Abstract
Cardiac angiosarcomas are rare, rapidly progressive tumours that often present as diagnostic dilemmas resulting in delayed diagnosis. They should be considered in patients with recurrent pericardial effusions.
A 33-year-old man presented for evaluation of a recurrent pericardial effusion. Infectious and rheumatological workups were negative. Pericardial fluid cytology and pericardial biopsy were unremarkable. Imaging, including echocardiogram and magnetic resonance imaging, were nondiagnostic.
While awaiting surgical intervention, the patient developed respiratory failure requiring urgent intubation. Intraoperatively, he experienced significant hemorrhage from the myocardium. Hemostasis could not be achieved and the patient expired. Pathology reports revealed metastatic angiosarcoma.
The present case illustrates a rare case of primary cardiac angiosarcoma posing a diagnostic dilemma in a young man. The authors present the challenges in diagnosis, and review the most current diagnostic and therapeutic strategies in the care of patients with this condition.
Keywords: Angiosarcoma, Pericardial effusion
Pericardial effusions may be due to an array of medical conditions including infection, malignancy, collagen vascular disease and chest radiation. Those of unclear etiology may represent a diagnostic challenge. Primary cardiac angiosarcomas are rare entities that may present as a recurrent pericardial effusion in a patient with nonspecific symptoms and no pertinent medical history.
The authors report on a young, healthy man who presented with three months of recurrent pericardial effusions. Postmortem, he was diagnosed with metastatic cardiac angiosarcoma. The authors review the clinical challenges raised by patients presenting with recurrent pericardial effusions of unknown etiology, as well as the current diagnostic and therapeutic modalities involved in their management.
CASE PRESENTATION
A 33-year-old man was referred for the evaluation of recurrent pericardial effusions.
Ten weeks earlier, he presented to an outside hospital with chest pain and dypsnea on exertion. His medical and surgical histories were unremarkable, he was not taking prescription or over-the-counter medications, and his family and social history were noncontributory. Initial laboratory values revealed a mildly elevated troponin-I level of 0.046 ng/mL (normal 0.000 ng/mL to 0.033 ng/mL) and d-dimer level of 1.41 mg/L (normal <0.6 mg/L). Computed tomography (CT) of the chest, abdomen and pelvis was remarkable for a 3.5 cm pericardial effusion, a right pleural effusion and a small amount of ascites.
The patient underwent a pericardial biopsy and pericardial window after a transthoracic echocardiogram (TTE) revealed a posterior pericardial effusion with tamponade physiology. Microscopic examination of the pericardial fluid revealed blood with rare reactive mesothelial cells. Cytology was negative for malignancy. Pericardial fluid gram stain and cultures were negative for infection. The pericardial biopsy showed fibrovascular tissue without atypia, significant inflammation or fibrosis. Serologies were negative for hepatitis B, hepatitis C, HIV and Lyme antibody. The patient was discharged home after two days, free from cardiovascular symptoms and with close outpatient follow-up.
Two weeks before his presentation, he developed recurrent dypsnea on exertion and sharp upper abdominal pain. A repeat TTE revealed reaccumulation of the pericardial effusion and a new pericardial mass abutting the right heart chambers. He was taken to the operating room for pericardiectomy. Intraoperative frozen biopsies revealed a severe inflammatory process. A follow-up TTE showed resolution of the pericardial effusion, no evidence of pleural effusion and a preserved left ventricular ejection fraction of 65%. He was again discharged with planned close outpatient follow-up.
One week before his transfer to our facility, the patient noted recurrent dypsnea on exertion. A chest x-ray revealed large bilateral pleural effusions. Thoracentesis was performed and produced gross blood. A chest CT showed interval reaccumulation of the pericardial effusion with compression of the left pulmonary veins. A pericardial drain was placed, which removed 1800 mL of bloody fluid.
On transfer to our institution, the patient was afebrile and normotensive with an oxygen saturation of 99% on room air. The physical examination was notable for a prominent friction rub over the left sternal border and for diffuse rhonchi throughout the lung fields. He had no peripheral edema and no jugular venous distension.
On initial laboratory evaluation, his white blood cell count was 11.3 k/mm3 (normal 4 k/mm3 to 10 k/mm3) and his hemoglobin level was 9.7 g/dL (normal 13.0 g/dL to 17.3 g/dL). His cardiac enzyme levels were within normal limits. A chest CT revealed scattered 2 mm to 4 mm noncalcified lung nodules and a large 4.2 cm × 6.5 cm heterogenous density within the pericardial space with apparent compression of the right-sided cardiac chambers. These findings were confirmed with TTE. Left ventricular function was preserved.
Gated cardiac magnetic resonance imaging (MRI) revealed a large pericardial effusion with heterogenous signal, consistent with blood product. Pleural fluid from a thoracentesis suggested a transudative process with no evidence of infection.
Serologies for HIV, hepatitis, tuberculosis and endemic fungi were negative. A screening laboratory rheumatological evaluation was negative, and a screening malignancy work-up including cancer antigen 19-9, alpha-fetoprotein, the beta subunit of human chorionic gonadotropin, prostate-specific antigen and carcinoembryonic antigen was also negative.
He was discharged nine days later on a prednisone taper (at 60 mg daily) with close outpatient follow-up and a presumptive diagnosis of autoimmune serositis.
One week postdischarge, he presented to his primary care physician with complaints of dypsnea, peripheral edema, increased abdominal girth and a 9 kg weight gain. He was readmitted to our facility for further evaluation. His initial admission vital signs included a heart rate of 108 beats/min, a blood pressure of 140/82 mmHg and 100% oxygen saturation with a 2 L nasal cannula.
A physical examination was significant for a 3+ pitting edema to the mid-calf and an elevated jugular venous pressure. An urgent TTE revealed a recurrence of echodense material within the pericardial space, extrinsic compression of the right atrium and tamponade physiology (Figure 1). Cardiac MRI showed a heterogenous signal compatible with blood products of different ages and a pericardial effusion (Figure 2). Right cardiac catheterization indicated elevated biventricular filling pressures and ventricular interdependence consistent with constrictive physiology. Cardiac output was normal (3.95 L/min thermodilution, 4.07 L/min, Fick principle).
Figure 1).
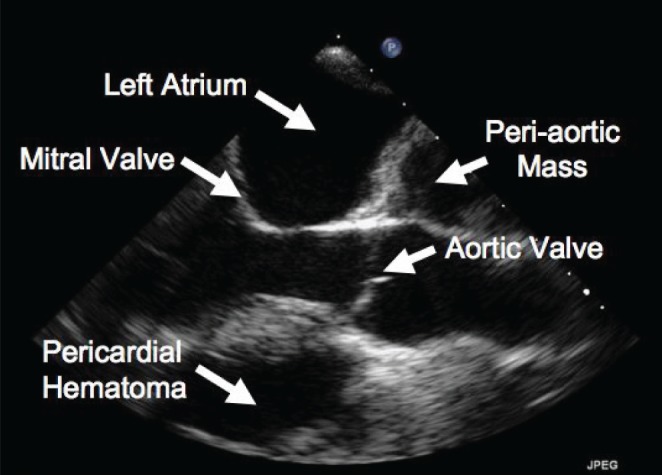
Transthoracic echocardiogram, parasternal long axis view, showing a pericardial hematoma and peri-aortic mass
Figure 2).
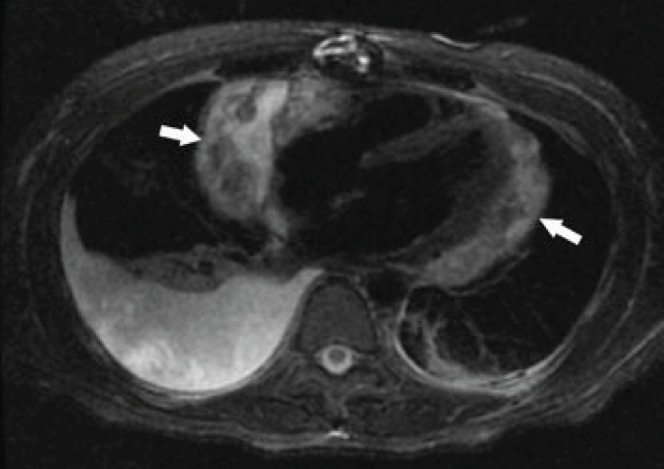
Axial magnetic resonance image of the chest (triple inversion recovery sequence with nulling of blood and fat) at the level of the left ventricle, showing a heterogenous signal within the pericardial effusion which is compatible with blood products of different ages. Arrows indicate a large pericardial effusion
Surgical exploration with pericardial space evacuation and thrombus extraction was planned. Before this procedure, the patient developed acute respiratory failure and circulatory compromise requiring emergent endotrachael intubation and hemodynamic vasopressor support. Right cardiac catheterization revealed a right atrial pressure of 17 mmHg, a right ventricular pressure of 38/24 mmHg and a pulmonary capillary wedge pressure of 24 mmHg.
The patient was urgently taken to the operating room. Laboratory data revealed a hemoglobin level of 5.9 g/dL (normal 13.0 g/dL to 17.3 g/dL), a platelet level of 76×109/L (normal 150×109/L to 450×109/L), a partial thromboplastin time of 67 s (normal 21 s to 30 s), a fibrinogen level of 107 mg/dL (normal 150 mg/dL to 450 mg/dL), and a D-dimer level of 41 mg/L (normal 0.4 mg/L to 2.5 mg/L), consistent with disseminated intravascular coagulation. Arterial blood gas on 100% fraction of inspired O2 revealed a pH of 7.31 (normal 7.35 to 7.45), PCO2 of 52.7 mmHg (normal 35 mmHg to 45 mmHg), PO2 of 72.6 mmHg (normal 80 mmHg to 100 mmHg), a bicarbonate level of 16 mmol/L and a lactic acid level of 10.7 mmol/L (normal 0.5 mmol/L to 2.2 mmol/L).
Intraoperatively, the patient experienced significant hemorrhage without a localizing source. Hemostasis could not be achieved. Pericardial exploration revealed a large hemorrhagic thrombus overlying a largely unrecognizable right atrium and right ventricle. Due to ongoing blood loss, the operative procedure was stopped, and the patient returned to the surgical intensive care unit for aggressive blood product resuscitation. Hemostasis was not achieved and he expired the next morning. Postmortem, he was diagnosed with metastatic angiosarcoma (Figure 3).
Figure 3).

Gross specimen revealing intact cardiac muscle (left), adipose tissue (centre), and hemorrhagic sponge-like tissue characteristic of angiosarcoma (right)
DISCUSSION
Primary cardiac tumours are extremely rare, with an incidence estimated at 0.0017% to 0.05% (1). Two-thirds are benign, 50% of which are myxomas (2). The remaining 25% are malignant, and the majority are angiosarcomas (3). These tumours typically occur in the third to fifth decade of life, with a male to female ratio of 3:1 (4). Although primary cardiac tumours can originate from any of the cardiac chambers, they typically originate from within the right atrium (5). The etiology of cardiac angiosarcoma is unknown, with one report suggesting a familial pattern of inheritance (6).
Cardiac angiosarcoma has a poor prognosis, partially due to its rapidly progressive nature. In a review by Janigan et al (7), the lung was the most common site of metastasis. Approximately 80% of patients have metastatic disease at the time of diagnosis and the average survival is less than nine months. Other common sites of metastasis include the liver, lymph nodes, bone and adrenals. Early diagnosis is often difficult because patients typically only experience symptoms late in the disease process (8). The most common presenting symptom is dyspnea, occurring in 59% to 88% of individuals (9). In one series, 86% of patients presented with pericardial disease or right-sided congestive heart failure caused by obstruction of either the vena cava or the left ventricular outflow tract (10). Other common findings at presentation include valvular dysfunction, arrhythmia, pericardial effusion, tamponade and pulmonary or systemic thromboembolism (11). Cardiac rupture is a rare event, with only a few cases reported in the literature (12–14).
Although TTE is often the initial diagnostic tool, transesophageal echocardiography has a sensitivity of 97% for detecting cardiac masses (11,15). Echocardiography is advantageous because it is inexpensive, widely available and noninvasive. Moreover, it can reveal the location and extent of the tumour, show whether cardiac function is compromised and, potentially, can aid in the initial differential diagnosis of the tumour (11). Echocardiographically, angiosarcomas are described as large, inhomogenous echogenic masses with poor border definition. Tumour malignancy is suggested by evidence of tumour invasion into extracardiac structures, right-sided lesions or the presence of a pericardial effusion (16). The limitations of echocardiography are its inability to adequately characterize different tissue types, and its dependence on operator experience and technique (15).
When compared with echocardiography, CT and MRI reveal more detail in the cardiac soft tissues and can define extracardiac involvement and metastasis. Calcifications associated with tumours can be visualized with CT, which may be an advantage over MRI. Additionally, CT does not have the imaging limitations associated with MRI and may also be used for transthoracic biopsies.
Cardiac MRI is superior to CT for soft tissue characterization and for evaluating abnormalities intrinsic to the myocardium. Cardiac MRI can differentiate between thrombus and neoplastic disease by means of delayed enhancement with gadolinium (17). Thrombi tend to remain dark, whereas cardiac tumours, which tend to have increased vascularity, enhance brightly with this contrast material (17). Primary cardiac tumours are characterized, by MRI, by heterogeneity or isointensity on T1-weighted images and hyperintensity on T2-weighted images. A noted heterogenous signal on T2-weighted imaging may be related to infiltration of the pericardial space with hemorrhagic and necrotic material (16). Areas of increased signal intensity have also been described on T1 images and may be secondary to the presence of blood products (17). Additional descriptions have been proposed and consist of local nodular areas of increased signal intensity interspersed within areas of intermediate signal intensity characterized as a ‘cauliflower’ appearance (18). Cardiac MRI is, however, subject to significant motion artefact (17).
Histological diagnosis of these tumours is challenging. In a review of 108 cases by Rettmar et al (19), only 42 of 108 (38%) cases were diagnosed intra vitam and 10 of these patients died shortly after diagnosis. Pericardial fluid cytology is typically unreliable (20). The fluid is characteristically bloody and frequently does not include malignant cells, even with direct tumour invasion of the pericardium (21). In the review by Rettmar et al (19), only 3.1% of pericardial fluid analyses performed were successful in obtaining a diagnosis. Poor diagnostic utility of pericardial and endomyocardial biopsies were also demonstrated with diagnosis achieved in only 23% and 50% of these samples, respectively (19). On microscopic visualization, cardiac angiosarcomas are characterized by the presence of anastomotic vascular channels formed by malignant cells, solid areas of spindle cells and other areas of primarily anaplastic cells (22). Immunohistochemical stains for CD31, CD34 and factor VIII-related protein can be used to identify the endothelial origin of sarcomas when the primary location is in question (23).
Treatment for cardiac angiosarcomas remains controversial. Given its low incidence, standard treatment guidelines do not exist; however, treatment typically involves surgical resection with or without additional chemotherapy and radiation therapy.
Surgical resection is typically indicated in cases without evidence of metastasis and when the tumour is curatively resectable (14). Complete resection is rarely possible due to the aggressive nature of the tumour and the high prevalence of metastasis at diagnosis. In cases where complete resection is achieved, local recurrence is common, with one-third of subsequent mortality being attributed to it (24).
Mean survival after surgical excision is estimated to be 10 months (5). In many cases, surgical resection has been used in combination with chemotherapy, with or without radiation therapy. However, the addition of chemotherapy after surgical resection has not been shown to increase survival when compared with surgery alone (5).
Heart transplantation has been performed on a select number of individuals with cardiac angiosarcomas; however, available data indicates that the long-term survival of patients receiving transplants is similar to that of patients undergoing other forms of cancer therapy (25). Interleukin-2, which has been used in the treatment of pulmonary angiosarcoma, has been used in isolated reports for cardiac angiosarcomas and may lead to improved survival (14). Kakizaki et al (21) reported on one patient who was treated with a regimen of chemotherapy plus interleukin-2 and survived 30 months post-surgery. However, more data are required before this can be recommended as a standard treatment protocol.
In summary, primary cardiac angiosarcoma is a rare but life-threatening disease that commonly has an indolent course early on and is often associated with recurrent pericardial effusions. Echocardiography, CT and cardiac MRI, are all excellent imaging tools that may enable early detection and diagnosis of cardiac angiosarcoma. Clinicians should remember to keep this rare disease in the differential diagnosis of patients being evaluated for recurrent pericardial effusions of unknown etiology.
REFERENCES
- 1.Silverman NA. Primary cardiac tumors. Ann Surg. 1980;191:127–38. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610–7. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 3.McAllister H, Fenoglio J. Tumors of the cardiovascular system. In: Hartmann WH, editor. Atlas of Tumor Pathology. Washington DC: Armed Forces Institute of Pathology; 1978. p. 81. [Google Scholar]
- 4.Sabatine M, Colucci W, Schoen F. Primary tumors of the heart. In: Braunwald E, editor. Heart Disease. 7th edn. Philadelphia: WB Saunders; 2005. pp. 1741–55. [Google Scholar]
- 5.Herrmann MA, Shankerman RA, Edwards WD, Shub C, Schaff HV. Primary cardiac angiosarcoma: A clinicopathologic study of six cases. J Thorac Cardiovasc Surg. 1992;103:655–64. [PubMed] [Google Scholar]
- 6.Casha AR, Davidson LA, Roberts P, Nair RU. Familial angiosarcoma of the heart. J Thorac Cardiovasc Surg. 2002;124:392–4. doi: 10.1067/mtc.2002.122314. [DOI] [PubMed] [Google Scholar]
- 7.Janigan DT, Husain A, Robinson NA. Cardiac angiosarcomas. A review and a case report. Cancer. 1986;57:852–9. doi: 10.1002/1097-0142(19860215)57:4<852::aid-cncr2820570428>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Brandt RR, Arnold R, Bohle RM, Dill T, Hamm CW. Cardiac angiosarcoma: Case report and review of the literature. Z Kardiol. 2005;94:824–8. doi: 10.1007/s00392-005-0296-0. [DOI] [PubMed] [Google Scholar]
- 9.Chidel M. Tumors of the heart and great vessels. In: Perez CBL, Halperin EC, SChmidit-Ullrich RK, editors. Principles and practice of radiation oncology. Philadelphia: Lippincott Williams and Wilkins; 2004. [Google Scholar]
- 10.Strohl KP. Angiosarcoma of the heart. A case study. Arch Intern Med. 1976;136:928–9. [PubMed] [Google Scholar]
- 11.Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathologic characteristics of primary cardiac tumors: A study of 149 cases. Int J Cardiol. 2002;84:69–75. doi: 10.1016/s0167-5273(02)00136-5. [DOI] [PubMed] [Google Scholar]
- 12.Corso RB, Kraychete N, Nardeli S, et al. Spontaneous rupture of a right atrial angiosarcoma and cardiac tamponade. Arq Bras Cardiol. 2003;81:611–3. doi: 10.1590/s0066-782x2003001400008. [DOI] [PubMed] [Google Scholar]
- 13.Mukohara N, Tobe S, Azami T. Angiosarcoma causing cardiac rupture. Jpn J Thorac Cardiovasc Surg. 2001;49:516–8. doi: 10.1007/BF02919548. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi M, Minato N, Katayama Y, Nakashima A. Cardiac angiosarcoma with right atrial perforation and cardiac tamponade. Ann Thorac Cardiovasc Surg. 2006;12:145–8. [PubMed] [Google Scholar]
- 15.Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc Intervent Radiol. 2004;27:459–69. doi: 10.1007/s00270-004-0123-4. [DOI] [PubMed] [Google Scholar]
- 16.Syed IS, Feng D, Harris SR, et al. MR imaging of cardiac masses. Magn Reson Imaging Clin N Am. 2008;16:137–64. vii. doi: 10.1016/j.mric.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Araoz PA, Eklund HE, Welch TJ, Breen JF. CT and MR imaging of primary cardiac malignancies. Radiographics. 1999;19:1421–34. doi: 10.1148/radiographics.19.6.g99no031421. [DOI] [PubMed] [Google Scholar]
- 18.Kim EE, Wallace S, Abello R, et al. Malignant cardiac fibrous histiocytomas and angiosarcomas: MR features. J Comput Assist Tomogr. 1989;13:627–32. doi: 10.1097/00004728-198907000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Rettmar K, Stierle U, Sheikhzadeh A, Diederich KW. Primary angiosarcoma of the heart. Report of a case and review of the literature. Jpn Heart J. 1993;34:667–83. doi: 10.1536/ihj.34.667. [DOI] [PubMed] [Google Scholar]
- 20.Adachi K, Tanaka H, Toshima H, Morimatsu M. Right atrial angiosarcoma diagnosed by cardiac biopsy. Am Heart J. 1988;115:482–5. doi: 10.1016/0002-8703(88)90504-2. [DOI] [PubMed] [Google Scholar]
- 21.Kakizaki S, Takagi H, Hosaka Y. Cardiac angiosarcoma responding to multidisciplinary treatment. Int J Cardiol. 1997;62:273–5. doi: 10.1016/s0167-5273(97)00259-3. 19. [DOI] [PubMed] [Google Scholar]
- 22.Frota Filho JD, Lucchese FA, Leaes P, Valente LA, Vieira MS, Blacher C. Primary cardiac angiosarcoma. A therapeutical dilemma. Arq Bras Cardiol. 2002;78:586–91. doi: 10.1590/s0066-782x2002000600006. [DOI] [PubMed] [Google Scholar]
- 23.Adem C, Aubry MC, Tazelaar HD, Myers JL. Metastatic angiosarcoma masquerading as diffuse pulmonary hemorrhage: Clinicopathologic analysis of 7 new patients. Arch Pathol Lab Med. 2001;125:1562–5. doi: 10.5858/2001-125-1562-MAMADP. [DOI] [PubMed] [Google Scholar]
- 24.Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–95. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Uberfuhr P, Meiser B, Fuchs A, et al. Heart transplantation: An approach to treating primary cardiac sarcoma? J Heart Lung Transplant. 2002;21:1135–9. doi: 10.1016/s1053-2498(02)00409-6. [DOI] [PubMed] [Google Scholar]


