Abstract
PURPOSE
Diffuse intrahepatic tumors are difficult to control. Whole liver radiotherapy has been limited by toxicity, most notably radiation-induced liver disease (RILD). Amifostine is a prodrug free-radical scavenger that selectively protects normal tissues and, in a preclinical model of intrahepatic cancer, systemic amifostine reduced normal liver radiation damage without compromising tumor effect.(1) We hypothesized that amifostine would permit escalation of whole liver radiation dose to potentially control microscopic disease. We also aimed to characterize the pharmacokinetics of amifostine and its active metabolite WR-1065 to optimize timing of radiotherapy.
METHODS AND MATERIALS
We conducted a radiation dose escalation trial for patients with diffuse, intrahepatic cancer treated with whole liver radiation and intravenous amifostine. Radiation dose was assigned using the Time-to-Event Continual Reassessment Method. A companion pharmacokinetic study was performed.
RESULTS
23 patients were treated, with a maximum dose of 40 Gy. Using a logistical regression model, compared to our previously treated patients, amifostine increased liver tolerance by 3.3 ± 1.1 Gy (p=0.007) (approximately 10%) with similar response rates. Peak concentrations of WR-1065 were 25 μM with an elimination half life of 1.5 hours; these levels are consistent with radioprotective effects of amifostine in patients.
CONCLUSION
These findings demonstrate for the first time that amifostine is a normal liver radioprotector. They further suggest that it may be useful to combine amifostine with fractionated or stereotactic body radiation therapy for patients with focal intrahepatic cancer.
Keywords: Amifostine, Liver Radiation, Radioprotector
Introduction
We and others have demonstrated that high dose external beam radiotherapy can be delivered safely and can control focal intrahepatic tumors.(2–4) Unfortunately, many patients with liver cancer present with diffuse liver tumors. For these patients, therapeutic radiation is limited by the whole liver tolerance of only about 30 Gy.(5)
Amifostine is an organic thiophosphate that was developed to selectively protect normal tissues against the toxicities of chemotherapy and radiation. It is a pro-drug that is dephosphorylated at the tissue site to its active metabolite (WR-1065) by alkaline phosphatase. Differences in the alkaline phosphatase concentration in normal tissues and tumor cells result in differential concentrations of active WR-1065. In addition, differences in the pH of normal and tumor tissues lead to preferential uptake of WR-1065 by normal tissues. Once inside the cell, WR-1065, a free thiol, acts as a potent scavenger of oxygen free radicals induced by ionizing radiation and some chemotherapies.(6)
Amifostine has been used in several clinical trials for protection of normal tissues.(7, 8) Brizel et al. demonstrated a salivary gland radiation protection factor of approximately 1.4 with amifostine.(7) If this same radiation protection factor were achievable in the whole liver setting, then patients would be able to tolerate 42 Gy or more, possibly enough to control microscopic disease and prevent recurrences in other parts of the liver after high dose focal radiation.
Using a rat liver model of intrahepatic cancer, we found that systemic administration of amifostine decreased radiation damage of normal hepatocytes by as much as 50% without protecting the tumor.(1) We therefore hypothesized that amifostine would allow for a higher dose of whole liver radiation to be safely administered. The primary aim of the study was to assess the maximum tolerated dose (MTD) of whole liver radiation with the protection of systemic amifostine. We also sought to characterize the pharmacokinetics of amifostine and its active metabolite, WR-1065, for optimal timing of therapy in this patient population.
Methods and Materials
Patients
This was an IRB approved prospective protocol; all patients signed an approved consent form describing the study's experimental nature. Eligible patients were ≥18 years old, with histologically or alpha-fetoprotein (AFP) confirmed multi-focal primary or metastatic intrahepatic cancers. Adequate liver, renal, and bone marrow function was required: INR < 1.2, LFTs ≤ grade 4, absolute granulocytes ≥ 1500/mm3, platelets > 80,000/mm3, BUN ≤ 40 mg/dl and creatinine < 2.0 mg/dl. Patients with neuroendocrine tumors and those treated with prior radiation were ineligible. After observing toxicity in 2 patients who had previously received alkylator or taxane chemotherapy, these patients were excluded from the remainder of this study.
Study Design
The primary endpoint was to determine the maximum tolerated dose of radiation that resulted in an estimated risk of radiation induced liver disease (RILD) of approximately 15%. The secondary endpoint was disease response as measured by the RECIST criteria. Dose was assigned using the time-to-event continual reassessment method (TITE-CRM),(9) specifically using the Lyman dose-toxicity model with an additional parameter representing the protective effect of amifostine, so that, for a patient treated at radiation dose D: where Φ is the cumulative standard normal probability function;(10) m is a steepness parameter of the curve; TD50 is a function of the patient's dose-volume histogram and an implicit dose-volume effect parameter, n; and α represents the protective effect of amifostine, such that α=1.0 represents no protection, α =0.0 represents complete protection.
Based on previous data(11)we fixed m=0.1, n=0.92 and TD50=39.7 Gy and 49 Gy in primary and metastatic patients respectively. TITE-CRM was used to continually re-estimate α during the trial using a Bayesian paradigm. Given the expected value of the posterior distribution, α, based on available data and a beta prior distribution, a patient was assigned to the highest dose in Table 1 such that the probability that a patient developed RILD was ≤15%. Pre-clinical data indicated that amifostine could reduce the toxicity of whole-liver radiation by 50%, represented by α=0.5, but the parameters of α's prior distribution were set to 7 and 2.5, so that the initial value of α was 0.74, which was felt to be conservative. These parameters resulted in baseline estimates of the probability of developing RILD. The first patients in the primary and metastatic cancer strata were treated at 32 Gy and 36 Gy, respectively, with lower doses given to patients with primary intrahepatic tumors, who generally had already compromised liver function in the setting of cirrhosis.
Table 1.
Baseline estimates of the probability of RILD
| Primary | Metastatic | |||
|---|---|---|---|---|
| Assigned Dose (Gy) | Predicted %risk of RILD without amifostine10 | Predicted %Risk of RILD with Amifostine | Predicted %risk of RILD without amifostine10 | Predicted %Risk of RILD with Amifostine |
| 32 | 2.6 | <0.1 | · | · |
| 34 | 7.6 | 0.1 | · | · |
| 36 | 17.6 | 0.3 | 2.3 | <0.1 |
| 38 | 33.4 | 1.0 | 6.0 | <0.1 |
| 40 | 53.0 | 2.6 | 13.3 | 0.2 |
| 42 | 71.9 | 6.2 | 25.2 | 0.6 |
| 44 | 86.1 | 12.9 | 41.2 | 1.5 |
| 46 | 94.4 | 23.3 | 58.8 | 3.4 |
| 48 | · | · | 74.8 | 7.1 |
| 50 | · | · | 86.7 | 13.3 |
| 52 | · | · | 94.0 | 22.5 |
Dose limiting toxicity was defined as developing grade 4 changes in AST, ALT, alkaline phosphatase, albumin, total bilirubin, or platelet count, or any other ≥ grade 3 toxicity attributable to radiation treatment (i.e. in the absence of tumor progression).
Whole Liver Radiation
Patients all underwent CT simulation and 3D conformal radiation treatment planning to minimize dose to the heart, kidneys, intestines, and lung. Whole liver radiation was delivered in 2 Gy fractions, 5 days a week, using high energy (15 MV) photon beams.
Amifostine Administration
Amifostine was given 340 mg/m2, IV push over 10 seconds, through a PICC line, approximately 15–30 minutes before each daily radiation treatment. Antihypertensive medications, including diuretics, were held 24 hours prior to amifostine administration. Blood pressure was measured before and after infusion. Patients were kept supine, and they received IV hydration afterward.
Amifostine and WR1065 Assays
To allow for optimization of combination therapy with amifostine and radiotherapy, we conducted a pharmacokinetic study on 5 patients on this trial. After informed consent, the pharmacokinetic parameters of amifostine and its active metabolite WR-1065 were determined on the first day of radiotherapy in 3 female and 2 male patients. Serial blood samples were collected from a peripheral vein distant from the injection site at 0 (predose), 1, 3, 5, 15, 30, 45 and 60 min, and hourly through 6 hours after amifostine administration. Amifostine plasma samples were analyzed using reversed-phase high-performance liquid chromatography (HPLC), with electrochemical detection, as described previously by Shaw et al.(12) Using this method, the limit of quantification for amifostine was 0.5μM. The peak height ratios of amifostine to internal standard (WR-80855) were linear over a concentration range of 0.5 to 50 μM (r2 = 0.999). The inter-day accuracy and precision of the assay were < 8% using spiked concentrations of amifostine at 1, 10 and 40 μM. For the pharmacokinetic analyses, plasma concentrations of amifostine were converted to blood concentrations of drug given its blood-to-plasma ratio of 0.5.(13) WR-1065 blood samples were analyzed using reversed-phase HPLC, with coulometric detection, according to published methods.(14) For WR-1065, the limit of quantification was 0.05 μM and WR-1065 peak heights were linear over a concentration range of 0.05 to 10 μM (r2 = 0.999). The inter-day accuracy and precision were < 2% and < 10%, respectively, using spiked concentrations of WR1065 at 0.1, 1.5 and 7.5 μM.
Pharmacokinetic Model and Analysis
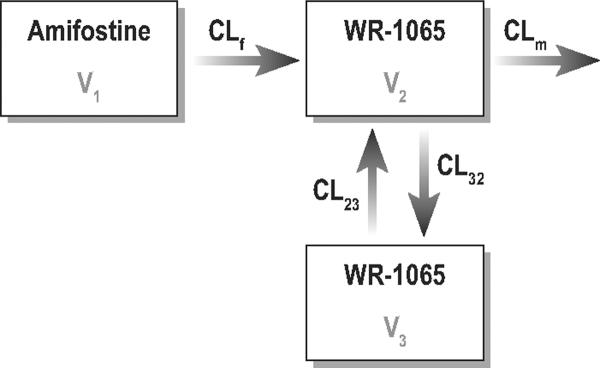
The statistical package NONMEM VI 1.0 (GloboMax LLC, Hanover, MD) was used to fit the amifostine and WR-1065 data using a first-order conditional estimation with η-ε interaction. Log-transformed data were used for model analysis. The disposition of amifostine was described by a one-compartment model with first-order elimination, and the disposition of WR-1065 was described by a two-compartment model (Figure 1).
Figure 1.
Schematic representation of the pharmacokinetic model for amifostine (parent drug) and WR-1065 (active metabolite). CLf =clearance of amifostine; CLm is the clearance of WR-1065; V1=the volume of distribution of amifostine; V2 =the volume of distribution of WR-1065 in the central compartment; V3=the volume of distribution of WR-1065 in the peripheral compartment, CL23, CL32=the inter-compartmental clearances.
Patient follow-up for toxicity and evaluation of response
Toxicity assessments were performed weekly during treatment, every 2 weeks for the first 2 months after treatment, and every three months thereafter. Disease response was assessed 2 months after treatment by CT or MRI and compared to pre-treatment measurements, using RECIST criteria.
Estimation of amifostine effect
To assess the effect of amifostine at the end of the trial, all the patients from the series described in Dawson et al(11) who underwent whole-liver radiation therapy with concurrent floxuridine for primary liver cancer were identified, and a logistic regression model estimating the probability of RILD as a function of mean radiation dose to normal liver was fit to these patients and the primary liver cancer patients who received amifostine. Separate regression parameters were estimated for patients treated with and without amifostine, and the statistical significance of the difference between those was assessed using a Wald χ2 test. The tolerance doses for 50% (TD50) and 15% (TD15) complication risk for uniform whole organ irradiation for patients treated without and with amifostine were estimated from the logistic regression parameters, and the standard errors of these estimates calculated by means of the delta method.
Results
Patients and treatment
23 patients were treated from April 2003 through October 2007; 10 with hepatocellular carcinoma, 6 with intrahepatic cholangiocarcinoma, and 7 with metastatic disease (colon/rectal, breast, melanoma, and unknown primary). Median age was 60 years (range 35–78). 19 patients were Child-Pugh Class A and 4 Class B. Nine patients (39%) received systemic therapy prior to enrollment, including 5-FU, leucovorin, capecitabine, gemcitabine, irinotecan, oxaliplatin, doxorubicin, paclitaxel, docetaxel, vinorelbine, cyclophosphamide, temozolomide, thalidomide, and interleukin-2. After noting toxicity in 2 patients previously treated with alkylators and taxanes, these were added to the exclusion criteria.
Amifostine and WR1065 Systemic Profiles
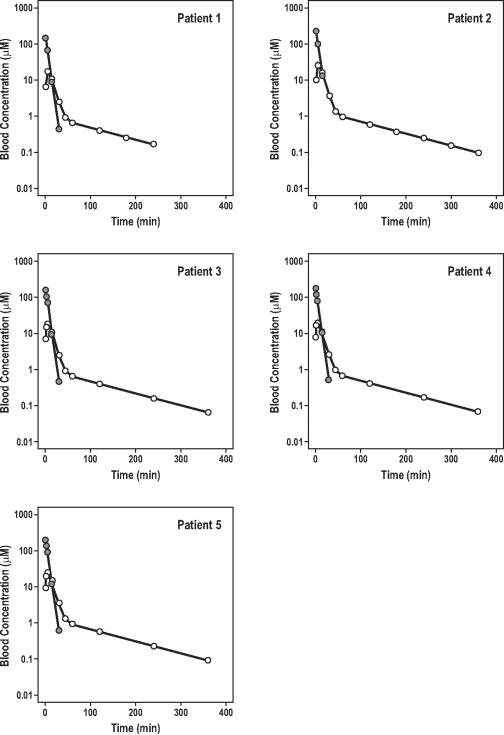
Blood concentration-time curves for amifostine, after intravenous bolus dosing, are displayed in Figure 2. Amifostine concentrations declined rapidly over 30 minutes, indicating the quick conversion of parent drug to WR-1065. This rapid decline was consistent with amifostine's large clearance (66.2 ml/min/kg), relatively small volume of distribution (0.3 L/kg) and very short elimination half-life (3.3 min). The inter-subject variability of clearance and volume of distribution for amifostine was low after including the covariate “weight” into the model. The formation and disposition of WR-1065 in blood are also shown in Figure 2. Peak WR-1065 concentrations of 25 μM were achieved at about 4 min, confirming that amifostine was rapidly converted to WR-1065.
Figure 2.
Blood concentration-time profiles of amifostine (dark circles) and its active metabolite WR-1065 (open circles) in five patients with intrahepatic cancer.
NONMEM estimates of amifostine and WR-1065 pharmacokinetics are shown in Table 2. The clearance (90.8 ml/min/kg) and volume of distribution estimates (V2=0.8 l/kg and V3=1.4 l/kg) of WR-1065 were larger than those of amifostine, indicating that WR-1065 was metabolized very quickly and was extensively bound in tissue compartments. The elimination half-life of WR-1065 (1.5 hr) was much longer than that of amifostine. The fits from 100 bootstrapped replicates of data demonstrate the stability of the model.
Table 2.
Population pharmacokinetics of amifostine and WR-1065 in patients with intrahepatic cancera
| Parameterb | NONMEM Method | Bootstrap Method | ||
|---|---|---|---|---|
| Estimate | % CV | Estimate | % CV | |
| CLf (ml/min/kg) | 66.2 | 5.8 | 65.7 | 6.7 |
| V1 (L/kg) | 0.3 | 11.6 | 0.3 | 11.4 |
| CLm (ml/min/kg) | 90.8 | 14.1 | 91.3 | 14.9 |
| V2 (L/kg) | 0.8 | 23.3 | 0.8 | 24.3 |
| V3 (L/kg) | 1.4 | 24.9 | 1.4 | 27.8 |
| CL23=CL32 (ml/min/kg) | 14.1 | 17.5 | 14.2 | 18.7 |
| Inter-individual Variability | ||||
| η CLf (%) | < 1 | < 1 | < 1 | < 1 |
| η V1 (%) | 24.8 | 59.5 | 22.1 | 65.1 |
| η CLm (%) | 7.1 | 58.2 | 6.0 | 73.2 |
| η V2 (%) | < 1 | < 1 | < 1 | < 1 |
| η V3 (%) | < 1 | < 1 | < 1 | < 1 |
| η CL23=CL32 (%) | < 1 | < 1 | < 1 | < 1 |
| Residual Variability | ||||
| ε Amifostine (μM) | 0.5 | 13.5 | 0.5 | 13.8 |
| ε WR-1065 (μM) | 0.4 | 5.1 | 0.4 | 9.2 |
Patient characteristics (mean ± SD) include the following: age = 54 ± 10 yr; body weight = 86 ± 24 kg; height = 170 ± 8 cm; and body surface area = 2.0 ± 0.3 m2.
CLf is the clearance of amifostine; CLm is the clearance of WR-1065; V1 is the volume of distribution of amifostine; V2 is the volume of distribution of WR-1065 in the central compartment; V3 is the volume of distribution of WR-1065 in the peripheral compartment, CL23, CL32 are the inter-compartmental clearances, ηi is the inter-subject variability, and εi is the residual error.
Toxicities
Radiation induced liver disease occurred in 4 patients. Of the 13 patients with primary intrahepatic cancer, one occurred at 38 Gy, and one at 40 Gy. In the group with metastatic disease, RILD occurred at 36 Gy in two patients, and led to closure of this arm of the study.
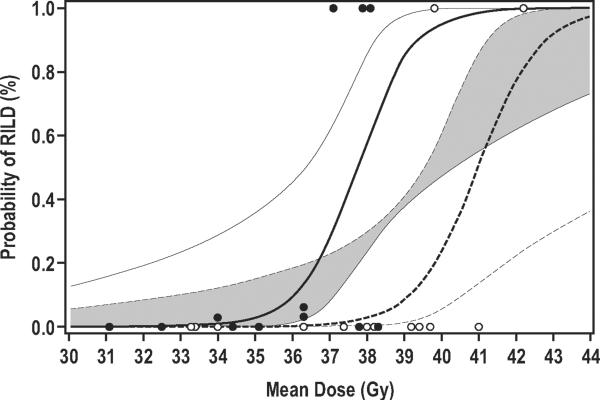
Based on these data, the logistic regression estimate of TD50 when patients are treated with amifostine is 41.0 Gy (±0.93 Gy), versus 37.7 Gy (±0.65 Gy) for 12 similar patients treated without amifostine in the series published in 2002 (3). The difference between these estimates (3.3±1.1 Gy) is statistically significant (p=0.007). Estimates of TD15 are 39.6 Gy (±0.89 Gy) and 36.4 Gy (±0.83 Gy) for patients treated with and without amifostine, respectively. The estimated dose-toxicity curves for RILD as a function of mean dose to normal liver, for patients treated with and without amifostine, are presented in Figure 3.
Figure 3.
Estimated probability of RILD given mean dose to normal liver for primary liver cancer treated with amifostine (dashed line) in this study and without amifostine (solid line), from the control series. Circles indicate mean dose and if patient experienced RILD (1.0) or not (0.0). Open circles represent patients from the current study, and closed circles from the control series. The thick lines are expected probabilities of toxicity from the logistic regression model. 90% confidence intervals are presented, with the overlap of the confidence regions shaded gray.
In patients with metastatic disease, we were unable to accurately estimate the MTD, as RILD occurred in 2 patients at the initial 36 Gy dose level. This was possibly due, in retrospect, to prior alkylating chemotherapies (cyclophosphamide, temozolomide) that these patients received. Besides RILD, other toxicities of treatment are listed in Table 3.
Table 3.
Other Toxicities
| Adverse Effect | Grade 1–2 | Grade 3–4 |
|---|---|---|
| Fatigue | 15 (65%) | 1 (4%) |
| Hypotension | 16 (70%) | 0 |
| Nausea/vomiting | 17 (74%) | 0 |
| Hypocalcemia | 7 (30%) | 1 (4%) |
| Hyponatremia | 0 | 3 (13%) |
| Pericarditis | 0 | 1 (4%) |
| Anaphylaxis | 0 | 1(1%) |
Disease Response
At 2 months, 2 patients had objective partial responses (one of which lasted 2 years), 8 had stable disease and 13 had progressive disease. Additionally, all 5 patients with hepatocellular carcinoma and pre-treatment alpha-fetoprotein (AFP) elevation showed AFP decreases of approximately 60% from baseline. These responses are not substantially different from those reported previously after whole liver radiation, although our power to detect a clinically significant improvement is small.
Discussion
Amifostine has already been demonstrated to be a radiation protector of the salivary glands, lungs, and esophagus.(7, 8) Based on our preclinical studies,(1) we had hypothesized that amifostine would protect the normal liver from radiation if sufficient plasma levels were achieved. In our study, WR1065 was found at levels consistent with radioprotective effects, and amifostine conferred modest protection of the normal liver from radiation damage. With amifostine, whole liver radiation doses of up to 38 Gy may be safely administered for primary liver cancer. Although the radiation protection factor of 1.1 was lower than we had predicted in our preclinical studies, our results demonstrate for the first time that amifostine is a normal liver radiation protector. This opens the possibility of using amifostine in combination with radiation for patients with focal liver disease undergoing fractionated or stereotactic body radiotherapy for focal liver tumors, as dose is often limited by the risk of RILD due to irradiation of uninvolved portions of the liver. For patients with diffuse primary or metastatic liver tumors, the use of amifostine combined with regional therapies including radioembolization and chemoembolization needs to be further explored.
One possible explanation for the modest radioprotection observed may be related to the pathophysiology of radiation induced liver disease. RILD is a form of veno-occlusive disease, which is thought to result from damage to liver endothelial cells. Alkaline phosphatase, which is instrumental in converting amifostine into active WR-1065, may be predominantly located in the hepatocytes, and it is possible that the endothelial cells may not have had adequate WR-1065 to confer greater protection.(15) Furthermore, endothelial cells may also be exquisitely sensitive to radiation. Our timing of amifostine administration, 15–30 minutes prior to radiotherapy, was according to other studies demonstrating radioprotective effects,(7) but may not have been optimized. According to pharmacokinetic data from our study, peak WR-1065 concentrations of 25 μM were achieved at about 4 min, suggesting that shortening the interval between amifostine administration and RT may increase its protective effects. Indeed, Kouloulias, et al found a significantly improved effect if RT was administered within 15 minutes after amifostine infusion, as compared with 25–40 minutes after infusion.(16)
There is little data currently available on the pharmacokinetics and disposition of amifostine and its active metabolite WR-1065 in clinically relevant settings. One study was performed in 12 healthy volunteers,(17) a second study was performed in a heterogeneous group of 13 cancer patients,(12) and a third study was performed in 9 cancer patients with solid tumors,(18) where 3 subjects were evaluated for amifostine kinetics and 6 patients were evaluated for WR-1065 kinetics in the presence of either cisplatin or carboplatin. Results from the current study represent the first “complete” model in which both amifostine and WR-1065 pharmacokinetics were examined simultaneously, and in a single patient population of intrahepatic cancer. We demonstrated that amifostine was eliminated rapidly from the body due to its rapid conversion to WR-1065, which in turn was quickly metabolized. Both the clearances of amifostine and WR-1065 (66.2 and 90.8 ml/min/kg, respectively) were on the order of cardiac output (about 75–80 ml/min/kg). Whereas amifostine was confined to the extracellular fluid (based on a plasma V1 of 12.9 liters), WR-1065 was more extensively distributed into peripheral tissues. As a result, amifostine was largely removed from the body over 30 min while WR-1065 levels persisted for a much longer period of time, with a half-life of 1.5 hours. Thus, amifostine's protective effect through WR-1065 will likely last long enough for stereotactic body radiotherapy or intensity-modulated radiotherapy, both of which are typically delivered over a protracted period of time, sometimes close to an hour.
In our study, amifostine was administered intravenously to ensure systemic drug delivery to the liver. Subcutaneous (SC) administration has also been studied, for convenience and reduced side effects, namely hypotension. (19) This route may be a more tolerable route of administration in the future, although pharmacokinetic data demonstrating levels similar to those found after IV administration are lacking, and are thus still necessary.
The maximal tolerated dose of whole liver radiation in our metastatic patients could not be estimated due to early toxicity in two patients who had previously received alkylating chemotherapy, which has been implicated in the development of veno-occlusive disease of the liver.(20) Therefore, whole liver radiation should be used very cautiously, if at all, for patients who have previously received alkylating chemotherapy, as this likely predisposes patients to RILD. In the absence of previous alkylating agent chemotherapy, we would have expected to find the liver of a patient with metastatic disease to be more tolerant of radiation than that of a patient who developed hepatocellular carcinoma in the setting of cirrhosis.(10)
In summary, we have demonstrated for the first time that amifostine has radioprotective effects on the normal liver. We have also characterized the pharmacokinetics and disposition for amifostine, and its active metabolite WR-1065 in a uniform group of patients with intrahepatic malignancies. While amifostine is unlikely to substantially benefit patients who require whole liver radiation, it may provide enough radiation protection to the normal liver during focal liver radiation to allow for the safe delivery of ≥75 Gy, or the biologic equivalent using stereotactic body radiotherapy, which, based on our previous experience could possibly improve survival.(2)
Acknowledgments
Supported by P01-CA-59827, RO1 CA098502, P30CA047904 and a grant from MedImmune – AstraZeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the American Society for Therapeutic Radiology and Oncology Annual Meeting, September 21–25, 2008, Boston, MA.
References
- 1.Symon Z, Levi M, Ensminger WD, et al. Selective radioprotection of hepatocytes by systemic and portal vein infusions of amifostine in a rat liver tumor model. Int J Radiat Oncol Biol Phys. 2001;50:473–478. doi: 10.1016/s0360-3016(01)01522-x. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 3.Seong J, Park HC, Han KH, et al. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329–336. doi: 10.1016/s0360-3016(02)03929-9. [DOI] [PubMed] [Google Scholar]
- 4.Mornex F, Girard N, Beziat C, et al. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies--mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66:1152–1158. doi: 10.1016/j.ijrobp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 6.Peters GJ, van der Vijgh WJ. Protection of normal tissues from the cytotoxic effects of chemotherapy and radiation by amifostine (WR-2721): preclinical aspects. Eur J Cancer. 1995;31A(Suppl 1):S1–7. doi: 10.1016/0959-8049(95)00145-9. [DOI] [PubMed] [Google Scholar]
- 7.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 8.Antonadou D, Coliarakis N, Synodinou M, et al. Randomized phase III trial of radiation treatment +/− amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:915–922. doi: 10.1016/s0360-3016(01)01713-8. [DOI] [PubMed] [Google Scholar]
- 9.Normolle D, Lawrence T. Designing dose-escalation trials with late-onset toxicities using the time-to-event continual reassessment method. J Clin Oncol. 2006;24:4426–4433. doi: 10.1200/JCO.2005.04.3844. [DOI] [PubMed] [Google Scholar]
- 10.Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 11.Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 12.Shaw LM, Turrisi AT, Glover DJ, et al. Human pharmacokinetics of WR-2721. Int J Radiat Oncol Biol Phys. 1986;12:1501–1504. doi: 10.1016/0360-3016(86)90203-8. [DOI] [PubMed] [Google Scholar]
- 13.Souid AK, Newton GL, Dubowy RL, et al. Determination of the cytoprotective agent WR-2721 (Amifostine, Ethyol) and its metabolites in human blood using monobromobimane fluorescent labeling and high-performance liquid chromatography. Cancer Chemother Pharmacol. 1998;42:400–406. doi: 10.1007/s002800050836. [DOI] [PubMed] [Google Scholar]
- 14.Bai F, Kirstein MN, Hanna SK, et al. New liquid chromatographic assay with electrochemical detection for the measurement of amifostine and WR1065. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;772:257–265. doi: 10.1016/s1570-0232(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 15.Fiorentini G, Giovanis P, Leoni M, et al. Amifostine (Ethyol) as modulator of hepatic and biliary toxicity from intraarterial hepatic chemoembolization: results of a phase I study. Hepatogastroenterology. 2001;48:313–316. [PubMed] [Google Scholar]
- 16.Kouloulias VE, Kouvaris JR, Kokakis JD, et al. Impact on cytoprotective efficacy of intermediate interval between amifostine administration and radiotherapy: a retrospective analysis. Int J Radiat Oncol Biol Phys. 2004;59:1148–1156. doi: 10.1016/j.ijrobp.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Bonner HS, Shaw LM. New dosing regimens for amifostine: a pilot study to compare the relative bioavailability of oral and subcutaneous administration with intravenous infusion. J Clin Pharmacol. 2002;42:166–174. doi: 10.1177/00912700222011201. [DOI] [PubMed] [Google Scholar]
- 18.Korst AE, Eeltink CM, Vermorken JB, et al. Pharmacokinetics of amifostine and its metabolites in patients. Eur J Cancer. 1997;33:1425–1429. doi: 10.1016/s0959-8049(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 19.Bardet E, Martin L, Calais G, et al. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: final results of the GORTEC2000-02 phase III randomized trial. Clin Oncol. 2011;29:127–133. doi: 10.1200/JCO.2009.25.5638. [DOI] [PubMed] [Google Scholar]
- 20.Bearman SI. Veno-occlusive disease of the liver. Curr Opin Oncol. 2000;12:103–109. doi: 10.1097/00001622-200003000-00001. [DOI] [PubMed] [Google Scholar]