Abstract
BACKGROUND:
An inflammatory response and systemic oxidative stress are directly caused by coronary artery bypass grafting (CABG) surgery. Cytokines, such as interleukin (IL) 1β, IL-6 and tumour necrosis factor-α (TNF-α), can also be stimulated. Reducing the release of pro-inflammatory cytokines plays an important role in limiting the postoperative inflammatory response. Silymarin has strong anti-inflammatory, antioxidant and cytoprotective properties.
OBJECTIVE:
To investigate the protective anti-inflammatory and antioxidant properties of silymarin against the inflammation and oxidative stresss inherent to CABG surgery.
METHODS:
Of the 102 patients undergoing elective first-time CABG surgery that were recruited, 50 (49.02%) received silymarin treatment and 52 (50.9%) were controls. Plasma cytokine levels (IL-1β, IL-6 and TNF-α) were measured preoperatively, 6 h and 24 h after CABG surgery. C-reactive protein (CRP) levels, trolox equivalent antioxidant capacity (TEAC) and glutathione (GSH) and malondialdehyde (MDA) levels were analyzed.
RESULTS:
Postoperative cytokine levels in the silymarin group were significantly lower compared with preoperative levels, and were significantly lower compared with postoperative control group levels. The area under the curve for cytokines and CRP for the silymarin group were significantly lower compared with preoperative levels, and were significantly lower compared with postoperative control group levels. Postoperative levels of TEAC and MDA in the silymarin-treated group were significantly lower than in the control group. GSH levels were significantly elevated in the silymarin group compared with control. No side effects or mortality were associated with the use of silymarin.
CONCLUSION:
The anti-inflammatory and antioxidant effects of silymarin treatment provided protection against reperfusion injury and inflammation after CABG surgery.
Keywords: CABG, Cytokines, Silymarin
Coronary artery bypass grafting (CABG) surgery with cardiopulmonary bypass (CPB) and cardioplegic cardiac arrest evokes an inflammatory response that may cause temporary organ dysfunction and affect the postoperative course (1,2). The inflammatory response after conventional CABG surgery is closely associated with low blood pressure, increased heart rate, increased body temperature, leukocytosis and tissue edema. These phenomena are accompanied by the activation of the cytokine system, including interleukin (IL)-6, IL-10, IL-2 receptor and tumour necrosis factor-alpha (TNF-α) (3). The inflammatory response and systemic oxidative stress are reported to be directly caused by the procedure (4). The mechanisms explaining these observations may be related to several events that occurr during CPB that are material dependent (eg, exposure of blood to nonphysiological surfaces) or material independent (eg, surgical trauma, ischemia-reperfusion and changes in body temperature) (5). The systemic inflammation observed during and after cardiac surgery and, particularly in CABG procedures, is related to the secretion of a large number of proinflammitory mediators, and to the activation of certain natural defense mechanisms (6). Complement activation and proinflammatory cytokines (eg, IL-1β, TNF-α, IL-6, IL-8 and monocyte chemotactic protein-1) are the major proinflammatory mediators that are involved in the acute inflammatory response to CPB (7). It is conceivable that C-reactive protien (CRP) levels are elevated as a nonspecific surrogate for IL-6 and acts as a sensitive marker of inflammatory response to injury. This highlights the possibility that some other factor, apart from IL-6 (eg, IL-1β, TNF-α, hepatocyte growth factor, etc), might also have a regulating influence (8). The inflammatory response is initiated by a large number of processes that act on both the cellular and humoral elements of blood. Some cytokines, such as IL-1β, IL-6 or TNF-α, which can be stimulated by a broad spectrum of agents, are able to act on a large number of effectors (9), thus, they may reflect the status of the inflammatory response in the situation where multiple initiating processes are involved. Cytokines appear to mediate a large number of cellular events that contribute to injurious processes after CBP (10). Several strategies, including the use of steroids (11), aprotinin (12), heparin-coated CPB circuits (13) and hemofiltration (14), have been reported to reduce the inflammatory reaction induced by CPB and its consequences. Silymarin is a polyphenolic flavonoid extracted from milk thistle that has strong antioxidant activity and exhibits cytoprotective, anti-inflammatory and anticarcinogenic properties (15,16). In addition to its free radical scavenging properties, silymarin increases antioxidant enzyme levels, such as superoxide dismutase and catalase, and inhibits lipid peroxidation (16–18). Silymarin has a good safety profile (19). The aim of the present study was to determine the anti-inflammatory and antioxidant effects of silymarin, using markers of inflammation (IL-1β, TNF-α, IL-6) and oxidative stress (glutathione [GSH]), trolox equivalent antioxidant capacity [TEAC], malondialdehyde [MDA]) in patients undergoing CABG surgery, and also to investigate the presence of any side effects and mortality associated with its use. To date, the protective effects and the efficacy of silymarin on pre- and post-CABG cytokine (IL-1β, IL-6, TNF-α) responses has not been investigated. To the best of our knowledge, the present study was the first to use silymarin as a protective, anti-inflammatory, antioxidant agent to decrease or abolish the reperfusion injury and inflammation resulting from CABG surgery.
METHODS
Patients
Patients undergoing elective first-time CABG surgery in the Iraqi Centre for Heart Diseases (Baghdad, Iraq) were invited to participate in the present study; all patients gave written informed consent. Patients undergoing additional surgical procedures (eg, valvular surgery or aneurysmectomy) were excluded. The protocol was approved by the institutional review committee.
All patients were taking 75 mg/day of acetylsalicylic acid, but stopped 10 days before surgery. All other drugs were withheld on the morning of surgery. Silymarin treatment (140 mg × three capsules per day) was given three days before surgery. None of the patients had any clinical evidence of active inflammatory or immunomodulatory disease (eg, acute coronary syndromes, malignancy, intercurrent infection, renal failure or autoimmune disease) at recruitment. No anti-inflammatory or antioxidant agents were given before surgery. Data from patients who experienced significant postoperative complications, even if apparent after the 6 h sample time point, were excluded from analysis because cytokine levels can rise within hours of an inflammatory stimulus. Study exclusion criteria thus included infections requiring antibiotic treatment, prolonged respiratory support, circulatory support or renal failure requiring hemofiltration.
Surgical procedure
CABG surgery was performed using on-pump and off-pump techniques through a midline sternotomy by one of two consultant surgical staff, with subsequent hypothermic CPB using right atrial and ascending aortic cannulation. Myocardial protection was maintained by intermittent cold cross-clamp fibrillation and heparin anticoagulation, which was reversed postoperatively by protamine sulfate. A blood sample was drawn before surgery (with the patient supine and resting) and again 6 h and 24 h after CPB. Venous blood was immediately centrifuged (at 3500 g for 10 min), and the plasma was separated and stored at −20°C until analysis. Apart from routine clinical and biochemical investigations performed by staff blinded to all subject data, specific evaluation of cytokine release (IL-1β, IL-6 and TNF-α) was performed using ELISA with a minimum detectable dose. Interassay and intra-assay coefficients of variation (CV) for cytokine release were 5% and 3%, respectively, and the assay sensitivity was <0.70 pg/mL. CRP levels were measured in patients pre- and postoperatively using a commercially available kit and according to manufacturer instructions (Behring Diagnostics, USA), with an interassay and intra-assay CV were <4% and <2%, respectively, with a detection limit of 0.20 mg/L. Plasma TEAC was measured according to the Rice-Evans and Miller method (20), with an interassay and intra-assay CV of 5% and 14%, respectively. GSH from whole blood was measured using a Ransel Glutathione Peroxidase kit (Randox Laboratories Ltd, United Kingdom). Interassay and intra-assay CV were 2% and 6%, respectively. MDA levels were analyzed according to standard methods (20).
Statistical analysis
All data were analyzed using SPSS version 9 (IBM Corporation, USA) for Windows (Microsoft Corporation, USA). Data are shown as means ± SD. The effect of silymarin treatment on cytokines was assessed by ANOVA and the Student’s t test for paired data. One-way analysis of covariance was performed using age, sex, smoking, diabetic status, left ventricular ejection fraction, duration of operation, and CPB and aortic cross-clamp times as covariates. Following backward stepwise linear regression modelling, only variables that had a significant effect on cytokine concentrations were included in the final model. Correlations between variables were tested by Pearson’s analysis. To compare the predictive role of different values of the studied parameters, ROC curves were constructed and the area under the curve (AUC) was calculated. Statistical significance was set at P<0.05.
RESULTS
A total of 106 CABG patients who fulfilled the inclusion criteria participated in the present study. Four patients were excluded because of complications. The final study group was comprised of 102 patients; 50 patients (49.02%) received silymarin treatment before CABG surgery and 52 patients (50.9%) had no treatment.
Clinical and patient characteristics, including age, sex, and body mass index for the two groups, are compared in Table 1. Perioperative surgical data showed no significant differences (Table 2). There were significant differences between diabetic and hypertensive patients, smokers and nonsmokers, but none of these factors were associated with a significant effect on cytokine concentrations (data not shown).
TABLE 1.
Patient characteristics
|
Group
|
P | ||
|---|---|---|---|
| Silymarin | Control | ||
| Patients, n | 50 | 52 | >0.05 |
| Male/female, n/n | 42/8 | 45/7 | >0.05 |
| Body mass index, kg/m2 | 27.5±3 | 27.9±4 | >0.05 |
| Age, years, median (range) | 60 (44–76) | 66 (58–74) | >0.05 |
| Body surface area (m2) | 2.4±0.2 | 2.5±0.1 | >0.05 |
| Left ventricle ejection fraction | 54±2 | 48±5 | <0.05 |
| Current smoker, % | 15 | 19 | >0.05 |
| Treated hypertension, % | 49 | 58 | <0.05 |
| Diabetes mellitus, % | 15 | 20 | <0.05 |
Data presented as mean ± SD unless otherwise indicated
TABLE 2.
Surgical parameters
|
Group
|
P | ||
|---|---|---|---|
| Silymarin | Control | ||
| Operative time, min | 208±22 | 200±35 | >0.05 |
| Grafts, n | 2.7±0.1 | 2.8±0.1 | >0.05 |
| Intubation time, h | 9.5±2.5 | 8.5±2 | >0.05 |
| Longest clamp time, min | 12±3 | 10±1 | >0.05 |
| Cross-clamp time, min | 33±12 | 25±16 | >0.05 |
| Pump time, min | 120±16 | 110±20 | >0.05 |
| Reperfusion time, min | 51±12 | 53±15 | >0.05 |
| Hospitalization, days, median (range) | 6 (5–7) | 10 (9–11) | <0.05 |
Data presented as mean ± SD unless otherwise indicated
Surgery was associated with a significant increase in cytokine concentrations, P<0.0005 (paired t test). The correlation between pre- and post-CABG cytokine levels (IL-1β, IL-6 and TNF-α) between the silymarin-treated and control groups, showed significant differences (Figures 1, 2 and 3). Postoperative cytokine concentrations were lowered significantly in the silymarin-treated group compared with preoperative levels. There were no significant differences between pre- and postoperative values in the control group. The effect of silymarin treatment on post-CABG cytokine concentrations remained significant after multivariate analysis (P=0.008 [analysis of covariance]). The area under the ROC curve for the silymarin-treated group was significantly lower than the AUC for the control group (Figure 4). CRP levels decreased significantly in the silymarin-treated group postoperatively compared with the control group (Figure 5). TEAC decreased in the control group throughout the surgery, with a peak 6 h after surgery, while there were significantly elevated values post-CABG in the silymarin-treated group (Figure 6). GSH levels showed significant elevation in the silymarin-treated group (P=0.001) postoperatively, however; the control group showed no significant change in GSH values postoperatively (P>0.05) (Figure 7). MDA levels showd a significant reduction postoperatively in the silymarin-treated group (P=0.005), while the control group had increased MDA levels, although not statistically significant (P=0.7) (Figure 8). Results demonstrating significantly lower cytokine levels in the silymarin-treated group compared with the control group are shown in Figure 9.
Figure 1).
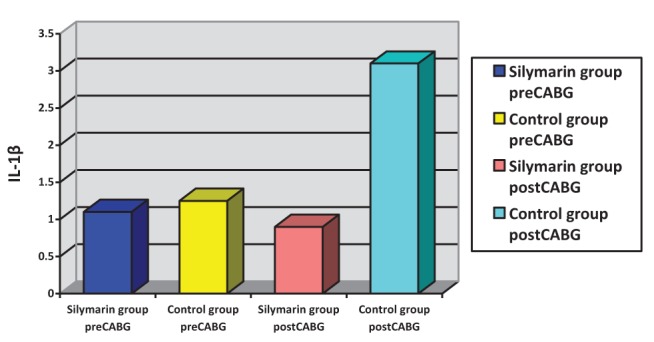
Interleukin (IL)-1β plasma levels, pre- and postcoronary artery bypass grafting (CABG), for silymarin-treated and control groups
Figure 2).
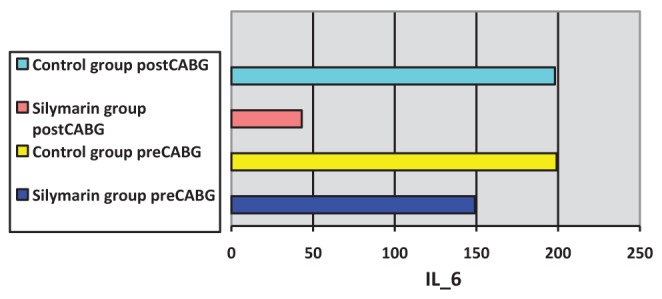
Interleukin (IL)-6 plasma levels pre- and postcoronary artery bypass grafting (CABG), for silymarin-treated and control groups
Figure 3).
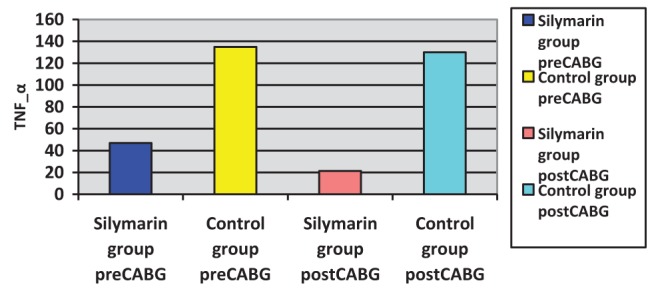
Tumour necrosis factor-alpha (TNF-α) plasma levels pre- and postcoronary artery bypass grafting (CABG), for silymarin-treated and control groups
Figure 4).
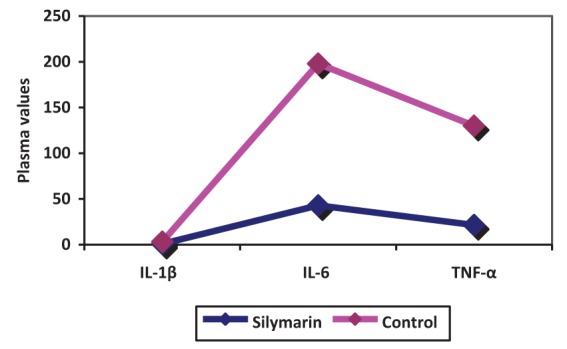
Interleukin (IL)-1ß, IL-6 and tumour necrosis factor-alpha (TNF-α) plasma levels pre- and postcoronary artery bypass grafting (CABG), for silymarin-treated and control groups
Figure 5).
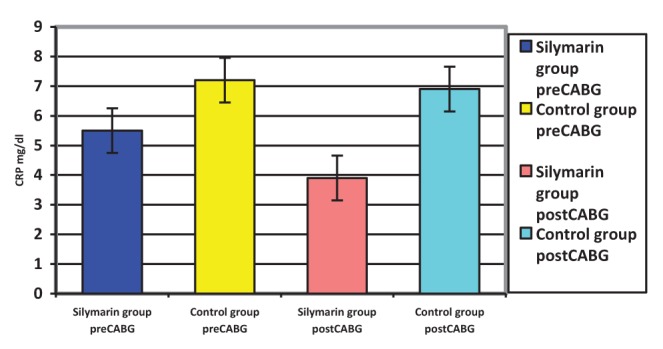
Serum C-reactive protein (CRP) levels pre- and postcoronary artery bypass grafting (CABG), for silymarin-treated and control groups
Figure 6).
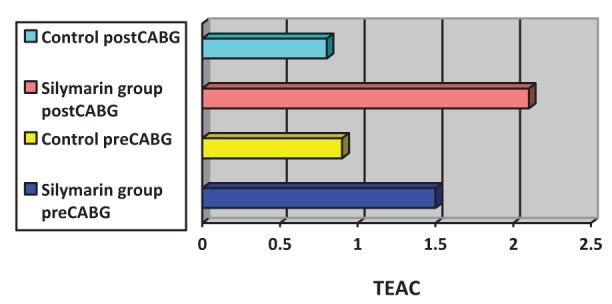
Plasma trolox equivalent antioxidant capacity (TEAC) pre- and postcoronary artery bypass grafting (CABG), for silymarin-treated and control groups
Figure 7).
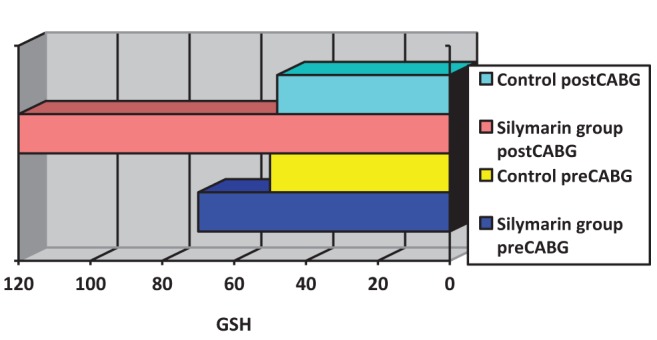
Blood glutathione (GSH) levels pre- and postcoronary artery bypass grafting (CABG), for silymarin-treated and control groups
Figure 8).
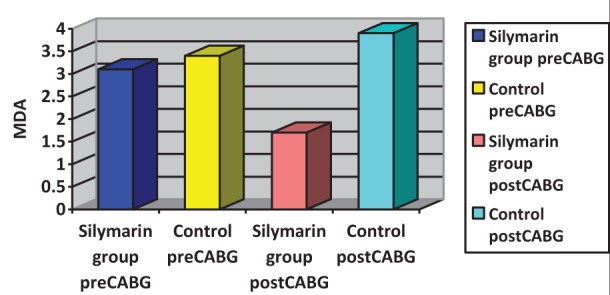
Malondialdehyde (MDA) plasma levels pre- and postcoronary artery bypass grafting (CABG), for silymarin-treated and control groups
Figure 9).
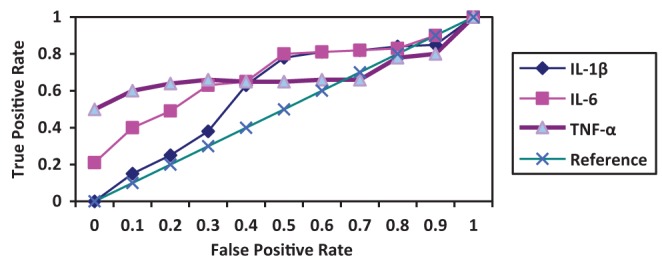
ROC curve of the performance of plasma cytokines, which were significantly lower following coronary artery bypass grafting in the silymarin-treated group compared with controls. IL Interleukin; TNF Tumour necrosis factor
There were no deaths, complications, or side effects among the two groups of patients.
DISCUSSION
The acute-phase response following CABG surgery is associated with the induction and release of cytokines (21–23). Investigations have shown that reduction of the inflammatory response is important (24), because the inflammatory response affects morbidity and mortality. Silymarin has cytoprotective, antioxidant and anti-inflammatory properties, and it can raise the activity levels of the free radical scavenging enzymes superoxide dismutase and glutathione peroxidase (25). It has been demonstrated that silymarin acts as an antioxidant by reducing free radical-mediated damage in tissues and inhibiting lipid peroxidation. It has been reported to have multiple pharmacological activities including hepatoprotectant, antibacterial, antiallergic, antiviral and antineoplastic effects (18). Cytokines are essential for immunological and physiological homeostasis, and they are produced in response to a variety of physiological and pathological stimuli (26). IL-1β and TNF-α are early response cytokines that are promptly produced at the site of infection or injury by resident macrophages (27). IL-6 is a proinflammatory cytokine with plasma concentrations that have been noted to increase in response to many major surgeries, as well as after cardiac surgery with CPB (28). The significantly increased levels of cytokines (ie, IL-1β, IL-6 and TNF-α) found during CABG surgery in the present study are consistent with those reported in previous studies. The use of the anti-inflammatory agent silymarin before CABG surgery significantly decreases cytokine release (Figures 1,2,3). The molecular bases of the anti-inflammatory and anticarcinogenic effect of silibinin/silymarin might be related to the inhibition of the transcription factor nuclear factor kappa B (NF-κB), which regulates and coordinates the expression of various genes involved in the inflammatory process, cytoprotection and carcinogenesis. In particular, NF-κB contributes to the production of IL-1 and IL-6, TNF-α, lymphotoxin, granulocyte-macrophage colony-stimulating factor and interferon gamma. Furthermore, some of these cytokines, including IL-1 and TNF-α, activate NF-κB themselves, thus creating a positive feedback loop. Interruption of this loop by silymarin might be responsible for its chronic anti-inflammatory activity (19). The area under the ROC curves of the three cytokines was significantly smaller for the silymarin-treated group compared with controls. In a nonrandomized, prospective study, Fransen et al (29) examined lipopolysaccharide binding protein and CRP as markers of the acute phase response in patients undergoing CABG, with or without CPB. There was a significant increase in lipopolysaccharide binding protein and CRP in both groups (29). In the present study, CRP levels were elevated in the control group, but there was a significant decrease of CRP levels in the silymarin group postoperatively compared with the control group, which is in agreement with the study by Fransen et al (29). After surgery, peak IL-6 levels were strongly correlated with peak CRP levels (r=0.34, P=0.0009). The systemic increase in oxidative stress during CABG is well-documented (30). All sources of reactive oxygen species during CABG surgery will ultimately lead to depletion of plasma antioxidants (31). The ischemic/reperfused heart is a major source of inflammatory cytokines and reactive oxidants (32). The production of reactive oxygen species during surgery decreased with the use of silymarin, which acts as an antioxidant, free radical scavenging agent. TEAC, a marker of total antioxidant capacity in the plasma used in the present study, decreased in the control group, demonstrating a depletion of plasma antioxidants, but increased significantly in the silymarin group because of the increased levels of plasma antioxidants (Figure 5). The activity of the enzymatic antioxidant glutathione peroxidase increased significantly and could explain the time course of depletion of GSH, because glutathione peroxidase uses GSH as a cofactor (33). This parallelism suggests that glutathione peroxidase forms a first barrier against the reactive oxygen species being formed during the operation. These findings confirm the results reported by Arduini et al (34), who also found an increase in glutathione peroxidase activity. The effect of silymarin on glutathione levels showed a significant increase in the silymarin-treated group compared with control (Figure 6). Direct measurements of oxygen free radicals are difficult in vivo, but various indirect methods have been used to show increased oxygen free radical activity during and after CPB (35–41). Prasad et al (38) showed increased levels of MDA, a lipid peroxidation product, indicating free radical-mediated damage to the lipid membrane. In the present study, treatment of CABG surgery patients with silymarin produced significant reduction of serum MDA levels compared with the control group (Figure 7). The present study is consistent with previous studies in that free radicals are produced during cardiac procedures, and that the reduction in the observed oxidative stress may be alleviated by the use of antioxidant agents, such as silymarin, which protects the cell from harmful free radicals (Figures 6, 7 and 8). These antioxidant effects, along with the anti-inflammatory effects, appear to be involved in the protective action of silymarin.
The period of hospitalization decreased significantly in the silymarin group (5 to 7 days) compared with the control group (nine to 11 days) after CABG surgery (Data not shown).
CONCLUSION
Preoperative silymarin treatment appears to reduce the inflammation and oxidative stresss inherent to CABG surgery. Further studies are needed to evaluate the effect of silymarin treatment on other cytokines and to compare the cardioprotection of silymarin compared with other anti-inflammatory, antioxidant agents.
Acknowledgments
The author thanks all the patients who so kindly participated, D Imad A Jamal consultant cardiac surgeon, the Cardiac centre and all the staff.
REFERENCES
- 1.Gu YJ, Mariani MA, van Oeveren W, et al. Reduction of the inflammatory response in patients undergoing minimally invasive coronary artery bypass grafting. Ann Thorac Surg. 1998;65:420–4. doi: 10.1016/s0003-4975(97)01127-2. [DOI] [PubMed] [Google Scholar]
- 2.Taylor KM. SIRS: the systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg. 1996;61:1607–8. doi: 10.1016/0003-4975(96)00225-1. [DOI] [PubMed] [Google Scholar]
- 3.Fransen EJ, Maessen JG, Hermens WT, Glatz JF, Buurman WA. Peri-operative myocardial tissue injury and the release of the inflammatory mediators in coronary artery bypass graft patients. Cardiovasc Res. 2000;45:853–9. doi: 10.1016/s0008-6363(99)00403-4. [DOI] [PubMed] [Google Scholar]
- 4.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: Mechanism involved and possible therapeutic strategies. Chest. 1997;112:676–92. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 5.Kotani N, Hashimoto H, Sessler DI, et al. Cardiopulmonary bypass produces greater pulmonary than systemic proinflammatory cytokines. Anesth Analg. 2000;90:1039–45. doi: 10.1097/00000539-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Massoudy P, Zahler S, Barankay A, Braun SL, Becker BF, Meisner H. Reperfusion of the lungs induces cytokine production and activation of leukocytes in CABG patients. Br J Anaesth. 1999;82:A50. (Abst) [Google Scholar]
- 7.Zahler S, Massoudy P, Becker BF, Hartl H, HaÈhnel C, Meisner H. Acute cardiac inflammatory responses to post ischemic reperfusion during cardiopulmonary bypass. Cardiovasc Res. 1999;41:722–30. doi: 10.1016/s0008-6363(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 8.Ping LI, Sanders J, Hawe E, Brull D, Montgomery H, Humphries S. Inflammatory response to coronary artery bypass surgery: Does the heme-oxygenase-1 gene microsatellite polymorphism play a role. Chin Med J. 2005;118:1285–90. [PubMed] [Google Scholar]
- 9.Drew CE, Cliffe P, Scurr CF, et al. Experimental approach to visual intracardiac surgery, using an extracorporeal circulation. BMJ. 1957;2:1323–9. doi: 10.1136/bmj.2.5057.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drew CE, Keen G, Benazon DB. Profound hypothermia. Lancet. 1959;1:746–7. doi: 10.1016/s0140-6736(59)91824-0. [DOI] [PubMed] [Google Scholar]
- 11.Hill GE, Alonso A, Spurzem JR, Stammers AH, Robbins RA. Aprotinin and methyprednisolone equally blunt cardiopulmonary bypass-induced inflammation in humans. J Thorac Cardiovasc Surg. 1995;110:1658–62. doi: 10.1016/S0022-5223(95)70027-7. [DOI] [PubMed] [Google Scholar]
- 12.Hill GE, Pohorecki R, Alonso A, Rennard SI, Robbins RA. Aprotinin reduces interleukin-8 production and lung neutrophil accumulation after cardiopulmonary bypass. Anesth Analg. 1996;83:696–700. doi: 10.1097/00000539-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Gu YJ, van Oeveren W, Akkerman C, Boonstra PW, Huyzen RJ, Wildervuur CRH. Heparin-coated circuits reduce the inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55:917–22. doi: 10.1016/0003-4975(93)90117-z. [DOI] [PubMed] [Google Scholar]
- 14.Millar AB, Armstrong L, van der Linden J, et al. Cytokine production and hemofiltration in children undergoing cardiopulmonary bypass. Ann Thorac Surg. 1993;56:1499–502. doi: 10.1016/0003-4975(93)90740-9. [DOI] [PubMed] [Google Scholar]
- 15.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:556–66. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Lahiri-Chatterjee M, Sharma Y, Agarwal R. Inhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skin. Carcinogenesis. 2000;21:811–6. doi: 10.1093/carcin/21.4.811. [DOI] [PubMed] [Google Scholar]
- 17.Soto C, Recoba R, Barron H, Alvarez C, Favari L. Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp Biochem Physiol C Toxicol Pharmacol. 2003;136:205–12. doi: 10.1016/s1532-0456(03)00214-x. [DOI] [PubMed] [Google Scholar]
- 18.Bosisio E, Benelli C, Pirola O. Effect of the flavanolignans of Silybum marianum L. on lipid peroxidation in rat liver microsomes and freshly isolated hepatocytes. Pharmacol Res. 1992;25:147–54. doi: 10.1016/1043-6618(92)91383-r. [DOI] [PubMed] [Google Scholar]
- 19.Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kB, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800–9. [PubMed] [Google Scholar]
- 20.Rice-Evans C, Miller N. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994;234:279–93. doi: 10.1016/0076-6879(94)34095-1. [DOI] [PubMed] [Google Scholar]
- 21.Buege JA, Aust SD. Microsomal lipid peroxidation. Method Enzymol. 1978;51:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 22.Whitten CW, Hill GE, Ivy R, et al. Does the duration of cardiopulmonary bypass or aortic cross-clamp, in the absence of blood and/or blood product administration, influence the IL-6 response to cardiac surgery? Anesth Analg. 1998;86:28–33. doi: 10.1097/00000539-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Liebold A, Langhammer T, Brunger F, et al. Cardiac interleukin-6 release and myocardial recovery after aortic crossclamping. Crystalloid versus blood cardioplegia. J Cardiovasc Surg. 1999;40:633–6. [PubMed] [Google Scholar]
- 24.Corbi P, Rahmati M, Delwail A, et al. Circulating soluble gp130, soluble IL-6R, and IL-6 in patients undergoing cardiac surgery, with or without extracorporeal circulation. Eur J Cardiothorac Surg. 2000;18:98–103. doi: 10.1016/s1010-7940(00)00388-2. [DOI] [PubMed] [Google Scholar]
- 25.Heinnen HA, Ebba H, Rodriguez JL, et al. Relationship of the pro-inflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108:626–35. [PubMed] [Google Scholar]
- 26.Altorjay I, Dalmi L, Sari B, Imre S, Balla G. The effect of silibinin (Legalon) on the free radical scavenger mechanisms of human erythrocytes in vitro. Acta Physiologica Hungarica. 1992;80:375–80. [PubMed] [Google Scholar]
- 27.Miller BE, Levy JH. The inflammatory response to cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:355–66. doi: 10.1016/s1053-0770(97)90106-3. [DOI] [PubMed] [Google Scholar]
- 28.Asimakopoulos G. Systemic inflammation and cardiac surgery: An update. Perfusion. 2001;16:353–60. doi: 10.1177/026765910101600505. [DOI] [PubMed] [Google Scholar]
- 29.Fromesa Yves, Gaillarda Didier, Ponzioa Olivier, et al. Reduction of the inflammatory response following coronary bypass grafting with total minimal extracorporeal circulation. Eur J Cardiothorac Surg. 2002;22:527–33. doi: 10.1016/s1010-7940(02)00372-x. [DOI] [PubMed] [Google Scholar]
- 30.Fransen E, Maessen J, Dentener M, et al. Systemic inflammation present in patients undergoing CABG without extracorporeal circulation. Chest. 1998;113:1290–5. doi: 10.1378/chest.113.5.1290. [DOI] [PubMed] [Google Scholar]
- 31.Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000;47:446–56. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 32.Pyles LA, Fortney JE, Kudlak JJ, Gustafson RA, Einzig S. Plasma antioxidants depletion after cardiopulmonary bypass in operations for congenital heart disease. J Thorac Cardiovasc Surg. 1995;110:165–71. doi: 10.1016/S0022-5223(05)80022-4. [DOI] [PubMed] [Google Scholar]
- 33.Schulze C, Conrad N, Schutz A. Reduced expression of systemic proinflammatory cytokines after off-pump versus conventional coronary artery bypass grafting. Thorac Cardiovasc Surg. 2000;48:364–9. doi: 10.1055/s-2000-8352. [DOI] [PubMed] [Google Scholar]
- 34.De Backer WA, Amsel B, Jorens PG, et al. N-acetylcysteine pretreatment of cardiac surgery patients influences plasma neutrophil elastase and neutrophil influx in bronchoalveolar lavage fluid. Intensive Care Med. 1996;22:900–8. doi: 10.1007/BF02044114. [DOI] [PubMed] [Google Scholar]
- 35.Arduini A, Mezzet A, Porecca E, et al. Effect of ischemia reperfusion on antioxidant enzymes and mitochondrial inner membrane proteins in perfused rat heart. Biochim Biophys Acta. 1988;970:113–21. doi: 10.1016/0167-4889(88)90169-3. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira R, Llesuy S, Milei J, et al. Assessment of myocardial oxidative stress in patients after myocardial revascularization. Am Heart J. 1988;115:307–12. doi: 10.1016/0002-8703(88)90475-9. [DOI] [PubMed] [Google Scholar]
- 37.Weisel RD, Mickle DAG, Finkle CD, et al. Myocardial free-radical injury after cardioplegia. Circulation. 1989;80(Suppl):14–8. [PubMed] [Google Scholar]
- 38.Davies SW, Underwood SM, Wickens DG, et al. Systemic pattern of free radical generation during coronary bypass surgery. Br Heart J. 1990;64:236–40. doi: 10.1136/hrt.64.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad K, Kalra J, Bharadwaj B, et al. Increased oxygen free radical activity in patients on cardiopulmonary bypass undergoing aortocoronary bypass surgery. Am Heart J. 1992;123:37–45. doi: 10.1016/0002-8703(92)90744-g. [DOI] [PubMed] [Google Scholar]
- 40.Davies SW, Duffy JP, Wickens DG, et al. Time-course of free radical activity during coronary artery operations with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;105:979–87. [PubMed] [Google Scholar]
- 41.Toivonen HJ, Ahotupa M. Free radical reaction products and antioxidant capacity in arterial plasma during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1994;108:140–7. [PubMed] [Google Scholar]
- 42.Pesonen EJ, Korpela R, Peltola K, et al. Regional generation of free oxygen radicals during cardiopulmonary bypass in children. J Thorac Cardiovasc Surg. 1995;110:768–73. doi: 10.1016/S0022-5223(95)70110-9. [DOI] [PubMed] [Google Scholar]


