Abstract
Enhanced external counterpulsation (EECP) is a non-invasive treatment for coronary artery disease (CAD) patients who have angina pectoris that is refractory to pharmacotherapy and revascularization. The popular concept is that EECP may promote collateral development and improve myocardial perfusion. We hypothesize that improvements in peripheral arterial function are responsible for the clinical benefits of EECP.
Keywords: angina pectoris, nitric oxide, endothelium, flow-mediated dilation, arterial stiffness
INTRODUCTION
Enhanced external counterpulsation (EECP) is a non-invasive outpatient therapy, approved by the United States Food and Drug Administration in 1995, for the treatment of coronary artery disease (CAD) patients with refractory angina pectoris who fail to respond to standard revascularization procedures and aggressive pharmacotherapy. Data from the International Patient Registry (IEPR) demonstrate that EECP effectively decreased angina episodes and nitrate usage, and increased exercise tolerance in patients with refractory angina (2, 31). The anti-ischemic benefits occur early and are sustained up to 5 years in patients with a favorable initial response (15, 28). However, the mechanisms by which EECP improves angina symptoms are not understood and remain controversial.
The popular hypothesis is that EECP promotes coronary collateral growth and improves myocardial perfusion. However, this understanding of EECP is a theory and remains unproven in randomized clinical trials. In an international trial (7 Centers; 175 chronic stable angina patients), EECP failed to elicit improved cardiac perfusion in 46% of study subject (29). In a recent US trial (6 US University Hospitals; 37 patients with stable chronic angina), EECP failed to improve myocardial blood flow to and within ischemic regions of the myocardium (19). However, despite negligible improvements in myocardial perfusion (ie. oxygen supply), approximately 85% of patients enrolled in EECP clinical trials experience reduction in angina and improvement in exercise tolerance (2, 29).
Over the past 6 years, we have studied the extra-cardiac vascular effects of EECP in CAD patients with refractory angina. In contrast to previous studies, our line of research has focused on EECP-induced improvement in peripheral arterial function and decreased myocardial oxygen consumption (i.e. oxygen demand) vs improvement in coronary blood flow (i.e. oxygen supply). We hypothesized that hemodynamic shear stress, with each inflation/deflation cycle of the compressive EECP cuffs (11), would improve endothelial function in peripheral muscular conduit arteries (5), decrease arterial stiffness (6), and thereby reduce myocardial oxygen demand and angina (6, 23). We further hypothesized that EECP would elicit commensurate changes in endothelial-derived vasoactive agents, plasma inflammatory cytokines, and indices of oxidative damage (5, 7). We speculated that these peripheral changes also beneficially influence the timing and amplitude of aortic reflected pressure waves, thereby reducing LV wasted energy and myocardial oxygen demand (6, 21).
The purpose of this review is to integrate a recent series of studies by the authors and others that identify the extra-cardiac mechanisms by which EECP decreases angina episodes and nitrate usage, while improving exercise tolerance in patients with symptomatic CAD.
EVOLUTION OF COUNTERPULSATION
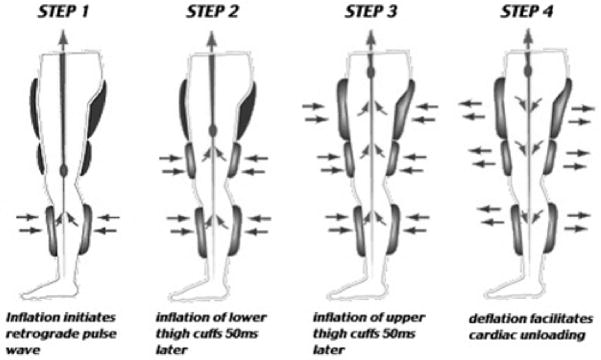
Counterpulsation as treatment for heart disease is not a new procedure. The concept of mechanically improving blood flow to the ischemic myocardium, by increasing coronary perfusion, can be traced back to the 1950’s. Early research led to development of the intra-aortic balloon pump (IABP). The IABP consists of an inflatable balloon catheter that is inserted into the femoral artery and advanced to the descending aorta. Rapid inflation of the balloon in diastole augments coronary artery perfusion. Modified and refined over the last 50 years, this device remains a primary therapy for assisting the heart function of patients in cardiogenic shock. In the mid-1960’s, Birtwell and others at Harvard developed a device for external counter pulsation (4). It was a hydraulic system that pumped water in and out of cuffs applied to the lower extremities. Though cumbersome, there were obvious advantages of the “Birtwell Device.” It was noninvasive and also increased venous return, thereby promoting cardiac output as it boosted coronary perfusion pressure. These early hydraulic devices reduced angina pectoris, and increased survival rates of patients with acute myocardial infarction and cardiogenic shock. However, in the United States, external counter pulsation was eclipsed by the emergence of coronary artery bypass graft surgery (CABG) and angioplasty. In the 1970’s, a more sophisticated external counter pulsation system was developed using pneumatic cuffs that inflated sequentially, rather than simultaneously as they had before (Figure 1). While pneumatic cuffs vastly improved EECP cycling speed vs the hydraulic system, sequential inflation (distal to proximal) introduced the possibility that rapid venous return and increased right ventricular loading might have adverse consequences in patients with symptomatic heart failure (HF) secondary to systolic dysfunction. However, EECP has been shown to improve exercise tolerance, quality of life, and New York Heart Association (NYHA) classification in stable HF patients without adverse consequences (2, 28).
Figure 1.
Enhanced external counterpulsation employs a series of 3 cuffs placed on the calves, lower thighs, and upper thighs/buttocks. The cuffs receive sequential distal-to-proximal pneumatic inflation upon onset of diastole, and simultaneous release of pressure at end-diastole. The hemodynamic result is increased retrograde aortic flow, increased venous return, and increased systolic unloading and cardiac output.
During an EECP treatment session using the modern ‘enhanced’ counterpulsation system, the patient lies on a padded table in which sets of electronically controlled inflation and deflation valves are located. These valves are connected to three pairs of pneumatic cuffs, wrapped around the calves, thighs, and buttocks. The cuffs are sequentially inflated to 300 mm Hg with compressed air from distal to proximal in early diastole and rapidly deflated at the onset of systole (Figure 1). Inflation and deflation of the cuffs are triggered by events in the cardiac cycle via microprocessor-interpreted electrocardiogram (EKG) signals. A standard course of EECP treatment consists of 35 1-hr sessions over a 7-week period.
INDICATIONS FOR EECP
A considerable segment of patients with CAD are not amenable to standard revascularization procedures, such as angioplasty and CABG, because of unsuitable coronary anatomy, multiple previous revascularization attempts, age, additional co-morbid conditions, or patient preference. Such difficult cases require physicians to entertain other options. In the United States alone, there are over 2.4 million patients with CAD not amenable to bypass or angioplasty (13). For those patients in whom repeat (or initial) revascularization procedures are not appropriate, and in whom aggressive medical therapy fails to reduce angina pain, the search for additional therapeutic options continues. New treatment modalities, which are at various stages of clinical evaluation, include minimally invasive bypass surgery, spinal cord stimulation, transcutaneous electrical nerve stimulation, transmyocardial laser revascularization, stem cell therapy, and EECP. Of these modalities, EECP is the only truly noninvasive, atraumatic technique for which clinical anti-ischemic improvements have been shown. In the United States alone, approximately 650 centers are currently providing EECP therapy.
THERAPEUTIC TARGET OF EECP: THE CENTRAL HYPOTHESIS
The often postulated mechanism of action of EECP describes robust diastolic pressure augmentation during the rapid cuff inflation, analogous to IABP counterpulsation (18). Indeed, acute coronary artery flow, determined by both Doppler and angiographic techniques, is increased during EECP suggesting that external compression may serve as a potential mechanical assist device (18).
Nonetheless, it remains unclear how diastolic augmentation could translate into the chronic anti-ischemic benefits of EECP. It is conceivable that diastolic augmentation and possible increased coronary shear forces could stimulate arteriogenesis, the maturation of epicardial vessels or opening of dormant collaterals, and/or angiogenesis, the formation of de novo vessels that perfuse the myocardium. However, this is a theory and has not been proven in humans, despite provocative evidence of improved myocardium perfusion in dogs(14). Indeed, EECP researchers using surrogate measures of cardiac perfusion such as quantitative gated single photon emission computed tomography (SPECT) have reported either little improvement (29), or no improvements in either resting or treadmill exercise stress myocardial perfusion in CAD patients after EECP treatment (19). In fact, SPECT imaging of target ischemic myocardial segments revealed small, but significant declines in myocardial blood flow after exercise stress at 1 month after EECP treatment in some regions of the heart (19). Notwithstanding the negative SPECT imaging results, all EECP studies to date uniformly report increased exercise time to angina symptoms and 85% of study subjects have an improvement in angina class (2, 29). These clinical benefits are in excess of any documented changes in myocardial perfusion, as measured by radionuclide imaging, and support the argument that cardiac angiogenesis cannot be the sole mechanism responsible for EECP-induced symptom reductions.
THERAPEUTIC TARGET OF EECP: PERIPHERAL HYPOTHESIS
Several years ago, Zhang and coworkers (35) used color Doppler imaging in a porcine EECP model and found that brachial artery blood flow velocity increased by 132% (59 versus 24 cm/second; p < 0.001) and brachial artery wall shear stress increased by > 200% (49 versus 23 dyne/cm2, p < 0.001) during lower body compression of EECP pneumatic cuffs. finding is intriguing and of great biological importance because blood flow induced endothelial shear stress (ESS) is the major physiological stimulus that maintains vascular endothelial function (9). Currently, however, there are few therapeutic interventions that could create episodic increases in arterial shear stress in humans, although selected modes of exercise are known to create pulsatile, hemodynamic shear forces and improve endothelial function (10). The remainder of this review summarizes support for our hypothesis that peripheral arterial function may be the therapeutic target for EECP in patients with symptomatic CAD.
ACUTE EFFECTS OF EECP ON ENDOTHELIAL FUNCTION
We recently sought to confirm the findings of Zhang and coworkers (35), observed in a porcine EECP model, by designing the first study to record acute EECP-induced endothelial shear stress in both femoral and brachial arteries during ‘real time’ EECP using high definition ultrasound images and color Doppler spectrum in humans. Moreover, we desired to go a step further and assess the acute effects of EECP on endothelial function in both femoral and brachial arteries using the flow mediated dilation (FMD) technique immediately after a single session of EECP (11). Eighteen healthy, young men (25±4 years) were randomly assigned to either one 45-minute session of EECP or Sham EECP. We observed that acute EECP treatment produces 2 opposite blood flow patterns: antegrade flow in the brachial artery, and retrograde flow in the femoral artery. During EECP, shear stress in the brachial artery was increased by 75% (29.0±6.6 vs. 16.6±4.1 dynes•cm−2; p<0.05) and proximal femoral artery shear stress was increased by 402% (−19.1±5.1 vs. −3.8±0.3 dynes•cm−2; p<0.01) when compared to Sham. Both brachial artery FMD (from 7.0 to 10.6%; p<0.05) and femoral artery FMD (from 7.8 to 13.1%; p<0.05) were increased significantly after a single session of EECP, compared to baseline(11). data suggested to us that dramatic increases in shear stress created by EECP, whether antegrade in the arms or retrograde in the legs, improve endothelium-dependent arterial vasodilation in muscular conduit arteries. This study, however, was not designed to assess whether changes in conduit artery function are accompanied by commensurate changes in the microcirculation. The significant increases in pulsatile and oscillatory flow during EECP treatment may provide a form of “massage” on the endothelium and improve its function. That is, each session of EECP may be thought of as providing a direct dose of vascular medicine.
CHRONIC EFFECTS OF EECP ON ENDOTHELIAL FUNCTION
We conducted the first randomized sham-controlled investigation to determine the chronic effects of EECP (ie. episodic changes in shear stress, as delivered during repeated bouts of EECP) on endothelial function in peripheral muscular conduit arteries and commensurate changes in plasma levels of endothelial derived vasoactive agents, proinflammatory cytokines, markers of lipid peroxidation and antioxidant capacity (5). Forty-two consecutive patients with refractory angina were randomized in a 2:1 manner into either EECP treatment groups or Sham-EECP control groups. The majority were men (81%) and all had a long history of CAD and interventions, and were not candidates for further revascularization therapy. All vasodilator drugs were discontinued 12 hours before laboratory testing. Patients followed the National Institutes of Health low-nitrate diet guidelines a minimum of 48 hours before each blood draw.
EECP and Flow Mediated Dilation
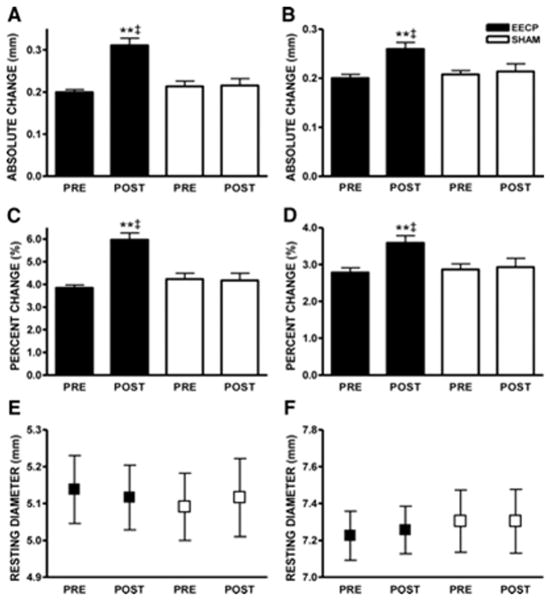
At study entry, resting diameter and FMD (absolute diameter and Δ%) of the brachial and femoral arteries did not differ between groups. EECP treatment improved absolute brachial artery FMD by 51% (Figure 2A) and percent change in dilation (Figure 2C). EECP also improved absolute femoral artery FMD by 39% (Figure 2B) and percentage change in dilation (Figure 2D). No changes in brachial or femoral FMD occurred in the Sham group.
Figure 2.
Absolute (mm) brachial artery (A) and femoral artery (B) FMD diameter; brachial artery FMD (Δ%) (C) and femoral artery FMD (Δ%)(D); brachial artery (E) and femoral artery (F) absolute diameter before (PRE) and after (POST) 35 sessions of EECP. Data are expressed as mean ± SEM (**p < 0.01 versus pretreatment values; ‡p < 0.01 EECP versus SHAM). (Reprinted from (5). Copyright © 2010 American Heart Association. Used with permission.)
EECP and Endothelial Derived Vasoactive Agents
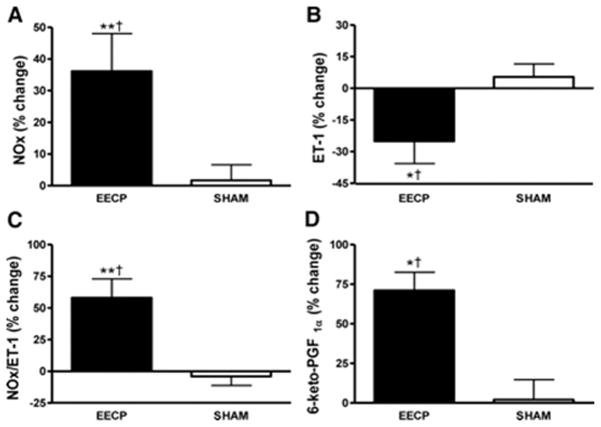
Endothelial dysfunction in patients with CAD is manifest by either decreased bioavailability of paracrine vasodilators including nitric oxide and 6-keto-PGF1α, the major metabolite of prostacyclin, or an increased production of the potent vasoconstrictor endothelin-1 (ET-1), and/or resistance of vascular smooth muscle to nitric oxide. Thirty-five sessions of EECP increased plasma levels of nitrate and nitrite (NOx), the stable metabolites of nitric oxide, by 36%, whereas ET-1 levels were concurrently decreased by 25% (Figure 3). EECP-induced increases in pulsatile endothelial shear stress is the putative mechanism responsible for the observed changes in NOx and ET-1. It is well documented that low endothelial shear upregulates ET-1 and downregulates endothelial nitric oxide synthase (eNOS) (9). ET-1 values after EECP treatment compare favorably with reference values (1 to 1.5 pg/ml) in normal healthy humans (16). Previously, Zhang and coworkers (35) reported that EECP increased the expression of eNOS in hypercholesterolemic pigs, and small uncontrolled studies have also reported increased plasma NOx levels in CAD patients after EECP (1). Akhtar et al. (1) found that NOx remained significantly above baseline (+12%) and ET-1 remained significantly below baseline (−11%) at 3 months after completion of EECP treatment in CAD patients.
Figure 3.
Percentage (%) change in plasma NOx (A); ET-1 (B); NOx/ET-1 ratio (C); and 6-keto-PGF1α (D) after 35 sessions of EECP. Data are expressed as mean ± SEM (*p < 0.05 versus pretreatment values; **p < 0.01 versus pretreatment values; †p < 0.05 EECP versus SHAM). (Reprinted from (5). Copyright © 2010 American Heart Association. Used with permission.)
EECP and Pro-Inflammatory Cytokines and Endothelial Adhesion Molecules
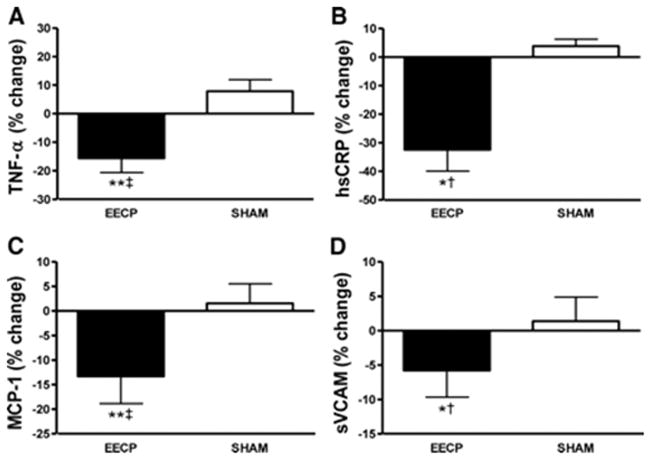
Endothelial function is adversely affected by inflammation, and extensive evidence indicates that patients with CAD have elevated levels of proinflammatory cytokines and adhesion molecules, including tumor necrosis factor-α (TNF-α), high-sensitivity C-reactive protein (hsCRP), monocyte chemoattractant protein-1 (MCP-1), and soluble vascular cell adhesion molecule-1 (sVCAM)(3). Moreover, CAD patients with refractory angina symptoms have proinflammatory biomarker levels that are elevated even further (3). Increased plasma levels of TNF-α, hsCRP, and MCP-1 are known to predict future coronary events. In vitro, CRP and TNF-α also actively participate in the atherogenic process by contributing to decreased nitric oxide synthase production and increased endothelial cell activation characterized by expression of sVCAM(32). After 35 sessions of EECP treatment, we observed reductions in plasma levels of TNF-α (− 16%), hsCRP (− 32%), MCP-1 (− 13%), and sVCAM (− 6%) (Figure 4)(5). changes in inflammatory cytokines or endothelial adhesion molecules were observed in the Sham group.
Figure 4.
Percentage (%) change in plasma TNF-α (A); hsCRP (B); MCP-1 (C); and sVCAM (D) after 35 sessions of EECP. Data are expressed as mean ± SEM (*p < 0.05 versus pretreatment values; **p < 0.01 versus pretreatment values; †p < 0.05 EECP versus SHAM; ‡p < 0.01 EECP versus SHAM). (Reprinted from (5). Copyright © 2010 American Heart Association. Used with permission.)
The anti-inflammatory effects of EECP may be clinically relevant with regard to decreasing the risk for future cardiovascular events in this patient population. For instance, sVCAM levels above ~1130 ng/ml are associated with increased ischemic events and mortality in patients with CAD (24). In our largest study examining the anti-inflammatory effects of EECP, 6 of 28 patients in the intervention group had baseline sVCAM > 1130 ng/ml. After EECP, only 1 of 28 patients had sVCAM values > 1130 ng/ml (5). Similarly, EECP shifted mean CRP values from the high- risk (> 3 mg/L) to the moderate-risk category (1 to 3 mg/L)(26). Despite significant reductions, TNFα levels after EECP remained high and approximated the 95th percentile of healthy control values (27). Increased bioavailability of nitric oxide after EECP therapy is the likely mechanism responsible for reduced plasma inflammatory markers. Nitric oxide serves an anti-inflammatory role by inhibiting the expression of MCP-1 and reducing VCAM-1 expression (12).
EECP and Lipid peroxidation and antioxidant capacity
F2-isoprostanes are a class of prostaglandin-like compounds that are produced by free radical-mediated lipid peroxidation of arachadonic acid, independent of cyclooxygenase. One of these isoprostanes, 8-isoprostaglandin F2α (8-iso-PGF2α), is a good plasma marker to assess oxidative stress(20). Moreover, 8-iso-PGF2α is a potent vasoconstrictor and an independent risk factor associated with CAD (34). We observed a 21% decrease in plasma levels of 8-iso-PGF2a after 35 1-hr sessions of EECP. The mechanisms responsible for decreased 8-iso-PGF2a are unclear. EECP did not change activity levels of the extracellular antioxidant enzyme superoxide dismutase (SOD), and changes in oxidant production by predominate enzyme systems such as NADPH oxidases, xanthine oxidase, and eNOS were not measured in that study. However, nitric oxide has been shown to have antioxidative properties and our observed 36% increase in plasma NOx following EECP could suppress oxidative stress.
We were also the first to measure the effects of EECP on plasma levels of asymmetric dimethylarginine (ADMA), the major endogenous competitive inhibitor of nitric oxide synthase (5). ADMA can be increased up to 10-fold greater than normal in patients with CAD (30). We observed a 28% reduction in plasma ADMA levels after 35 1-hr sessions of EECP. This finding is similar to what has been reported following exercise training in CAD patients (25). ADMA contributes to endothelial dysfunction by displacing the substrate L-arginine from the catalytic site of nitric oxide synthase, thus inhibiting nitric oxide formation and reducing nitric oxide bioavailability. Oxidative stress is a key modulator of ADMA levels (30), suggesting that our observed 21% reduction in the marker of oxidative stress (8-iso-PGF2α) may have led to decreased levels of ADMA.
CHRONIC EFFECTS OF EECP ON ARTERIAL STIFFNESS
Arterial stiffness is increasingly recognized as a surrogate end point for cardiovascular disease. Central aortic stiffness, expressed as aortic pulse wave velocity (PWV), is a strong predictor of future cardiovascular events and all-cause mortality based on the results of individual studies (33). In contrast to widely held, but largely unsubstantiated beliefs, abnormalities in arterial stiffness are modifiable. Elastic conduit artery (aorta) function is modifiable passively by lowering mean arterial pressure and reflection. Muscular conduit artery (brachial, femoral) properties are modifiable actively by changes in endothelial function and/or vascular smooth muscle.
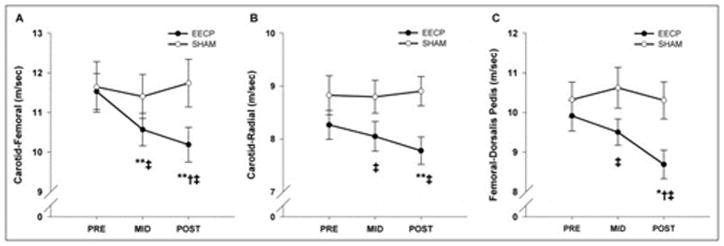
We conducted the first randomized sham-controlled study to test the hypothesis that EECP treatment would decrease both central and peripheral arterial stiffness in patients with CAD, as determined by PWV techniques (6). Pressure waveforms were recorded at the following 3 sets of sites sequentially---carotid-radial (arm), carotid-femoral (aorta), and femoral-dorsalis pedis (leg)---using a SphygmoCor Pulse Wave Velocity Vx system and SCOR-2000 6.31 software (AtCor Medical). Our results demonstrated that central aortic stiffness is decreased after short-and long-term EECP treatment (17 and 35 sessions, respectively) (Figure 5). EECP treatment also decreased peripheral arterial stiffness in the upper (arm) and lower (leg) body, thus suggesting that improvements in arterial stiffness are not limited to vascular regions directly exposed to external mechanical compression (Figure 5).
Figure 5.
Pulse-wave velocity between the carotid and femoral artery (A), carotid and radial artery (B), and femoral and dorsalis pedis artery (C) before (PRE) and after 17 (MID) and 35 (POST) sessions of enhanced external counterpulsation and sham treatment. Data are presented as mean ± SEM (*p <0.05 vs before treatment; **p <0.01 vs before treatment; †p <0.05 vs sham). (Reprinted from (6). Copyright © 2011 Elsevier. Used with permission.)
Stiffness of a vessel is commonly thought to be controlled by the distending (mean arterial) pressure and structural elements within the vessel wall. Changes in distending pressure can impact stiffness in large elastic arteries as well as muscular arteries. Therefore, the possibility exists that the changes in arterial stiffness (i.e. PWV) after EECP are caused by the passive effect of a decreased mean arterial pressure (MAP). After controlling for change in MAP, change in carotid-femoral PWV in the EECP group remained highly significant (P < 0.001). However, changes in carotid-radial (P = 0.15) and femoral-dorsalis pedis (P = 0.08) PWV after treatment were no longer significant. This suggests that the improvements in central PWV (i.e. aorta) were independent of BP changes, whereas decreases in peripheral PWV after EECP were partly due to a decrease in pressure.
We reasoned that the decreases in regional and systemic arterial stiffness after EECP may be explained by the improvements in endothelial function reported above. EECP has been shown by us and others to have a dose-related sustained effect in stimulating endothelial production of nitric oxide and decreasing the release of the potent vasoconstrictor ET-1(1, 5). EECP-induced improvements in endothelial function likely decreases smooth muscle tone and arterial stiffness and consequently alters wave reflection from peripheral arteries. It is also possible that improvements in the capacitive or reservoir function of the aorta after EECP contributes to the improved aortic wave characteristics (9). Aortic reservoir pressure, a major contributor to central augmented pressure and the timing and amplitude of reflected pressure waves, increases with aging as a result of decreasing compliance and increasing impedance to outflow in the aorta.
LEFT VENTRICULAR WORK and INDEXES OF MYOCARDIAL OXYGEN DEMAND
EECP-induced improvements in peripheral endothelial function and arterial stiffness should cause a significant reduction in left ventricular afterload and consequently, a reduction in myocardial oxygen demand. We have performed 2 studies investigating whether these EECP-induced reductions in LV afterload are indeed associated with improvement in indexes of myocardial oxygen demand in CAD patients with chronic ischemic symptoms (6, 21).
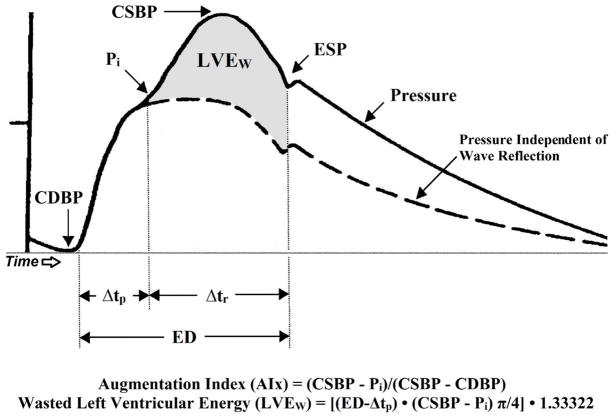
In our lab, indexes of LV afterload and myocardial oxygen demand are derived noninvasively from the assessment of arterial wave reflection characteristics. This technique was well described previously (22) and shown to estimate central arterial pressures to ≤ 0.2 ± 3.8 mm Hg error (8). A typical aortic pressure waveform synthesized from radial pressure waves using applanation tonometry and the generalized transfer function is shown in Figure 6. The central aortic pressure wave is composed of a forward traveling wave, generated by LV ejection and a reflected wave that is returning to the ascending aorta from the periphery. When the reflected wave returns during systole, as seen in Figure 6, the aortic pressure is augmented and, therefore, the LV must generate enough energy to overcome this added boost in pressure and opposition to emptying. This energy is wasted, because it does not contribute to blood flow production, and can be estimated as [(ED−Δtp)•(CSBP−Pi) π/4•1.3332], where 1.3332 is the conversion factor for mm Hg/s to dynes•sec/cm2.
Figure 6.
A typical central aortic pressure waveform synthesized from the radial artery pressure waveform using applanation tonometry, with superimposed waveform of aortic blood flow. The dotted line is representative of the theoretical aortic pressure waveform independent of wave reflection. Augmentation index is the ratio of augmented pressure (CSBP – Pi) and central aortic pulse pressure (CSBP – CDBP). Wasted left ventricular pressure energy (LVEw) is defined as the portion of area under the pulse pressure curve attributed to amplitude and duration of wave reflection where, even though there is an increased systolic pressure, blood flow through the aorta decreases. LVEw, expressed in dynes•sec/cm2, is directly related to augmented pressure (calculated as CSBP – Pi) and to the time duration of the reflected aortic pressure wave, (Δtr). Δtp, time to arrival of the reflected pressure wave; CDBP, central aortic diastolic blood pressure; CSBP, central aortic systolic blood pressure; ED, ejection duration; ESP, end systolic pressure; Pi, pressure at the first inflection point marking the onset of reflected aortic pressure wave return from the periphery. (Reprinted from (17). Copyright © 2011 Nature Publishing. Used with permission.
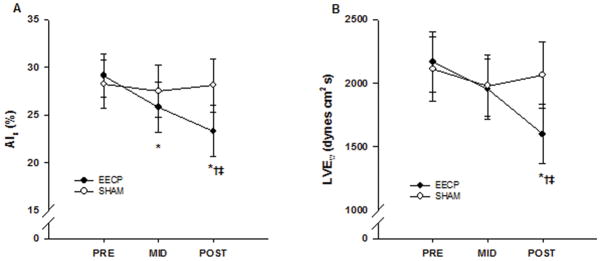
In our initial open-label uncontrolled study, we demonstrated that 34 sessions of EECP resulted in a significant decrease in aortic augmentation index (from 18 ± 9% to 12 ± 8%; p < 0.01), wasted left ventricular pressure energy (LVEw)(from 1,560 ± 162 to 1,360 ± 166 dynes•sec/cm2; p < 0.001) and an increase in round trip travel time of pressure wave from the heart to the periphery and back (from 68 ± 8 ms to 74 ± 6 ms; p < 0.001) in CAD patients with refractory angina (21). We’ve recently expanded on our pilot research by conducting the first randomized sham-controlled study investigating the effects of EECP treatment on indexes of myocardial oxygen demand in CAD patients with chronic angina symptoms refractory to conventional medical and revascularization treatment (6). Pulse wave analysis results demonstrated progressive decrease in aortic augmentation index after 17 (25.8 ± 2.7%) and 35 (23.3 ± 2.7%) sessions of EECP compared to baseline (29.1 ± 2.3%, p ≤ 0.01; Figure 7). Likewise, there was a progressive increase in round trip travel time of pressure wave from the heart to the periphery and back after 17 (143 ± 2 ms) and 35 (145 ± 2 ms) sessions of EECP compared to baseline (140 ± 2 ms, p < 0.01). Aortic systolic tension-time index (AsTTI) was also decreased after 35 sessions of EECP (from 2,287 ± 93 to 2,071 ± 73 mmHg•s•min−1; p ≤ 0.05). The difference in AsTTI resulted in a significantly higher subendocardial viability ratio (from 147 ± 6 to 172 ± 8; p ≤ 0.01) after EECP treatment. In turn, these vascular adaptations lead to a substantial decrease in LVEw after 35 sessions of EECP compared to pretreatment values (from 2,167 ± 372 to 1,598 ± 233 dynes•sec/cm2, p < 0.01; Figure 7). These modifications in wave reflection characteristics and indexes of myocardial oxygen demand were accompanied by a 14% increase in peak oxygen consumption (VO2peak), improvements in Canadian cardiovascular Society angina classification from 3.1 to 1.2 (mean ± SEM), and decreases in daily angina episodes by ~72%, and daily nitrate usage by 81% (6).
Figure 7.
Changes in aortic augmentation index (AIx) (A) and wasted left ventricular pressure energy (LVEw) (B) before (PRE) and after 17 (MID) and 35 (POST) sessions of enhanced external counterpulsation and sham treatment. Data are presented as means ± SEM (*p <0.01 vs PRE; †p <0.05 vs MID; ‡p <0.05 vs sham). (Reprinted from (6). Copyright © 2011 Elsevier. Used with permission.)
SUMMARY and CONCLUSIONS
Only through identification of the specific mechanisms responsible for the anti-ischemic clinical benefits of EECP, will this treatment option receive acceptance by the medical community for the treatment of symptomatic CAD. There is inadequate data to support the hypothesis that EECP increases myocardial perfusion and oxygen supply. In this review, the authors have proposed an alternative hypothesis and presented novel data from studies that investigated peripheral vascular adaptations to EECP. In aggregate, results support the hypothesis that EECP improves peripheral arterial function which serves to reduce myocardial oxygen demand. Our hypothesis is further supported by novel neurohormonal evidence that EECP markedly increases plasma NOx and 6-keto-PGF1α, and simultaneously decreases ET-1, 8- iso-PGF2α, and the pro-inflammatory markers TNF-α, hsCRP, MCP-1, and sVCAM.
As suggested previously by others (15), EECP treatment appears to exert a “training” effect. Indeed, the results from our studies with EECP treatment (5–7, 11, 21) are similar to those reported by others as a result of exercise training (10) produced by running, which increases blood flow and wall shear stress in the muscular arteries of the legs. EECP creates significant increases in shear stimulus via pulsatile retrograde flow and antegrade flow in the femoral and brachial arteries, respectively. Moreover, we calculate that with a resting heart rate of 60 beats/min, 35 1-hr EECP sessions cause ~150,000 hyperemic episodes (stimuli) in the arteries of both the lower and upper extremities. These chronic maneuvers increase release of nitric oxide from endothelial cells and thus cause vasodilation, with a delayed return of the reflected wave from the lower body to the heart and reduced LVEw.
Acknowledgments
Disclosure of Funding: These projects were funded by the National Institutes of Health, NIH/HLB Grant #R01 HL077571 to Randy W. Braith, PhD
Footnotes
There are no conflicts of interest. None of the authors have professional relationships with companies or manufacturers who will benefit from the results of the present study.
References
- 1.Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. The American journal of cardiology. 2006;98(1):28–30. doi: 10.1016/j.amjcard.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 2.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, Mckiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. Journal of the American College of Cardiology. 1999;33(7):1833–40. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 3.Aukrust P, Berge RK, Ueland T, Aaser E, Damas JK, Wikeby L, Brunsvig A, Muller F, Forfang K, Froland SS, Gullestad L. Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. Journal of the American College of Cardiology. 2001;37(2):485–91. doi: 10.1016/s0735-1097(00)01110-4. [DOI] [PubMed] [Google Scholar]
- 4.Birtwell WC, Giron F, Ruiz U, Norton RL, Soroff HS. The regional hemodynamic response to synchronous external pressure assist. Trans Am Soc Artif Intern Organs. 1970;16:462–5. [PubMed] [Google Scholar]
- 5.Braith RW, Conti CR, Nichols WW, Choi CY, Khuddus MA, Beck DT, Casey DP. Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: a randomized sham-controlled study. Circulation. 2010;122(16):1612–20. doi: 10.1161/CIRCULATIONAHA.109.923482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey DP, Beck DT, Nichols WW, Conti CR, Choi CY, Khuddus MA, Braith RW. Effects of enhanced external counterpulsation on arterial stiffness and myocardial oxygen demand in patients with chronic angina pectoris. The American journal of cardiology. 2011;107(10):1466–72. doi: 10.1016/j.amjcard.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey DP, Conti CR, Nichols WW, Choi CY, Khuddus MA, Braith RW. Effect of enhanced external counterpulsation on inflammatory cytokines and adhesion molecules in patients with angina pectoris and angiographic coronary artery disease. The American journal of cardiology. 2008;101(3):300–2. doi: 10.1016/j.amjcard.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95(7):1827–36. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 9.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature clinical practice. 2009;6(1):16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37(4):196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 11.Gurovich AN, Braith RW. Three-dimension blood flow classification scheme better describes NO-mediated arterial vasodilation. Medicine and science in sports and exercise. 2011;42(10 Suppl 2):7. [Google Scholar]
- 12.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. Journal of internal medicine. 2006;259(4):351–63. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 13.Holmes DR., Jr Treatment options for angina pectoris and the future role of enhanced external counterpulsation. Clinical cardiology. 2002;25(12 Suppl 2):II22–5. doi: 10.1002/clc.4960251407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobey JA, Taylor WJ, Smith GT, Gorline R, Harken DE. A new therapeutic approach to acute coronary occlusion: Opening dormant coronary collateral channels by counterpulsation. Am J Card. 1963 Feb;:218–27. [Google Scholar]
- 15.Lawson WE, Hui JC, Lang G. Treatment benefit in the enhanced external counterpulsation consortium. Cardiology. 2000;94(1):31–5. doi: 10.1159/000007043. [DOI] [PubMed] [Google Scholar]
- 16.Maeda S, Miyauchi T, Iemitsu M, Sugawara J, Nagata Y, Goto K. Resistance exercise training reduces plasma endothelin-1 concentration in healthy young humans. J Cardiovasc Pharmacol. 2004;44 (Suppl 1):S443–6. doi: 10.1097/01.fjc.0000166319.91623.b0. [DOI] [PubMed] [Google Scholar]
- 17.Martin JS, Casey DP, Gurovich AN, Beck DT, Braith RW. Association of age with timing and amplitude of reflected pressure waves during exercise in men. American journal of hypertension. 2011;24(4):415–20. doi: 10.1038/ajh.2010.261. [DOI] [PubMed] [Google Scholar]
- 18.Michaels AD, Accad M, Ports TA, Grossman W. Left ventricular systolic unloading and augmentation of intracoronary pressure and Doppler flow during enhanced external counterpulsation. Circulation. 2002;106(10):1237–42. doi: 10.1161/01.cir.0000028336.95629.b0. [DOI] [PubMed] [Google Scholar]
- 19.Michaels AD, Raisinghani A, Soran O, De Lame PA, Lemaire ML, Kligfield P, Watson DD, Conti CR, Beller G. The effects of enhanced external counterpulsation on myocardial perfusion in patients with stable angina: a multicenter radionuclide study. American heart journal. 2005;150(5):1066–73. doi: 10.1016/j.ahj.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 20.Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. Faseb J. 2004;18:1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 21.Nichols WW, Estrada JC, Braith RW, Owens K, Conti CR. Enhanced external counterpulsation treatment improves arterial wall properties and wave reflection characteristics in patients with refractory angina. Journal of the American College of Cardiology. 2006;48(6):1208–14. doi: 10.1016/j.jacc.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 22.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Current opinion in cardiology. 2002;17(5):543–51. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Novo G, Bagger JP, Carta R, Koutroulis G, Hall R, Nihoyannopoulos P. Enhanced external counterpulsation for treatment of refractory angina pectoris. Journal of cardiovascular medicine (Hagerstown, Md) 2006;7(5):335–9. doi: 10.2459/01.JCM.0000223255.24309.fa. [DOI] [PubMed] [Google Scholar]
- 24.Postadzhiyan AS, Tzontcheva AV, Kehayov I, Finkov B. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and their association with clinical outcome, troponin T and C-reactive protein in patients with acute coronary syndromes. Clin Biochem. 2008;41(3):126–33. doi: 10.1016/j.clinbiochem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Richter B, Niessner A, Penka M, Grdic M, Steiner S, Strasser B, Ziegler S, Zorn G, Maurer G, Simeon-Rudolf V, Wojta J, Huber K. Endurance training reduces circulating asymmetric dimethylarginine and myeloperoxidase levels in persons at risk of coronary events. Thromb Haemost. 2005;94(6):1306–11. doi: 10.1160/TH05-03-0158. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101(18):2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 28.Soran O, Kennard ED, Kfoury AG, Kelsey SF. Two-year clinical outcomes after enhanced external counterpulsation (EECP) therapy in patients with refractory angina pectoris and left ventricular dysfunction (report from The International EECP Patient Registry) The American journal of cardiology. 2006;97(1):17–20. doi: 10.1016/j.amjcard.2005.07.122. [DOI] [PubMed] [Google Scholar]
- 29.Stys TP, Lawson WE, Hui JC, Fleishman B, Manzo K, Strobeck JE, Tartaglia J, Ramasamy S, Suwita R, Zheng ZS, Liang H, Werner D. Effects of enhanced external counterpulsation on stress radionuclide coronary perfusion and exercise capacity in chronic stable angina pectoris. The American journal of cardiology. 2002;89(7):822–4. doi: 10.1016/s0002-9149(02)02191-4. [DOI] [PubMed] [Google Scholar]
- 30.Sydow K, Munzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4(4):41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 31.Urano H, Ikeda H, Ueno T, Matsumoto T, Murohara T, Imaizumi T. Enhanced external counterpulsation improves exercise tolerance, reduces exercise-induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. Journal of the American College of Cardiology. 2001;37(1):93–9. doi: 10.1016/s0735-1097(00)01095-0. [DOI] [PubMed] [Google Scholar]
- 32.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439–41. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 33.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55(13):1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis. 2006;184(2):425–30. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, He X, Chen X, Ma H, Liu D, Luo J, Du Z, Jin Y, Xiong Y, He J, Fang D, Wang K, Lawson WE, Hui JC, Zheng Z, Wu G. Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation. 2007;116(5):526–34. doi: 10.1161/CIRCULATIONAHA.106.647248. [DOI] [PubMed] [Google Scholar]