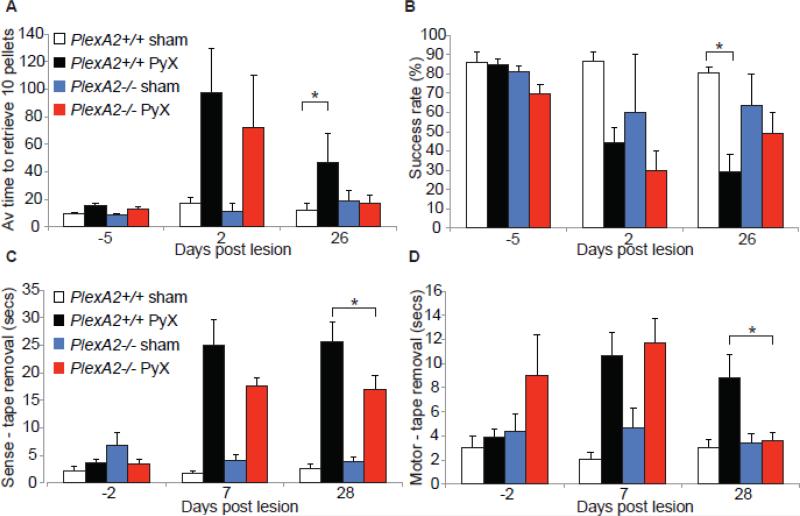
Given the increased growth from the intact CST into the denervated spinal cord of PlexinA2-/- mice with the presence of synaptic profiles, an improved functional recovery of fine motor control may be expected. To assess behaviour, both wild type and PlexinA2-/- mice were trained in a food pellet retrieval task (Cafferty and Strittmatter, 2006) and a forepaw tape removal task (Cafferty et al., 2007). Both of these behaviours are significantly impaired after PyX. Baseline measurements were made 1 week before PyX, within 1 week of PyX and then 4 weeks after PyX. Both wild type and PlexinA2-/- mice demonstrate a significant impairment in pellet retrieval time and success rate shortly after PyX (Figs. 5A, 5B), in comparison to sham-lesioned controls. After 4 weeks, wild type mice maintain significant deficits in retrieval time and retrieval success (Figs. 5A, 5B). In contrast, the PlexinA2-/- mice recover fully, so that they are not significantly different from their sham-lesioned controls (Figs. 5A, 5B). We also assessed the mice on an untrained task, namely their ability to sense and remove sticky tape from the paw. Both wild type and PlexinA2-/- mice display significant impairments in their ability to sense and to remove the tape during the first week after PyX (Figs. 5C, 5D). By the end of the testing period, mice of both genotypes reduce their time to sense the tape, while remaining impaired relative to pre-injury or sham-lesion. Importantly, the PlexinA2-/- mice are significantly quicker than wild type mice at 28 days (Fig. 5C, P<0.05 ANOVA). We also assessed the time taken to remove the tape from the affected forepaw after sensing. This parameter recovers fully to baseline levels in PlexinA2-/- mice, but remains significantly impaired in wild type mice at 4 weeks (Fig. 5D, P<0.05 ANOVA). Thus, the enhanced CST growth after injury of adult PlexinA2-/- mice is correlated with improved behaviour.
Figure 5. Pyramidotomized PlexinA2-/- mice recover fine forelimb function.
PyX and sham lesioned wild type and PlexinA2 -/- mice were pre-trained and assessed post lesion in a forepaw pellet retrieval task (A, B) and a forepaw sticky tape removal task (C, D). Paw usage prior to injury was identical between genotypes in average pellet retrieval time and success rate (A, B). Within the first week post lesion wild type and PlexinA2-/- mice displayed robust deficits in pellet retrieval time (A) and success rate (B) with affected right forepaw. By the end of the testing period PlexinA2-/- mice demonstrated forepaw dexterity insignificantly different from sham lesioned controls in pellet retrieval time and success rate. In contrast, wild type mice maintained a significant deficit in retrieval time (A, * P<0.05, ANOVA) and success rate (B, * P<0.05, ANOVA). The tape removal task allows for assessment of both sensory (C) and motor (D) function by the affected forepaw. There was no significant difference between genotypes during the pre-testing period (C, D). Seven days post lesion both wild type and PlexinA2-/- mice demonstrated robust deficits in their ability to sense (C) the presence of the tape on their forepaws and an elevation in the time taken to remove the tape (D). By the end of the testing period PyX PlexinA2-/- mice had recovered significant sense (C, * P<0.05, ANOVA) and motor (D, * P<0.05, ANOVA) function in comparison to PyX wild type mice.
DISCUSSION
The primary finding of this study is that PlexinA2 limits neurological recovery and axonal growth after adult CNS injury. After unilateral pyramid transection in the medulla, mice lacking PlexinA2 recover substantially greater ability to retrieve food pellets with affected limb and to perform other skilled tasks. The behavioral recovery is accompanied by enhanced growth of uninjured corticospinal fibers that cross the midline below the lesion to enter the denervated spinal cord and form synaptic specializations.
The pyramidotomy lesion severs only the corticospinal tract and does so unilaterally. No other systems are directly injured. This provides a discrete lesion in which deficits dependent on the function of this tract rather than multiple tracts as in most spinal cord injury models. The CST has special relevance clinically because human voluntary movements depend on its function. In the mouse, locomotion and general health are altered minimally, if at all, by pyramidotomy, but fine motor tasks such as food pellet retrieval is persistently and severely impaired on one side after injury. In PlexinA2 null mice, deficits are noted immediately after injury but recovery of neurological function is much more robust than in wild type mice. The recovery may in principle occur via alteration in neuronal function at multiple sites within the nervous system. We focused on the spared CST and observed increased sprouting from the uninjured side into the denervated side in the upper cervical spinal cord. Such growth has the potential for re-crossing CST fibers to transmit signals from the cerebral cortex to the motoneurons mediating impaired function in the affected forelimb. The expression of PlexinA2 is widespread, so injury-induced sprouting at several locations is likely to contribute to neurological improvements in PlexinA2-/- mice. For example, our preliminary data demonstrate enhanced sprouting of transected CST fibers in the midbrain rostral to the lesion across the midline (data not shown). Through polysynaptic connections to rubrospinal systems these fibers could contribute to functional recovery.
During embryonic and early postnatal brain development, Sema6A has selective expression in neuronal populations and contributes to axonal guidance. Consistent with previous reports, we find that Sema6A is expressed at high levels in adult oligodendrocytes (Cohen et al., 2003; Goldberg et al., 2004; Runker et al., 2008), and can inhibit adult axonal growth. Thus, together with ephrinB3, Nogo-A, MAG, OMgp, CSPGs and RGM-A, Sema6A contributes to the inhibitory nature of mammalian CNS myelin.
PlexinA2 is known to function as a receptor for certain Sema6A-mediated guidance events during development (Kerjan et al., 2005; Matsuoka et al., 2011; Runker et al., 2008; Suto et al., 2007). Here, we show that it is essential for the Sema6A-mediated axonal growth inhibition in adult DRGs. Our results for adult DRG contrast with studies of embryonic day E13.5 DRG outgrowth, in which DRG expression of PlexinA4 and Sema6A play key roles in determining Sema6A responsiveness (Haklai-Topper et al., 2010). This is most likely explained by altered expression patterns as a function of age. While the expression of many axonal guidance molecules are suppressed in the adult CNS (Harel and Strittmatter, 2006), we demonstrate that PlexinA2 expression remains high and might mediate adult neuronal responses to Sema6A.
Because PlexinA2 can function as a part of a complex with Neuroplilin for secreted class 3 Semaphorins (Rohm et al., 2000; Takahashi et al., 1999; Takahashi and Strittmatter, 2001; Tamagnone et al., 1999), additional Sema molecules may be contribute as ligands to the reparative PlexinA2 phenotype reported here. For example, Sema3A expression has been reported within scars derived from meningeal fibroblasts after CNS injury (De Winter et al., 2002; Kaneko et al., 2006; Pasterkamp et al., 1999), and to a lesser extent in motoneurons of the adult injured spinal cord (Lindholm et al., 2004). One putative Sema3A antagonist promoted recovery from SCI (Kaneko et al., 2006), while another did not (Mire et al., 2008).
The results presented here demonstrate that a specific Plexin family member mediates axonal growth inhibition and limits recovery after adult CNS injury. Coupled with the implication of Sema6A as an oligodendrocyte-derived growth inhibitor, the data provide cellular and molecular parallels between axonal repulsion during development and adult neurological recovery. Future work will determine the relative role of PlexinA2 transduction in the growth of other axonal pathways after adult CNS injury.
ACKNOWLEDGMENTS
We thank Yiguang Fu for excellent technical assistance and Dr. Fumikazu Suto for providing PlexinA2-/- mice. This work was supported by grants from the Christopher and Dana Reeve Foundation, the Wings for Life Foundation, the Dr. Ralph and Marion Falk Medical Research Trust, and the National Institutes of Health to S.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RI, Rottkamp DM, Maric D, Barker JL, Hudson LD. A role for semaphorins and neuropilins in oligodendrocyte guidance. Journal of neurochemistry. 2003;85:1262–1278. doi: 10.1046/j.1471-4159.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-Specific Molecular Switch for RPTP{sigma} Clustering and Neuronal Extension. Science. 2011 doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, Pasterkamp RJ, Gispen WH, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989–4999. doi: 10.1523/JNEUROSCI.4390-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haklai-Topper L, Mlechkovich G, Savariego D, Gokhman I, Yaron A. Cis interaction between Semaphorin6A and Plexin-A4 modulates the repulsive response to Sema6A. Embo J. 2010;29:2635–2645. doi: 10.1038/emboj.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner EA, Kim BG, Duffy PJ, Brown RH, Strittmatter SM. A multi-domain fragment of nogo-a is a potent inhibitor of cortical axon regeneration via nogo receptor 1. J Biol Chem. 2011 doi: 10.1074/jbc.M110.208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature. 2010;467:1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, Nakayama C, Kumagai K, Kimura T, Sato Y, Goshima Y, Taniguchi M, Ito M, He Z, Toyama Y, Okano H. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Kerjan G, Dolan J, Haumaitre C, Schneider-Maunoury S, Fujisawa H, Mitchell KJ, Chedotal A. The transmembrane semaphorin Sema6A controls cerebellar granule cell migration. Nat Neurosci. 2005;8:1516–1524. doi: 10.1038/nn1555. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, Skarnes WC, Tessier-Lavigne M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410:174–179. doi: 10.1038/35065539. [DOI] [PubMed] [Google Scholar]
- Lindholm T, Skold MK, Suneson A, Carlstedt T, Cullheim S, Risling M. Semaphorin and neuropilin expression in motoneurons after intraspinal motoneuron axotomy. Neuroreport. 2004;15:649–654. doi: 10.1097/00001756-200403220-00015. [DOI] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire E, Thomasset N, Jakeman LB, Rougon G. Modulating Sema3A signal with a L1 mimetic peptide is not sufficient to promote motor recovery and axon regeneration after spinal cord injury. Mol Cell Neurosci. 2008;37:222–235. doi: 10.1016/j.mcn.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, Toyofuku T, Uchiyama S, Goshima Y, Kumanogoh A, Takagi J. Structural basis for semaphorin signalling through the plexin receptor. Nature. 2010;467:1123–1127. doi: 10.1038/nature09473. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- Rohm B, Ottemeyer A, Lohrum M, Puschel AW. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev. 2000;93:95–104. doi: 10.1016/s0925-4773(00)00269-0. [DOI] [PubMed] [Google Scholar]
- Runker AE, Little GE, Suto F, Fujisawa H, Mitchell KJ. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev. 2008;3:34. doi: 10.1186/1749-8104-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci. 2005;25:3628–3637. doi: 10.1523/JNEUROSCI.4480-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, Chedotal A, Mitchell KJ, Manabe T, Fujisawa H. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron. 2007;53:535–547. doi: 10.1016/j.neuron.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Strittmatter SM. Plexina1 autoinhibition by the plexin sema domain. Neuron. 2001;29:429–439. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming G.-l., Song H.-j., Chedotal A, Winberg ML, Goodman CS, Poo M.-m. Plexins Are a Large Family of Receptors for Transmembrane, Secreted, and GPI-Anchored Semaphorins in Vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thallmair M, Metz GA, Z'Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annual review of cell and developmental biology. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Xu XM, Fisher DA, Zhou L, White FA, Ng S, Snider WD, Luo Y. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J Neurosci. 2000;20:2638–2648. doi: 10.1523/JNEUROSCI.20-07-02638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]