Abstract
In vivo data have been unable to provide conclusive results with regard to the relative impact of circumferential and longitudinal shortening on stroke volume. The objective of the present study was to assess the relative contribution of circumferential and longitudinal myocardial shortening to left ventricular stroke volume and ejection fraction, and to evaluate the effect of left ventricular hypertrophy. A two-shell, three-dimensional mathematical model was used to assess the individual contributions of longitudinal and midwall circumferential shortening (or strain) to stroke volume and ejection fraction. Reducing either circumferential or longitudinal shortening resulted in a reduced ejection fraction and stroke volume. The stroke volume fell by 43% when circumferential strain was reduced from −20% to −5%, but only by 19% when longitudinal strain was similarly reduced. The sole contribution of circumferential and longitudinal shortening to stroke volume was 67% and 33%, respectively. These proportions were independent of wall thickness. The present study demonstrated that both longitudinal and midwall circumferential shortening contribute to different extents depending on the degree of abnormality of myocardial shortening. Contrary to most previous studies, the present study shows that circumferential shortening has a relatively greater contribution to stroke volume (ie, two-thirds) and ejection fraction than longitudinal shortening. These observations have important clinical and research implications in the assessment of left ventricular function.
Keywords: Ejection fraction, Mathematical modelling, Myocardial mechanics, Myocardial strain, Stroke volume
The left ventricle is comprised of three continuous layers of muscle bundles (1). The endocardial and epicardial fibres originate near the base of the heart, loop around the apex and then return to the base forming a helical structure; the circumferential fibres are situated in the midwall of the left ventricle (2,3). The left ventricle ejects its stroke volume during systole using a combination of longitudinal and circumferential shortening associated with twisting of the ventricle. The apex remains relatively stationary; therefore, the longitudinal shortening results in mitral annular motion toward the apex. This shortening produces radial thickening causing an inward displacement of the endocardium and a reduction in left ventricular cavity volume (Figure 1) (4). For any given end-diastolic volume, ejection fraction is predominantly determined by absolute wall thickening rather than relative wall thickening (ie, radial strain) (5). Moreover, absolute wall thickening is determined by both end-diastolic wall thickness and radial strain. During diastole, myocardial fibre relaxation, untwisting and lengthening occurs, resulting in ventricular wall thinning during refilling of the ventricle.
Figure 1).
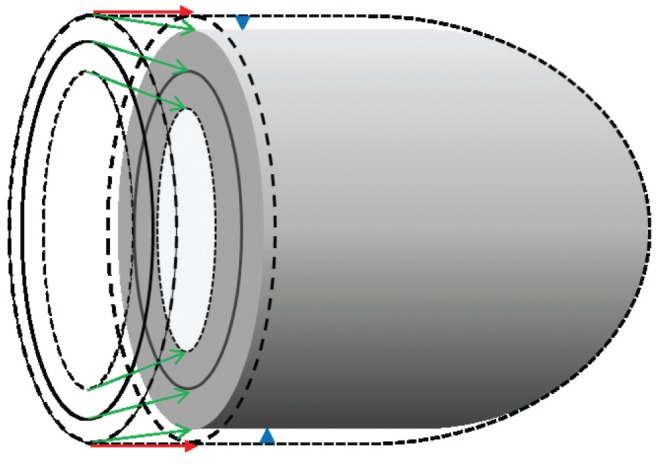
Left ventricular contraction. Figure represents diastole (external dashed lines) and systole (shaded figure). Stroke volume is determined from the difference in internal end-diastolic and end-systolic volumes. It is also determined by the change in total external volume assuming the myocardium is incompressible. Therefore, the stroke volume is the sum of the volume of atrioventricular displacement (red arrows) and is disc shaped. In addition, there is a smaller contribution from external epicardial displacement (blue arrow heads) and is approximately cylindrically shaped). Note that epicardial fractional shortening is less than midwall fractional shortening and midwall fractional shortening is less than endocardial fractional shortening (green arrows)
Normally, midwall fractional shortening (FSm) and longitudinal fractional shortening (FSl) are similar (21%) (Table 1) (6). However, in hypertensive-hypertrophic left ventricular disease, both FSm and FSl are significantly reduced despite a normal ejection fraction (6,7). In aortic stenosis, FSl is also reduced while ejection fraction is preserved (8). There is a reduced FSm and FSl in concentric left ventricular hypertrophy in spite of a normal ejection fraction (Table 1) (9). Reduced FSm occurs even though endocardial fractional shortening (FSen) is normal in hypertensive heart disease due to a relatively greater contribution of ventricular wall thickening (10). In patients with heart failure and a preserved ejection fraction, FSm is significantly lower than in control pateints despite mean endocardial fractional shortening being unchanged (11). Concentric left ventricular hypertrophy with a normal ejection fraction is common in heart failure (2,12–14). A reduced FSm and FSl, and yet normal ejection fraction, may be best explained by an increase in end-diastolic wall thickness (15). Furthermore, epicardial FS (FSep) is lower and FSen is higher than FSm (9,16).
TABLE 1.
Studies showing fractional shortening of epicardium (FSep), midwall (FSm), endocardium (FSen) and longitudinal (FSl) in hypertrophic left ventricular disorders
| Cohort |
FSl, %
|
FSep, %
|
FSm, %
|
FSen, %
|
EF, %
|
Author (reference) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Patient | Control | Patient | Control | Patient | Control | Patient | Control | Patient | ||
| CRF | NA | NA | NA | NA | 21.4 | 14.6* | NA | NA | 64.2 | 56.0* | Koh et al (43) |
| DM | NA | NA | NA | NA | 21.4 | 15.3* | NA | NA | 64.2 | 60.8 (NS) | |
| HBP | NA | NA | NA | NA | 21.4 | 16.7* | NA | NA | 64.2 | 64.7 (NS) | |
| HBP | 21 | 18* | NA | NA | 21 | 18* | 37 | 42* | 63 | 69* | Aurigemma et al (6) |
| HBP | 18 | 12* | 22 | 11* | 30 | 21* | 44 | 30* | NA | 64 | Palmon et al (9) |
| AS | 21 | 9* | NA | NA | 21 | 23 (NS) | 36 | 42* | NA | NA | Dumesnil et al (8) |
P<0.05 versus control. AS Aortic stenosis; CRF Chronic renal failure; DM Diabetes mellitus; EF Ejection fraction; HBP High blood pressure; NA Data not available; NS Not statititically significant. FSm and FSl are generally reduced despite relatively preserved FSen and EFs. FSen are greater than FSm and FSep
There is a reduced longitudinal strain and decreased radial strain in heart failure patients with a preserved ejection fraction compared with control subjects (Table 2) (17,18,22). There is also a trend toward lower (ie, less negative) circumferential strain (17). In patients with concentric left ventricular hypertrophy, longitudinal, circumferential and radial strain are decreased despite a normal ejection fraction (19). These findings of reduced strain, even in the presence of a normal ejection fraction, have recently been shown to be independent of wall stress (20). Similar abnormalities occur in other hypertrophic conditions such as aortic stenosis (21), hypertension (21), cardiac amyloidosis (22) and hypertrophic cardiomyopathy (23).
TABLE 2.
Peak resting systolic myocardial strain in heart failure and hypertrophic left ventricular disease
| Cohort |
Longitudinal strain, %
|
Circumferential strain, %
|
Radial strain, %
|
EF, %
|
Author (reference) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Patient | Control | Patient | Control | Patient | Control | Patient | ||
| LVH | −22.9 | −17.9** | −23.7 | −20.4** | +74.4 | 62.7** | 77 | 70* | Mizuguchi et al (19) |
| AS | −20.3 | −14.6** | −19.5 | −15.2** | +38.9 | +33.9 (NS) | 62 | 61 (NS) | Delgado et al (21) |
| HBP | −20.3 | −17.2** | −19.5 | −17.0 (NS) | +38.9 | +34.4 (NS) | 62 | 61 (NS) | Delgado et al (21) |
| HCM | −20.3 | −15.1** | −19.6 | −16.8** | +36.8 | +25.2** | 67 | 69 (NS) | Serri et al (23) |
| HFPEF | −19.0 | −12.0* | −20.0 | −15.0 (NS) | +47.0 | +28.0* | 64 | 63 (NS) | Wang et al (17) |
| HFPEF | −20.9 | −18.9* | NA | NA | +49.2 | +41.8* | 62 | 61 (NS) | Tan et al (18) |
| HFPEF | −20.9 | −15.9** | −26.4 | −20.8 (NS) | +44.5 | +32.9** | 68 | 61 (NS) | Yip et al (20) |
| HFREF | −19.0 | −4.0* | −20.0 | −7.0* | +47.0 | +14.0* | 64 | 24* | Wang et al (17) |
| HFREF | −20.9 | −9.6** | −26.4 | −9.5** | +44.3 | +18.0** | 68 | 31* | Yip et al (20) |
P<0.01;
P<0.001. AS Aortic stenosis; EF Ejection fraction; HBP High blood pressure; HCM Hypertrophic cardiomyopathy; HFPEF Heart failure with preserved ejection fraction; HFREF Heart failure with reduced ejection fraction; LVH Concentric left ventricular hypertrophy; NA Data not available; NS Not statistically significant (ie, P>0.05)
These results suggest that a generalized reduction in myocardial shortening in left ventricular hypertrophy is common despite a normal ejection fraction (Table 2). Normal resting peak longitudinal and circumferential strains are approximately −20%, and radial strain is generally approximately +50%. Notably, lower strain values occur in all three directions with both reduced ejection fraction and in hypertrophic left ventricular disease even when the ejection fraction is normal (Table 2). Reduced circumferential and longitudinal strains also correlate with elevated filling pressure and severity of myocardial disease despite a preserved ejection fraction (24).
Stroke volume is determined by the change in internal volume of the heart during systole. The myocardium is nearly noncompressible and so the muscle volume is virtually constant (25). Therefore, the absolute change in total external left ventricular volume also determines stroke volume (see Supplementary Data section for mathematical proof). The reduction in external volume consists of two inter-related changes: the change in longitudinal length (measured by, for example, atrioventricular plane displacement); and external (epicardial) displacement (Figure 1). Consequently, the stroke volume is determined by the summation of the displaced volume as a result of longitudinal shortening and the volume derived from epicardial displacement.
Previous studies have produced inconsistent data regarding the relative contribution of circumferential and longitudinal annular displacement to stroke volume (26). Carlsson et al (27) concluded that annular displacement accounted for approximately 60% of the stroke volume. Two other studies suggested that annular displacement contributed 75% and 82%, respectively (28,29). Ballo et al (30) suggested that changes in ventricular wall thickness and ejection fraction also determined the relative contribution. Conversely, Carlhäll et al (31) calculated that mitral annular excursion volume represented only 19% of the total stroke volume. Similar findings have been published elsewhere (32) and the contradictory findings have been debated (33,34). Recently, it was even suggested that epicardial shortening is the best way to assess the contribution of circumferential function to stroke volume, particularly in the presence of hypertrophy (35,36).
There is only a minor reduction in external diameter of the left ventricle during systole (9,28,29,37,38); therefore, these differing results are difficult to reconcile. These variances may reflect the methods used. For example, Carlhäll et al (31) measured the internal dimensions under the mitral valve rather than the external cross sectional area of the left ventricle (Figure 1). Some of these studies also assumed that circumferential ‘function’ was determined by epicardial rather than midwall shortening of the left ventricle. There is an important need to improve our understanding of left ventricular myocardial mechanics. Previous in vivo studies have attempted to clarify the relative contribution of longitudinal and circumferential function to stroke volume and ejection fraction, with inconclusive results.
Mathematical modelling complements existing investigational in vitro, experimental and observational methods, and often has several distinct advantages. For example, it enables the exclusion of confounding factors (eg, body size, valvular disease, inotropic effects, heart rate, changes in filling pressure, blood pressure, abnormal ventricular-arterial interaction, reflected waves and peripheral vascular resistance). More importantly, modelling is particularly helpful in studying complex systems in which multiple, often linked, processes occur and to study the specific effects of certain changes. Specifically, it would not be possible to examine the independent effects of differing longitudinal and circumferential strain in either a clinical or experimental study.
The present study addresses the hypothesis that midwall circumferential shortening and longitudinal shortening have different effects on stroke volume and ejection fraction. The limitations of in vivo studies are avoided by using a mathematical model to assess the relative contribution of longitudinal and circumferential strain in different pathological conditions such as reduced myocardial strain and concentric left ventricular hypertrophy.
METHODS
The endocardium of the left ventricle is represented by a cylindrical-hemispheroidal shape (39), which has been shown to be a good approximation of reality (r=0.97) (40). The midwall and epicardium are assumed to have the equivalent shape. The middle layer of the left ventricle is the site of the circumferential fibres, with longitudinal fibres present in the subendocardial (inner shell) and subepicardial (outer shell) layer.
To measure the independent effects of changes in circumferential and longitudinal shortening, a two-shell approach was used whereby the inner and outer shell muscle volumes can be calculated independently (5). Left ventricular end-diastolic length was assumed to be 10 cm (29,41). The inner, outer and midwall volumes were calculated using the following formula:
in which V represents volume, A represents the area perpendicular to the long-axis at the level of the mitral valve and L represents the length of long-axis. Short-axis area (A) was calculated from width in which, A=πr2 (Figure 1). Total midwall volume (intraventricular volume plus inner shell volume) was obtained from the short-axis area (A) (middle ring in diagram) and length (L) in diastole. The volumes of outer and inner shells were then calculated and the diastolic external and internal ventricular volumes were obtained, followed by the total left ventricular myocardial volume derived from the difference. The midwall short-axis width (W) and longitudinal length (L) were reduced to simulate circumferential and longitudinal shortening respectively and the new midwall volume derived. Myocardium is assumed to be a noncompressible elastomer (25). The internal end-systolic volume was calculated by subtracting the total muscle volume from the external end-systolic volume. Stroke volume and ejection fraction were calculated in the usual manner.
This model allows for both longitudinal shortening and midwall circumferential shortening to be adjusted independently. The percentage of longitudinal and midwall circumferential shortening (strain) used were as follows: normal (−20%); mildly reduced (−15%); moderately reduced (−10%); and severely reduced (−5%). The other variable altered was end-diastolic wall thickness from normal (0.9 cm) to hypertrophic (2.1 cm).
All other factors, such as external end-diastolic long-axis length, were kept constant. It was assumed that heart rate, body habitus, valvular regurgitation, blood pressure, systemic vascular resistance and all other physiological variables were identical.
RESULTS
The modelling shows a curvilinear relationship between ejection fraction and end-diastolic wall thickness for a constant strain (Figure 2A). Worsening longitudinal strain (less negative) resulted in a decrease in ejection fraction; however, more marked changes were seen with reducing circumferential compared with longitudinal strain (Figure 2B). Stroke volume was also reduced by deteriorating longitudinal (Figure 3A) and circumferential strain (Figure 3B). The fall in stroke volume was greater with abnormalities of circumferential rather than longitudinal strain.
Figure 2).
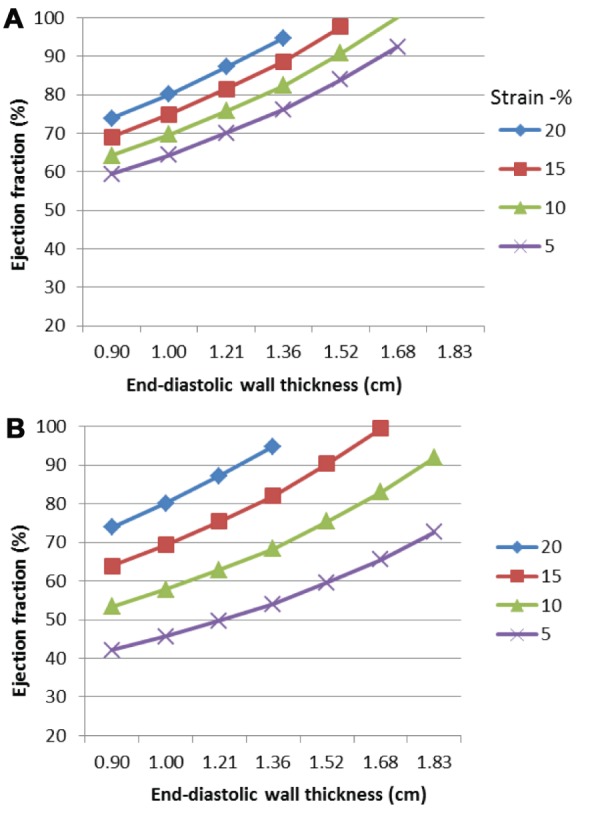
Effect of myocardial shortening (peak systolic strain) and left ventricular end-diastolic wall thickness on ejection fraction. The ejection fraction increases with increasing left ventricular end-diastolic wall thickness. Worsening longitudinal strain leads to a reduction in ejection fraction. Reducing circumferential strain results in a relatively greater fall in ejection fraction compared with longitudinal strain. A Effect of peak systolic longitudinal strain on ejection fraction (midwall circumferential strain normal). B Effect of peak systolic midwall circumferential strain on ejection fraction (longitudinal strain normal)
Figure 3).
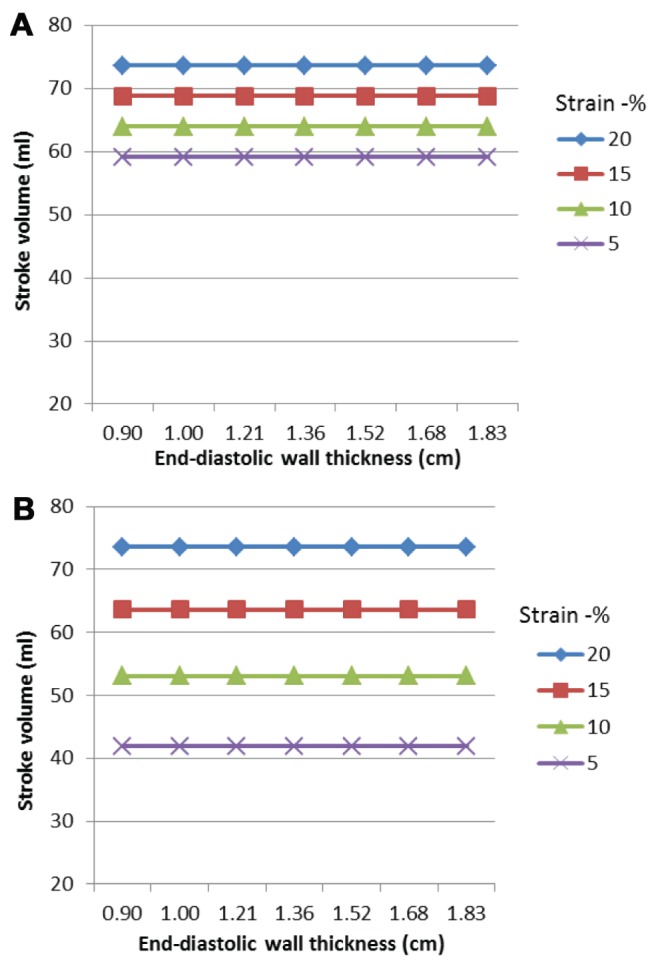
Effects of peak systolic strain on stroke volume. Stroke volume remains constant as end-diastolic wall thickness increases. Reducing longitudinal strain resulted in a fall in stroke volume. A relatively greater reduction in stroke volume occurs when midwall circumferential strain is reduced compared with longitudinal strain. A Effect of reducing peak systolic longitudinal strain on stroke volume. B Effect of reduction in peak systolic mid-wall circumferential strain on stroke volume
Figure 4A shows the independent effects of longitudinal and circumferential strain on ejection fraction assuming a normal wall thickness. The effect on stroke volume is greater with changes in midwall strain compared with longitudinal strain (Figure 4B). Figure 5 shows the relative per cent reduction in stroke volume. Reducing longitudinal strain from −20% to −5% resulted in a decrease in stroke volume by 20%, whereas a fall in midwall circumferential strain resulted in a decrease in stroke volume by 43%. The sole contribution of midwall circumferential shortening was 67% and longitudinal shortening was 33%. These proportions did not change with increasing left ventricular wall thickness.
Figure 4).
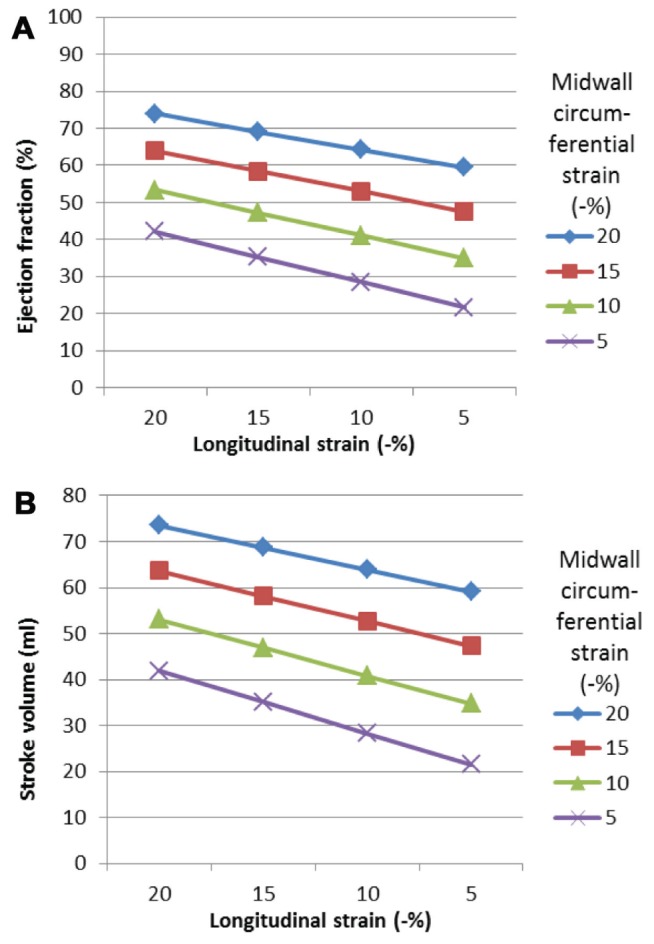
Effects of peak systolic strain on ejection fraction. Ejection fraction falls with reducing strain. Reducing longitudinal strain resulted in a fall in ejection fraction. A relatively greater reduction in ejection fraction occurs when midwall circumferential strain is reduced compared with longitudinal strain. A Effects of altering longitudinal and circumferential peak systolic strain on ejection fraction (normal wall thickness). B Effect of altering longitudinal and circumferential peak systolic strain on stroke volume (normal wall thickness)
Figure 5).
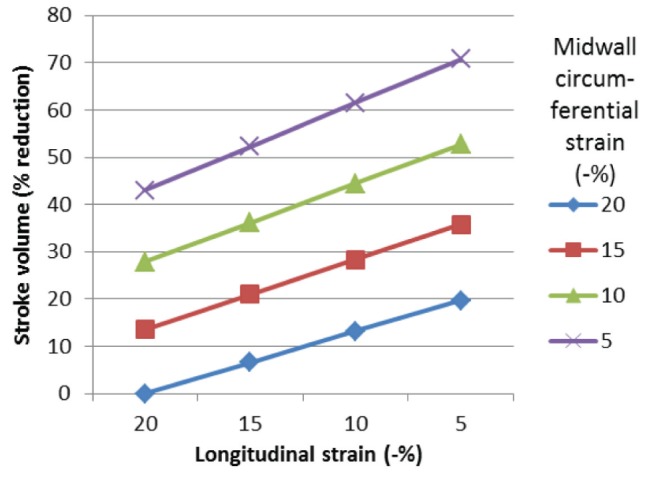
Effect of altering longitudinal and midwall circumferential peak systolic strain on the percentage reduction in stroke volume (normal wall thickness). Reducing circumferential strain results in a greater percentage reduction in stroke volume than longitudinal strain
Quantified changes in fractional shortening are shown in Figure 6. Increasing myocardial strain resulted in increasing fractional shortening. However, the fractional shortening change was much greater in the endocardium than in the epicardium.
Figure 6).
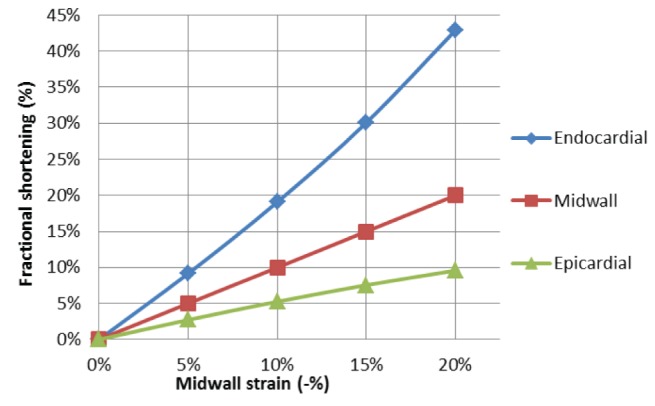
Effect of strain on endocardial, midwall and epicardial fractional shortening (normal wall thickness). Note longitudinal and midwall strains are the same and midwall fractional shortening is identical to circumferential strain. As strain increases, endocardial fractional shortening rises to a greater extent than epicardial fractional shortening (see Table 1 for clinical trial results)
DISCUSSION
The present study used mathematical modelling to accurately assess the independent effects of peak systolic longitudinal and circumferential strain on left ventricular stroke volume and ejection fraction while controlling for both confounding factors and epiphenomena. The modelling shows that midwall circumferential shortening has a greater influence on ejection fraction and stroke volume than longitudinal shortening.
The results of the present study differ from those reported in previous studies that have used either epicardial or endocardial shortening as a surrogate for circumferential function. The current study challenges earlier studies that used epicardial fractional shortening to assess circumferential ‘function’ (27–29,35,36). Using the FSep will result in the underestimation of the contribution of circumferential shortening to stroke volume, as is shown in Figure 1. In contrast, studies using the endocardial cross-sectional area (31,32) will underestimate the contribution of longitudinal shortening. Because the circumferential muscle fibres are situated in the midwall, the current approach would appear to be more appropriate.
These findings may be explained as follows: a normal size left ventricle with a length of 10 cm, an end-diastolic dimension of 4.5 cm and a wall thickness of 0.9 cm, will have a midwall circumference of 17 cm. The relative shortening of the midwall circumferential fibres and longitudinal strain are similar at −20%; therefore, the absolute shortening is 34 mm and 20 mm, respectively. Hence, midwall circumferential shortening has a greater influence on absolute wall thickening and, therefore, stroke volume and ejection fraction, than longitudinal shortening. Furthermore, when the left ventricle dilates with a lower ejection fraction, it becomes more spherical so that any contribution from circumferential shortening will increase further relative to longitudinal shortening. However, because longitudinal muscle fibres actually originate near the base and loop around the apex and return to the base, they are longer than the circumferential fibres. Using an unconventional definition of longitudinal shortening, ie, the entire length of the muscle fibres, then this would mean that longitudinal shortening would have a greater effect and exceed that of circumferential shortening.
Subepicardial and subendocardial longitudinal myocardial fibres sandwich the midwall circumferential fibres but with a continuous variation in fibre angle across the wall (3). This supports the concept that the angulated longitudinal fibres could contribute to circumferential shortening as well as longitudinal shortening. It should be emphasised that myocardial shortening occurs as a consequence of a complex interaction between these indistinct ‘layers’ as well as geometric (eg, wall thickness) and loading factors in all three dimensions. The ejection fraction and stroke volume depend on all muscle fibres, regardless of orientation and include a twisting motion. Therefore, it is an oversimplification to assume that either longitudinal or circumferential myocardial shortening alone represents only shortening of aligned myocardial fibres. Myocardial muscle fibres are not directed in an endocardial-epicardial orientation because this would hinder radial thickening. In addition to an inward epicardial movement, the overall myocardial fibre shortening in two orthogonal planes (and all angles between) results in wall thickening in a third direction, ie, radially. This results in endocardial displacement and the generation of the stroke volume. Endocardial displacement and, therefore, FSen are not direct measures of myocardial shortening but arise dominantly as a consequence of absolute wall thickening. Similarly, ejection fraction is only partly determined by relative myocardial shortening (strain) independent of loading conditions.
Clinical implications
These conclusions are of significant clinical relevance. Strain and fractional shortening each measure the same biomechanical process. Both fractional shortening and strain vary gradually and significantly between the epicardial and endocardial surfaces (Figure 1). Global circumferential strain measured by tissue tracking will average the strain across the wall, potentially leading to important measurement errors. This may explain the lack of significance reported in clinical trials (17). It is often assumed that longitudinal shortening is more important than circumferential shortening, perhaps because it is more at risk of subendocardial ischaemia. However, the clinical data presented in Tables 1 and 2 do not support this notion. The misconception may have arisen because longitudinal strain and long-axis shortening velocities are easier to measure and less prone to errors. Radial strain results from the combination of longitudinal and circumferential fibre shortening and, therefore, could be a better measure of either of these measurements alone or even in combination. Measuring FSep will underestimate the circumferential contribution and FSen will overestimate the contribution to ejection fraction and stroke volume. An increased left ventricular wall thickness will result in an amplified FSen. Endocardial fractional shortening and, therefore, ejection fraction are measures of absolute wall thickening and will overestimate true myocardial fibre shortening in hypertrophic left ventricular disease.
Limitations
The present study was based on mathematical modelling with an idealized left ventricular shape that has been shown to mimic findings in clinical practice (5). Variables such as heart rate, body size and presence of valvular regurgitation were not modelled. The modelling does not take into account the potential compensatory mechanism, such as remodelling/dilation, that may arise following the development of reduced stroke volume (14). The study only assessed the relative myocardial shortening rather than cardiomyocyte contractility or other measures of function such as velocity or force of contraction. However, this is also a limitation of in vivo studies. Rotational motion of the left ventricle has not been specifically considered; however, changes in torsion correlate closely with longitudinal strain (42) and stress-corrected strain and torsion are similarly related to ejection fraction (20). Future studies could model twist and assess wall stress in combination with the longitudinal and circumferential shortening. It was unnecessary to model torsion because it would not have an impact on endocardial motion, stroke volume or ejection fraction independently of longitudinal and circumferential shortening.
SUMMARY
The present study is the first to demonstrate the independent effects of the relative contributions of midwall circumferential and longitudinal strains to left ventricular ejection fraction and stroke volume. It is also the only study showing the independent effects of left ventricular hypertrophy on these physiological measures.
By using mathematical modelling, it is possible to assess the independent effects of the variables under investigation and adjust for all other confounding factors, thus circumventing the problems of in vivo studies. The modelling showed a 43% relative reduction in stroke volume when circumferential strain was altered from −20% to −5%, but only a 19% relative reduction in stroke volume when longitudinal strain was similarly reduced. The sole contribution of midwall circumferential shortening was two-thirds and longitudinal shortening was one-third; importantly these values did not change with increasing concentric hypertrophy. The present study’s findings are consonant with observational studies and theoretical considerations (44). The stroke volume is determined by endocardial motion and the volume displaced. Endocardial motion is determined by absolute wall thickening and, to a lesser extent, epicardial motion. Absolute wall thickening is the dominant factor, which in turn is determined by both myocardial strain (relative radial thickening) and end-diastolic wall thickness. Radial thickening is determined by both circumferential and longitudinal myocardial fibre shortening. Absolute midwall circumferential shortening is greater than absolute longitudinal shortening, even though their relative shortening (strain) may be similar. The present study quantifies the larger impact of midwall circumferential shortening on stroke volume and ejection fraction compared with longitudinal shortening.
SUPPLEMENTARY DATA
Stroke volume may be determined by either a change in external or internal volume
SV=IVd−IVs
SV=(EVd−MV)−(EVs−MV); therefore,
SV=EVd−EVs and
SV=(LS×EWd)+(external volume change through epicardial displacement) (see Figure 1)
EVd External volume at end-diastole; EVs External volume at end-systole; EWd External short-axis width at end-diastole; IVd Internal volume at end-diastole; IVs Internal volume at end-systole; MV Muscle volume; LS Absolute longitudinal shortening; SV Stroke volume
Midwall circumferential shortening is the same as midwall fractional shortening.
Assuming circular midwall then circumference = 2πR
Midwall strain= (2πRd−2πRs)/2πRd, therefore
= (Dd−Ds)/Dd
= midwall fractional shortening
Dd Midwall diameter, Ds Midwall diameter end-systole; Rd Midwall radius in end-diastole; Rs Midwall radius at end-systole
Figure 7).
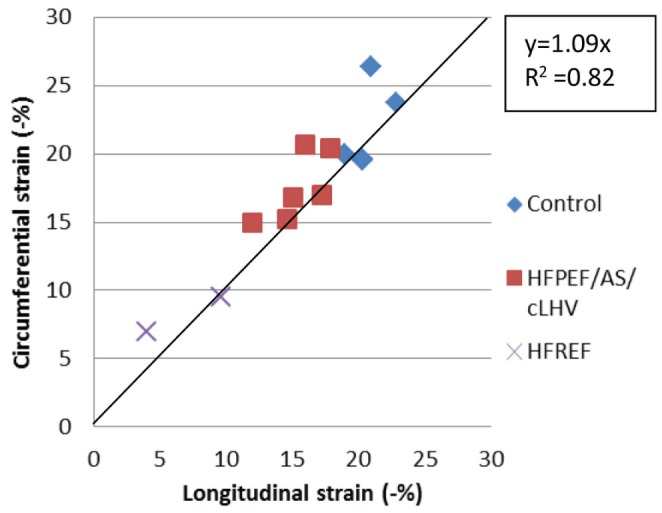
Association of circumferential and longitudinal strain using speckle tracking. AS Aortic stenosis; cLVH Concentric left ventricular hypertrophy; HFPEF Heart failure with a preserved ejection fraction; HFREF Heart failure with a reduced ejection fraction. Plot derived from data presented in Table 2
REFERENCES
- 1.MacIver DH. A mathematical model of left ventricular contraction and its application in heart disease. In: Atherton M, Collins M, Dayer M, editors. Repair and redesign of physiological systems. Boston: WIT Press; 2008. pp. 65–86. [Google Scholar]
- 2.MacIver DH. Current controversies in heart failure with a preserved ejection fraction. Fut Cardiol. 2010;6:97–111. doi: 10.2217/fca.09.56. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RH, Ho SY, Redmann K, Sanchez-Quintana D, Lunkenheimer PP. The anatomical arrangement of the myocardial cells making up the ventricular mass. Eur J Cardiothorac Surg. 2005;28:517–25. doi: 10.1016/j.ejcts.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 4.MacIver DH. Understanding myocardial deformation, ‘global systolic function’ and abnormal geometry. Heart. 2009. eletter:Re: [DOI]
- 5.MacIver DH. A new method for quantification of left ventricular systolic function using a corrected ejection fraction. Eur J Echocardiog. 2011;12:228–34. doi: 10.1093/ejechocard/jeq185. [DOI] [PubMed] [Google Scholar]
- 6.Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995;26:195–202. doi: 10.1016/0735-1097(95)00153-q. [DOI] [PubMed] [Google Scholar]
- 7.Ballo P, Mondillo S, Guerrini F, et al. Midwall mechanics in physiologic and hypertensive concentric hypertrophy. J Am Soc Echocardiogr. 2004;17:418–27. doi: 10.1016/j.echo.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation. 1979;59:1024. doi: 10.1161/01.cir.59.5.1024. [DOI] [PubMed] [Google Scholar]
- 9.Palmon LC, Reichek N, Yeon SB, et al. Intramural myocardial shortening in hypertensive left ventricular hypertrophy with normal pump function. Circulation. 1994;89:122–31. doi: 10.1161/01.cir.89.1.122. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu G, Hirota Y, Kita Y, et al. Left ventricular midwall mechanics in systemic arterial hypertension. Myocardial function is depressed in pressure-overload hypertrophy. Circulation. 1991;83:1676–84. doi: 10.1161/01.cir.83.5.1676. [DOI] [PubMed] [Google Scholar]
- 11.Vinch CS, Aurigemma GP, Simon HU, et al. Analysis of left ventricular systolic function using midwall mechanics in patients >60 years of age with hypertensive heart disease and heart failure. Am J Cardiol. 2005;96:1299–303. doi: 10.1016/j.amjcard.2005.06.076. [DOI] [PubMed] [Google Scholar]
- 12.Maurer MS, Burkhoff D, Fried LP, et al. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction. The cardiovascular health study. J Am Coll Cardiol. 2007;49:972–81. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 13.García EH, Perna ER, Farías EF, et al. Reduced systolic performance by tissue doppler in patients with preserved and abnormal ejection fraction: New insights in chronic heart failure. Int J Cardiol. 2006;108:181–8. doi: 10.1016/j.ijcard.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 14.MacIver DH. Is remodeling the dominant compensatory mechanism in both chronic heart failure with preserved and reduced left ventricular ejection fraction? Basic Res Cardiol. 2010;105:227–34. doi: 10.1007/s00395-009-0063-x. [DOI] [PubMed] [Google Scholar]
- 15.MacIver DH, Townsend M. A novel mechanism of heart failure with normal ejection fraction. Heart. 2008;94:446–9. doi: 10.1136/hrt.2006.114082. [DOI] [PubMed] [Google Scholar]
- 16.Clark NR, Reichek N, Bergey P, et al. Circumferential myocardial shortening in the normal human left ventricle. Assessment by magnetic resonance imaging using spatial modulation of magnetization. Circulation. 1991;84:67. doi: 10.1161/01.cir.84.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–9. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 18.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction: Exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Mizuguchi Y, Oishi Y, Miyoshi H, et al. Concentric left ventricular hypertrophy brings deterioration of systolic longitudinal, circumferential, and radial myocardial deformation in hypertensive patients with preserved left ventricular pump function. J Cardiol. 2010;55:23–33. doi: 10.1016/j.jjcc.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Yip GW-K, Zhang Q, Xie J-M, et al. Resting global and regional left ventricular contractility in patients with heart failure and normal ejection fraction: Insights from speckle-tracking echocardiography. Heart. 2011;97:287–94. doi: 10.1136/hrt.2010.205815. [DOI] [PubMed] [Google Scholar]
- 21.Delgado V, Tops LF, van Bommel RJ, et al. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur Heart J. 2009;30:3037–47. doi: 10.1093/eurheartj/ehp351. [DOI] [PubMed] [Google Scholar]
- 22.Sun JP, Stewart WJ, Yang XS, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103:411–5. doi: 10.1016/j.amjcard.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 23.Serri K, Reant P, Lafitte M, et al. Global and regional myocardial function quantification by two-dimensional strain application in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1175–81. doi: 10.1016/j.jacc.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen JS, Lakkis NM, Bobek J, Goswami R, Dokainish H. Systolic and diastolic myocardial mechanics in patients with cardiac disease and preserved ejection fraction: Impact of left ventricular filling pressure. J Am Soc Echocardiogr. 2010;23:1273–80. doi: 10.1016/j.echo.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Papademetris X, Sinusas AJ, Duncan JS. A coupled deformable model for tracking myocardial borders from real-time echocardiography using an incompressibility constraint. Med Image Anal. 2010;14:429–48. doi: 10.1016/j.media.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballo P, Bocelli A, Mondillo S. What is the actual contribution of atrioventricular plane displacement to left ventricular stroke volume? Am J Physiol Heart Circ Physiol. 2007;293:H1315. doi: 10.1152/ajpheart.00437.2007. [DOI] [PubMed] [Google Scholar]
- 27.Carlsson M, Ugander M, Mosen H, Buhre T, Arheden H. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2007;292:H1452. doi: 10.1152/ajpheart.01148.2006. [DOI] [PubMed] [Google Scholar]
- 28.Carlsson M, Cain P, Holmqvist C, et al. Total heart volume variation throughout the cardiac cycle in humans. Am J Physiol Heart Circ Physiol. 2004;287:243–50. doi: 10.1152/ajpheart.01125.2003. [DOI] [PubMed] [Google Scholar]
- 29.Emilsson K, Brudin L, Wandt B. The mode of left ventricular pumping: Is there an outer contour change in addition to the atrioventricular plane displacement? Clin Physiol. 2001;21:437–46. doi: 10.1046/j.1365-2281.2001.00343.x. [DOI] [PubMed] [Google Scholar]
- 30.Ballo P, Quatrini I, Giacomin E, Motto A, Mondillo S. Circumferential versus longitudinal systolic function in patients with hypertension: A nonlinear relation. J Am Soc Echocardiogr. 2007;20:298–306. doi: 10.1016/j.echo.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Carlhäll C, Wigstrom L, Heiberg E, et al. Contribution of mitral annular excursion and shape dynamics to total left ventricular volume change. Am J Physiol Heart Circ Physiol. 2004;287:H1836. doi: 10.1152/ajpheart.00103.2004. [DOI] [PubMed] [Google Scholar]
- 32.Toumanidis ST, Sideris DA, Papamichael CM, Moulopoulos SD. The role of mitral annulus motion in left ventricular function. Acta Cardiol. 1992;47:331. [PubMed] [Google Scholar]
- 33.Wandt B, Brodin LA, Lundback S. Misinterpretation about the contribution of the left ventricular long-axis shortening to the stroke volume. Am J Physiol Heart Circ Physiol. 2006;291:H2550. doi: 10.1152/ajpheart.00530.2006. [DOI] [PubMed] [Google Scholar]
- 34.Carlhäll C, Wigström L, Heiberg E, et al. Misinterpretation about the contribution of the left ventricular long-axis shortening to the stroke volume reply. Am J Physiol Heart Circ Physiol. 2006;291:H2551–H2552. doi: 10.1152/ajpheart.00530.2006. [DOI] [PubMed] [Google Scholar]
- 35.Ugander M, Carlsson M, Arheden H. Short-axis epicardial volume change is a measure of cardiac left ventricular short-axis function, which is independent of myocardial wall thickness. Am J Physiol Heart Circ Physiol. 2010;298:H530. doi: 10.1152/ajpheart.00153.2009. [DOI] [PubMed] [Google Scholar]
- 36.Carlsson M, Ugander M, Heiberg E, Arheden H. The quantitative relationship between longitudinal and radial function in left, right and total heart pumping in humans. Am J Physiol Heart Circ Physiol. 2007;293:H636–44. doi: 10.1152/ajpheart.01376.2006. [DOI] [PubMed] [Google Scholar]
- 37.Assmann PE, Slager CJ, Dreysse ST, et al. Two-dimensional echocardiographic analysis of the dynamic geometry of the left ventricle: The basis for an improved model of wall motion. J Am Soc Echocardiogr. 1988;1:393–405. doi: 10.1016/s0894-7317(88)80021-x. [DOI] [PubMed] [Google Scholar]
- 38.Lundbäch S. Cardiac pumping and function of the ventricular septum. Acta Physiol Scand Suppl. 1986;50:1–101. [PubMed] [Google Scholar]
- 39.Folland ED, Parisi AF, Moynihan PF, et al. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60:760–6. doi: 10.1161/01.cir.60.4.760. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt HL, Heng MK, Meerbaum S, et al. Cross-sectional echocardiography. II. Analysis of mathematic models for quantifying volume of the formalin-fixed left ventricle. Circulation. 1980;61:1119–25. doi: 10.1161/01.cir.61.6.1119. [DOI] [PubMed] [Google Scholar]
- 41.Emilsson K, Egerlid R, Nygren B, Wandt B. Mitral annulus motion versus long-axis fractional shortening. Exp Clin Cardiol. 2006;11:302–4. [PMC free article] [PubMed] [Google Scholar]
- 42.Carreras F, Garcia-Barnes J, Gil D, et al. Left ventricular torsion and longitudinal shortening: Two fundamental components of myocardial mechanics assessed by tagged cine-MRI in normal subjects. Int J Cardiovasc Imaging. 2011:1–12. doi: 10.1007/s10554-011-9813-6. [DOI] [PubMed] [Google Scholar]
- 43.Koh YS, Jung HO, Park MW, et al. Comparison of left ventricular hypertrophy, fibrosis and dysfunction according to various disease mechanisms such as hypertension, diabetes mellitus and chronic renal failure. J Cardiovasc Ultrasound. 2009;17:127–34. doi: 10.4250/jcu.2009.17.4.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacIver DH, Dayer MJ, Harrison AJI. A general theory of acute and chronic heart failure. Int Cardiology. 2012 doi: 10.1016/j.ijcard.2012.03.093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stroke volume may be determined by either a change in external or internal volume
SV=IVd−IVs
SV=(EVd−MV)−(EVs−MV); therefore,
SV=EVd−EVs and
SV=(LS×EWd)+(external volume change through epicardial displacement) (see Figure 1)
EVd External volume at end-diastole; EVs External volume at end-systole; EWd External short-axis width at end-diastole; IVd Internal volume at end-diastole; IVs Internal volume at end-systole; MV Muscle volume; LS Absolute longitudinal shortening; SV Stroke volume
Midwall circumferential shortening is the same as midwall fractional shortening.
Assuming circular midwall then circumference = 2πR
Midwall strain= (2πRd−2πRs)/2πRd, therefore
= (Dd−Ds)/Dd
= midwall fractional shortening
Dd Midwall diameter, Ds Midwall diameter end-systole; Rd Midwall radius in end-diastole; Rs Midwall radius at end-systole


