Abstract
BACKGROUND:
Several allelic variants of matrix γ-carboxyglutamic acid protein (MGP) can differentially affect the development of certain forms of ischemic heart disease depending on specific characteristics of each population.
OBJECTIVE:
To study the distribution of allelic variants of MGP promoter T−138→C (rs1800802) and G−7→A (rs1800801), and Thr83→Ala exon 4 (rs4236) polymorphisms in a Ukrainian population of patients with acute coronary syndrome (ACS).
METHODS:
Polymerase chain reaction and restriction fragment length polymorphism (RFLP) analysis were used to detect the above-mentioned variants of the MGP gene in 115 patients with ACS and in 140 essentially healthy individuals.
RESULTS:
The distribution of homozygous carriers of a major allelic variant, and heterozygous and homozygous minor allele variants of the T−138→C MGP promoter polymorphism in patients with ACS were 59.8%, 32.7% and 7.5%, respectively. The corresponding distributions of variants in the control group were 54.0%, 41.0% and 5.0%, respectively (P>0.05 [χ2 test]). With respect to the G−7→A polymorphism, the respective distributions were 42.1%, 45.6% and 12.3%, compared with 50.7%, 45.0% and 4.3% in the control group, respectively (P<0.05). Finally, the respective distributions according to the Thr83→Ala exon 4 polymorphism were 42.6%, 43.5% and 13.9%, respectively, compared with 45.3%, 43.0% and 11.7% in the control group. Using logistic regression analysis, it was estimated that the A/A genotype (G−7→A polymorphism) was significantly (P=0.02) associated with ACS (OR 4.302 [95% CI 1.262 to 14.673]).
CONCLUSION:
The allelic A/A promoter variant of MGP G−7→A polymorphism can be considered a risk factor for ACS in the Ukrainian population.
Keywords: Acute coronary syndrome, Matrix Gla protein, Single nucleotide polymorphism
Extracellular calcification is a common and clinically significant component of several important human diseases including atherosclerosis (1–3). In most instances, myocardial infarction (MI) is the consequence of a thrombus forming on a ruptured atherosclerotic plaque, which is frequently associated with calcification (4). Coronary calcification in asymptomatic patients is known to increase the risk of coronary artery disease (CAD), with mineral deposits found in more than 90% of patients with CAD (5,6). Recent studies suggest that in addition to modifiable coronary risk factors, such as hypertension, hyperlipidemia and cigarette smoking, there is a strong genetic background for the development of arterial calcification. The contribution of genetic predisposition to coronary artery calcification has been estimated to be as high as 50% (7).
Matrix γ-carboxyglutamic acid protein (MGP) is known to be a potent inhibitor of calcification in blood vessels and is highly expressed on calcified atherosclerotic plaques in humans; MGP can modify calcification in such plaques and the risk of CAD (8–12). It has been shown that the anticalcifying activity of MGP depends on the γ-carboxylation of specific glutamic acid residues in MGP. This vitamin K-dependent reaction yields γ-carboxyglutamic acid residues, which are then able to bind calcium (13).
The human MGP gene is located on chromosome 12p (14). Among the large number of identified MGP single nucleotide polymorphisms (SNPs) that have been indentified, eight are under the most intensive investigation: two SNPs are located in exons, and six in the upstream region of the MGP gene (15,16). In vitro studies suggest that SNPs in MGP are associated with altered promoter activity (15–17). In addition, there is some evidence that MGP SNPs are associated with arterial calcification (15,18,19), although these data are not consistent (17,20).
There is only one study in which the association of MGP SNPs with acute coronary syndrome (ACS) has been investigated (15). It was shown that some MGP SNPs (G−7→ A, Thr83→Ala) are associated with acute MI, but only in a subgroup of individuals with a low risk for CAD. Such a weak association was revealed by screening MGP polymorphisms among populations in Northern Ireland and France.
The purpose of the present study was to investigate the association of three MGP SNPs (G−7→A, T−138→C and Thr83→Ala) in a population of Ukrainian patients with ACS.
METHODS
Patients
The study recruited 115 ACS patients (70% men and 30% women) 40 to 83 years of age (mean [± SD] age 58.5±0.7 years) admitted to the Reanimation and Intensive Therapy Department of the National Scientific Center “M.D. Strazhesko Institute of Cardiology”, National Medical Academy of Science, Kiev, Ukraine. A final diagnosis of unstable angina pectoris and acute MI was established in 41% and 59% of the patients, respectively. In 23 patients, non-Q wave MI was established on electrocardiogram (ECG), while in 45 patients with MI, peak Q was present on ECG. Diagnosis of acute MI and unstable angina pectoris were established on the basis of clinical, ECG and biochemical examinations according to the recommendations of WHO experts, and also according to recommendations of the European and American cardiology societies (21–23). Excluded from the study group were patients with hereditary and innate diseases, severe metabolic pathologies including severe diabetes mellitus, marked renal and liver failure, deficiencies of the hemostatic system, oncological and systemic pathologies, chronic stage IIB–III heart failure and true cardiogenic shock. The control group consisted of 110 clinically healthy individuals with the absence of cardiovascular pathologies, as confirmed by medical history, ECG, and measurement of arterial pressure and biochemical data. Blood collection was performed under sterile conditions into 2.7 mL tubes (S-Monovette [Sarstedt, Germany]) containing EDTA potassium salt as an anticoagulant (samples were frozen and stored at −20°C.
Identification of allelic MGP gene variants: Polymerase chain reaction with subsequent restriction fragment length polymorphism analysis of the MGP gene
DNA for genotyping was extracted from venous blood using commercially available kits (Isogene Lab Ltd, Russia) according to the manufacturer’s protocol.
MGP promoter T−138→C polymorphism (rs1800802):
The MGP T−138→C polymorphism was determined using polymerase chain reaction (PCR) with subsequent analysis of restriction fragment length. To accomplish this, a specific region of the MGP gene promoter was amplified using a pair of specific primers (Table 1). PCR was performed for 33 cycles in a 25 μL volume containing 50 ng to 100 ng of DNA, 5 μL 5× PCR buffer, 1.5 mM magnesium sulfate, 200 μM of each dNTP, 20 pM of each primer and 0.5 U of Taq DNA polymerase (Fermentas, Lithuania). PCR was performed in a thermocycler GeneAmp PCR System 2700 (Applied Biosystems, USA). Six microlitres (6 μL) of the PCR products (142 bp) were subjected to digestion with 3U BseNI (Fermentas, Lithuania) and incubated at 37°C for 18 h. The presence of thymine at position −138 of the promoter prevented restriction and, in the case of substitution for cytosine, BseNI cleaved the amplified fragment of the promoter into two fragments 118 bp and 24 bp in length (Figure 1).
TABLE 1.
Details of polymerase chain reaction (PCR) and restriction fragment length polymorphism analysis
| Polymorphism | Primers | Annealing temperature (time) | PCR product size, bp | Restriction enzyme | Fragment length after restriction, bp |
|---|---|---|---|---|---|
| T−138→C (rs1800802) | F: 5′-AAGCATACGAТGGCCAAAACTTCTGCA-3′ R: 5′-GAACTAGCAТТGGAACTTTTCCCAACC-3′ |
57°C (1 min) | 142 | BseNI | 118 and 24 |
| G−7→A (rs1800801) | F: 5′-CTAGTTCAGTGCCAACCCTTCCCCACC-3′ R: 5′-TAGCAGCAGTAGGGAGAGAGGCTCCCA-3′ |
64.5°C (45 s) | 500 | NcoI | 240 and 260 |
| Thr83→Ala (rs4236) | F: 5′-TCAATAGGGAAGCCTGTGATG-3′ R: 5′-AGGGGGATACAAAATCAGGTG-3′ |
64.5°C (45 s) | 173 | Eco477 | 127 and 46 |
bp Base pairs; F Forward; R Reverse
Figure 1).
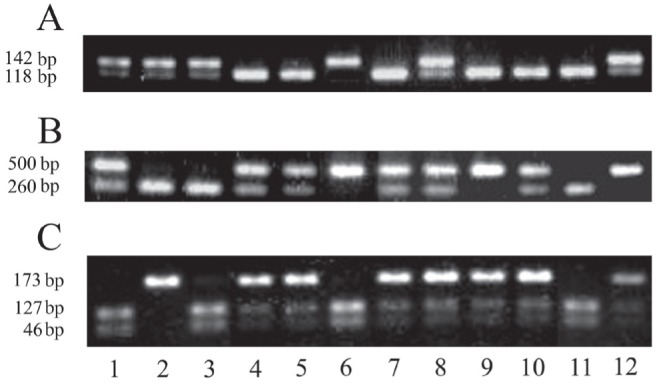
Results of restriction fragment electrophoresis of polymerase chain reaction amplification products of matrix γ-carboxyglutamic acid polymorphisms. A T138→C: lanes 4,5,7,9–11 (T/T genotype); lanes 1–3,8,12 (T/C genotype); 6(C/C genotype). B G7→A: lanes 2,3,11 (G/G genotype); lanes 1,4,5,7,8,10 (G/A genotype); lanes 6,9,12 (A/A genotype). C Thr83→Ala: lanes 1,3,6,11 (Thr/Thr genotype); lanes 4,5,7–10,12 (Thr/Ala genotype); lane 2 (Ala/Ala genotype)
MGP Promoter G−7→A polymorphism (rs1800801):
PCR was performed using primers in 33 cycles in a 25 μL volume (Table 1) containing 50 ng to 100 ng of DNA, 5 μL 5× PCR buffer, 1.5 mM magnesium sulfate, 200 μM of each dNTP, 20 pM of each primer and 0.5 U of Taq DNA polymerase. Six microlitres (6 μL) of the PCR products (500 bp) were subjected to digestion with 2 U NcoI (Fermentas, Lithuania) and incubated at 37°C for 18 h. In the presence of guanine at position −7 of the promoter, the NcoI restriction enzyme produced two fragments 240 bp and 260 bp in length. Substitution of guanine for adenine prevented restriction, thereby precluding cleaving of the amplified fragment of the promoter (500 bp) (Figure 1).
MGP exon 4 Thr83→Ala polymorphism (rs4236):
PCR was performed using specific primer sequences (Table 1) in 33 cycles in a 25 μL volume containing 50 ng to 100 ng of dry DNA, 5 μL 5× PCR buffer, 1.5 mM magnesium sulfate, 200 μM of each dNTP, 20 pM of each primer and 0.5 U of Taq DNA polymerase. Six microlitres (6 μL) of the PCR products (173 bp) were subjected to digestion with 3 U Eco477 (Fermentas, Lithuania) and incubated at 37°C for 18 h. The presence of adenine at position 3748 of exon 4 prevented digestion and, in the case of substitution for thymine, Eco477 cleaved the amplified fragment of exon 4 into two fragments 127 bp and 46 bp in length (Figure 1).
The restriction fragments were separated by electrophoresis and analyzed on an ethidium bromide-stained 2.5% agarose gel visualized using ultraviolet transillumination.
Statistical Analysis
Clinical data were tested for normal distribution using the Shapiro-Wilk test, with assumption of equality of variances analyzed with the Levine test using SPSS version 17.0 (IBM Corporation, USA). The Online Encyclopedia for Genetic Epidemiology studies (www.oege.org/software/hwe-mr-calc.shtml) was used to examine Hardy-Weinberg equilibrium. Additional statistical analysis was performed to assess the independent main and joint effects of all analyzed SNPs. Logistic regression analysis (using SPSS version 17.0) was used to detect the strongest main effect of three SNPs of the MGP gene.
RESULTS
Clinical characteristics
The clinical characteristics of 115 patients with ACS and 140 healthy controls are summarized in Table 2. No differences between the groups were noted with respect to sex, age or glucose concentration. Atherogenic risk factors (including cigarette smoking, overweight, hypertension and total cholesterol) were significantly more prevalent in the patient group.
TABLE 2.
Clinical parameters of acute coronary syndrome (ACS) and healthy (control) subjects
| Parameter |
Subjects
|
|
|---|---|---|
| ACS (n=115) | Control (n=140) | |
| Sex, male/female, n/n | 93/22 | 98/42 |
| Age, years | 58.7±0.5 | 54.0±0.8 |
| Current smokers, % | 36.5 | 25.7 |
| BMI, kg/m2 | 28.9±0.41 | 23.8±1.20* |
| SBP, mmHg | 145.8±2.6 | 131.6±8.3* |
| DBP, mmHg | 88.4±1.5 | 72.8±9.7* |
| TC, mmol/L | 6.3±0.13 | 5.6±0.25* |
| HDL-C, mmol/L | 1.3±0.05 | 1.4±0.10 |
| Glucose, mmol/L | 5.8±0.6 | 4.7±0.7 |
Data presented as mean ± standard error unless otherwise indicated.
P<0.05. BMI Body mass index; DBP Diastolic blood pressure; HDL-C High-density lipoprotein cholesterol; SBP Systolic blood pressure; TC Total cholesterol
Association between manifestations of ACS and MGP polymorphisms
Results of patient genotyping at three sites of the MGP gene are summarized in Table 3. As shown, the distribution of major allele homozygotes, heterozygotes and minor allele homozygotes while analyzing the G−7→A polymorphism of the promoter wwere 42.1%, 45.6% and 12.3% respectively (control group: 50.7%, 45.0% and 4.3%). Analysis of T−138→C promoter polymorphism showed respective figures of 59.8%, 32.7% and 7.5% (control group: 54.0%, 41.0% and 5.0%). Corresponding values for the Thr83→Ala polymorphism (exon 4) were 42.6%, 43.5% and 13.9% (control group: 45.3%, 43.0% and 11.7%). In the ACS group, a minor allele variant A/A (G−7→A polymorphism) was found to occur 2.9-fold more frequently than in controls. Using logistic regression analysis (Table 4), it was estimated that the A/A genotype (G−7→A polymorphism) was significantly associated with ACS (OR 4.302 [95% CI, 1.262 to 14.673]; P=0.02).
TABLE 3.
Genotype comparisons for several MGP gene polymorphisms in patients with acute coronary syndrome (ACS) and control subjects
| Genotype |
Promoter T−138→C
|
Promoter G−7→A
|
Exon 4 Thr83→Ala
|
|||
|---|---|---|---|---|---|---|
| Control (n=139) | ACS (n=107) | Control (n=140) | ACS (n=114) | Control (n=128) | ACS (n=115) | |
| AA | 75 (54.0) | 64 (59.8) | 71 (50.7) | 48 (42.1) | 58 (45.3) | 49 (42.6) |
| Aa | 57 (41.0) | 35 (32.7) | 63 (45.0) | 52 (45.6) | 55 (43.0) | 50 (43.5) |
| aa | 7 (5.0) | 8 (7.5) | 6 (4.3) | 14 (12.3) | 15 (11.7) | 16 (13.9) |
Data presented as n (%). A Major allele; a Minor allele; MGP Matrix γ-carboxyglutamic acid protein
TABLE 4.
Results of logistic regression analysis
| Single-nucleotide polymorphism | Genotype | Coefficient of regression | Standard error | Wald statistic | P | OR (95% CI) |
|---|---|---|---|---|---|---|
| Promoter T−138→C | T/C | −0.185 | 0.287 | 0. 416 | 0.519 | 0.831 (0.474–1.458) |
| C/C | 0.502 | 0.584 | 0.738 | 0.390 | 1.651 (0.526–5.189) | |
| Promoter G−7→A | G/A | 0.431 | 0.351 | 1.502 | 0.220 | 1.538 (0.773–3.064) |
| A/A | 1.459 | 0.626 | 5.434 | 0.020 | 4.302 (1.262–14.673) | |
| Exon 4 Thr83→Ala | Thr/Ala | −0.247 | 0.358 | 0.479 | 0.489 | 0.781 (0.387–1.574) |
| Ala/Ala | −0.402 | 0.509 | 0.623 | 0.430 | 0.669 (0.247–1.814) |
Homozygotes according to major allele were considered as a reference group
DISCUSSION
In the present study, we explored associations between genetic variation in the MGP gene and ACS risk. Analyzing MGP SNPs, we found the G−7→A promoter polymorphism to be associated with ACS in males but not in females. We found no relationship between the other two studied polymorphisms (T−138→C, Thr83→Ala) and ACS in the Ukrainian population.
Herrman et al (15) analyzed MGP polymorphisms in the Etude Cas-Témoin de l’Infarctus du Myocarde study (ECTIM) study, which included MI patients and control subjects from Northern Ireland and France, and the AXA study, which consisted of health volunteers from France. In the ECTIM Study, the genotype distributions did not differ between patients with MI and control subjects. Only in a group of low-risk subjects were the Ala83 and A−7 alleles more frequent in case subjects than in controls. In the AXA study, none of the MGP polymorphisms were related to calcification or atherosclerosis of the carotid artery. On the other hand, the A−7 and Ala83 alleles were associated with femoral calcification in the presence of atherosclerotic plaques. The differences in findings between the carotid and femoral arteries are probably explained by the significantly lower frequency of atherosclerotic/calcified plaques in the carotid than in the femoral arteries. According to these observations, the G−7→A or Thr83→Ala polymorphism could influence the calcification process affecting atherosclerotic plaques, which may contribute to the risk of MI in low-risk individuals. The authors proposed that it would be necessary to verify these results in other studies before any definitive conclusion could be drawn, especially because the associations were observed in subgroups of patients and not in the entire population.
In another study, associations between MGP SNPs and coronary artery calcification (CAC) in older men and women of European descent from Massachusetts (USA) were examined (19). Various methods of analysis revealed that in men, homozygous carriers of the minor allele of G−7→A, T−138→C and Thr83→Ala polymorphisms were associated with a decreased level of CAC relative to major allele carriers. This association was not found in women. In addition, genetic variation in MGP was shown to associate with serum MGP concentrations, but there were no association between serum MGP levels and CAC.
In the Coronary Artery Risk Development in Young Adults (CARDIA) study, a population-based investigation of cardiovascular disease in younger African-American and non-Hispanic white participants, the T−138→C polymorphism of the MGP gene was analyzed for association with the presence or absence of CAC (20). This SNP was also studied in autopsy samples for an association with several measures of atherosclerotic calcification (17). However, no association with the T−138→C polymorphism and measures of vascular calcification was found in either of these studies.
Italian scientists defined the distribution of two MGP polymorphisms (G−7→A, T−138→C) in patients with chronic kidney disease (CKD) and age- and sex-matched healthy controls (18). It was shown that the frequency of the minor A allele (G−7→A polymorphism) and the major T allele (T−138→C polymorphism) was significantly higher in the CKD group versus controls. A/A and T/T homozygotes were associated with cardiovascular events in CKD patients. It was concluded that altered MGP gene polymorphisms may be a negative prognostic factor for the progression to end-stage renal disease and for cardiovascular events in patients with CKD.
The findings presented in the current study are inconsistent with data reported in the literature. In some studies, MGP polymorphisms were shown to be associated with arterial calcification and MI (15,18,19), while in others (17,20), no association between MGP SNPs and cardiovascular events was found. Moreover, in the studies in which such associations were reported, the relationship between the type of MGP polymorphism and arterial calcification was different. For example, in the AXA study, the minor alleles −7A and 83Ala were associated with increased femoral artery calcification (15), while in the study by Crosier et al (19), the same alleles were linked to a decreased level of CAC.
The results of in vitro studies do not clarify the question. In one investigation (15), analysis of MGP promoter activity revealed that the −138C allele reduced promoter activity by 20% in rat vascular smooth muscle cells (VSMC), and by up to 50% in a human fibroblast cell line. Moreover, it was demonstrated that a nuclear protein specifically binds to the region covering the T−138→C polymorphic site, and that binding is enhanced in the presence of the T allele. Thus, the difference in promoter activity might be explained by differential binding of a nuclear protein that is important in MGP transcription. With regard to the G−7→A polymorphism, the findings did not suggest that this SNP may be functional in vitro.
In another study (16), the influence of G−7→A and T−138→C polymorphisms on gene expression was examined by using reporter gene constructs transiently transfected into VSMCs. It was demonstrated that both common polymorphisms independently impacted transcriptional activity of the MGP gene. The −7A variant had 1.5-fold higher activity than the −7G variant, whereas the −138C variant had four-fold higher activity than the −138T variant. On the other hand, it was shown that the −138T allelic variant binds AP-1 complexes and is induced following phorbol 12-myristate 13-acetate treatment, while the −138C variant is refractory to this compound, confirming that AP-1 factors preferentially bind to the −138T variant. It was suggested that the −138C variant provided protection against tissue calcification in VSMC by resulting in higher levels of MGP transcription. Equally, the responsiveness of the −138T site to extracellular stimuli mediated via AP-1 may result in altered susceptibility to calcification. Clearly, these results are not consistent with above-mentioned findings (15).
CONCLUSION
We defined a significant association between the G−7→A promoter polymorphism of the MGP gene and ACS (unstable angina pectoris + acute MI) in the Ukrainian population. The observed association suggests involvement of this polymorphism in CAD; however, these results must be substantiated in other studies focusing on appropriate end points.
REFERENCES
- 1.Bostrom K, Watson KE, Horn S, et al. Bone morphogenetic expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–9. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohr W, Görz E. Imitiert die arteriosklerotische Gefässwandverkalkung die Osteogenese? Pathomorphologische Untersuchungen an arteriosklerotischen Beeten. Z Kardiol. 2002;91:212–32. doi: 10.1007/s003920200015. [DOI] [PubMed] [Google Scholar]
- 3.Bobryshev YV. Calcification of elastic fibers in human atherosclerotic plaque. Atherosclerosis. 2005;180:293–303. doi: 10.1016/j.atherosclerosis.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;326:242–50. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 5.Loecker TH, Schwartz RS, Cotta CW, Hickman JRJ. Fluoroscopic coronary artery calcification and associated coronary disease in asymptomatic young men. J Am Coll Cardiol. 1992;19:1167–72. doi: 10.1016/0735-1097(92)90319-i. [DOI] [PubMed] [Google Scholar]
- 6.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 7.Post W, Bielak LF, Ryan KA, et al. Determinants of coronary artery and aortic calcification in the Old Order Amish. Circulation. 2007;115:717–24. doi: 10.1161/CIRCULATIONAHA.106.637512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanahan CM, Cary NR, Metcalfe JC, et al. High expression of genes for calcification regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallin R, Cain D, Sane DC. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells: A cell system which resembles the system in bone cells. Thromb Haemostat. 1999;82:1764–7. [PubMed] [Google Scholar]
- 10.Shearer MJ. Role of vitamin K and Gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Metab Care. 2000;3:433–8. doi: 10.1097/00075197-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 12.Doherty TM, Fitzpatrick LA, Inoue D, et al. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocrine Rev. 2004;25:629–72. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 13.Murshed M, Schinke T, McKee MD, Karsenty G. Extra cellular matrix mineralization is regulated locally: Different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–30. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancela L, Hsiehg CL, Francket U, Price PA. Molecular structure, chromosome assignment, and promoter organization of the human matrix Gla protein gene. J Biol Chem. 1990;265:15040–8. [PubMed] [Google Scholar]
- 15.Herrmann SM, Whatling C, Brand E, et al. Polymorphisms of the human matrix Gla protein (MGP) gene, vascular calcification, and myocardial infarction. Arterioscler Thromb Vasc Biol. 2000;20:2386–93. doi: 10.1161/01.atv.20.11.2386. [DOI] [PubMed] [Google Scholar]
- 16.Farzaneh-Far A, Davies JD, Braam LA, et al. A polymorphism of the human matrix γ-carboxyglutamic acid protein promoter alters binding of an activating protein-1 complex and is associated with altered transcription and serum levels. J Biol Chem. 2001;276:32466–73. doi: 10.1074/jbc.M104909200. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N, Kitazawa R, Maeda S, et al. T-138C polymorphism of matrix Gla protein promoter alters its expression but is not directly associated with atherosclerotic vascular calcification. Kobe J Med Sci. 2004;50:69–81. [PubMed] [Google Scholar]
- 18.Brancaccio D, Biondi ML, Gallieni M, et al. Matrix Gla protein gene polymorphisms: Clinical correlates and cardiovascular mortality in chronic kidney disease patients. Am J Nephrol. 2005;25:548–52. doi: 10.1159/000088809. [DOI] [PubMed] [Google Scholar]
- 19.Crosier MD, Booth SL, Peter I, et al. Matrix Gla protein polymorphisms are associated with coronary artery calcification. J Nutr Sci Vitaminol. 2009;55:59–65. doi: 10.3177/jnsv.55.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor BC, Schreiner PJ, Doherty TM, et al. Matrix Gla protein and osteopontin genetic associations with coronary artery calcification and bone density: The CARDIA study. Hum Genet. 2005;116:525–8. doi: 10.1007/s00439-005-1258-3. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand ME, Simoons ML, Fox KAA, et al. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. The Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology. Eur Heart J. 2002;23:1809–40. doi: 10.1053/euhj.2002.3385. [DOI] [PubMed] [Google Scholar]
- 22.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. J Am Coll Cardiol. 2004;44:E1–211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert J, White H, et al. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–38. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]


