Abstract
Breast cancer is the most commonly diagnosed cancer among women worldwide. Many women have become more aware of the benefits of increasing fruit consumption, as part of a healthy lifestyle, for the prevention of cancer. The mechanisms by which fruits, including berries, prevent breast cancer can be partially explained by exploring their interactions with pathways known influence cell-proliferation and evasion of cell-death. Two receptor pathways- estrogen receptor (ER) and tyrosine kinase receptors, especially the epidermal growth factor receptor (EGFR) family- are drivers of cell-proliferation and play a significant role in the development of both primary and recurrent breast cancer. There is strong evidence to show that several phytochemicals present in berries such as cyanidin, delphinidin, quercetin, kaempferol, ellagic acid, resveratrol and pterostilbene, interact with and alter the effects of these pathways. Further, they also induce cell death (apoptosis and autophagy) via their influence on kinase signaling. In this review, we summarize in vitro data regarding the interaction of berry polyphenols with the specific receptors and the mechanisms by which they induce cell death. Further, we also present in vivo data of primary breast cancer prevention by individual compounds and whole berries. Finally, we present a possible role for berries and berry compounds in the prevention of breast cancer and our perspective on the areas that require further research.
Keywords: Berries, Berry Polyphenols, Breast Cancer, Ellagic Acid, Cyanidin, Delphinidin, Quercetin, Kaempherol, Resveratrol, Estrogen Receptor, Epidermal Growth Factor Receptor, Kinase Signaling, Apoptosis, Autophagy, ACI rats
Introduction
Cancer development and metastasis is a multistep process and a result of the dysfunction of several regulatory features that keep the cells in check (1). Although food has not been posited as a cure for cancer, several lines of evidence exist to believe that components of food can affect the development of cancer both in beneficial and detrimental ways (2–3). The reason is the intense interaction of the food components with the cells at different stages during cancer development. Breast cancer development follows this rule as well. Healthy changes in the lifestyle, including a better diet may prevent up to 40% of breast cancers (4). Increasing the consumption of fruits and vegetables is one part of a healthy dietary modification. Berries contain phytochemicals that have been shown to interfere, in a beneficial way, with multiple pathways linked to the development of cancer. These compounds are bioavailable and can potentiate each others’ effect. In addition, they taste good, are a part of the Western culinary repertoire, grown locally and available year-round as fresh or frozen varieties. They are also a constant media presence, leading to increased awareness regarding their effects among consumers. A recent study reports that there is a high correlation between increased fruit and vegetable consumption in cancer survivors that actively seek health information in the media (5). Thus a general case can be made for the study of berries, as fruits, in the prevention cancer. A particular case can be made for breast cancer because women form a large part of the media consumers and are more likely to change their dietary behavior based on such information. One purpose of this review is to present to the scientific community the evidence that is available regarding the benefits of berries for breast cancer prevention.
The health benefits of berries have been linked mostly to its antioxidant effects. While this is an important contributor, berry-phytochemicals also interact with other pathways, especially receptor-signaling. In this review, we focus on two receptor signaling pathways known to play key roles in breast cancer development- Estrogen receptor (ER) and Tyrosine Kinase Receptor (TKR). These two pathways are important for bestowing proliferative potential to breast cancer cells and allowing the evasion of cell-death. Berry phytochemicals can interfere with both ER and TKR signaling. They can also induce apoptotic and/or autophagic cell-death by modulating Kinase signaling, which is also involved in the cross-talk between ER and TKR pathways. We evaluate the in vitro data available in the literature for these modulations and apply it to breast cancer prevention. The mechanism by which well-studied compounds such as resveratrol and quercetin induce cell-death in breast cancer cell lines is available. But there is a lack of such information for compounds such as cyanidin, delphinidin and pterostilbene. Since there is a generality among various cell-lines in how a phytochemical induces cell-death, we present mechanistic data generated in other cell-lines for the latter. However, we emphasize the importance of confirming these in breast cancer specific cell-lines. We also present the in vivo data available from existing animal models of breast cancer. Finally, we provide a perspective on how whole berries and berry compounds can be used for the prevention of primary and recurrent breast cancer and the areas of research that need to be explored further.
Estimated intake and plasma levels of berry polyphenols
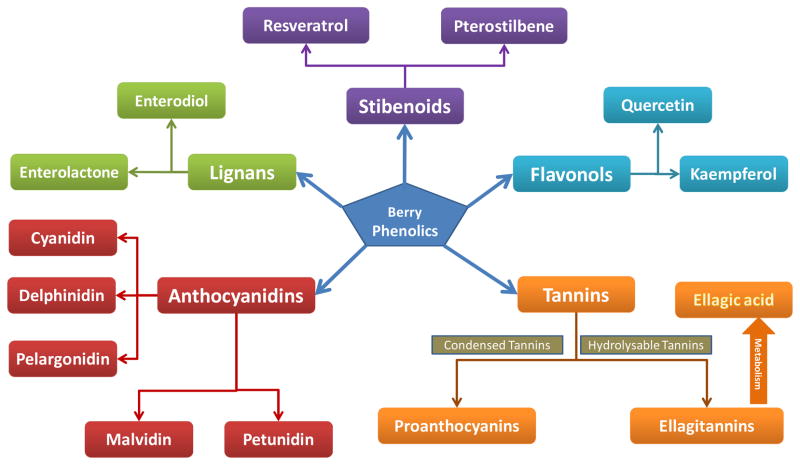
We begin by discussing the berries that are commonly consumed, the different classes of polyphenols present in them and the typical plasma concentrations of these phytochemicals. It is useful to understand the physiological levels that can be achieved by oral administration in order to contrast it with those in in vitro studies that tend to use supra-physiological doses. The berries most commonly consumed in the American diet are blackberry, blueberry, cranberry, raspberry, including black raspberry and strawberry. While they are good sources of micronutrients such as vitamin C and selenium, they are richer sources of polyphenols (6). Berries vary greatly in their chemical composition, which is affected by agricultural and geographical variations as well as the species. Instead of focusing on a single type of berry or a single polyphenol, this review will evaluate several polyphenols typically found in the most commonly consumed berries. We will consider five classes of polyphenols- anthocyanins, flavonols, tannins, stilbenes and lignans, predominantly found in berries (Figure 1). Of these, anthocyanins are the most ubiquitous and the primary flavonoid responsible for their vibrant colors (7). Table 1 lists the various polyphenols often found in commonly consumed berries, their primary berry sources and estimated levels of consumption in the US population.
Figure 1.
Classification of berry phenolics and representative polyphenols from each class.
Table 1.
Intakes and plasma concentration of select polyphenols found in commonly consumed berries.
| Polyphenol Class | Berry polyphenol | Berry sourcea | Quantity present (mg/Kg fresh weight)b | Estimated intake (mg/person/day)c | Plasma levelsd | Other food source | Reference |
|---|---|---|---|---|---|---|---|
| Anthocyanidins | Cyanidin Delphinidin Malvidin Pelargonidin Petunidin |
Blackberry Black raspberry Blueberry Cranberry Raspberry Strawberry |
417–6870 | 12.5 | 1.4–115 nM | Red cabbage, Grapes Pomegranate Red wine |
(119) (8) |
| Flavonols | Quercetin | Cranberry | 6–263 (Total) Quercetin (6–121) ≫ Kaempferol (5–8) |
25–35 | 0.08–7.6 μM | Apples, onions, | (90, 119) |
| Kaempferol | Strawberry | ||||||
| Ellagitannins | Ellagic acid | Blackberry Black raspberry Raspberry Strawberry |
57–537 | 1 | 60–100nM | Pomegranate Walnuts Oak-aged Whisky |
(120– 122),(123) |
| Urolithins (Ellagic acid metabolites) | |||||||
| Proanthocyanidins | Procyanidin Prodelphinidin Propelargonidin |
Blackberry Blueberry Cranberry Strawberry |
302–4188 | 57.7 | 40 nM | Chocolate Apples Grapes Tea |
(121, 124–125) |
| Phytoalexins-Stilbenes | Resveratrol | Blueberry Cranberry |
0.7–85 μg/kg | 0.9e | 2–3.3μMf | Wine Grapes Peanuts |
(126–129) |
| Pterostilbene | Blueberry | 9.9–15.1 μg/kg | Grapes | ||||
| Lignans | Secoisolarisinol | Blackberry Blueberry Raspberry Strawberry |
13.9–371 | 0.6 | 1.7 μM (enterolact one) | Flax seed Rye bread Sesame seed |
(82, 130) |
Berries with the highest reported content of the selected polyphenols. Neither polyphenols nor berries are arranged in order of specific contents.
Some quantities (in bold) have been converted from dry weight values using the formula 1g dry wt = 10 g fresh weight for most berries
All estimated intakes are for US population unless specifically stated
Plasma levels are collected from a wide variety of human intervention studies. They are representative of peak plasma levels and are not representative of US population
Estimated intake for Spanish population cohort from the EPIC study (129).
Calculated from raw value 2.7 mg/3.6L plasma using formula μM = μg/L ÷ molecular weight of resveratrol (228.4) (126)
The relative abundance of various polyphenols present in berries is of the order anthocyanins > proanthocyanins > ellagitannins > flavonols > lignans > stilbenes (Table 1). However, the relative intake in the US population is proanthocyanins > flavonols > anthocyanins > ellagitannin > stilbenes/lignans (Table 1). This is reflective of the contributions from other food sources to the intake. Although proanthocyanins are consumed at a higher level, they are less bioavailable than anthocyanins. This is reflected in the peak plasma levels seen for anthocyanidins (115 nM) versus proanthocyanidins (40 nM). The average intake of anthocyanins is 12.5 mg/person/day, whereas that of proanthocyanin is 57 mg/person/day (8). Due to limited bioavailability and extensive clearance/metabolism in the body, the typical plasma concentrations of these compounds occur in the nanomolar range. Nevertheless, low micromolar ranges have been reported for resveratrol, quercetin and enterolactone (Table 1). These micromolar levels were achieved by feeding foods other than berries. Both the gut (gastric, intestinal and colonic) and the liver metabolites of these compounds play a significant role in mediating the physiological effects of these compounds. The important role of metabolites is briefly discussed later. It must be kept in mind that although individual compounds may affect a pathway one way, the presence of several polyphenols together may change these effects. In this review, we have chosen to focus on a select group of polyphenols and their metabolites where applicable, based either on the abundance of the polyphenol in berries (ellagic acid, anthocyanidins) or the data available for their interaction with the cell-signaling pathways mentioned (resveratrol, quercetin and lignans).
Receptor pathways that play a key role in breast cancer development
The development of primary and secondary neoplasia is a highly complex process involving multiple pathways and bi-directional cross-talk amongst these. In this section, we discuss the alterations in two cell-signaling pathways known to play a role in both primary cancer and acquired antiestrogen (AE)-resistant secondary cancer. These are Estrogen Receptor (ER) and Tyrosine Kinase Receptor (TKR) pathways. Kinase signaling, the key connector of these pathways, is also discussed. The effect of individual berry phytochemicals, berry extracts and whole berries on cancer cells mediated via these pathways is summarized. The mechanisms by which molecules of these pathways can influence cancer development include but are not limited to alterations in expression, activity, regulation by upstream and downstream molecules, phosphorylation, and epigenetic modifications.
Role of Estrogen Receptor (ER) signaling in breast cancer cells
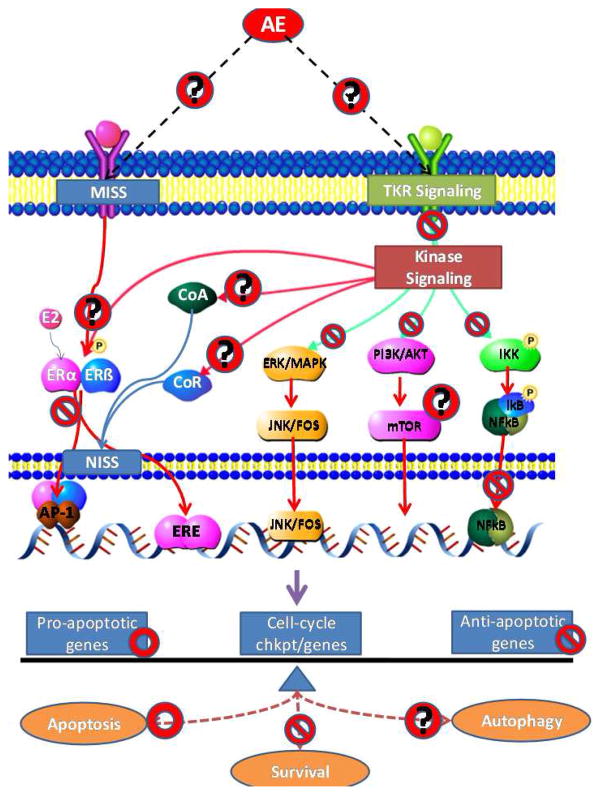
Estrogen receptors (ER) are central to the development of the normal mammary gland, as well as primary and secondary breast cancers. ER action in the breast epithelium can be classified into two: Nuclear initiated steroid signaling (NISS)- classic action via estrogen response elements (ERE) and non-classic action via AP-1, SP1 and other transcription factors; and membrane initiated steroid signaling (MISS) (Figure 2) (9–10). The complexity of how ER expression and signaling affects carcinogenesis is summarized in Table 2 and reviewed in (11). In the normal mammary gland ER-positive cells are fewer and do not proliferate, instead they produce paracrine growth factors that signal proliferation in adjacent cells (Reviewed in (12–13). During primary ER+ neoplasia, many proliferating cells are ER+ and possibly convert paracrine to autocrine growth signaling. Over 70% of breast tumors diagnosed are ER+ (14). ER+ status is a key diagnostic criterion for the choice of AE or aromatase inhibitor (AI) treatment in patients. Many AE-treated tumors retain their ER expression during recurrence, but signaling through the ER pathway is altered in these resistant tumors (10, 14). Thus, it is clear that targeting this pathway using berry-polyphenols may affect the development of both primary and secondary cancers.
Figure 2. A simplified schematic of the influence of berry polyphenols on cell-signaling pathways in breast cancer.
This simplified schematic shows the effect of berry compounds on cell-signaling pathways involved in breast cancer development.
 indicates inhibition;
indicates inhibition;
 indicates activation and
indicates activation and
 indicates that the interaction needs to be explored further.
indicates that the interaction needs to be explored further.
Table 2.
Effect of Estrogen Receptor Signaling on Breast Carcinogenesis
| ER signaling component | Mechanism by which it affects carcinogenesis | Effect of berry polyphenols | Reference |
|---|---|---|---|
| Type of ER (α or β) | Expression pattern in tumors ERα/ERβ ratio |
Some bind more avidly to ERβ than to α (Table 3) | (11, 131–132) |
| Mutant forms of ER | Altered ligand and DNA binding | NR | (11, 133) |
| Coactivators | Increased expression | NR | (134–135) |
| Corepressors | Decreased expression | NR | (29, 136) |
| Phosphorylation | Can cause constitutive activation of ER | NR |
NR- Not reported in literature to the best of our knowledge.
Effect of berry-polyphenols on ER-signaling
Several berry phytochemicals interact with the ER, Table 3 summarizes these interactions. It is currently accepted that ERα plays a pro-proliferative role and is involved in the growth-promoting actions, while ERβ may counteract its effects (15–17). Most dietary polyphenols bind to these receptors with 100- to 1000-fold weaker affinity than estrogen (18–20). Studies presented in Table 3 were performed using either direct ligand binding or ERE-reporter assays, which measure the ability of phytochemicals to bind to either ERα or β and cause downstream effects. Polyphenols do not bind equally avidly to both receptors and often show preference for one or the other. In ligand binding assays, quercetin and kaempferol have a higher affinity for ERβ (21–23), whereas resveratrol and enterolactone have a higher affinity for ERα (24–25). In breast cancer cells, anthocyanidins- cyanidin, delphinidin and pelargonidin, bind to ERs and significantly reduce 17β estradiol (E2)-induced ERE-luciferase expression acting like an antiestrogen (26). However, whether they preferentially bind to ERα or β is not reported. In HeLa cells transfected with either ERα or β, ellagic acid acts like an estrogen in ERα-transfected cells, but like an antiestrogen in ERβ cells (27). In MCF-7 cells, metabolites of ellagic acid- urolithins A and B bind avidly to ERα and β, respectively (28). This suggests that the effect of ellagic acid on the tissue may be dependent on the type of ER primarily expressed and also on the metabolite generated. Many of these phytochemicals are agonists of ER, in that they can induce cell proliferation in MCF-7 cells in the absence of E2. However in the presence of E2, they antagonize E2 action, thus acting as antiestrogens (28). In such in vitro studies, the dose used may have a specific effect on the outcome. For example, if a particular polyphenol is used at a very high concentration in culture, it could simply compete for the receptor and displace E2, the natural ligand, resulting in an antiestrogenic activity. Quercetin, kaempferol and resveratrol show distinct dose-dependent biphasic effects (Table 3). It has been postulated that since these compounds bind to ERβ with a greater affinity than ERα and their growth retarding effects are ERβ-mediated (21, 24). However, quercetin stimulates MCF7 cell-proliferation at 10 μM, whereas inhibits it significantly at 100 μM (22), supporting our theory of dose-dependent displacement of the natural ligand.
Table 3.
Interaction of berry polyphenols with ER signaling
| Polyphenol | ER binding | Assay type | Effect | Dose | Cell type | Expression changes | Reference |
|---|---|---|---|---|---|---|---|
| Cyanidin | Y | Ligand binding; ERE-Luciferase | Antiestrogenic | MCF-7 | ↓ ERβ expression | (26, 137) | |
| Delphinidin | Y | Antiestogenic | ↓ ERα expression | ||||
| Pelargonidin | Y | Antiestrogenic | - | ||||
| Quercetin | Y (ERβ≫E Rα) | Estrogenic/Antiestrogenic | Biphasic effects | MCF-7 MDA-MB-231 |
↑ ER α and β mRNA | (21–22, 138) | |
| Kaempferol | Y (ERβ≫E Rα) | Ligand binding; Yeast transactivation | Estrogenic/Antiestrogenic | Biphasic effects | MCF-7 | ↓ ERα mRNA and protein ↓ PGR, Cyclin D1 and IRS-1 |
(21, 23, 139) |
| Resveratrol | Y (ERα > ERβ) | ERE-Luciferase | Estrogenic/Antiestrogenic | Biphasic effects | Ishikawa cells with stable ERs; MCF-7 |
↓ ERα by proteasomal degradation ↓ Cathepsin D and pS-2 Exhibits biphasic effects on EGFR-ER cross talk via dose-dependant induction of AKT |
(24, 140) |
| Ellagic Acid | Y | ERE-Luciferase | Estrogenic (via ERα)/Antiestrogenic (via ERβ) | HeLa | ↑ IGFBP3 levels similar to ICI 182,780 Co-treatment with ICI abrogates EA effect |
(27) | |
| Urolithin Aa | Y (ERα≫E Rβ) | Ligand binding assay | Antiestrogenic | MCF-7 | (28) | ||
| Urolithin Ba | Y (ERβ≫E Rα) | Antiestrogenic | |||||
| Enterolactonea | Y (ERα≫E Rβ) | ERE-Luciferase | Partial agonist | Ishikawa cells with stable ERs; MCF7; HeLa |
↑ ERβ ↓ E2-induced VEGF |
(24–25, 85) | |
Cell line origins: Breast -MCF-7(ER+), MDA-MB-231 (ER−); cervical-HeLa (ER−); endometrial-Ishikawa (ER−)
Urolithins A and B are gut metabolites of Ellagic acid and enterolactone is a gut metabolite of lignans.
ER- estrogen receptor; ERE- estrogen receptor response elements; PGR- progesterone receptor; IRS1- insulin receptor substrate 1; IGFBP3- insulin like growth factor binding protein-3; VEGF- vascular endothelial growth factors
There is evidence to suggest than many of these phytochemicals act as selective estrogen receptor modulators (SERM) similar to Tamoxifen (TAM) and its active metabolite 4-hydroxy tamoxifen (4-OHT). TAM/4OHT can act as an agonist in the absence of E2 and an antagonist in its presence (14). Further, similar to 4OHT, these compounds bind to ERβ with a higher affinity than to ERα (29). In addition, several berry phytochemicals can act as pure agonists in cell types other than mammary epithelial cells (27, 30–31). Many studies have blocked ER-activity using either 4-OHT or ICI 182,780 (ICI; an ER degrading AE) to show that the effect of these phytochemicals is ER-mediated (26–27, 32). However, data on whether these compounds antagonize, synergize or potentiate the effects of AE in breast cancer cells is very limited. Our preliminary data show that the ellagitannin- punicalagin and ellagic acid cause a synergistic cytotoxicity in combination with subtoxic levels of 4OHT and ICI in MCF-7 cells (Woode and Aiyer, unpublished data). We know of no such information for other berry compounds or their respective metabolites.
Coregulators are molecules that either potentiate (coactivator) or repress (corepressors) the transcriptional activity of the steroid receptors. Very little data is available on whether polyphenols modulate the selective recruitment of coregulators to ER α or β. It can be inferred from Klinge et al., (33) that the estrogenic action of resveratrol requires co-regulator recruitment since resveratrol fails to elicit a response in a yeast-expression system but shows a dose-dependent activation of ERβ in mammalian cells. Indeed, resveratrol does differentially recruit ER co-activators SRC-1 and GRIP-1 to either ERα or β depending on the dose used. At a dose of 1–10μM, it preferentially recruits SRC-1 to ERβ (24). This effect is not seen at 100 μM at which dose it doesn’t recruit either coactivator to ERα or β. At this dose, it simply acts as an ER-antagonist. Such results underscore the importance of using physiological concentrations in culture to glean mechanistic insights. Regardless, the effect of berry polyphenols on differential recruitment of coregulators to the ER is an area that still needs much research.
The effect of berry polyphenols on ER-signaling is a sum of several mechanisms such as direct interaction with receptor, specificity for receptor isoform (α or β), competition with ligand for receptor binding and differential recruitment of coregulators. The mechanism likely varies depending on the cell type, dose and the polyphenol involved. Given that pharmaceutical agents used for endocrine therapy of breast cancer such as TAM and ICI often utilize similar mechanisms, it is important to study the drug-nutrient interaction and how this may affect the outcome of treatment.
Role of Growth Factor Receptor (GFR) signaling in breast cancer cells
Membrane growth factors are activated by extracellular ligands and activate targets through phosphorylation of upstream kinases, which then leads to activation of downstream transcription factors. One of their functions during normal development is to stimulate cell-proliferation in response to external growth factors (12). In breast cancer, growth factor receptors (GFR) become overactivated via gene amplification, chromosome translocation and mutations, leading to a constant stimulation of cell-proliferation. Although many members of GFR signaling affect mammary tumorigenesis, the epidermal growth factor receptor (EGFR) family in particular is discussed here. The EGFR family consists of- EGFR (ErbB1), HER2/ErbB2, HER3/ErbB3, and HER4/ErbB4 and the activation of downstream targets depends on the dimerization of any two members of the family in response to ligand binding. Expression of EGFR is increased in 30–60% of triple-negative (ER−, PR− and HER2-negative) breast cancers (12). Growth factor signaling induces the non-genomic activation of the ER pathway by phosphorylation of both the ER as well as its co-factors (34–35). This cross-talk between GFR and ER pathways, facilitated via the kinase signaling cascade is thought to play an important role in conferring TAM-resistance. Inhibition of this cross-talk using the EGFR-inhibitor Gefitinib, abolishes the stimulatory effect of TAM on HER2 overexpressing cells (36).
Effect of berry-polyphenols on GFR signaling
Berry polyphenols inhibit the tyrosine kinase activity of EGFR and HER2 leading to reduced autophosphorylation, a key step in receptor activation (Table 4). The inhibition of this step leads to reduced phosphorylation of several kinases such as Phosphotydyl Inositol-3-phosphate Kinase/Protein Kinase B (PI3K/AKT) and Extracellular signal Regulated Kinase (ERK)/Mitogen Activated Protein Kinase (MAPK). Table 4 summarizes the effect of various berry polyphenols on EGFR, HER2 and their downstream targets. Polyphenols can interfere with TKR action by two possible mechanisms- inhibiting tyrosine kinase activity and preventing the dimerization between the various EGFR family members. There is clear evidence that many berry polyphenols inhibit the tyrosine kinase activity of EGFR and HER2 purified from the cell membrane. This also occurs in intact cells, albeit at considerably higher doses (Table 4) (37–38). However, evidence of whether these polyphenols inhibit the dimerization of GFRs is not known. In a well-designed study Weinstein and co-workers show that the green tea polyphenol epigallocatechin gallate (EGCG) and extract polyphenon E can inhibit the EGF-induced dimerization of EGFR in HT29 cells by disruption of lipid-order in the plasma membrane (39). They also found that cyanidin and delphinidin (50μM) do not cause disruption of lipid-order, but their effect on EGFR dimerization was not tested. The authors report exposure times of 5 to 30m. Thus it is not clear whether plasma-membrane incorporation of EGCG plays any role in the observed effects. In a separate study, it has been shown that elderberry anthocyanins are incorporated into the plasma membrane of endothelial cells after 4h (40). It would be interesting to explore whether berry polyphenols inhibit the dimerization and subsequent activation of the TKR either by their ability to incorporate into the cell membrane or by direct binding to the receptors.
Table 4.
Berry polyphenol interaction with growth factor receptor signaling and its downstream effects
| Polyphenol | GFR type | Cell line | Dose | Effect observed | Reference |
|---|---|---|---|---|---|
| Cyanidin | EGFR | A431 (Human vulva carcinoma) | 0.8 μM | ↓ tyrosine kinase activity ↓ phosphorylation of ELK-1 and MAPK-1 |
(38) |
| HER2 | MDA-MB231 BT474 MCF-7ErbB2 (ethanol mediated) |
10–40 μM | ↓ cell migration ↓ autophosphorylation ↓ phosphorylation of FAK and p130cas ↓ association of FAK and p130cas to ErbB2 |
(141) | |
| Delphinidin | EGFR | AU565 MCF-10 A |
40 μM | ↓ autophosphorylation ↓ phosphorylation of AKT, ERK1/2, JNK1/2/P38 |
(142) |
| A431 | 1.3 μM | ↓ tyrosine kinase activity | (38) | ||
| HER2 | SKBR3 | 12.5–100 μM | ↓ autophosphorylation ↓ phosporylation of ERK1/2 ≫ AKT |
(41) | |
| Quercetin | EGFR | HT29 (Colon carcinoma) | 0.5–10μM | ↓ autophosphorylation ↓ phosphorylation of ERK1/2 |
(143) |
| HER2 | SKBR3 MCF7 |
100–200 μM | ↓ tyrosine kinase activity ↑ ubiquitination ↓ expression ↓ phosphorylation of PI3K and AKT |
(144) | |
| Ellagic Acid | EGFR | HT29 | 65nM 100 μM |
↓ tyrosine kinase activity ↔ autophosphorylation ↓ cell growth |
(37) |
| Resveratrol | EGFR | MDA-MB-231 | 50 μM | ↓ phosphorylation of FAK ↓ cell migration and invasion |
(145) |
| HER2 | MCF7 (Heregulin B1 induced) | 2–10μM | ↔ autophosphorylation ↓ phosphorylation of ERK1/2 ↓ ERK-mediated MMP9 activation |
(146) | |
| Pterostilbene | HER2/3 | MCF7 (Heregulin B1 induced) | 5–20 μM | ↓ phosphorylation of AKT and p38 ↓ MMP-9 ↔ phosphorylation of ERK1/2 |
(147) |
| Procyanidins | EGFR | HT29 | 5–50 μM | ↓ tyrosine kinase activity ↓ autpphosphorylation |
(148) |
Abbreviations: ELK-1- E twenty-six (ETS)-like transcription factor 1; MAPK-1- Mitogen activated protein kinase 1; FAK- focal adhesion kinase; p130cas- Crk-associated protein; ERK- extracellular signal regulated kinase; JNK- c-Jun N-terminal kinase; PI3K- phosphatydylinositol-3-phosphate kinase; MMP- matrix metalloproteinase.
Many studies presented in Table 4 have been conducted in cell lines that overexpress EGFR or HER2 with or without ER. However, the cross-talk between EGFR activation and ER-phosphorylation has rarely been explored in these. With respect to TAM co-treatment, it is unknown whether any of the selected berry-compounds selectively potentiate or inhibit its effects and how this may affect the development of TAM-resistance. Co-treatment of BT474 or SKBR cells with delphinidin (6–24μg/ml) significantly reduces the effect of HER2-inhibitors Herceptin and Lapatinib (41). Although the authors report significant inhibition of HER2-signaling in these cells, they do not mention whether delphinidin actually binds to the HER2 receptor. So it can only be speculated that the reduced efficacy of the drugs is due to the binding of HER2 receptor by delphinidin, but the exact mechanism is unknown.
It is clear that many berry polyphenols can inhibit the tyrosine kinase signaling component of the EGFR family of TKRs. Constitutive activation of TKR and the subsequent activation of ER through phosphorylation are involved in the development of TAM-resistant recurrent tumors (42). However, very little is known about whether the presence of one or more polyphenols can inhibit the development of TAM-resistance and whether their effect on EGFR/HER2 pathway plays a significant role in this. More research is warranted to understand the mechanisms by which berry-polyphenols affect EGFR/HER2 pathway and its cross-talk with ER-signaling, both in the presence and absence of TAM.
Evasion of cell death mechanisms in breast cancer
Programmed cell death in cancer cells can be classified into at least three- apoptosis, autophagy and necroptosis (43–44). They play important and defined roles in the normal development during ductal elongation, alveolar development and especially during the post-lactional involution of mammary gland (45–46). The balance between survival and death in breast epithelial cells is determined by induction of one or more of these mechanisms by various signaling molecules (Figure 2). The two mechanisms pertaining to breast cancer development- apoptosis and autophagy are discussed herein. While apoptosis has been studied extensively, autophagy is a relatively new field, whose possible correlation is being explored further. In primary breast cancer, cancer cells evade apoptosis to survive. Resistance to apoptosis caused by increased expression of anti-apoptotic and/or reduced expression of pro-apoptotic molecules is often seen in breast cancer. Induction of apoptosis by a drug leads to reduction in cell survival and tumor growth (47–48). Autophagy is a mechanism of both cell-survival and cell-death and its role and regulation are of significant interest. The Beclin 1 (BECN1) gene, a mediator of autophagy, is deleted in 50% of breast tumors. In primary breast cancer cells resistant to apoptosis, induction of autophagy results in cell-death and heterozygous knockout of BECN1 gene leads to accelerated malignancy (49). On the other hand, recent studies point to a role for autophagy in AE-resistance, wherein inhibition of autophagy leads to sensitization of resistant cells to AE and subsequent cell death (50–52). Thus, autophagy may play a dual role and whether the induction of autophagy is pro- or anti-cancer is dependent on the cellular context and the stage of cancer development (51).
Kinases act as connectors of upstream signaling to downstream transcription factors and are central to modulating the effects of growth factors, hormones, and cytokines on the breast epithelium. They are key players in determining the cell’s decision to live or die. The ERK/MAPK and PI3K/AKT pathways play an important role in linking the GFR and ER− signaling. Kinase signaling is significantly altered during breast cancer development and its inhibition can reduce cancer cell growth (53). Ultimately the kinases converge on to key transcription regulators, Jun/Fos for the MAPK and mTOR for the PI3K (Figure 2). The function of transcription regulators in a cancer cell is to overcome self-limiting growth and evade cell-death. Evasion of apoptosis by increased expression of anti-apoptotic/pro-proliferative molecules (e.g. BCL2, BCLxl, BCLw, Cyclins A, B, D) and down regulation of pro-apoptotic/anti-proliferative molecules (BAD, BAX, BIK, p53) is commonly seen in cancer cells.
Effect of berry-polyphenols on cell-death
If the goal of the cancer cell is to avoid cell-death mechanisms, then the objective of a chemopreventive agent is to enforce these. Data summarized in Table 5 suggests that many berry compounds can induce both apoptosis and autophagy. Although all of the studies have not been done in breast cancer cells, the aim of this section is to highlight the mechanisms by which berry compounds cause cell-death. Since autophagic cell-death has not been extensively studied for the selected berry polyphenols in breast cancer cells, evidence from other cell-lines is presented. Several mechanisms have been proposed regarding the apoptotic effect of these compounds, while ROS generation has been suggested for compounds including delphinidin, kaempferol and resveratrol; caspase- and p53-dependent apoptosis can be induced by other compounds (Table 5). Regardless of the mechanisms by which these compounds induce cell-death, kinase signaling is involved in most cases, except for cyanidin and quercetin (Table 5). Although a likely mechanism is the inhibition of TKRs and the subsequent phosphorylation of downstream kinases (Table 3), it appears that in some cases the opposite is true. For example, delphinidin-induced apoptosis in HepG2 cells involves increased p-JNK (54); and resveratrol and kaempferol induce apoptosis in breast cancer cells by sustained ERK1/2 activation (55–56). Berry compounds also induce autophagy by different mechanisms (Table 5). AE-resistant cells may utilize autophagy as a mechanism of cell-survival (51, 57). Feng et al., show that although delphindin has no effect on HCC cells at 24h, sustained induction of autophagy ultimately results in reduced cell-survival at 120h (58). Pterostilbene, a blueberry stilbene, induces both apoptosis and autophagy in drug-resistant bladder cancer cells (59). Since, the utilization of autophagy for cell-survival is highly dependent on cellular context, it is imperative to explore the effects of berry compounds on autophagy and how the interplay affects the development of drug-resistance in breast cancer cells.
Table 5.
Control of kinase signaling by berry polyphenols and effects on cell cycle, apoptosis and autophagy
| Polyphenol | Cell line/Dose | Cell cycle arrest | Cell death pathway | Markers | Proposed mechanisms | Kinase pathway | Reference |
|---|---|---|---|---|---|---|---|
| Cyanidin | HS578T/10μM | G2/M | Apoptosis | ↓ CDK1, CDK2, cyclin B, cyclin D PARP cleavage | Caspase-dependant | - | (149) |
| Delphinidin | SMMC7721/150μM | Autophagy | ↑ LC3 II. Inhibited by 3MA and Bafilomysin. No ↑ in caspase activity. | No ER stress. | Class III PI3K | (58) | |
| AU565/40μM | Apoptosis | ↑ PARP cleavage ↓ BCL2, ↑ BAX |
Inhibition of EGFR signaling | PI3K/AKT MAPK |
(142) | ||
| Hep G2/100μM | Apoptosis | ↑ caspase 3, PARP cleavage ↓ BCL2, ↑ BAX |
Oxidative stress mediated | JNK ↑ cJun mRNA; ↑ p-JNK | (54) | ||
| PC3/180 μM | G2/M | Apoptosis | ↑ caspases, PARP cleavage, BAX, p21, p27 ↓ BCL2 |
Inhibition of NFkB signaling | IKK⌈ (↓ p-IKK) | (150) | |
| Quercetin | MCF-7/150μM | S | Apoptosis | ↓ BCL2, Cyclin A, B, procaspase 8,12 and CDK2 ↑ PERK, GRP78, CHOP/GADD153, p53 and p57 ↓ mitochondrial membrane potential ↑ Caspases 6,8,9 |
ER stress and UPR ↑ Ca2+ influx, ↔ ROS |
- | (151) |
| MCF-10A, MDA-MB-231/100μM | G1 | Apoptosis | ↑ p-p53, p21 ↓ BCL-xl, cyclin B1 ↔ PTEN, Cyclin D, p27, cdc2 and p-cdc2 |
P53 dependant | - | (152) | |
| AGS, MKN28 | Autophagy | ↑ LC3 II, AVO | Beclin-1 mediated, HIF1-α | AKT/mTOR | (153) | ||
| Kaempferol | MCF-7, MDA-MB-231/30μM | G1 | Apoptosis | ↑ PARP cleavage ↑ MEK1 and p-ELK1 |
ROS mediated | ERK1/2 (sustained activation) | (55) |
| Ellagic acid (Urolithins) | Caco-2/10μM | G1 | Apoptosis | ↓ cyclins A and B, BCL-xl ↑ Cyclin E |
Fas-dependent Caspase 8-independent, mitochondrial membrane mediated | ERK1/2 (↑ activation) | (154–155) |
| Resveratrol | MDA-MB-231/50μM | Apoptosis | ↓ BCL2 ↔ BAX, BAD, BCL-xl JNK and p38 inhibition |
MEK1/2 mediated | ERK1/2 (sustained activation) | (56) | |
| MCF-7/150μM | Apoptosis | ↓ BCL2 ↔ caspase 8 activity ↔ PARP cleavage ↓Δ ψm |
ROS mediated NFkB dependent |
PI3K | (140) | ||
| MCF-7vc and MCF-7casp3/64μM | Autophagy/Apoptosis | Cell death in MCF-7vc ≫MCF-7Cap3 Caspase expression ↓ autophagy LC3 II and GFP-LC3 not ↓ by BECN1 or hvP534 knockdown |
Non-canonical BECN1 – independent atg7-dependent | AKT/mTOR (↓ p-AKT; inhibition of mTOR) | (156) | ||
| Pterostilbene | T24 and T24R/100μM) | S (50–75μM) G1 (100μM) |
Apoptosis/Autophagy | ↓ Cyclins A, B, D1, p-Rb ↓ caspase 3 activity ↑ LC3 II, AVO ↑ BCL2. BCL-xl ↔ BAX, BAD |
BECN1-dependent (3MA and BECN1 shRNA inhibit action) | AKT/mTOR (↓ p-AKT/mTOR) ERK 1/2 activation |
(59) |
Cell line origins: Breast- AU565, HS578T, MCF-10A, MCF-7, MDA-MB-231, MCF-7vc, MCF-7casp8; Liver- SMMC7721, HepG2; Gastric cancer- AGS, MKN28; Prostate- PC3; bladder- T24, T24R; Colon- Caco-2.
Abbreviations: CDK- cyclin dependent kinase; PARP- Poly (ADP-ribose) polymerase; BCL2- B-cell lymphoma 2; BAX- Bcl-2 associated X protein; BAD- Bcl-2 associated death promoter; PERK- Protein kinase RNA-like endoplasmic reticulum kinase; GRP78- glucose regulated protein 78 (also called- BiP or HSPA5); CHOP- ccaat enhancer binding protein homologous protein; PTEN- phosphatase and tensin homolog; cdc- cell division cycle; LC3- microtubule associated protein light chain 3; AVO- autophagic vacuoles; ELK- E twenty-six (ETS)-like transcription factor 1
Limitations of in vitro models to study breast cancer prevention
In vitro models available to study breast cancer usually consists of cancer-cell lines that are derived from tumors of varied origins and kept in culture continuously. Although these models are helpful in describing the molecular mechanisms of breast cancer and in the discovery of drugs used to effectively target particular pathways, they fall short when it comes to studying cancer prevention. The reason is that cancer cell-lines are already transformed and thus do not represent a typical population for “primary prevention”. Immortalized normal cells can be cultured with physiological concentrations of polyphenols for several passages and then tested for transformation upon challenge with a carcinogen. Although this would simulate primary prevention, it must be kept in mind that even these “normal” cells have altered their molecular behavior to adapt to the culture conditions and hence are poor substitutes for mimicking the real nature of the developing breast epithelium. In vitro studies also do not take into account interaction between various cell-types in the breast. Various 3-D cultures have been used to mimic the stromal-epithelial interactions (60–61). However, we are not aware of any study that has used a 3-D culture to study berry-polyphenols.
Another limitation of many in vitro studies reported is their use of concentrations of pure compounds in the micromolar range (Tables 3–5). Yet, it is clear that the actual plasma concentrations of many of these agents are in the nanomolar range (Table 1). Thus the doses used in these studies largely limit their usefulness in the translational setting. The primary paradigm of research with bioactive compounds has been to find agents that “kill” cancer cells, regardless of the concentration at which this occurs. It is easy to understand that killing a cancer cell is not the same as preventing cancer development. Nonetheless, this flawed concept has often supported the use of high in vitro concentrations. Further, due to their relatively non-toxic nature the maximum tolerated dose (MTD) for many food bioactive are high in rodents (62). This has in turn supported the use of high doses of a purified compound, rather than a natural food source, in animal models. The culmination of this paradigm is evident in the clinical outcome of the ATBC trial, wherein supra-physiological doses of vitamins A (30 mg) and E (25, 000 IU) caused an increase in lung-cancer incidence in an at-risk population (63). This has led to a critical rethinking of our approach to food and food component research (64–66). Thus, currently there is a heightened awareness among scientists to use more physiologically relevant concentrations and an appreciation for the synergy among food components as well as the effects of a food matrix.
Berry extracts form an intermediate system between testing individual berry compounds in vitro and whole berries in vivo. Typically, alcohol extracts enriched in berry polyphenolics have been tested on various cancer cell lines to assess their effects in reducing cell-proliferation. The IC50 for these extracts for various cell-lines range from 27μg/ml to 4 mg/ml (67–68). Studies show that extracts reduce cell proliferation using similar mechanisms of action as pure compounds but at a much lower concentration (69–71). Extracts also may account for the synergistic action of different compounds as present in the food matrix. Seeram et al., show that extracts and fruit juices achieve significantly higher anti-proliferative effects on various cancer cells than when their individual components are used (72–73).
Effect of metabolites on the biological activity of berries
The complexity of studying berry phytochemicals is further increased by the presence of many metabolites. The metabolism of polyphenols plays an important role in their bioavailability. Also the gut (gastric, intestinal and colonic) and liver metabolites affect the effectiveness of these compounds in vivo. Gut metabolites of ellagic acid- urolithins A and B, interact with the ER-signaling in very different ways (Table 3). The anthocyanidins are conjugated with many types of sugar moieties to form anthocyanins. This can influence both the gut metabolism and absorption of these compounds (74). Furthermore, the type and quantity of metabolites generated varies widely depending on the gut microbiota and the gene polymorphisms of the consumer. Only a few of these metabolites have been discovered. Thus, an in vitro study reporting the effects of a pure berry-compound will provide only a partial picture of its full potential. The best way to take into account the contribution of metabolites is to use in vivo approaches.
In vivo Models for studying breast cancer prevention
Animal models are necessary tools for understanding the effects of both pure compounds and whole berries, given through the diet, on mammary tumorigenesis. Models currently being used consists of 7,12- dimethyl benze(a)anthrazene (DMBA)-induced tumors in Sprague Dawley (SD) rats, E2-induced tumors in August-Copenhagen-Irish hooded (ACI) rats, transgenic mice containing specific gene alterations and xenograft of human breast cancer cells in immunodeficient mice (75–78). Although they provide a better system for evaluating the preventive aspects of dietary components, certain key points have to be kept in mind regarding the use of these in vivo models in chemoprevention studies. First, in rat models of mammary tumorigenesis, it is important to deliver the initiating insult (DMBA or E2) to the terminal end buds, which are the most susceptible structures, at a specific moment of glandular development. In the DMBA model, this is a very narrow period (ca. postnatal day 50 ± 2) beyond which tumor incidence is greatly altered. In ACI rats, this window is slightly larger (7–8 weeks), however dramatic differences in tumor volume can still be seen when E2 implantation is done at 7 versus 9 weeks. Dietary phytochemicals fed prior to this window (as typically done in prevention-intervention) can alter mammary gland development and thus potentially change this window. Second, breast cancer xenografts cannot be used for prevention studies since they assess the growth retardation of already tumorigenic cells and studies in transgenic mice usually focus on a single gene or molecular pathway. Third, the bioavailability of various berry polyphenols changes depending on the species used (79–80). These factors may influence the data derived from rodent studies and the investigators must be aware of these when they are interpreting chemoprevention data.
Berries in the prevention of primary mammary tumors in vivo
Individual berry-compounds and whole berries have been used in animal models to illustrate their effect in preventing or reducing tumor growth. Pterostilbene (10 μM) reduces the formation of DMBA-induced mammary transformation in an ex-vivo organ culture (81). Lariciresinol (LR) is a precursor of secoisolarisiresinol, a lignan found in some berries (82). Saarinen et al., treated rats with 3 or 15 mg/kg of LR ten weeks after mammary tumors were induced with DMBA. While there was no reduction in incidence or multiplicity of these already established tumors, LR significantly reduced tumor growth and surface area after 9 weeks of administration (83). Dietary LR (100 mg/kg) also reduces the growth of orthotopic MCF-7 tumors in nude mice (84) (85). Quercetin shows a dose dependant reduction of 30–50%, of DMBA-induced mammary tumors, at dietary doses of 2 and 5%, respectively (86). However, another study in the same model with a similar treatment protocol showed no effect of a 2% diet (87). In a recent study, Singh et al., show that a 2.5% quercetin in the diet can increase E2-induced mammary tumors by inhibition of the phase II P450 enzyme catechol-O-methyl transferase (COMT) that detoxifies harmful E2-metabolites (88). These authors clearly show that both phase I and II metabolism of E2 play an important role in the development of E2-induced mammary tumors in ACI rats (89). However, the dose of 2.5%, translating to 25 g/kg diet is relatively high. Considering the fact that the highest amount of quercetin to be found in any berry species is only 158 mg/kg of bog whortleberry (90), such doses are unachievable using natural berry sources. This also underscores the importance of using biologically relevant doses in animal models. Although individual compounds are effective at varying levels in reduction of mammary tumor indices, the question of the food-matrix effect remains. Whole-berries (blueberry and black raspberry) supplemented at 2.5% (w/w) dose inhibit E2-induced mammary tumorigenesis in ACI rats (91–93). The interest in berries as a chemopreventive agent stemmed from the pioneering work of Gary Stoner and colleagues in using berries for prevention of esophageal cancer (94–96). When these studies were initiated, whole-berries had not been tested in any cancer other than GI-tract cancers. Nevertheless, sound scientific and translational reasoning lay behind the choice of the type of berries, their respective doses, and the pre-clinical animal model used.
In initial studies, ellagic acid showed a dose-dependent decrease in 4-hydroxy estradiol-mediated DNA damage in vitro as well as a significant up-regulation of DNA–damage repair genes in vivo (97). Black raspberries had been tested successfully against carcinogen-induced esophageal tumors (98) and are an excellent source of ellagic acid (>1500 ppm) and anthocyanins (≈ 7000 ppm) primarily in the form of cyanidin-glycosides (76). On the other hand, blueberry contains moderate levels of anthocyanins (≈ 4000 ppm) derived from 5 different anthocyanidins – delphinidin, malvidin, petunidin, peonidin and cyanidin but almost no ellagic acid (<100 ppm). They are also cultivated at a much larger scale than black raspberries and are more easily available to the consumers (99). To further delineate the chemopreventive effects of anthocyanins versus ellagic acid, a group of rats were fed ellagic acid (400 ppm) alone. The equivalent concentration of ellagic acid in 2.5% (w/w) black raspberry diet is 80 ppm, thus the ellagic acid only diet also compares the effectiveness of delivering a whole food matrix versus individual components. A dose of 2.5% (w/w) for the berries was selected to enable translation to reasonably achievable human doses and is the lowest dose of berries to be tested in an animal model to date. With regard to the animal model, ACI rats implanted subcutaneously with silastic implants containing E2 develop mammary adenocarcinoma in 6–8 months (93, 100–101). The plasma levels of E2 and progesterone (Pg), in these animals ranges between 35–230 pg/ml and 35 pg/ml, compared to 15–53 pg/ml and 35 pg/ml, respectively, in untreated rats (93, 100, 102). In premenopausal women, the E2 levels range between 20–1500 pg/ml depending on the ovulatory phase, while the progesterone levels are 1–9 ng/ml during ovulation with a very wide inter-individual variation (103). Since higher circulating levels of these steroid hormones are linked to increased breast cancer risk (104–105), this pre-clinical model is to some extent clinically relevant. Further, the combination of low-dose dietary berries and high-dose E2 provides a higher translational quotient.
Dietary black raspberry, blueberry (2.5% w/w) and ellagic acid (400 ppm), significantly reduce E2-induced mammary tumor indices such as tumor latency, incidence, volume, multiplicity and mortality to varying extents in the ACI rat model. The data from three individual tumorigenesis studies summarized in Table 6 show that these berries are consistently effective in the prevention of mammary tumors in this animal model. Black raspberry is the most effective in reducing tumor indices, followed by ellagic acid then blueberry diet. However, in studies 1 and 2, where mortality due to pituitary-hyperplasia was higher, blueberry significantly reduced this mortality (93). Berry diets decrease E2-induced mammary cell-proliferation in these rats (106), which may either be due to the promotion of cell-death or due to an antiestrogenic effect. There is evidence for the latter since both dietary berries and ellagic acid counteract the E2-induced increase in the uterine weight (76). Of interest pertaining to the previous discussion of quercetin, Aiyer and Gupta show that dietary berries and ellagic acid decrease the expression of COMT in the mammary epithelium at 18w after E2-treatment but not at 6w. This temporal effect is linked to the expression of CYP1A1, an enzyme whose product (2-hydroxy E2) is the substrate for COMT. CYP1A1 expression is significantly downregulated by berries at 6w (91). Even though COMT expression is reduced, there is no increase in tumor indices as reported by the previous authors (88, 91). This further highlights the importance of the food-matrix as well as using a physiologically relevant dose. Berries also reverse E2-induced hepatic oxidative DNA damage (107). However, the effect of dietary berries on hepatic enzymes involved in E2 metabolism has not been explored yet.
Table 6.
Summary of three in vivo studies performed with berry-intervention in ACI rats
| Serum E2 level (pg/ml) | Implant size (E2 dose mg) | Length of treatment (wks) | % reduction compared to control diet | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5% Black raspberry dieta | 2.5% Blueberry diet | 400 ppm Ellagic acid diet | Reference | ||||||||||
| V | M | Burden | V | M | Burden | V | M | Burden | |||||
| Study 1a | NA | 30 mm (27 mg) | 24 | 76 | 33 | 60 | 70 | 0 | 84 | 37a | 0 | 67a | (92) |
| Study 2 | 236 ± 24 | 30 mm (27 mg) | 24 | 69 | 40 | 67 | 40 | 0 | 81 | 75 | 43 | 70 | (93) |
| Study 3 | 200 ± 44 | 12 mm (9 mg) | 32 | 56 | 41 | 41 | 46 | 38 | 43 | 45 | 38 | 47 | (91, 100) |
V- Tumor volume (mm3); M- Tumor multiplicity; Burden= tumor volume/multiplicity; NA- not available.
Study 1 was a pilot study, some data for ellagic acid group is missing and hence the true effect of intervention cannot be clearly assessed. Also, the first column is 2.5% mixed-berry diet (0.5% each of blackberry, black-raspberry, blueberry, red-raspberry and strawberry) instead of 2.5% black raspberry diet.
More recently, Wu et al., have shown that maternal exposure to 5% blueberry diets significantly affects both mammary branching and the size of terminal end buds in pups. This is indicative of a reduced mammary tumor susceptibility (108) (109). However, these investigators have not yet reported mammary tumor incidences in these pups. In another study, blueberry juice (100μL/day; gavage) significantly inhibited the growth of MDA-MB-231 tumors in nude mice mediated by the inhibition of PI3K/AKT pathway (70). In a follow up study, a 5 and 10%, blueberry diet reduced the volume of orthotopic MDA-MB-231 tumors by 75 and 60%, respectively, suggesting that a lower dose is more effective. Inhibition of Wnt-signaling is involved in the tumor retardation (110).
These data collectively show that whole berries and berry constituents have significant preventive effect on development of mammary tumors in pre-clinical animal models. Allometric conversion to human equivalents from the rodent studies suggests that a daily intake of as little as 1–2 cups fresh berries may provide significant benefits (76) (110). These preclinical studies offer a strong evidence to pursue a pilot clinical trial among women at a high-risk to develop breast cancer. Although long-term follow-ups through the course of a life-time are not feasible, changes in biomarkers after short- to medium-term exposures can be assessed. The period between initial diagnostic core-biopsy and the surgical removal of tumor has been suggested as a potential window to evaluate the effects of chemopreventive agents on breast tissue biomarkers (111). This window can be effectively used to assess the effect of different types of berries on biomarkers of breast cancer risk.
Perspective-Berries in the prevention of breast cancer
To put the information gathered from the interactions of berry-phenolics with cell-signaling pathways in context of breast cancer prevention, we will consider the three “windows” of prevention available during a woman’s lifetime (84). The primary prevention window occurs during in utero to peri-pubertal period. Epigenetic modification in the mammary gland during this developmental period has an effect on breast cancer risk (112–115). Further, exposure to dietary phytoestrogens during this developmental period affects breast cancer risk (116). The only support for effectiveness of berries in this prevention phase comes from a study showing that in utero blueberry diet reduces the number of terminal end buds, the glandular structures most susceptible to transformation by a carcinogen (109). Epigenetic regulation is a possible mechanism by which this is accomplished. At present only ellagic acid has been shown to inhibit DNA methyltransferase, an enzyme involved in the methylation of DNA and hence cause epigenetic modifications, in MCF7 cells (117). Data on the effect of other berry-polyphenols in the epigenetic regulation of mammary gland development is yet to be explored. Although this may be a good approach to chemoprevention, it may not be directly applicable to the larger at-risk population that currently exists. More studies on the effects of dietary berry exposure at other stages of mammary gland development (in utero, pre-pubertal, peri-pubertal) are needed to assess which may be the best stage for preventive intervention.
The secondary prevention window occurs from adulthood to the first diagnosis of breast cancer. Much data discussed in this review pertain to this window. Although there is no direct evidence that berry-consumption throughout adulthood will prevent breast cancer incidence, the mechanistic action of berry-compounds provides support for this. The data from in vitro and in vivo studies suggest that berry-polyphenols act in an antiestrogenic fashion in the presence of E2 (Table 3) (76). Berry polyphenols inhibit GFR activation, which is also involved in the growth of primary tumors. These compounds also beneficially modify many other pathways involved in breast cancer development. Pre-clinical studies presented in the previous section provide evidence to support this. Thus consuming berries during adulthood could be protective for high-risk women (early menarche, late menopause, high premenopausal circulating E2). Clinical trials can be conducted in high-risk women and the changes in circulating E2 levels, antioxidant status, plasma and urinary polyphenols/metabolites, and other relevant biomarkers can be assessed as an indication of risk and benefit.
There is much less pre-clinical data available on whether berries will be effective during the tertiary prevention phase-the period after the breast cancer diagnosis, when a woman is on adjuvant or neo-adjuvant endocrine-therapy. This is the chemoprevention of recurrent breast cancer. So far an animal model that mimics endocrine-resistance development is not yet available to researchers. However, based on the signaling effects and what is known about AE-resistance development, one can speculate the effects of berry and its compounds in tertiary prevention of breast cancer. If these agents potentiate drug activity, perhaps a lower dose can be used leading to reduced drug side-effects and/or resistance. Finally, the cross-talk between GFR and ER signaling plays an important role in the development of AE-resistance. Inhibition of GFR/kinase signaling by berry polyphenols may reduce the development of resistance arising due to this crosstalk. The effect of polyphenols on the metabolism and clearance of tamoxifen is also an area that requires further research. However, these are only the speculated benefits of the berries. The effectiveness of berries in the tertiary prevention phase still needs to be explored. There is a paucity of data even at the in vitro level, since not many studies have focused on how berry polyphenols may affect AE-activity. This research is even more imperative since, in the internet era, much information is available to the public regarding the possible “beneficial” effects of these compounds that may or may not be substantiated by original research (118). The onus is on the scientific community to provide persuasive evidence for the protective effect of berries and berry polyphenols in prevention of recurrent breast cancer, especially when provided alongside routine endocrine therapies.
Berry bioactives have a high potential for breast cancer chemoprevention as they act on multiple pathways involved in carcinogenesis. Studies from preclinical animal models strongly support the preventive role of berries in primary breast cancer. Interaction with the ER− and TKR-signaling may play a role in this beneficial effect. However, their effect on other pathways such as the inflammatory- and angiogenic-signaling cannot be dismissed. Berries may also reduce tumor growth by promoting cell-death. Their role in inducing apoptotic cell-death is well studied. However, autophagic cell-death is emerging as an important mechanism by which a population of cells can survive drug-treatment and thus lead to drug-resistance. The study of how various berry polyphenols affect autophagy during the development of AE-resistance can illuminate their usefulness for the prevention of drug-resistance.
Finally, there is very limited data in the literature for the anthhocyanins and anthocyanidins. Considering that anthocyanins are most ubiquitous polyphenol present in berries and likely play an important role in the in vivo effects of whole berries, more research if required on how anthocyanins interact with and alter the various molecular pathways of breast cancer development.
With heightened media awareness regarding a healthy lifestyle, berries are fast becoming the go to super fruits for their health benefits. Based on the evidence presented, the case for using berries as preventive intervention in primary breast cancer is strong; whether this is true in recurrent cancer remains to be determined. There is much that needs to be explored before firm recommendations can be set forth for breast cancer survivors.
Acknowledgments
HSA is profoundly grateful to RC and LHC for providing an intellectually stimulating and collaborative environment to train in.
Financial Support
HSA is funded in part by a postdoctoral fellowship award 09A123 from the American Institute for Cancer Research, Washington D.C. and in part by the NIH award CA149147 to RC. DRW is a Sophomore majoring in Biochemistry at Columbia University, New York, NY.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011:144. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Bode AM, Dong Z. Cancer prevention research - then and now. Nat Rev Cancer. 2009:9. doi: 10.1038/nrc2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross SA. Evidence for the relationship between diet and cancer. Exp Oncol. 2010:32. [PubMed] [Google Scholar]
- 4.AICR. Prevent Breast Cancer. http://preventcancer.aicr.org/site/News2?page=NewsArticle&id=16505&printer_friendly=1.
- 5.Lewis N, Martinez LS, Freres DR, Schwartz JS, Armstrong K, Gray SW, Fraze T, Nagler RH, Bourgoin A, Hornik RC. Seeking Cancer-Related Information From Media and Family/Friends Increases Fruit and Vegetable Consumption Among Cancer Patients. Health Commun. 2011 doi: 10.1080/10410236.2011.586990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeram N, Berries P. In: Nutritional Oncology. Heber D, Blackburn G, Go V, Milner J, editors. Elsevier, Inc; Amsterdam, The Netherlands: 2006. pp. 615–628. [Google Scholar]
- 7.Veberic R, Jakopic J, Stampar F. Flavonols and anthocyanins of elderberry fruits (SAMBUCUS NIGRA L.) Acta Hort (ISHS) 2009:841. [Google Scholar]
- 8.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006:54. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 9.Acconcia F, Marino M. The Effects of 17beta-estradiol in Cancer are Mediated by Estrogen Receptor Signaling at the Plasma Membrane. Front Physiol. 2011:2. doi: 10.3389/fphys.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiff R, Massarweh SA, Shou J, Bharwani L, Arpino G, Rimawi M, Osborne CK. Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulators. Cancer Chemother Pharmacol. 2005;56(Suppl 1) doi: 10.1007/s00280-005-0108-2. [DOI] [PubMed] [Google Scholar]
- 11.Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O’Brien K, Wang Y, Hilakivi-Clarke LA. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003:22. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 12.Hynes NE, Watson CJ. Mammary gland growth factors: roles in normal development and in cancer. Cold Spring Harb Perspect Biol. 2010:2. doi: 10.1101/cshperspect.a003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo J, Hu YF, Silva ID, Russo IH. Cancer risk related to mammary gland structure and development. Microsc Res Tech. 2001:52. doi: 10.1002/1097-0029(20010115)52:2<204::AID-JEMT1006>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Clarke R, Skaar TC, Bouker KB, Davis N, Lee YR, Welch JN, Leonessa F. Molecular and pharmacological aspects of antiestrogen resistance. J Steroid Biochem Mol Biol. 2001:76. doi: 10.1016/s0960-0760(00)00193-x. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi SI, Eguchi H, Tanimoto K, Yoshida T, Omoto Y, Inoue A, Yoshida N, Yamaguchi Y. The expression and function of estrogen receptor alpha and beta in human breast cancer and its clinical application. Endocr Relat Cancer. 2003:10. doi: 10.1677/erc.0.0100193. [DOI] [PubMed] [Google Scholar]
- 16.Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S. Estrogen receptor (ER) beta1 and ERbetacx/beta2 inhibit ERalpha function differently in breast cancer cell line MCF7. Oncogene. 2003:22. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- 17.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011:11. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 18.Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000:141. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 19.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998:139. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt E, Dekant W, Stopper H. Assaying the estrogenicity of phytoestrogens in cells of different estrogen sensitive tissues. Toxicol In Vitro. 2001:15. doi: 10.1016/s0887-2333(01)00048-0. [DOI] [PubMed] [Google Scholar]
- 21.Moutsatsou P. The spectrum of phytoestrogens in nature: our knowledge is expanding. Hormones. 2007:6. [PubMed] [Google Scholar]
- 22.van der Woude H, ter Veld MGR, Jacobs N, van der Saag PT, Murk AJ, Rietjens IMCM. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Molecular nutrition & food research. 2005:49. doi: 10.1002/mnfr.200500036. [DOI] [PubMed] [Google Scholar]
- 23.Zoechling A, Reiter E, Eder R, Wendelin S, Liebner F, Jungbauer A. The Flavonoid Kaempferol Is Responsible for the Majority of Estrogenic Activity in Red Wine. Am J Enol Vitic. 2009:60. [Google Scholar]
- 24.Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004:80. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 25.Penttinen P, Jaehrling J, Damdimopoulos AE, Inzunza J, Lemmen JG, van der Saag P, Pettersson K, Gauglitz G, Makela S, Pongratz I. Diet-derived polyphenol metabolite enterolactone is a tissue-specific estrogen receptor activator. Endocrinology. 2007:148. doi: 10.1210/en.2007-0289. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt E, Stopper H. Estrogenic activity of naturally occurring anthocyanidins. Nutr Cancer. 2001:41. doi: 10.1080/01635581.2001.9680625. [DOI] [PubMed] [Google Scholar]
- 27.Papoutsi Z, Kassi E, Tsiapara A, Fokialakis N, Chrousos GP, Moutsatsou P. Evaluation of estrogenic/antiestrogenic activity of ellagic acid via the estrogen receptor subtypes ERalpha and ERbeta. J Agric Food Chem. 2005:53. doi: 10.1021/jf0510539. [DOI] [PubMed] [Google Scholar]
- 28.Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006:54. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 29.Pearce ST, Jordan VC. The biological role of estrogen receptors alpha and beta in cancer. Crit Rev Oncol Hematol. 2004:50. doi: 10.1016/j.critrevonc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Rassi CM, Lieberherr M, Chaumaz G, Pointillart A, Cournot G. Modulation of osteoclastogenesis in porcine bone marrow cultures by quercetin and rutin. Cell Tissue Res. 2005:319. doi: 10.1007/s00441-004-1053-9. [DOI] [PubMed] [Google Scholar]
- 31.Rucinski M, Ziolkowska A, Hochol A, Pucher A, Macchi C, Belloni AS, Nussdorfer GG, Malendowicz LK. Estradiol and resveratrol stimulating effect on osteocalcin, but not osteonectin and collagen-1alpha gene expression in primary culture of rat calvarial osteoblast-like cells. Int J Mol Med. 2006:18. [PubMed] [Google Scholar]
- 32.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005:280. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 33.Klinge CM, Risinger KE, Watts MB, Beck V, Eder R, Jungbauer A. Estrogenic activity in white and red wine extracts. Journal of agricultural and food chemistry. 2003:51. doi: 10.1021/jf0259821. [DOI] [PubMed] [Google Scholar]
- 34.Murphy LC, Skliris GP, Rowan BG, Al-Dhaheri M, Williams C, Penner C, Troup S, Begic S, Parisien M, Watson PH. The relevance of phosphorylated forms of estrogen receptor in human breast cancer in vivo. J Steroid Biochem Mol Biol. 2009:114. doi: 10.1016/j.jsbmb.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Wu RC, Smith CL, O’Malley BW. Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocrine reviews. 2005:26. doi: 10.1210/er.2004-0018. [DOI] [PubMed] [Google Scholar]
- 36.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004:96. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 37.Fridrich D, Glabasnia A, Fritz J, Esselen M, Pahlke G, Hofmann T, Marko D. Oak ellagitannins suppress the phosphorylation of the epidermal growth factor receptor in human colon carcinoma cells. J Agric Food Chem. 2008:56. doi: 10.1021/jf073427z. [DOI] [PubMed] [Google Scholar]
- 38.Meiers S, Kemeny M, Weyand U, Gastpar R, von Angerer E, Marko D. The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J Agric Food Chem. 2001:49. doi: 10.1021/jf0009100. [DOI] [PubMed] [Google Scholar]
- 39.Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, Weinstein IB. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007:67. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 40.Youdim KA, Martin A, Joseph JA. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic Biol Med. 2000:29. doi: 10.1016/s0891-5849(00)00329-4. [DOI] [PubMed] [Google Scholar]
- 41.Ozbay T, Nahta R. Delphinidin Inhibits HER2 and Erk1/2 Signaling and Suppresses Growth of HER2-Overexpressing and Triple Negative Breast Cancer Cell Lines. Breast cancer: basic and clinical research. 2011:5. doi: 10.4137/BCBCR.S7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev. 2001:53. [PubMed] [Google Scholar]
- 43.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004:4. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 44.Wrighton KH. Cell death: A new platform for death. Nat Rev Mol Cell Biol. 2011:12. doi: 10.1038/nrm3174. [DOI] [PubMed] [Google Scholar]
- 45.Gajewska M, Sobolewska A, Kozlowski M, Motyl T. Role of autophagy in mammary gland development. J Physiol Pharmacol. 2008;59(Suppl 9) [PubMed] [Google Scholar]
- 46.Strange R, Metcalfe T, Thackray L, Dang M. Apoptosis in normal and neoplastic mammary gland development. Microsc Res Tech. 2001:52. doi: 10.1002/1097-0029(20010115)52:2<171::AID-JEMT1003>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 47.Riggins RB, Bouton AH, Liu MC, Clarke R. Antiestrogens, aromatase inhibitors, and apoptosis in breast cancer. Vitam Horm. 2005:71. doi: 10.1016/S0083-6729(05)71007-4. [DOI] [PubMed] [Google Scholar]
- 48.Simstein R, Burow M, Parker A, Weldon C, Beckman B. Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp Biol Med (Maywood) 2003:228. doi: 10.1177/153537020322800903. [DOI] [PubMed] [Google Scholar]
- 49.Chen N, Karantza-Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta. 2009:1793. doi: 10.1016/j.bbamcr.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clarke R, Shajahan AN, Riggins RB, Cho Y, Crawford A, Xuan J, Wang Y, Zwart A, Nehra R, Liu MC. Gene network signaling in hormone responsiveness modifies apoptosis and autophagy in breast cancer cells. J Steroid Biochem Mol Biol. 2009:114. doi: 10.1016/j.jsbmb.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook KL, Shajahan AN, Clarke R. Autophagy and endocrine resistance in breast cancer. Expert Review of Anticancer Therapy. 2011:11. doi: 10.1586/era.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawford AC, Riggins RB, Shajahan AN, Zwart A, Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS One. 2010:5. doi: 10.1371/journal.pone.0008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest. 2008:118. doi: 10.1172/JCI36898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeh CT, Yen GC. Induction of apoptosis by the Anthocyanidins through regulation of Bcl-2 gene and activation of c-Jun N-terminal kinase cascade in hepatoma cells. J Agric Food Chem. 2005:53. doi: 10.1021/jf048955e. [DOI] [PubMed] [Google Scholar]
- 55.Kim BW, Lee ER, Min HM, Jeong HS, Ahn JY, Kim JH, Choi HY, Choi H, Kim EY, Park SP, Cho SG. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol Ther. 2008:7. doi: 10.4161/cbt.7.7.6164. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen TH, Mustafa FB, Pervaiz S, Ng FS, Lim LH. ERK1/2 activation is required for resveratrol-induced apoptosis in MDA-MB-231 cells. Int J Oncol. 2008:33. [PubMed] [Google Scholar]
- 57.Schoenlein PV, Periyasamy-Thandavan S, Samaddar JS, Jackson WH, Barrett JT. Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance. Autophagy. 2009:5. doi: 10.4161/auto.5.3.7784. [DOI] [PubMed] [Google Scholar]
- 58.Feng R, Wang SY, Shi YH, Fan J, Yin XM. Delphinidin induces necrosis in hepatocellular carcinoma cells in the presence of 3-methyladenine, an autophagy inhibitor. J Agric Food Chem. 2010:58. doi: 10.1021/jf9025458. [DOI] [PubMed] [Google Scholar]
- 59.Chen RJ, Ho CT, Wang YJ. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol Nutr Food Res. 2010:54. doi: 10.1002/mnfr.201000067. [DOI] [PubMed] [Google Scholar]
- 60.Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su G, Chen B, Dai J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials. 2012:33. doi: 10.1016/j.biomaterials.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Lu Y. Optimizing a 3D Culture System to Study the Interaction between Epithelial Breast Cancer and Its Surrounding Fibroblasts. J Cancer. 2011:2. doi: 10.7150/jca.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rao CV, Tokumo K, Rigotty J, Zang E, Kelloff G, Reddy BS. Chemoprevention of colon carcinogenesis by dietary administration of piroxicam, -difluoromethylornithine, 16 -fluoro-5-androsten-17-one, and ellagic acid individually and in combination. Cancer research. 1991:51. [PubMed] [Google Scholar]
- 63.Forman MR, Hursting SD, Umar A, Barrett JC. Nutrition and cancer prevention: a multidisciplinary perspective on human trials. Annual review of nutrition. 2004:24. doi: 10.1146/annurev.nutr.24.012003.132315. [DOI] [PubMed] [Google Scholar]
- 64.Cooper DA. Carotenoids in health and disease: recent scientific evaluations, research recommendations and the consumer. The Journal of nutrition. 2004:134. doi: 10.1093/jn/134.1.221S. [DOI] [PubMed] [Google Scholar]
- 65.Meyskens FL, Jr, Szabo E. Diet and cancer: the disconnect between epidemiology and randomized clinical trials. Cancer Epidemiol Biomarkers Prev. 2005:14. doi: 10.1158/1055-9965.EPI-04-0666. [DOI] [PubMed] [Google Scholar]
- 66.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992:18. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 67.Katsube N, Iwashita K, Tsushida T, Yamaki K, Kobori M. Induction of apoptosis in cancer cells by Bilberry (Vaccinium myrtillus) and the anthocyanins. J Agric Food Chem. 2003:51. doi: 10.1021/jf025781x. [DOI] [PubMed] [Google Scholar]
- 68.Li L, Adams LS, Chen S, Killian C, Ahmed A, Seeram NP. Eugenia jambolana Lam. berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J Agric Food Chem. 2009:57. doi: 10.1021/jf803407q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai T, Huang C. Berries and Cancer Prevention. 2011. The Effects of Berry Extracts on Cell Signaling Pathways: Leading to Cellular Transformation. [Google Scholar]
- 70.Adams LS, Phung S, Yee N, Seeram NP, Li L, Chen S. Blueberry phytochemicals inhibit growth and metastatic potential of MDA-MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2010:70. doi: 10.1158/0008-5472.CAN-09-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madhusoodhanan R, Natarajan M, Singh JV, Jamgade A, Awasthi V, Anant S, Herman TS, Aravindan N. Effect of black raspberry extract in inhibiting NFkappa B dependent radioprotection in human breast cancer cells. Nutr Cancer. 2010:62. doi: 10.1080/01635580903191494. [DOI] [PubMed] [Google Scholar]
- 72.Seeram NP, Adams LS, Hardy ML, Heber D. Total cranberry extract versus its phytochemical constituents: antiproliferative and synergistic effects against human tumor cell lines. Journal of agricultural and food chemistry. 2004:52. doi: 10.1021/jf0352778. [DOI] [PubMed] [Google Scholar]
- 73.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. The Journal of nutritional biochemistry. 2005:16. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Wu X, Pittman HE, 3rd, McKay S, Prior RL. Aglycones and sugar moieties alter anthocyanin absorption and metabolism after berry consumption in weanling pigs. J Nutr. 2005:135. doi: 10.1093/jn/135.10.2417. [DOI] [PubMed] [Google Scholar]
- 75.Warri AM, Saarinen NM, Makela SI. Can modulation of mammary gland development by dietary factors support breast cancer prevention? Horm Res. 2007:68. doi: 10.1159/000102869. [DOI] [PubMed] [Google Scholar]
- 76.Aiyer H, Ravoori S, Gupta R. Chemopreventive Effects of Berries and Berry Components in Animal Models: Prevention of Estrogen-Mediated Mammary Tumors in ACI Rats by Berries. In: Stoner GD, Seeram NP, editors. Berries and Cancer Prevention. Springer; New York, NY: 2011. pp. 163–187. [Google Scholar]
- 77.Hilakivi-Clarke L, Cho E, Cabanes A, DeAssis S, Olivo S, Helferich W, Lippman ME, Clarke R. Dietary modulation of pregnancy estrogen levels and breast cancer risk among female rat offspring. Clinical cancer research. 2002:8. [PubMed] [Google Scholar]
- 78.Clarke R. Animal models of breast cancer: their diversity and role in biomedical research. Breast Cancer Res Treat. 1996:39. doi: 10.1007/BF01806073. [DOI] [PubMed] [Google Scholar]
- 79.Felgines C, Krisa S, Mauray A, Besson C, Lamaison JL, Scalbert A, Mérillon JM, Texier O. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse. British Journal of Nutrition. 2010:103. doi: 10.1017/S0007114510000061. [DOI] [PubMed] [Google Scholar]
- 80.Talavára S, Felgines C, Texier O, Besson C, Lamaison JL, Rémésy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. The Journal of nutrition. 2003:133. doi: 10.1093/jn/133.12.4178. [DOI] [PubMed] [Google Scholar]
- 81.Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem. 2002:50. doi: 10.1021/jf0116855. [DOI] [PubMed] [Google Scholar]
- 82.Mazur WM, Uehara M, Wahala K, Adlercreutz H. Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br J Nutr. 2000:83. [PubMed] [Google Scholar]
- 83.Saarinen NM, Warri A, Dings RP, Airio M, Smeds AI, Makela S. Dietary lariciresinol attenuates mammary tumor growth and reduces blood vessel density in human MCF-7 breast cancer xenografts and carcinogen-induced mammary tumors in rats. Int J Cancer. 2008:123. doi: 10.1002/ijc.23614. [DOI] [PubMed] [Google Scholar]
- 84.Saarinen NM, Warri A, Airio M, Smeds A, Makela S. Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Food Res. 2007:51. doi: 10.1002/mnfr.200600240. [DOI] [PubMed] [Google Scholar]
- 85.Saarinen NM, Power KA, Chen J, Thompson LU. Lignans are accessible to human breast cancer xenografts in athymic mice. Nutr Cancer. 2008:60. doi: 10.1080/01635580701649669. [DOI] [PubMed] [Google Scholar]
- 86.Verma AK, Johnson JA, Gould MN, Tanner MA. Inhibition of 7,12-dimethylbenz(a)anthracene- and N-nitrosomethylurea-induced rat mammary cancer by dietary flavonol quercetin. Cancer Res. 1988:48. [PubMed] [Google Scholar]
- 87.Pereira MA, Grubbs CJ, Barnes LH, Li H, Olson GR, Eto I, Juliana M, Whitaker LM, Kelloff GJ, Steele VE, Lubet RA. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis. 1996:17. doi: 10.1093/carcin/17.6.1305. [DOI] [PubMed] [Google Scholar]
- 88.Singh B, Mense SM, Bhat NK, Putty S, Guthiel WA, Remotti F, Bhat HK. Dietary quercetin exacerbates the development of estrogen-induced breast tumors in female ACI rats. Toxicol Appl Pharmacol. 2010:247. doi: 10.1016/j.taap.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mense SM, Singh B, Remotti F, Liu X, Bhat HK. Vitamin C and alpha-naphthoflavone prevent estrogen-induced mammary tumors and decrease oxidative stress in female ACI rats. Carcinogenesis. 2009:30. doi: 10.1093/carcin/bgp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hakkinen SH, Karenlampi SO, Heinonen IM, Mykkanen HM, Torronen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999:47. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- 91.Aiyer HS, Gupta RC. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prev Res (Phila) 2010:3. doi: 10.1158/1940-6207.CAPR-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aiyer HS, Ravoori S, Vadhanam MV, Schultz DJ, Gupta RC. Effect of dietary berries and ellagic acid on cell proliferation, estrogen-metabolizing enzymes and tumor indices in an estrogen-induced rat mammary tumor model. 2009 [Google Scholar]
- 93.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008:60. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 94.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res (Phila) 2009:2. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001:22. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 96.Stoner GD, Wang LS, Seguin C, Rocha C, Stoner K, Chiu S, Kinghorn AD. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm Res. 2010:27. doi: 10.1007/s11095-010-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aiyer HS, Vadhanam MV, Stoyanova R, Caprio GD, Clapper ML, Gupta RC. Dietary berries and ellagic acid prevent oxidative DNA damage and modulate expression of DNA repair genes. Int J Mol Sci. 2008:9. doi: 10.3390/ijms9030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, Blackwood M, Stoner GD. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001:61. [PubMed] [Google Scholar]
- 99.Aiyer HS, Li Y, Lasso JN, Schiffman SC, Slone SC, Martin RC. Effect of freeze-dried berries on the development of reflux-induced esophageal adenocarcinoma. Nutrition and Cancer: An International Journal. 2011 doi: 10.1080/01635581.2011.609307. In Press. [DOI] [PubMed] [Google Scholar]
- 100.Ravoori S, Vadhanam MV, Sahoo S, Srinivasan C, Gupta RC. Mammary tumor induction in ACI rats exposed to low levels of 17beta-estradiol. Int J Oncol. 2007:31. [PubMed] [Google Scholar]
- 101.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997:18. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 102.Ruhlen RL, Willbrand DM, Besch-Williford CL, Ma L, Shull JD, Sauter ER. Tamoxifen induces regression of estradiol-induced mammary cancer in the ACI.COP-Ept2 rat model. Breast Cancer Res Treat. 2009:117. doi: 10.1007/s10549-008-0169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berne RM, Levy MN. Female Reproduction. In: Berne RM, Levy MN, editors. Principles of Physiology. 2. Mosby; St Louis, MO: 1996. [Google Scholar]
- 104.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. Journal of the National Cancer Institute. 2004:96. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 105.Walker K, Bratton DJ, Frost C. Premenopausal endogenous oestrogen levels and breast cancer risk: a meta-analysis. Br J Cancer. 2011 doi: 10.1038/bjc.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aiyer H, Ravoori S, Schultz D, Gupta RC. Effect of dietary berries and ellagic acid on cell proliferation, estrogen-metabolizing enzymes and tumor indices in an estrogen-induced rat mammary tumor model. Proceedings of the International Conference on New Developments in Drug Discovery from Natural Products and Traditional Medicines.,; SAS Nagar, Chadigarh, India. 2008. [Google Scholar]
- 107.Aiyer HS, Kichambare S, Gupta RC. Prevention of oxidative DNA damage by bioactive berry components. Nutr Cancer. 2008;60(Suppl 1) doi: 10.1080/01635580802398448. [DOI] [PubMed] [Google Scholar]
- 108.de Assis S, Warri A, Cruz MI, Hilakivi-Clarke L. Changes in mammary gland morphology and breast cancer risk in rats. J Vis Exp. 2010 doi: 10.3791/2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu X, Rahal O, Kang J, Till SR, Prior RL, Simmen RC. In utero and lactational exposure to blueberry via maternal diet promotes mammary epithelial differentiation in prepubescent female rats. Nutr Res. 2009:29. doi: 10.1016/j.nutres.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 110.Adams LS, Kanaya N, Phung S, Liu Z, Chen S. Whole Blueberry Powder Modulates the Growth and Metastasis of MDA-MB-231 Triple Negative Breast Tumors in Nude Mice. J Nutr. 2011:141. doi: 10.3945/jn.111.140178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singletary E, Lieberman R, Atkinson N, Sneige N, Sahin A, Tolley S, Colchin M, Bevers T, Stelling C, Fornage B, Fritsche H, Hittelman W, Kelloff G, Lippman SM. Novel translational model for breast cancer chemoprevention study: accrual to a presurgical intervention with tamoxifen and N-[4-hydroxyphenyl] retinamide. Cancer Epidemiol Biomarkers Prev. 2000:9. [PubMed] [Google Scholar]
- 112.Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006:17. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Hilakivi-Clarke L, Clarke R, Lippman ME. Perinatal factors increase breast cancer risk. Breast Cancer Res Treat. 1994:31. doi: 10.1007/BF00666160. [DOI] [PubMed] [Google Scholar]
- 114.Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010:140. doi: 10.3945/jn.110.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hilakivi-Clarke L. Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets. 2007:7. doi: 10.2174/156800907781386641. [DOI] [PubMed] [Google Scholar]
- 116.Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer. 2008:98. doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paluszczak J, Krajka-Kuzniak V, Baer-Dubowska W. The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol Lett. 2010:192. doi: 10.1016/j.toxlet.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 118.Espin JC, Garcia-Conesa MT, Tomas-Barberan FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007:68. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 119.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American journal of clinical nutrition. 2005:81. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 120.Allen C, Peden-Adams M, EuDaly J, Keil D. Subchronic exposure to ellagic acid impairs cytotoxic T-cell function and suppresses humoral immunity in mice. Immunopharmacology and immunotoxicology. 2003:25. doi: 10.1081/iph-120024508. [DOI] [PubMed] [Google Scholar]
- 121.Del Rio D, Borges G, Crozier A. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. Br J Nutr. 2010:104. doi: 10.1017/S0007114510003958. [DOI] [PubMed] [Google Scholar]
- 122.Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clinica Chimica Acta. 2004:348. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 123.Berry Health Benefits Network. http://berryhealth.fst.oregonstate.edu/
- 124.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Gebhardt S, Prior RL. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. The Journal of nutrition. 2004:134. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 125.Rasmussen SE, Frederiksen H, Struntze Krogholm K, Poulsen L. Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Molecular nutrition & food research. 2005:49. doi: 10.1002/mnfr.200400082. [DOI] [PubMed] [Google Scholar]
- 126.Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. Journal of agricultural and food chemistry. 2004:52. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 127.Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. Journal of agricultural and food chemistry. 2004:52. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 128.Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug metabolism and disposition. 2004:32. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 129.Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventós RM, Berenguer T, Jakszyn P, Martinez C, Sanchez MJ, Navarro C, Chirlaque MD, Tormo MJ. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. British Journal of Nutrition. 2008:100. doi: 10.1017/S0007114507882997. [DOI] [PubMed] [Google Scholar]
- 130.de Kleijn MJJ, Van der Schouw YT, Wilson PWF, Adlercreutz H, Mazur W, Grobbee DE, Jacques PF. Intake of Dietary Phytoestrogens Is Low in Postmenopausal Women in the United States: The Framingham Study1–4. The Journal of nutrition. 2001:131. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- 131.Lofgren L, Sahlin L, Von Schoultz B, Fernstad R, Skoog L, Von Schoultz E. Expression of sex steroid receptor subtypes in normal and malignant breast tissue - a pilot study in postmenopausal women. Acta Oncol. 2006:45. doi: 10.1080/02841860500371865. [DOI] [PubMed] [Google Scholar]
- 132.Younes M, Honma N. Estrogen receptor beta. Arch Pathol Lab Med. 2011:135. doi: 10.5858/2010-0448-RAR.1. [DOI] [PubMed] [Google Scholar]
- 133.Barone I, Brusco L, Fuqua SA. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin Cancer Res. 2010:16. doi: 10.1158/1078-0432.CCR-09-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv. 2005:5. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- 135.Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000:65. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 136.Skliris GP, Nugent ZJ, Rowan BG, Penner CR, Watson PH, Murphy LC. A phosphorylation code for oestrogen receptor-alpha predicts clinical outcome to endocrine therapy in breast cancer. Endocr Relat Cancer. 2010:17. doi: 10.1677/ERC-10-0030. [DOI] [PubMed] [Google Scholar]
- 137.Fernandes I, Faria A, Azevedo J, Soares S, Calhau C, De Freitas V, Mateus N. Influence of anthocyanins, derivative pigments and other catechol and pyrogallol-type phenolics on breast cancer cell proliferation. Journal of agricultural and food chemistry. 2010:58. doi: 10.1021/jf903714z. [DOI] [PubMed] [Google Scholar]
- 138.Notas G, Nifli AP, Kampa M, Pelekanou V, Alexaki VI, Theodoropoulos P, Vercauteren J, Castanas E. Quercetin accumulates in nuclear structures and triggers specific gene expression in epithelial cells. The Journal of nutritional biochemistry. 2011 doi: 10.1016/j.jnutbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 139.Hung H. Inhibition of estrogen receptor alpha expression and function in MCF 7 cells by kaempferol. Journal of cellular physiology. 2004:198. doi: 10.1002/jcp.10398. [DOI] [PubMed] [Google Scholar]
- 140.Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, Centeno F, Alvarez-Barrientos A, Fernandez-Salguero PM. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 2005:115. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 141.Xu M, Bower KA, Wang S, Frank JA, Chen G, Ding M, Shi X, Ke Z, Luo J. Cyanidin-3-Glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Molecular Cancer. 2010:9. doi: 10.1186/1476-4598-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Afaq F, Zaman N, Khan N, Syed DN, Sarfaraz S, Zaid MA, Mukhtar H. Inhibition of epidermal growth factor receptor signaling pathway by delphinidin, an anthocyanidin in pigmented fruits and vegetables. International Journal of Cancer. 2008:123. doi: 10.1002/ijc.23675. [DOI] [PubMed] [Google Scholar]
- 143.Fridrich D, Teller N, Esselen M, Pahlke G, Marko D. Comparison of delphinidin, quercetin and (−)-epigallocatechin-3-gallate as inhibitors of the EGFR and the ErbB2 receptor phosphorylation. Mol Nutr Food Res. 2008:52. doi: 10.1002/mnfr.200800026. [DOI] [PubMed] [Google Scholar]
- 144.Jeong JH, An JY, Kwon YT, Li LY, Lee YJ. Quercetin induced ubiquitination and down regulation of Her 2/neu. Journal of cellular biochemistry. 2008:105. doi: 10.1002/jcb.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia (New York, NY) 2005:7. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang FY, Su YC, Chen NC, Hsieh HS, Chen KS. Resveratrol inhibits migration and invasion of human breast-cancer cells. Mol Nutr Food Res. 2008:52. doi: 10.1002/mnfr.200700325. [DOI] [PubMed] [Google Scholar]
- 147.Pan M-H, Lin Y-T, Lin C-L, Wei C-S, Ho C-T, Chen W-J. Suppression of Heregulin β-1/HER2-Modulated Invasive and Aggressive Phenotype of Breast Carcinoma by Pterostilbene via Inhibition of Matrix Metalloproteinase-9, p38 Kinase Cascade and Akt Activation. Evidence-Based Complementary and Alternative Medicine. 2011:2011. doi: 10.1093/ecam/nep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fridrich D, Kern M, Fritz J, Pahlke G, Köhler N, Winterhalter P, Marko D. The epidermal growth factor receptor and human topoisomerases represent potential cellular targets of oligomeric procyanidins. Molecular nutrition & food research. 2007:51. doi: 10.1002/mnfr.200600186. [DOI] [PubMed] [Google Scholar]
- 149.Chen PN, Chu SC, Chiou HL, Chiang CL, Yang SF, Hsieh YS. Cyanidin 3-glucoside and peonidin 3-glucoside inhibit tumor cell growth and induce apoptosis in vitro and suppress tumor growth in vivo. Nutrition and cancer. 2005:53. doi: 10.1207/s15327914nc5302_12. [DOI] [PubMed] [Google Scholar]
- 150.Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM, Saleem M, Din M, Mukhtar H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-B signaling. Cancer research. 2008:68. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC, Lin JP, Tang NY, Chung JG, Chou MJ. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Archives of pharmacal research. 2010:33. doi: 10.1007/s12272-010-0808-y. [DOI] [PubMed] [Google Scholar]
- 152.Seo HS, Ju J, Jang K, Shin I. Induction of apoptotic cell death by phytoestrogens by up-regulating the levels of phospho-p53 and p21 in normal and malignant estrogen receptor [alpha]-negative breast cells. Nutrition Research. 2011:31. doi: 10.1016/j.nutres.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 153.Wang K, Liu R, Li J, Mao J, Lei Y, Wu J, Zeng J, Zhang T, Wu H, Chen L. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR-and hypoxia-induced factor 1-mediated signaling. Autophagy. 2011:7. doi: 10.4161/auto.7.9.15863. [DOI] [PubMed] [Google Scholar]
- 154.González Sarrías A, Espín JC, Tomás Barberán FA, García Conesa MT. Gene expression, cell cycle arrest and MAPK signalling regulation in Caco 2 cells exposed to ellagic acid and its metabolites, urolithins. Molecular nutrition & food research. 2009:53. doi: 10.1002/mnfr.200800150. [DOI] [PubMed] [Google Scholar]
- 155.Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006:17. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 156.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death & Differentiation. 2008:15. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]