Abstract
Rational selection of individual adjuvants can often be made based on innate molecular interactions of the foreign molecules with pattern recognition receptors such as Toll-like receptors. For example, monophosphoryl lipid A, a family of endotoxic TLR4 agonist molecules from bacteria, has recently been formulated with liposomes, oil emulsions, or aluminum salts for several vaccines. Combinations of antigens and adjuvants with particulate lipid or oil components may reveal unique properties of immune potency or efficacy, but these can sometimes be exhibited differently in rodents when compared to nonhuman primates or humans. New adjuvants, formulations, microinjection devices, and skin delivery techniques for transcutaneous immunization demonstrate that adjuvant systems can include combinations of strategies and delivery mechanisms for uniquely formulated antigens and adjuvants.
Introduction
Because of complex interactions between multiple large systems, immunology is all about complexity and control. However, vaccine development is all about controlled leverage of complexity. Modern vaccine development strives to render complexity into simplicity, and practical realization of immunological leverage for optimal vaccine development can often be influenced by vaccine adjuvants. A malaria vaccine (known as RTS,S) utilizing an adjuvant system comprising liposomes having a proprietary form of detoxified monophosphoryl lipid A (known as MPL®) and a detoxified saponin derivative (QS-21) as an adjuvant formulation, when mixed with a particle that displays both recombinant hepatitis B surface antigen and the circumsporozite antigen of Plasmodium falciparum, appears to represent a possible window to introduction of a successful pediatric vaccine to falciparum malaria [1–3]. If successful, this liposome-based vaccine would represent not only an important advance against one of the great scourges of mankind, but also a possible path to dealing with one of the most complex problems in modern vaccinology, the creation of a vaccine to a difficult and resilient parasitic disease. The emergence of novel potent and safe commercial adjuvants may herald an important advance that might lead to the creation of vaccines to other difficult chronic diseases such as HIV-1, tuberculosis, and cancer [4, 5].
Adjuvants may be molecules, compounds, or macromolecular complexes that boost the potency, quality, or longevity of specific immune responses to antigens, but which should cause minimal toxicity. In general, adjuvants can be classified as immune modulators or delivery vehicles, with some components sharing both properties. Adjuvants target antigen presenting cells (APC), central in both innate and adaptive immunity.
Recent advances in our understanding of innate immunity and antigen presentation now permit a rational approach to designing and selecting compounds with adjuvant activity based on molecular interactions. The immune system has evolved to respond to foreign molecules, including viral, bacterial, fungal, or parasitic molecules, including cell wall components, lipoproteins, proteins, lipopolysaccharides, DNA and RNA. Such molecules are able to stimulate innate immune responses via pattern recognition receptors, including the toll-like receptors (TLR). TLR agonists have been reviewed extensively during the past few years [6, 7]. Of the TLR 1-10, agonists of which were derived originally from bacterial or viral components, agonists of TLR 3, 4, 5, 7, 8, and 9 have been most widely studied and advanced to clinical development. Agonists of TLR3, an endosomal receptor, are based on viral double-stranded RNA, and include poly (I:C) which has been used extensively in clinical trials, particularly for cancer . TLR4, expressed on the plasma membrane of human macrophages and dendritic cells, as well as on other cell types in lower mammalian species, is engaged by bacterial lipopolysaccharide, monophosphoryl lipid A (MPLA), and synthetic derivatives. MPLA is the only TLR agonist in approved human vaccines and therapeutics, to be discussed later in this review. Agonists of TLR5 (bacterial flagellin), TLR7, 8 (single stranded RNA), and TLR9 (CpG oligonucleotides, ISS immunostimulatory sequences) have all been developed into products, and have been reviewed elsewhere [8, 9].
Formulations
The beneficial effects of adjuvant formulations (also known as adjuvant systems) have been described for many individual clinical vaccines [10–15], but important chemical and structural features of individual oil and lipid components can often be complex [16]. Adjuvant molecules must be appropriately formulated for both maximum effect and stability. Criteria involved in selecting the formulation for a given vaccine include: the nature of the antigenic components (soluble, particulate, charge etc.), the type of immune response desired, the anticipated route of delivery, the avoidance of side effects, and the stability of the vaccine. The optimally formulated adjuvant will be safe, stable prior to administration, readily biodegraded and eliminated, able to promote an antigen-specific immune response, inexpensive to produce, and easy to use. Examples of formulation components that are used alone or in combination with TLR agonists include alum, virosomes, and oil in water (O/W) emulsions.
An example of the importance of formulation can be illustrated with MPL®, the first TLR ligand and biological adjuvant approved for human use (Hepatitis B vaccine, Fendrix®, GSK, European approval 2005). Unformulated MPLA is insoluble and prone to aggregation, which adversely affects its bio-availability. Formulations that enhance its solubility enhance its efficacy and reliability; including an O/W emulsion (MPL-SE) (Figure 1) or combining MPL® with aluminum salts (AS04). Furthermore, MPLA in aqueous formulation enhances antibody responses, while MPLA in oil formulation stimulates T cell responses. Moreover, formulations that generate defined structures, such as liposomal AS01B (or AS01E), induce more potent CTL responses than formulations with similar components with smaller particle size, such as AS02A.
Figure 1.
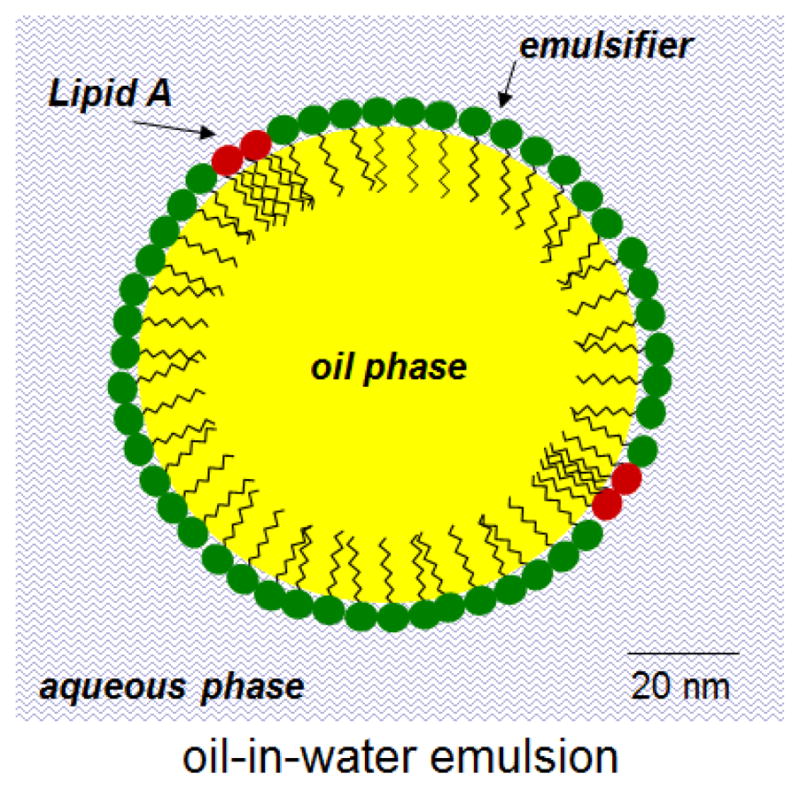
Schematic representation of O/W emulsion containing a TLR 4 Agonist (lipid A or derivatives). This type of adjuvant, referred to as MPL-SE (stable emulsion) or GLA-SE, has been used in vaccine trials against leishmaniasis, influenza, and other indications [6].
Formulations as Adjuvants
Liposomes
AS01E (or AS01B), the adjuvant system(s) used for the RTS,S malaria vaccine “comprises 3D-MPL and QS21 in a quenched form with cholesterol” and the liposomes “comprise dioleoyl phosphatidyl choline, cholesterol and 3D MPL” [17]. Cholesterol is included in the liposomes mainly to bind to and detoxify (i.e., quench) QS21 which, like other saponins, can exert toxicity by specifically binding to cholesterol and punching holes in the lipid bilayer membranes of cells such as erythrocytes. In essence, cholesterol in the liposomes blocks the toxic properties of QS21 by allowing holes to be punched in the liposomes rather than in cells, and this also allows simple mixing of the QS21 with preformed liposomes containing cholesterol and 3-deoxy MPLA. The inherent toxicity of MPLA itself is also known to be completely eliminated by incorporation into liposomes [18]. In the final vaccine formulation, the RTS,S antigen, which is a relatively hydrophobic protein, might spontaneously interact with hydrophobic sites revealed in the liposomes that contain holes punched by the QS21, thus allowing self-assembly and simplified manufacture of a complete detoxified adjuvant system containing associated antigen.
The ease of use of an adjuvant formulation can be an important factor to consider, particularly if the adjuvant formulation can be simply mixed with the antigen. In the RTS,S/AS01E (or AS01B) malaria vaccine, the AS01E (or AS01B) is simply mixed with the antigen during the manufacturing process. In another example, liposomes containing both MPLA and encapsulated recombinant anthrax protective antigen [L(MPLA + PA)] were compared with liposomes containing MPLA that were simply mixed with unencapsulated protective antigen [L(MPLA) + PA] for the ability to induce binding and neutralizing antibodies in nonhuman primates [19]. A stronger immune response was observed simply by mixing with liposomes compared to encapsulation.
Virosomes
Virosomes are unilamellar structures comprised of membrane lipids and viral membrane proteins. These empty enveloped particles are physically associated with vaccine antigen which results in enhanced immunogenicity. Virosome technology has been most advanced in influenza, in association with protein or peptides, but it is rapidly being applied to other antigens as well [20]. A potential advantage or application of this technology is to take advantage of the physical properties of virosomes, in terms of uptake by antigen presenting cells (APC), as well as the chemical composition, and compatibility with adjuvant molecules derived from lipid A.
Saponins
Saponin, a natural product derived from tree bark, was used to make ISCOMs, which are immunostimulatory complexes incorporating protein antigen into saponin. This technology has led to the development of ISCOMATRIXTM, which can be combined with a variety of antigens, and has been reported to induce CD8 responses via the MyD88 pathway [21]. Association of saponin with cholesterol to form ISCOMATRIXTM, reduces reactogenicity [22], and enhances its adjuvant effects possibly by improving bio-availability. As noted above, this approach is also used by GSK to detoxify the QS21 saponin in AS01B, or with AS02A where the QS21 interacts with squalene, a cholesterol-like molecule, reducing reactogenicity, enhancing stability, and improving adjuvant effect.
Emulsions
Oil-in-water (O/W) emulsions have been used successfully with influenza vaccines, primarily those produced in eggs. Inclusion of emulsion has enabled dose sparing and broadening of the immune response. Pandemic influenza vaccines with O/W emulsion adjuvants have been prioritized because of antigen dose-sparing and enhancing cross-reactive antibody titers which could be critical in the event of a pandemic. Examples include Focetria®, an H5N1 pandemic influenza vaccine which contains MF59, Prepandrix® and Pandemrix®, adjuvanted with AS03. Both of these emulsions contain 2% squalene; AS03 has α-d tocopherol (vitamin E) in addition to squalene. Molecular effects of MF59 have been described in the mouse model, following injection into the muscle [23], demonstrating recruitment of APC and up-regulation of multiple inflammatory cytokines, chemokines, and receptors. In addition, antigen presenting cells were recruited in response to MF59 and genes responsible for antigen processing and presentation were up-regulated. Recent effort has gone into developing TLR agonists that are more compatible with emulsions, including lipid-associated imidazoquinoline [24], leading to local adjuvant effects with decreased systemic immune activation.
Differential cytokine responses are observed following administration of different adjuvants. In general, when used with pure proteins, O/W emulsions up-regulate Th2 responses; addition of TLR agonists to the emulsions skews the response to Th1 [25]. Much of this work has been done with TLR4 agonists, including MPL®, E6020, and GLA O/W formulations [25,26]. GLA (a formulation containing PHAD, a synthetic MPLA from Avanti Polar Lipids, Alabaster, AL) has been characterized in this context, and dramatic reductions of Th2 responses normally induced by emulsion alone were observed, in which the addition of a small amount of TLR4 agonist completely blocked Th2 responses in mice.
Animal models for down-selection of adjuvants
Down-selection of an optimal adjuvant system for a human vaccine antigen by using an animal model is one of the most difficult problems in the adjuvant field [27]. Allometric scaling principles between species that are often used for calculating doses for parenteral drug delivery may not be relevant for predicting adjuvant effects and cannot explain that some novel adjuvants give similar levels of enhancement between species while others do not [28] For example, temporal or spatial separation of a CpG adjuvant and hepatitis B surface antigen was said to have no effect in mice as long as drainage to the same lymph nodes was retained, but slight spatial separation of the adjuvant and antigen, even in the same muscle, attenuated the adjuvant effect in pigs [28]. Recent evidence has strengthened the intuitive concept that NHPs may be consistently better as models than smaller animals in their ability to predict comparative efficacies of adjuvants for many human vaccines [19]. Even though liposomal MPLA is a potent formulation for inducing antibodies to co-encapsulated antigen in mice, rabbits, and humans [18, 29, 30], when liposomes lacking antigen but containing MPLA, or liposomal MPLA and saponin, are simply mixed with various soluble antigens, or with unadjuvanted liposomes containing antigen, they usually fail to stimulate significantly higher levels of antibodies than antigen alone in rodents [17, 31, 32]. However, in nonhuman primates mixing of recombinant protective antigen (PA) with liposomal MPLA lacking encapsulated antigen induced higher titers of binding or neutralizing antibodies than those induced by liposomes containing both MPLA and encapsulated PA [19].
Dramatic discrepancies in both innate and adaptive immunity between the immune systems of mice and humans have been previously summarized [33]. These differences thus highlight a substantial theoretical limitation in the use of animal models for adjuvant selection. Because of this it has been argued that although animal models may be poorly predictive of adjuvant effects for humans, NHPs are more similar than mice with regard to DC subsets and pathogen recognition receptors and are therefore likely to be more useful [7]. Thus, although NHPs may be a useful bridge between rodents and humans for adjuvant selection, comparative phase I clinical trials in which different adjuvants are compared using a single antigen is still the most reliable, and often the most cost-effective, prediction strategy for down-selection of vaccine adjuvants [27].
Transcutaneous immunization: the roles of carriers and adjuvants
The skin is the largest organ in the body and contains a rich assortment of immunologically important cells, including major innate and adaptive immune systems that monitor and react to environmental threats that breach the permeability barrier of the stratum corneum. Transcutaneous immunization (TCI) is defined as “antigen delivery into the epidermis and/or dermis through intact or pre-treated skin” [34]. The ability to perform TCI was originally enabled by the discovery that the co-application of a potent adjuvant that was simply mixed with an antigen on unbroken hydrated skin could allow TCI to occur in mice with a variety of antigens [34, 35]. The range of antibodies induced by TCI includes specificities against a wide variety of antigens [34]. Cytotoxic T cells (CTLs) are also induced by TCI [36–38], and the induction of CTLs as a cancer immunotherapeutic apparently can be achieved in the absence of exogenous adjuvant in humans simply by stringent removal of the stratum corneum [39]. In addition to parenteral immunity, it was discovered that TCI also induces mucosal immunity, and phase I and phase II human TCI vaccine trials have been conducted against diarrhea caused by Escherichia coli [40]. The emergence of TCI has led to a burgeoning number of proposed vaccines to bacterial and viral diseases, even including a preventative and therapeutic vaccine to otitis media caused by Haemophilus influenzae [41], and tumor vaccines [38].
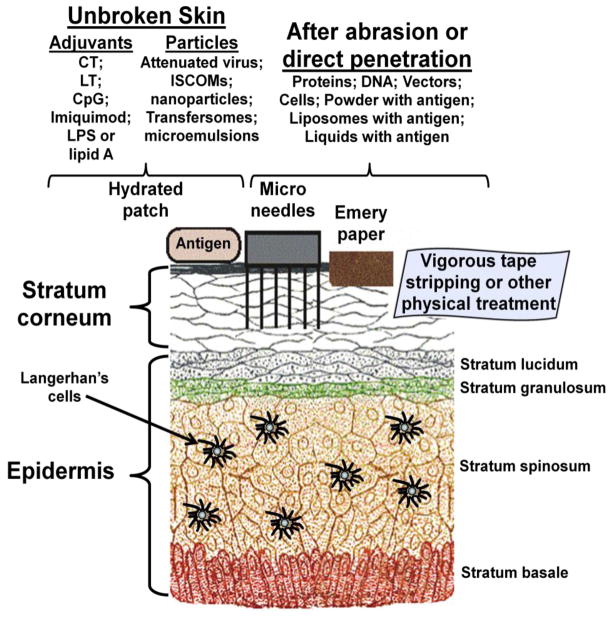
The mechanisms by which antigens and adjuvants manage to traverse intact stratum corneum are still not completely understood, but this problem can often overcome by a variety of adjuvants, particularly cholera toxin, heat-labile enterotoxin from Escherichia coli, CpG oligodeoxynucleotides, and Imiquimod [34, 42] (Figure 2). Because of this, there is considerable interest in the creation of novel adjuvants, devices, and strategies for expanding the repertoire of skin-breaching modalities for TCI [34, 42]. These have included a variety of types of microneedles, skin abrasion, ultrasound, electroporation, thermo-ablation, jet immunization [34], and even ultraviolet pre-exposure for enabling cell-mediated immunity [43]. The objective of such studies is generally to bypass or disrupt the permeability barrier of the stratum corneum in a safe manner to allow access to the underlying immune environment. An interesting observation was also made that direct intradermal injection of an established HPV vaccine with a microneedle patch caused dose-sparing of the vaccine in mice when compared to intramuscular injection [44]. In many instances a pre-treatment of the skin to remove some of the outer layers of the stratum corneum is employed [34], and sometimes ultra-short microneedles allow direct access by nanoparticles such as cationic liposomes, with or without associated adjuvants [45]. However, certain types of lipid particles, including highly flexible liposomes (Transfersomes ) can be used [34].
Figure 2.
Strategies for transcutaneous immunization. Although TCI was enabled by the use of the antigen together with an adjuvant applied with an aqueous patch to unbroken skin, numerous alternative strategies and methods have been proposed and developed for using adjuvants, particles, microneedles, and other devices for achieving delivery of antigen through the stratum corneum. See [34, 42] for details.
In summary, adjuvants may be used to improve vaccines in several different ways, including;
Enabling development of T cell vaccines. A good example is tuberculosis, in which formulated TLR agonists have led to excellent protection in animal models [38].
Antigen Dose Sparing. Important for use with vaccine antigens that are difficult to manufacture and/or which much be prepared on short notice, as in pandemic influenza or bio-defense priorities
Vaccine Dosage Sparing. Adjuvants can greatly accelerate the development of a protective immune response, resulting in protection with a single dose.
Immune Response Broadening. This has been demonstrated in egg-based influenza vaccines [14] as well as with recombinant proteins, in which incorporation of a TLR 4 agonist in an O/W emulsion greatly increased B cell sequence diversity [46].
Overcoming Immune Senescence. Certain adjuvants, particularly TLR ligands have been shown to increase immune responses in elderly populations [12].
Future directions
The science of formulations continues to advance in ways beneficial to adjuvant development. In the case of emulsions, evolution from high oil content (e.g. Freund's adjuvants) to low oil emulsions, led to the approval and wide use of the latter in influenza vaccines. Although alum has been used for decades, recent studies are now defining the mechanism(s) of action, and alum is being combined with other adjuvant components, such as MPL (AS04, GSK) to improve responses obtained with alum alone. Finally, although molecules that engage TLR have advanced furthest as adjuvant components, other PAMPs, including those that stimulate the RIG-I pathway [47] are in development.
Highlights.
Formulations of adjuvants can have different properties than individual adjuvants
Beneficial adjuvant formulations can be made with alum, oil emulsions and liposomes
Effectiveness, safety, stability, and cost are important features of formulations
Nonhuman primates are often more predictive than rodents for human adjuvant effects
Numerous adjuvants, materials, devices, and strategies are useful for skin immunization
Acknowledgments
This work was supported through a Cooperative Agreement contract (no. W81XWH-07-2-067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine and the U.S. Army Medical Research and Materiel Command.
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, Department of Defense, or the U.S. government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1**.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmüller B, et al. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. This landmark study announces positive results in a phase III vaccine trial that for the first time demonstrated significant, albeit modest, efficacy of a malaria vaccine in the prevention of Plasmodium falciparum malaria infection in infants. The vaccine was enabled by the use of the AS01 adjuvant system comprised of liposomes containing monophosphoryl lipid A and QS21 saponin. [DOI] [PubMed] [Google Scholar]
- 2.White NJ. A vaccine for malaria. N Engl J Med. 2011;365:1926–1927. doi: 10.1056/NEJMe1111777. [DOI] [PubMed] [Google Scholar]
- 3.Asante KP, Abdulla S, Agnandji S, Lyimo J, Vekemans J, Soulanoudjingar S, Owusu R, Shomari M, Leach A, Jongert E, et al. 2011 Safety and efficacy of the RTS,S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis. 2011;11:741–749. doi: 10.1016/S1473-3099(11)70100-1. [DOI] [PubMed] [Google Scholar]
- 4.Harandi AM, Medaglini D, Shattock RJ. Vaccine adjuvants: a priority for vaccine research. Vaccine. 2010;28:2363–2366. doi: 10.1016/j.vaccine.2009.12.084. [DOI] [PubMed] [Google Scholar]
- 5.Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 6.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2010;239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. Summarizes innate and adaptive immune roles of a variety of adjuvants, and also describes many of the limitations of both in vitro and animal models for down-selection of adjuvants for human vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011 Apr;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomai MA, Vasilakos JP. TLR-7 and -8 agonists as vaccine adjuvants. Expert Rev Vaccines. 2011 Apr;10(4):405–407. doi: 10.1586/erv.11.26. [DOI] [PubMed] [Google Scholar]
- 10*.Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2:15ra5. doi: 10.1126/scitranslmed.3000624. MF59 induced epitope spreading from HA2 to HA1 in hemagglutinin (HA) and neuraminidase and increased binding and avidity of antibodies to conformational epitopes relative to unadjuvanted or aluminum-adjuvanted vaccines. [DOI] [PubMed] [Google Scholar]
- 11.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 12.Behzad H, Huckriede AL, Haynes L, Gentleman B, Coyle K, Wilschut JC, Kollmann TR, Reed SG, McElhaney JE. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis. 2012;205(3):466–473. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, Baehner F, Borkowski A, Tsai TF, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406–1416. doi: 10.1056/NEJMoa1010331. [DOI] [PubMed] [Google Scholar]
- 14.O'Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines. 2011;10:447–462. doi: 10.1586/erv.11.23. [DOI] [PubMed] [Google Scholar]
- 15.Zollinger WD, Babcock JG, Moran EE, Brandt BL, Matyas GR, Wassef NM, Alving CR. Phase I study of a Neisseria meningitidis liposomal vaccine containing purified outer membrane proteins and detoxified lipooligosaccharide. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.11.084. [DOI] [PubMed] [Google Scholar]
- 16.Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine. 2011;29:9563–9572. doi: 10.1016/j.vaccine.2011.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Vandepapeliere P. Vaccine Compositions Comprising a Saponin Adjuvant. 13/020,045. US patent application No. 2011 Feb 3; Publ No. US 20/0206758, 25 Aug 2011. Gives a detailed current description of the composition of the AS01 series of adjuvants.
- 18.Fries LF, Gordon DM, Richards RL, Egan JE, Hollingdale MR, Gross M, Silverman C, Alving CR. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci USA. 1992;89:358–362. doi: 10.1073/pnas.89.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Rao M, Peachman KK, Li Q, Matyas GR, Shivachandra SB, Borschel R, Morthole VI, Fernandez-Prada C, Alving CR, Rao VB. Highly effective generic adjuvant systems for orphan or poverty-related vaccines. Vaccine. 2011;29:873–877. doi: 10.1016/j.vaccine.2010.11.049. Comparison is shown of the relative potencies of seven different adjuvants with anthrax protective antigen in non-human primates (NHP). This also illustrates the benefits of comparing adjuvant efficacy in NHP rather than rodents. Also demonstrates creative formulation of generic adjuvants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moser C, Amacker M, Zurbriggen R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev Vaccines. 2011 Apr;10(4):437–446. doi: 10.1586/erv.11.15. [DOI] [PubMed] [Google Scholar]
- 21.Wilson NS, Yang B, Morelli AB, Koernig S, Yang A, Loeser S, Airey D, Provan L, Hass P, Braley H, et al. ISCOMATRIX vaccines mediate CD8(+) T-cell cross priming by a MyD88-dependent signaling pathway. Immunol Cell Biol. 2011 Sep 6; doi: 10.1038/icb.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lövgren Bengtsson K, Morein B, Osterhaus AD. ISCOM technology-based Matrix M adjuvant: success in future vaccines relies on formulation. Expert Rev Vaccines. 2011;10:401–403. doi: 10.1586/erv.11.25. [DOI] [PubMed] [Google Scholar]
- 23.Mosca, Tritto F, Muzzi E, Monaci A, Bagnoli E, Iavarone F, O'Hagan C, Rappuoli DR, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc Nat Acad Sci. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011 Jul 26;29(33):5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 25.Coler RN, Bertholet S, Moutafsti M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, et al. Development and Characterization of Synthetic Glucopyranosyl Lipid Adjuvant System as a Vaccine Adjuvant. PLoS One. 2011;6(1):e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baudner BC, Ronconi V, Casini D, Tortoli M, Kazzaz J, Singh M, Hawkins LD, Wack A, O'Hagan DT. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020) Pharm Res. 2009;26(6):1477–1485. doi: 10.1007/s11095-009-9859-5. [DOI] [PubMed] [Google Scholar]
- 27.Alving CR. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine. 2002;20 (Suppl 3):S56–S64. doi: 10.1016/s0264-410x(02)00174-3. [DOI] [PubMed] [Google Scholar]
- 28.Davis HL. Novel vaccines and adjuvant systems. Hum Vaccin. 2008;4:246–250. doi: 10.4161/hv.4.3.5318. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Peachman KK, Sower L, Leppla SH, Shivachandra SB, Matyas GR, Peterson JW, Alving CR, Rao M, Rao VB. Anthrax LFn-PA Hybrid Antigens: Biochemistry, Immunogenicity, and Protection Against Lethal Ames Spore Challenge in Rabbits. Open Vaccine J. 2009;2:92–99. doi: 10.2174/1875035400902010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peachman KK, Li Q, Matyas GR, Shivachandra SB, Lovchik J, Lyons RC, Alving CR, Rao VB, Rao M. Anthrax Vaccine Antigen-Adjuvant Formulations Completely Protect New Zealand White Rabbits against Challenge with Bacillus anthracis Ames Strain Spores. Clin Vaccine Immunol. 2011 doi: 10.1128/CVI.05376–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma JN, Rao M, Amselem S, Krzych U, Alving CR, Green SJ, Wassef NM. Adjuvant effects of liposomes containing lipid A: enhancement of liposomal antigen presentation and recruitment of macrophages. Infect Immun. 1992;60(6):2438–2444. doi: 10.1128/iai.60.6.2438-2444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friede M, Muller S, Briand JP, Van Regenmortel MH, Schuber F. Induction of immune response against a short synthetic peptide antigen coupled to small neutral liposomes containing monophosphoryl lipid A. Mol Immunol. 1993;30:530–547. doi: 10.1016/0161-5890(93)90028-a. [DOI] [PubMed] [Google Scholar]
- 33.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 34.Bal SM, Ding Z, van Riet E, Jiskoot W, Bouwstra JA. Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J Control Release. 2010;148:266–282. doi: 10.1016/j.jconrel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Glenn GM, Rao M, Matyas GR, Alving CR. Skin immunization made possible by cholera toxin. Nature. 1998;391(6670):851. doi: 10.1038/36014. [DOI] [PubMed] [Google Scholar]
- 36.Kahlon R, Hu Y, Orteu CH, Kifayet A, Trudeau JD, Tan R, Dutz JP. Optimization of epicutaneous immunization for the induction of CTL. Vaccine. 2003;21:2890–2899. doi: 10.1016/s0264-410x(03)00141-5. [DOI] [PubMed] [Google Scholar]
- 37.Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J Clin Invest. 2004;113:998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, Kissenpfennig A, Hermans IF, Ronchese F. Tumor immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 39.Yagi H, Hashizume H, Horibe T, Yoshinari Y, Hata M, Ohshima A, Ito T, Takigawa M, Shibaki A, Shimizu H, Seo N. Induction of therapeutically relevant cytotoxic T lymphocytes in humans by percutaneous peptide immunization. Cancer Res. 2006;66 :10136–41014. doi: 10.1158/0008-5472.CAN-06-1029. [DOI] [PubMed] [Google Scholar]
- 40.Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind-Gerson J, Figueroa JF, Okhuysen PC, Guerrero NH, Martinez-Sandoval FG, Meléndez-Romero JH, et al. Use of a patch containing heat-labile toxin from Escherichia coli against travellers' diarrhoea: a phase II, randomised, double-blind, placebo-controlled field trial. Lancet. 2008;371(9629):2019–20.25. doi: 10.1016/S0140-6736(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 41.Novotny LA, Clements JD, Bakaletz LO. Transcutaneous immunization as preventative and therapeutic regimens to protect against experimental otitis media due to nontypeable Haemophilus influenzae. Mucosal Immunol. 2011;4:456–467. doi: 10.1038/mi.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N, Peng LH, Chen X, Nakagawa S, Gao JQ. Transcutaneous vaccines: novel advances in technology and delivery for overcoming the barriers. Vaccine. 2011;29(37):6179–6190. doi: 10.1016/j.vaccine.2011.06.086. [DOI] [PubMed] [Google Scholar]
- 43.Stein P, Rechtsteiner G, Warger T, Bopp T, Fuhr T, Prüfer S, Probst HC, Stassen M, Langguth P, Schild H, et al. UV exposure boosts transcutaneous immunization and improves tumor immunity. cytotoxic T-cell priming through the skin. J Invest Dermatol. 2011;131:211–219. doi: 10.1038/jid.2010.254. [DOI] [PubMed] [Google Scholar]
- 44.Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010;5(10):e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slütter B, Bal SM, Ding Z, Jiskoot W, Bouwstra JA. Adjuvant effect of cationic liposomes and CpG depends on administration route. J Control Release. 2011;154:123–130. doi: 10.1016/j.jconrel.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 46*.Wiley SR, Raman VS, Desbien A, Bailor HR, Bhardwaj R, Shakri AR, Reed SG, Chitnis CE, Carter D. Targeting TLRs Expands the Antibody Repertoire in Response to a Malaria Vaccine. Sci Transl Med. 2011;3(93):93ra69. doi: 10.1126/scitranslmed.3002135. Using deep sequencing, demonstrated the importance of TLR agonists in increasing B cell diversity. [DOI] [PubMed] [Google Scholar]
- 47.Loo Y-M, Gale M. Immune Signaling by RIG-I Receptors. Immunity. 2011;34:1–9. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]