Abstract
The delayed rectifier voltage-gated potassium channel Kv2.1 underlies a majority of the somatic K+ current in neurons and is particularly important for regulating intrinsic neuronal excitability. Various stimuli alter Kv2.1 channel gating as well as localization of the channel to cell-surface cluster domains. It has been postulated that specific domains within the C-terminus of Kv2.1 are critical for channel gating and sub-cellular localization; however, the distinct regions that govern these processes remain elusive. Here we show that the soluble C-terminal fragment of the closely related channel Kv2.2 displaces Kv2.1 from clusters in both rat hippocampal neurons and HEK293 cells, however neither steady-state activity nor N-methyl-D-aspartate (NMDA)-dependent modulation are altered in spite of this non-clustered localization. Further, we demonstrate that the C-terminus of Kv2.1 is not necessary for steady-state gating, sensitivity to intracellular phosphatase or NMDA-dependent modulation, though this region is required for localization of Kv2.1 to clusters. Thus, the molecular determinants of Kv2.1 localization and modulation are distinct regions of the channel that function independently.
Keywords: Ik, Kv2.1 clustering, gating, quantum dot, delayed rectifier, Kv2 channels
1.0 INTRODUCTION
Modulation of neuronal excitability is dependent in part on the activity of delayed rectifier voltage-gated K+ (Kv) channels. Although there are multiple neuronal Kv channels, a majority of the somatic K+ current (IK) is carried by a single channel, Kv2.1 (Murakoshi and Trimmer, 1999; Du et al., 2000; Malin and Nerbonne, 2002; Pal et al., 2003). Kv2.1 is especially notable for its characteristic localization on the soma and proximal dendrites, where it forms large cell-surface clusters (Lim et al., 2000; Antonucci et al., 2001; O'Connell and Tamkun, 2005; O'Connell et al., 2006). Furthermore, Kv2.1 exhibits an unusually depolarized voltage-dependence of activation, making it unlikely that the channel is significantly activated during a single spike (Murakoshi and Trimmer, 1999; Misonou et al., 2004; Misonou et al., 2005b; Mohapatra et al., 2009). Kv2.1 is sensitive to a variety of neuronal stimuli that cause both a hyperpolarizing shift in channel gating and dispersion of the somatodendritic clusters (Misonou et al., 2004; Misonou et al., 2005b; Misonou et al., 2006; Mohapatra and Trimmer, 2006; Mohapatra et al., 2007; Mulholland et al., 2008), suggesting that channel localization and activity are intimately linked. For these reasons, it has been proposed that Kv2.1 may be particularly important for modulating intrinsic excitability of neurons during periods of increased rates of firing (Surmeier and Foehring, 2004; Misonou et al., 2005a).
Despite the correlation between channel localization and function of Kv2.1, the exact mechanisms underpinning Kv2.1 clustering and modulation remain unclear. While “declustered” Kv2.1 localization has been proposed to be associated with hyperpolarized channel gating (Misonou et al., 2004; Misonou et al., 2005b; Mohapatra and Trimmer, 2006), we recently demonstrated that “clustered” Kv2.1 channels are actually non-conducting and that it is “non-clustered” channels that exhibit the depolarized voltage-dependence of channel activation underlying steady-state IK (O'Connell et al., 2010).
The C-terminus of Kv2.1 has been implicated as critical for both targeting and localization of Kv2.1 – a truncated Kv2.1 channel fails to form cell-surface clusters (Lim et al., 2000; Tamkun et al., 2007) and deletion of the entire C-terminal domain results in a channel that fails to traffic to the membrane (Mohapatra et al., 2008). Furthermore, mass spectrometry has demonstrated that multiple C-terminal sites are sensitive to calcineurin-dependent dephosphorylation indicating that this domain of the channel may mediate activity-dependent changes in Kv2.1 gating and localization (Park et al., 2006; Park et al., 2007). Interestingly, Mohapatra et al (2008) demonstrated that while co-expression of the soluble C-terminal fragment of Kv2.1 could rescue Kv2.1 trafficking and function, channel clustering was not restored, which indicates that Kv2.1 activity and localization may in fact be distinct processes.
In the present study, we show that the C-terminus of Kv2.2 can displace Kv2.1 from plasma membrane clusters. Moreover, we show that localization of Kv2.1 to clusters is not required for the modulation of channel gating by NMDA receptors (NMDARs) and furthermore, that while the distal C-terminus of Kv2.1 is necessary for the formation of Kv2.1 clusters, it is not required for modulation of Kv2.1 gating by either NMDARs or dephosphorylation.
2.0 EXPERIMENTAL PROCEDURES
2.1 Generation of channel constructs
The construction of vectors containing GFP-Kv2.1loopBAD, Kv2.1loopBAD-pBK and GFP-Kv2.1-ΔC318 was as previously described (O'Connell et al., 2006; Tamkun et al., 2007). Rat Kv2.2 in pBK was a gift from Dr. Jeanne Nerbonne (Washington University at St. Louis) and is the Kv2.2long isoform (Kihira et al., 2010). Green fluorescent protein (GFP) was tagged in frame at the N-terminus of rat Kv2.2 to permit live-cell visualization of Kv2.2. The soluble C-terminus of Kv2.2 (Kv2.2CT) was generated by restriction digest of Kv2.2 in pBK with BstXI and KpnI to generate a fragment encoding residues 491-907 of Kv2.2, with transcription likely beginning at Met542 to produce a soluble protein consisting of the last 367 amino acids of Kv2.2long (Figure 2). The resulting construct was confirmed by DNA sequencing. NMDAR subunits NR1, NR2a and NR2b were a gift of Dr. Steven Tavalin (University of Tennessee Health Science Center).
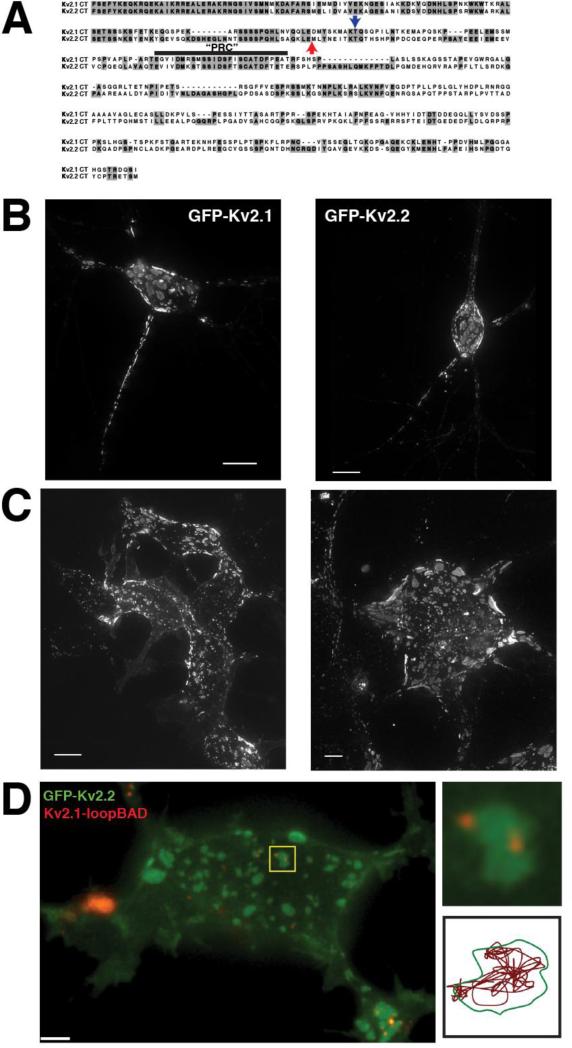
Figure 2. Clustering of Kv2.1 and Kv2.2 is governed by a mechanism common to both channels.
A. Sequence alignment of the C-termini of Kv2.1 and Kv2.2. The blue arrow indicates the point at which Kv2.1 is truncated in the Kv2.1ΔC318 construct. There are 98 residues remaining past the S6 domain (of a total of 416). The red arrow indicates the beginning of the sequence of the soluble Kv2.2CT. B. Expression of GFP-Kv2.1 (left) and GFP-Kv2.2 (right) in cultured rat hippocampal neurons. C. Expression of GFP-Kv2.1 (left) and GFP-Kv2.2 (right) in HEK293 cells. Images in B and C are maximum intensity projections of XYZ stacks. D. (left) Coexpression of GFP-Kv2.2 and Kv2.1-loopBAD (red) in HEK293 cell. Kv2.1-loopBAD was visualized by labeling with streptavidin-conjugated QD605. Image is a single Z-section of the basal surface of the cell. (Top right) Enlarged image of the cluster highlighted by the yellow box illustrating a GFP-Kv2.1 cluster containing 2 QD-labeled Kv2.1 channels. (Bottom right) Single particle track of one of the QD in the cluster illustrating that Kv2.1 channels obey the same cluster boundary as Kv2.2.
2.2 Cell culture and transfection
HEK293 cells (ATCC) were transfected with channel plasmids using Lipofectamine 2000 (Invitrogen) as previously described (O'Connell and Tamkun, 2005; O'Connell et al., 2006). For experiments using Kv2.2CT, plasmids were transfected at a ratio of 1:10 (Kv2.1:Kv2.2CT). NMDAR subunits combined with Kv2.1 were expressed at a 1:1:10 ratio and 200 μM D,L-2-amino-5-phosphonopentanoic acid (AP5) and 7-chlorokynurenic acid (7-CK) (Tocris) were added to the culture medium following transfection to block NMDAR activity. Cells were used for experiments 48 h post-transfection; for electrophysiology experiments, HEK293 cells were trypsinized and replated at low density 6-12 hours prior to use.
Rat hippocampal neurons (rHN) were cultured from E18 rat embryos (of either sex) as previously described (Brewer, 1997; Kaech and Banker, 2006; O'Connell et al., 2006). Cultured neurons were transfected with channel constructs at DIV5 as previously described (O'Connell et al., 2006) and used for experiments at DIV7-10. For electrophysiology experiments, neurons were transfected with Kv2.2CTpBK and peGFP-C1.
2.3 Fluorescence imaging
Widefield fluorescence imaging of GFP-Kv2.1 or GFP-Kv2.2 transfected HEK cells and cultured hippocampal neurons was performed using a personalDV widefield system (Applied Precision) equipped with a Coolsnap HQ2 CCD camera (Photometrics). Fluorescent images were acquired using a 60X, 1.4 NA objective and filter sets optimized for FITC and TRITC. Optically sectioned images were generated by acquiring Z-stacked images at a 0.2 μm interval followed by offline deconvolution and volume rendering using the Softworx analysis package.
For quantum-dot imaging of single Kv2.1 channels, HEK293 cells were transfected at a 1:1:1 ratio of Kv2.1loopBAD-pBK: GFP-Kv2.2 : BirA (biotin ligase) (O'Connell et al., 2006; Tamkun et al., 2007). Kv2.1 channels were labeled with streptavidin-conjugated QD655 quantum dots (Invitrogen) by incubating live cells with a 1:10,000 dilution of the quantum dots in imaging saline + 1% BSA at 37°C for 15 min. Cells were rinsed a minimum of 3x with imaging saline prior to imaging.
A Weather Station stage incubator was used to maintain the cells at 37°C during imaging experiments. All imaging experiments were performed in imaging saline consisting of (in mM): 145 NaCl, 5 KCl, 2.5 CaCl2, 0.6 MgSO4, 1.6 NaHCO3, 0.15 NaH2PO4, 0.1 ascorbic acid, 10 glucose and 10 HEPES, pH 7.4.
2.4.1 Electrophysiology
HEK293 cells were transfected with the channel constructs described above with or without NMDARs (NR1 + NR2a/NR2b). Outward K+ currents were recorded using the whole-cell voltage clamp technique on a Multiclamp 700B amplifier, digitized at 10 kHz (Digidata 1440) and lowpass filtered at 2 kHz. Patch pipettes were fabricated from borosilicate glass to give a resistance of 1-3 MΩ (HEK) or 3-5 MΩ (rHN) when filled with intracellular solution. Both capacitance and series resistance compensation (up to 80%) were used. K+ currents were online leak subtracted using a –P/3 protocol delivered from -80 mV prior to each test pulse. Currents were elicited by 200 ms test pulses to potentials from -80 mV to +80 mV in 10 mV steps from a holding potential of -80 mV. For rHN recording, a brief depolarizing prepulse (30 ms to -10 mV) was used to inactivate voltage-gated Na+ channels and A-type K+ channels. Tail currents were measured by repolarizing to -40 mV for 100 ms before returning to -80 mV for 10 s between sweeps to ensure complete channel deactivation. For HEK cells, the normalized tail current amplitude was plotted as a function of membrane potential to generate the current-voltage (I-V) relationship. For rHN, the current amplitude at the end of the test pulse was normalized to driving force using the calculated equilibrium potential for K+ for the solutions used here (EK = -83 mV), then used to generate conductance-voltage curves (G-V). The voltage dependence of activation was determined by fitting the I-V or G-V curves with the Boltzmann equation: where Imax is the peak current, V1/2 is the potential at which 50% of the channels are activated, Vm is the test potential and k is a slope factor. Results are presented as the mean ± SE. Data were acquired using pClamp 10 (Molecular Devices) and analyzed using pClamp 10 and SigmaPlot 11 (Systat, Inc.). Statistical analysis was performed using the Student's t-test; paired t-tests were used in the analysis of K+ currents from cells expressing NMDAR subunits. A p-value < 0.05 was considered to be statistically significant.
The following controls were performed in HEK293 cells to verify that there were no time-dependent shifts in Kv2.1 gating or non-specific effects of glutamate on Kv2.1 activation: 1) Currents were recorded from HEK cells expressing only Kv2.1 or Kv2.1ΔC318 for up to 40 minutes after establishing the whole-cell configuration; 2) Currents were recorded from HEK cells expressing Kv2.1 + NR2 subunits (NR1 was omitted) and 3) Currents were recorded from HEK cells expressing Kv2.1, NR1 and NR2 but glycine was omitted from the extracellular solution. There was no change in the voltage-dependence of activation under any of these conditions (data not shown).
2.4.2 Solutions
The HEK cell extracellular solution contained (in mM): 145 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 10 glucose and 10 HEPES, pH 7.4 with NaOH. For NMDAR experiments, 50 μM glycine was added to the extracellular solution and NMDARs were activated by addition of 10 μM glutamate for 60 s while stepping the membrane potential to +40 mV, followed by washout with glutamate-free extracellular solution. The intracellular solution contained (in mM): 135 mM KCl, 0.5 Na2EGTA, 1 MgCl2, 10 glucose and 10 HEPES, pH 7.4 with KOH. The liquid junction potential between the pipette and bath solutions was measured to be less than 5 mV and was not corrected.
For rHN, the extracellular solution contained (in mM): 135 NaCl, 5 KCl, 2.5 CaCl2, 0.1 MgCl2, 0.05 glycine, 10 glucose and 10 Hepes, pH 7.35 with NaOH. The intracellular solution contained (in mM): 130 K-gluconate, 10 KCl, 1 EGTA, 1 MgCl2, 0.3 CaCl2, 3 Mg-ATP, 0.3 Na-GTP, 10 Hepes and 0.005 QX-314, pH 7.35 with KOH. Glutamate (10 μM) was added as described above. All recordings were performed at room temperature (~25°C). A 10.6 mV liquid junction potential was measured for these solutions; all command potentials were corrected accordingly.
3.0 RESULTS
3.1 Expression of NR2a or NR2b-containing NMDARs in HEK293 cells modulates Kv2.1 channel function in response to glutamate
Like many ion channels, the neuronal delayed rectifier K+ channel Kv2.1 is highly regulated – both its function and localization are sensitive to a variety of neuronal stimuli, including glutamatergic input, hypoxia/ischemia and muscarinic activation (Misonou et al., 2004; Misonou et al., 2006; Mohapatra and Trimmer, 2006). Recent studies suggest that the modulation of Kv2.1 by glutamate is mediated at least in part by the activation of NMDARs (Mulholland et al., 2008). Since the subunit composition of NMDARs is variable, we investigated whether NR2a- or NR2b-containing NMDARs exert differential effects on the modulation of Kv2.1 function by co-expressing Kv2.1 with the obligate NR1 subunit together with either NR2a or NR2b in HEK293 cells and measuring the delayed rectifier K+ current from expressing cells before and after a brief (60 s) application of 10 μM glutamate in the presence of 50 μM glycine combined with voltage steps (+40 mV).
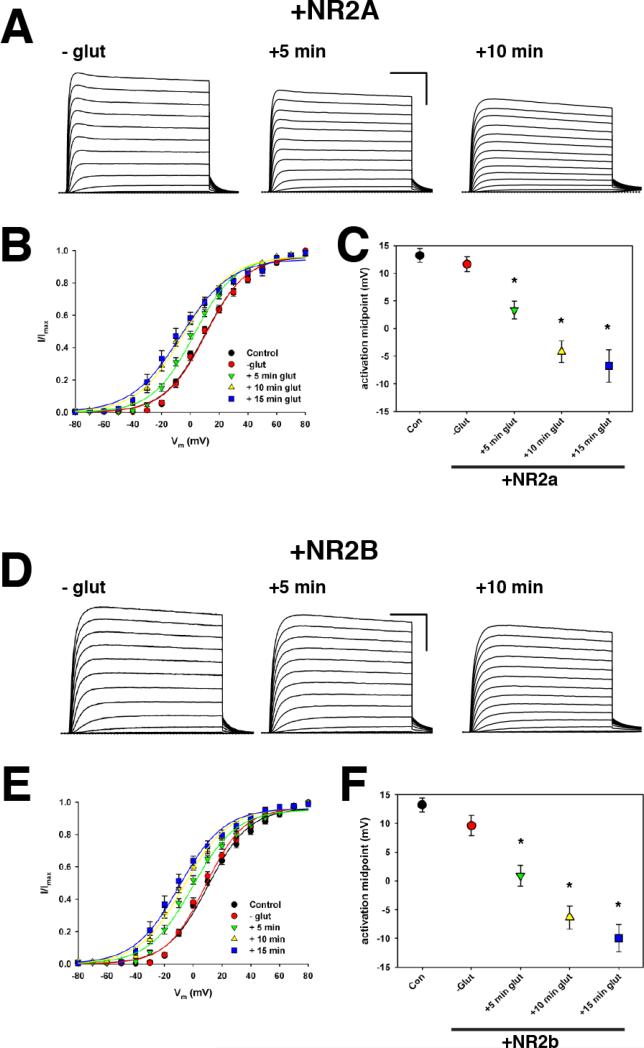
As shown in Figure 1A and B, expression of NMDARs consisting of NR1 and NR2a (NMDAR2a) did not significantly alter the gating of Kv2.1 channels in the absence of glutamate (Control V1/2 = +13.2 ± 1.2 mV, n = 12 vs. +NR2a V1/2 = +11.6 ± 1.4 mV, n = 24). However, a significant hyperpolarizing shift in the voltage-dependence of activation (V1/2) of Kv2.1 to +3.4 ± 1.6 mV (n = 24) was observed within 5 min of activation of NMDAR2a by glutamate (Figure 1 A-C). This shift became even more pronounced 10 min and 15 min after the application of glutamate (V1/2 = -4.2 ± 2.0 mV, n = 19 and -6.8 ± 3.0 mV, n = 12, respectively) (Figure 1 A-C). It should be noted that while glycine was present throughout the experiment, glutamate was applied for only ~ 60 s and was subsequently washed out with extracellular solution. Thus, the effects of NMDAR2a activation on Kv2.1 gating occur rapidly and persist for at least 15 minutes after the addition and washout of glutamate.
Figure 1. Modulation of Kv2.1 by NMDAR does not depend on the identity of the NR2 subunit.
A. Representative whole-cell currents from an HEK293 cell expressing GFP-Kv2.1 plus NR2a-containing NMDAR either before (-glut) or at the indicated time after the addition of 10 μM glutamate to the extracellular solution. Scale bars: 50 ms, 1 nA. B. Average normalized tail current-voltage (I-V) relationship for the timepoints described in A. C. Average V1/2 for all timepoints following glutamate addition. *, p < 0.001. D. Representative whole-cell recordings from HEK293 cell expressing GFP-Kv2.1 plus NR2b-containing NMDAR. Conditions are the same as in A. E. Average normalized tail I-V relationships for all timepoints. F. Average V1/2 for all timepoints after glutamate addition, *, p < 0.001.
To ascertain if modulation of Kv2.1 activity by NMDAR is dependent on the identity of the NR2 subunit, we next investigated if NMDAR comprised of NR1 and NR2b (NMDAR2b) could also induce a hyperpolarizing shift in the activation of Kv2.1. As with NMDAR2a, there was no significant effect of the expression of NMDAR2b alone on Kv2.1 activation (V1/2: +9.6 ± 1.7 mV, n = 12) and the addition of 10 μM glutamate induced a significant shift in the V1/2 within 5 min (V1/2 = +0.9 ± 1.8 mV, n = 12) (Figure 1D -F). As with NMDAR2a, the effect of NMDAR2b activation further shifted V1/2 at 10 min (-6.3 ± 2.0 mV, n = 11) and 15 min (-10.0 ± 2.3 mV, n = 6) following the application of glutamate. Although not statistically significant, there was a tendency for Kv2.1 activation to shift to more hyperpolarized potentials in NMDAR2b expressing cells than in NMDAR2a.
3.2 Localization of both Kv2.1 and Kv2.2 to plasma membrane clusters is governed by a common molecular determinant
Misonou and colleagues recently demonstrated that the 902-a.a. variant of Kv2.2 exhibits a clustered phenotype identical to Kv2.1 in both HEK cells and neurons ((Kihira et al., 2010) and our Figure 2B and C) and the two channels may be capable of heterotetramerization (Kihira et al., 2010). Although the N-termini and transmembrane domains of Kv2.1 and Kv2.2long share considerable homology (89% identity), the two channels are quite divergent in their C-termini, particularly in the distal C-terminus, where there is only ~ 36% identity homology (Figure 2A). However, the so-called “PRC” domain, considered essential for the clustering of Kv2.1 (Lim et al., 2000) is well-conserved between the two channels (17 of 26 residues, Figure 2A). Since the C-terminus of Kv2.1 has been demonstrated to be essential for the proper targeting, localization and modulation of channel function (Lim et al., 2000; Mohapatra and Trimmer, 2006; Park et al., 2006; Mohapatra et al., 2007; Park et al., 2007; Mohapatra et al., 2008), it likely plays the same role in the regulation of Kv2.2 and the two channels may use a common mechanism to establish and maintain the plasma membrane clusters.
We previously demonstrated that single Kv2.1 channels are quite mobile within the cluster domain itself even though they do not readily cross the boundary of the cluster (O'Connell et al., 2006; Tamkun et al., 2007). To determine if Kv2.1 and Kv2.2 obey a common cluster boundary, we co-expressed GFP-tagged Kv2.2 and Kv2.1loopBAD in HEK293 cells. Kv2.1loopBAD carries a biotin acceptor domain (BAD) in the S1-S2 loop that is a specific substrate for the bacterial biotin ligase BirA (Howarth et al., 2005; Tamkun et al., 2007), thus allowing the specific labeling of Kv2.1 channels on the plasma membrane with streptavidin-conjugated quantum dots (QD) to permit the visualization of single Kv2.1 channels (O'Connell et al., 2006; Tamkun et al., 2007). GFP-Kv2.2 forms plasma membrane clusters that are identical to Kv2.1-containing clusters in both cultured hippocampal neurons (Figure 2B) and in HEK293 cells (Figure 2C). As shown in Figure 2D, QD-labeled Kv2.1 channels are found primarily within the same domains as Kv2.2, suggesting that the mechanism underlying channel targeting to these domains is conserved between Kv2.1 and Kv2.2. Furthermore, single particle tracking of QD-Kv2.1 demonstrates that Kv2.1 channels do not cross the cluster boundary when the boundary is visualized using Kv2.2, indicating that a common mechanism governs the restriction of both channels to the cluster domain.
3.3 Overexpression of the soluble C-terminus of Kv2.2 disrupts clustering of Kv2.1 but does not affect Kv2.1 function
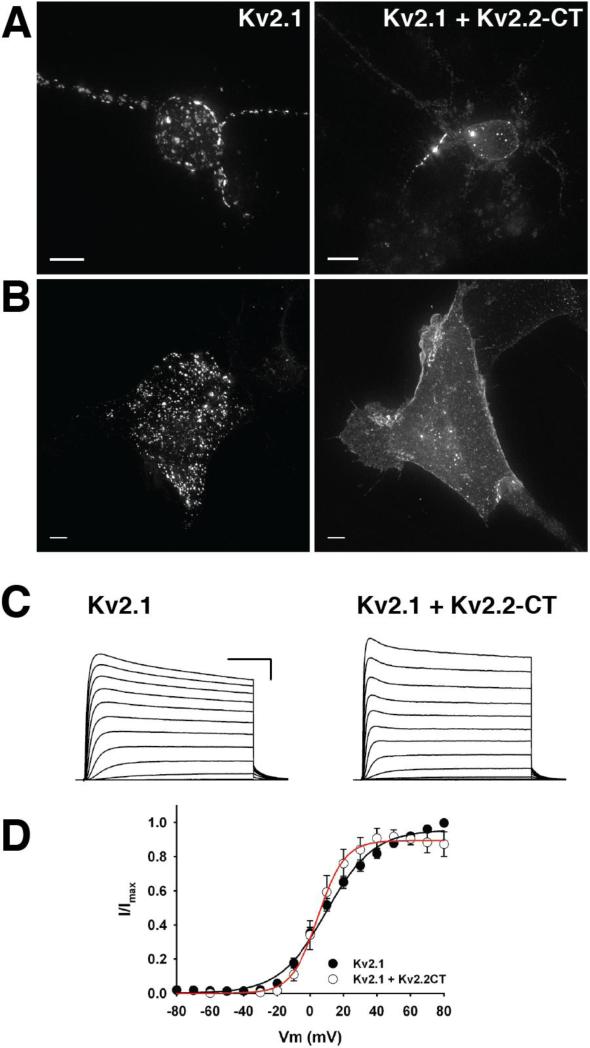
In a recent study, Mohapatra et al (2008) demonstrated that the surface expression and functional properties of a “tail-less” Kv2.1 (Kv2.1ΔC416) can be rescued by the expression of the soluble C-terminal domain of Kv2.1 (Kv2.1CT). However, although Kv2.1ΔC416+Kv2.1CT is trafficked to the plasma membrane, it fails to assemble into the large plasma membrane clusters. Possible reasons for this observation are that 1) the soluble Kv2.1CT does not efficiently adopt the required conformation to retain the tail-less Kv2.1 channels in the cluster or 2) unassembled Kv2.1CT that are not associated with a Kv2.1ΔC416 channel bind to and inhibit the channel from interacting with intracellular determinants of the cluster boundary. To distinguish between these possibilities, we overexpressed a construct consisting of only the last 367 a.a. of Kv2.2, including the “PRC” sequence (Kv2.2CT) with full-length GFP-tagged Kv2.1 (see Figure 2A). When expressed at a 1:1 ratio, GFP-Kv2.1 still formed typical large plasma membrane clusters and when Kv2.2CT was expressed at a 5-fold higher concentration than full-length Kv2.1, approximately 50% of cells exhibited a more dispersed, non-clustered channel localization (data not shown). When expressed at a 1:10 ratio, nearly 100% of cells exhibited a non-clustered phenotype (Figure 3B). Disruption of Kv2.1 clusters also occurred in cultured hippocampal neurons transfected with Kv2.2CT (Figure 3A). Consistent with previous reports, Kv2.1 localizes to the axon initial segment (Lim et al., 2000) (Sarmiere et al., 2008), but Kv2.2CT had little impact on Kv2.1 clusters in the putative AIS (Figure 3A), suggesting that a different mechanism mediates the clustering of Kv2.1 in this subcellular region. Taken together, these results support the hypothesis that retention of Kv2.1 within plasma membrane clusters requires an interaction of the channel's C-terminal domain with a saturable intracellular scaffold or fence and that the C-terminus of Kv2.2 can efficiently displace Kv2.1 from its binding partner.
Figure 3. The soluble C-terminus of Kv2.2 displaces full-length Kv2.1 from cell-surface clusters but does not affect the steady-state voltage-dependence of Kv2.1.
A. Representative maximum intensity projections of cultured hippocampal neurons expressing either full-length GFP-Kv2.1 only (left) or GFP-Kv2.1 + Kv2.2CT (right). B. Maximum intensity projections of either GFP-Kv2.1 only (left) or GFP-Kv2.1 + Kv2.2CT (right) expressed in HEK293 cells. C. Representative whole-cell recordings from HEK293 cells expressing either full-length GFP-Kv2.1 (left) or GFP-Kv2.1 + Kv2.2CT (right). Scale bars: 50 ms, 1 nA. D. Average normalized I-V relationships for full-length Kv2.1 and Kv2.1 + Kv2.2CT.
Localization of Kv2.1 is closely associated with channel function: cellular stimuli that induce “declustering” of Kv2.1, such as glutamate, hypoxia and muscarinic activation, also result in a hyperpolarizing shift in the voltage dependence of activation (Misonou et al., 2004; Misonou et al., 2005b; Mohapatra and Trimmer, 2006; Mohapatra et al., 2008). In spite of this, it remains unknown whether the localization of Kv2.1 within clusters is required for the extensive phosphorylation and relatively depolarized activation of Kv2.1 at steady-state, since all the stimuli that result in declustering also cause biochemical changes (e.g., phosphorylation) in the channel. On the other hand, it is likely that dispersion of Kv2.1 clusters by Kv2.2CT does not involve activation of intracellular signal transduction pathways and thus presents a means by which to dissect localization and function independently of these mechanisms.
The overexpression of Kv2.2CT with full-length Kv2.1 has no impact on the trafficking of functional Kv2.1 channels to the plasma membrane, as the maximal conductance (Gmax) of Kv2.1 is not significantly different from expression of full-length Kv2.1 alone (Control: 144.7 ± 19.1 nS, n = 12 vs. +Kv2.2CT: 127.3 ± 25.7 nS, n = 6). (Figure 3C and D). Furthermore, expression of Kv2.2CT had no effect on the voltage-dependence of activation of the Kv2.1-dependent delayed rectifier K+ current (V1/2 Control = +13.2 ± 1.2 mV, n = 12; V1/2 Kv2.2CT: +17.8 ± 3.7 mV, n = 6). These results indicate that targeting of Kv2.1 to clusters is not required for appropriate channel function at steady state and are consistent with our previous report that the bulk of the Kv2.1-dependent delayed rectifier K+ current is carried by “non-clustered” Kv2.1 channels (O'Connell et al., 2010).
3.4 Clustering of Kv2.1 is not required for NMDAR dependent modulation of channel gating
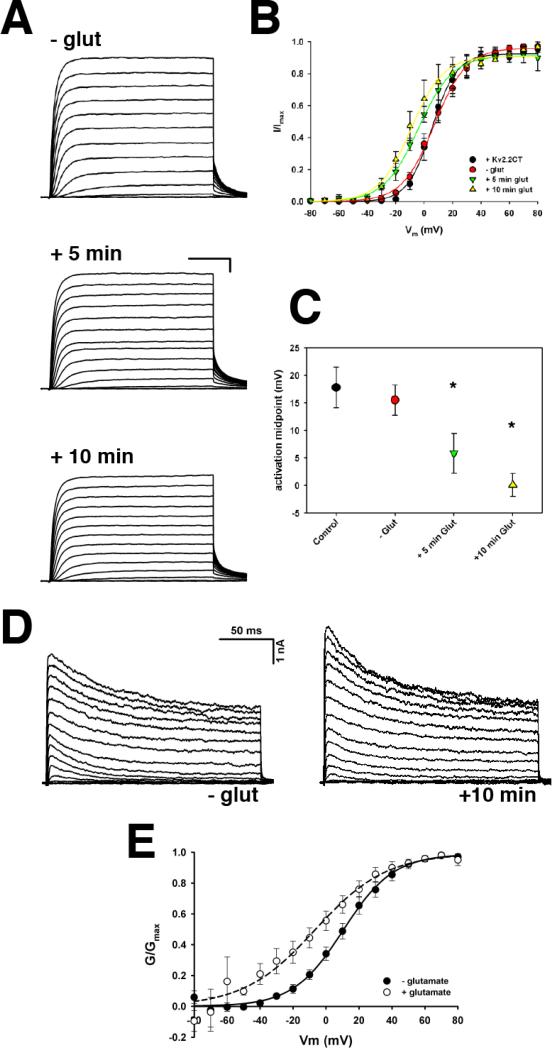
These results suggest that the localization of Kv2.1 within plasma membrane clusters is not a requirement for the appropriate steady-state gating of Kv2.1. However, given the correlation between localization and function for Kv2.1, it is possible that modulation of channel function by stimuli such as NMDAR activation (Mulholland et al., 2008) requires the targeting of Kv2.1 to cell surface cluster domains. Therefore, we investigated whether the expression of Kv2.2CT could also disrupt NMDAR-dependent modulation of Kv2.1. Since we observed no difference between NR2a- and NR2b-containing NMDARs, we did not differentiate between NMDARs containing NR2a or NR2b. As shown in Figure 4, addition of 10 μM glutamate to the extracellular solution for 60 s followed by washout with normal extracellular solution resulted in a significant hyperpolarizing shift in the V1/2 of activation within 5 min (V1/2: -glut = +15.5 ± 2.8 mV (n = 4) vs. +5 min glut = +5.9 ± 3.6 mV (n = 4), p = 0.002). Ten minutes after activation of NMDAR, the V1/2 shifted further to +0.11 ± 2.1 mV (p = 0.01). At both 5 min and 10 min post-glutamate, the magnitude of the shift in the activation midpoint was nearly identical to that observed in the absence of Kv2.2CT (ΔV1/2 = -8.3 ± 0.7 mV and -15.1 ± 1.4 mV vs. ΔV1/2 = -9.6 ± 1.0 mV and -17.3 ± 2.0 mV at +5 min and +10 min, respectively). Thus, these data strongly suggest that clustering of Kv2.1 on the plasma membrane is not required for the modulation of channel function by NMDAR.
Figure 4. Disruption of Kv2.1 clustering by the soluble C-terminus of Kv2.2 has no effect on Kv2.1 channel modulation by NMDAR activation.
A. Representative whole-cell currents from HEK293 cells expressing full-length GFP-Kv2.1 + Kv2.2CT plus NMDAR, either before (- glut) or at the indicated time following addition of 10 μM glutamate. B. Average normalized I-V relationship illustrating that even in the presence of Kv2.2CT, NMDAR activation induces a hyperpolarizing shift in the voltage-dependence of activation of Kv2.1. The average V1/2 for all conditions is presented in panel C. D. Whole-cell K+ currents from a representative rHN transfected with Kv2.2CT before (left) and 10 minutes after the addition of 10 μM glutamate+50 μM glycine (right). E. Average normalized G-V relationship for all neurons before (closed symbols) and after (open symbols) glutamate addition.
We next determined if the overexpression of Kv2.2CT altered the glutamatergic modulation of the endogenous neuronal delayed rectifier K+ current (which is primarily due to Kv2.1). As shown in Figure 4D and E, the addition of 10 μM glutamate and 50 μM glycine to cultured rHN expressing Kv2.2CT, induced a significant hyperpolarizing shift in the voltage-dependence of activation of the delayed rectifier current (Control V1/2 = +12.5 ± 4.9; +Glutamate V1/2 = -9.5 ± 7.8, n=7, p = 0.01), indicating that cell surface clustering of Kv2.1 is not required for glutamate receptor-mediated modulation of Kv2.1 gating.
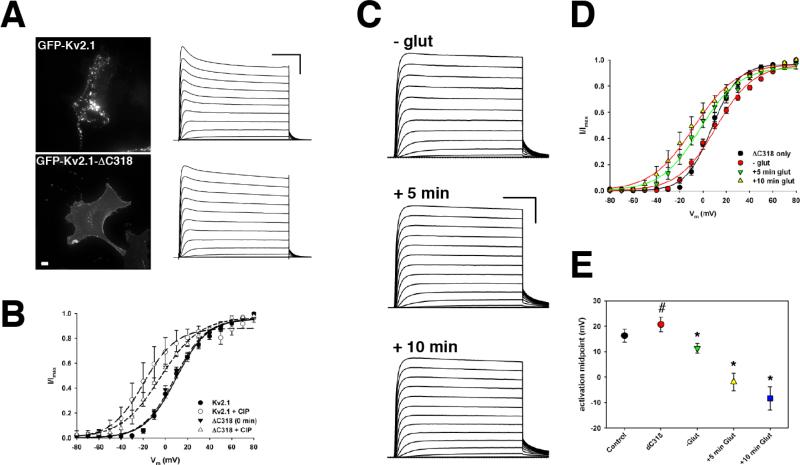
3.5 Deletion of the last 318 a.a. of Kv2.1 disrupts channel localization on the plasma membrane but does not affect either steady-state activity or modulation of Kv2.1 function by NMDAR
It has been well-established that truncation of Kv2.1 at residue 535, 98 amino acids from the end of S6, (Kv2.1ΔC318) results in a functional channel that efficiently traffics to the plasma membrane (VanDongen et al., 1990; Bentley et al., 1999) but does not form cell surface clusters and instead has an even distribution ((Lim et al., 2000), Figure 5A). In Xenopus oocytes (VanDongen et al., 1990) and COS-1 cells (Murakoshi et al., 1997), Kv2.1ΔC318 exhibits a more hyperpolarized voltage dependence of activation than full-length Kv2.1, although in both of these systems, full-length Kv2.1 activates at more hyperpolarized potentials than in HEK293 cells and is less extensively phosphorylated than in brain or HEK293 cells (Shi et al., 1994; Mohapatra and Trimmer, 2006). Notably, the biophysical properties of Kv2.1 expressed in HEK293 cells most closely resemble the properties of the neuronal delayed rectifier current, suggesting that HEK293 cells may be a more appropriate model system.
Figure 5. The distal C-terminus of Kv2.1 is required for clustering but not for modulation by NMDARs.
A. (Left) Maximum intensity projections of HEK293 cells expressing either full-length GFP-Kv2.1 or GFP-Kv2.1-ΔC318. (Right) Representative whole cell currents from GFP-Kv2.1 or GFL-Kv2.1-ΔC318 expressing HEK293 cells. Scale bars: 50 ms, 1 nA. B. Average normalized I-V curves for Kv2.1 and Kv2.1-ΔC318 ± calf intestinal phosphatase (CIP), illustrating that Kv2.1-ΔC318 retains functional sensitivity to phosphorylation state. C. Representative whole-cell currents from HEK293 cell expressing GFP-Kv2.1-ΔC318 plus NMDAR at the indicated time points before and after the addition of 10 μM glutamate. Average normalized I-V curves (D) and V1/2 (E) for Kv2.1-ΔC318 before and after activation of NMDAR. #, p < 0.05, compared to Control; *, p < 0.001, compared to –glut.
Consistent with previous reports in COS-1 cells (Shi et al., 1994) and cultured hippocampal neurons (Lim et al., 2000), Kv2.1ΔC318 exhibits a dispersed localization on the plasma membrane and does not form the clusters typical of full-length Kv2.1 (Figure 5A). The truncated channel is functional and exhibits robust delayed rectifier currents (Figure 5A), however, unlike in previous reports, the voltage-dependence of activation of gating for Kv2.1ΔC318 in HEK cells is similar to full-length Kv2.1 (Kv2.1: +16.3 ± 2.6 mV, n = 9; Kv2.1ΔC318: +20.7 ± 2.8 mV, n = 7) (Figure 5B). The 318 residues deleted in Kv2.1ΔC318 contain 10 of the 16 known phosphorylation sites in the Kv2.1 C-terminus, including 5 of the 7 residues sensitive to dephosphorylation by calcineurin (Park et al., 2006; Park et al., 2007), thus our results indicate that these residues are not an important determinant of the steady-state function of Kv2.1.
Intracellular dialysis with alkaline phosphatase has been used to demonstrate that dephosphorylation of Kv2.1 is the principle cause of the large hyperpolarizing shift in channel activation (Murakoshi et al., 1997; Mohapatra and Trimmer, 2006; Mohapatra et al., 2008). We therefore used this approach to determine if dephosphorylation of Kv2.1ΔC318 has a similar effect as the full-length Kv2.1 in HEK293 cells. As shown in Figure 5B, 10 min intracellular dialysis with 100 U/ml calf intestinal alkaline phosphatase (CIAP) resulted in a significant hyperpolarizing shift in the V1/2 of activation (Control V1/2: +20.7 ± 2.8 mV vs. +CIP V1/2: -0.64 ± 2.1 mV, p < 0.001; n = 7), suggesting that phosphorylation of residues outside of the distal C-terminus also substantially impact channel function. It should be noted that the CIAP-induced shift in the activation of full-length Kv2.1 is slightly larger than we observed for Kv2.1ΔC318 after similar periods of CIAP dialysis (FL-Kv2.1 ΔV1/2 = -25.5 mV vs. Kv2.1ΔC318 ΔV1/2 = -21.3 mV).
Since the activity of Kv2.1ΔC318 is regulated by phosphorylation (Figure 5B), we next investigated if Kv2.1ΔC318 is also sensitive to modulation by NMDARs. We transfected HEK293 cells with GFP-Kv2.1ΔC318 and NR1 + either NR2a or NR2b (since there was no significant difference between the NR2 subunits, we pooled all data from both NR2a and NR2b-expressing cells) and measured Kv2.1-dependent delayed rectifier currents in response to glutamate stimulation as described above. Interestingly, as with full-length Kv2.1, within 5 min of glutamate addition, we observed a significant hyperpolarizing shift in the midpoint of activation of Kv2.1ΔC318 from +11.3 ± 1.9 mV to -1.9 ± 3.4 mV (p < 0.001; n = 11) (Figure 5C-E). By 10 min, the V1/2 had shifted to -8.3 ± 4.6 mV (p < 0.001). These results clearly demonstrate that modulation of Kv2.1 is strongly influenced by channel domains outside of the distal C-terminus. Furthermore, the calcineurin-sensitive residues in the distal C-terminus of Kv2.1 are not required for the regulation of Kv2.1 activity by NMDARs, although our data do not exclude a potential role of these residues in the regulation of Kv2.1 localization.
4.0 DISCUSSION
In this study, we show that the distal C-terminus of Kv2.2 can displace full-length Kv2.1 from cell surface clusters and that this displacement does not alter either the steady-state activity of Kv2.1 or the sensitivity of channel gating to NMDAR-dependent modulation. We also demonstrate that although the distal C-terminus of Kv2.1 is required for retention of Kv2.1 within the cluster domain, it is not required for steady-state gating, sensitivity to intracellular phosphatase or NMDAR-dependent modulation.
We previously demonstrated that the “clustered” Kv2.1 channels are non-conducting and that neither intracellular phosphatase treatment nor depolymerization of cortical actin affects the conductance of these “silent” channels (O'Connell et al., 2010). We observed no change in the maximal conductance in this study for either Kv2.1ΔC318 or full-length Kv2.1+Kv2.2CT, suggesting that the mechanism that induces the clustered Kv2.1 channels to be non-conducting is intrinsic to the channel protein itself and does not require retention of Kv2.1 to the cluster domain.
Consistent with our previous study, in which we demonstrated that non-clustered Kv2.1 channels are responsible for the majority of the Kv2.1-dependent delayed rectifier current (with its characteristic depolarized voltage-dependence of activation), we saw no change in the voltage-dependence of activation of the Kv2.1 current in cells co-expressing Kv2.2CT. Taken together with our previous results, these data support the hypothesis that phosphorylation is not likely to be the mechanism for silencing the clustered Kv2.1 channels. It has been recently demonstrated that Kv2.1 is SUMOylated on its C-terminus (Dai et al., 2009; Plant et al., 2011). In neurons, SUMOylated Kv2.1 channels are required to generate an IDR with the characteristic depolarized voltage dependence seen at steady-state (Plant et al., 2011), indicating that post-translational modifications other than phosphorylation are essential for the normal function of Kv2.1 channels. Despite the fact that Kv2.1 channels are no longer clustered when co-expressed with Kv2.2CT, the channel is still highly sensitive to modulation by NMDARs and exhibits the same magnitude and time course for the shift in the voltage dependence of activation.
The C-terminus of Kv2.1 is required for the appropriate subcellular localization of Kv2.1, as the channel fails to form cell surface clusters when the distal 318 amino acids are deleted and changes in Kv2.1 gating are associated with “declustering” of the channel at the plasma membrane. It has been well-established that Kv2.1 is highly phosphorylated at a number of C-terminal sites and that glutamatergic activation of ionotropic glutamate receptors, such as NMDA receptors modulates Kv2.1 gating and localization via calcineurin-dependent dephosphorylation induced by Ca2+ influx through these receptors (Misonou et al., 2004; Park et al., 2006). However, we show here that truncation of the residues that are critical for channel localization have no effect on gating, as Kv2.1ΔC318 exhibits activation properties nearly identical to the full-length Kv2.1. Furthermore, Kv2.1ΔC318 is still sensitive to both intracellular phosphatase and modulation by NMDARs, indicating that channel domains beyond the distal C-terminus are responsible for NMDAR-dependent modulation of channel gating. Interestingly, 10 of the 16 phosphorylation sites identified using mass spectrometry are found within the 318 deleted residues, including 5 of the 7 sites shown to be sensitive to dephosphorylation by calcineurin (Park et al., 2006; Park et al., 2007). Calcineurin-dependent dephosphorylation has been implicated as a key mechanism in stimulus-induced modulation of Kv2.1 function and localization, thus raising the possibility that the role of these residues in modulating channel gating may not be as prominent as suggested. More perplexing is that individual Ser -> Ala mutation of residues within this region resulted in graded shifts in channel activation (Park et al., 2006), whereas their complete absence in the Kv2.1ΔC318 construct used in our study has no apparent affect on the steady-state gating of Kv2.1 (Figure 5). The steady-state activity of Kv2.1ΔC318 measured here is consistent with previous reports in which this truncation has only a small effect (if any) on channel gating (VanDongen et al., 1990; Bentley et al., 1999). These results are somewhat difficult to reconcile, although it appears likely that the regulation of Kv2.1 gating by phosphorylation is a complex phenomenon dependent on a specific pattern of phosphosites, rather than an “on/off” switch. Thus, some sites may exert more influence than others or mutation of a single site could disrupt Kv2.1 gating in a manner that does not accurately reflect that residue's specific role in modulating Kv2.1. Although the deletion of such a large segment of the Kv2.1 C-terminus might also result in perturbation of channel function, the fact that Kv2.1ΔC318 exhibits wild-type gating and is still highly sensitive to both phosphatase and NMDAR modulation indicates that this is not the case. More likely, phosphosites outside the distal 318 amino acids, a non-phosphorylation mechanism (residue K470 of Kv2.1 is SUMOylated and is still present in Kv2.1ΔC318, (Plant et al., 2011)) or a combination of both, are crucial for modulation of Kv2.1 gating.
It is also plausible that the distal regions of Kv2.1 play a more pronounced role in channel localization (hence the inability of Kv2.1ΔC318 to form clusters) while more membrane proximal regions (or regions more proximal to the transmembrane core of Kv2.1) are more dominant in regulating channel function. Nevertheless, our data clearly show that Kv2.1 localization and function are two distinct processes and that both channel function and modulation are, in fact, independent of the localization of Kv2.1 to clusters. This is entirely consistent with our previous finding that clustered Kv2.1 channels do not function as K+ conducting channels and likely have a separate role that does not depend on their K+ channel activity. Such a role has been postulated by Lotan's group, who have demonstrated that Kv2.1 plays a non-conducting role in dense-core vesicle release in chromaffin cells and dorsal root ganglion cells (Singer-Lahat et al., 2008; Feinshreiber et al., 2010).
Our results clearly demonstrate that Kv2.1 localization and function are governed by different amino acid domains which is consistent with our hypothesis that clustered Kv2.1 channels function in a capacity other than as K+ fluxing channels (O'Connell et al., 2010). It should be noted that it is clear that Kv2.1 function and localization are likely subject to the same modulatory pressures (Misonou et al., 2004; Misonou et al., 2005b; Mohapatra and Trimmer, 2006). Nevertheless, the precise domains for Kv2.1 cluster and function remain elusive.
Highlights.
Localization of Kv2.1 and Kv2.2 is governed by a common mechanism.
Both NR2A- and NR2B-containing NMDA receptors modulate Kv2.1.
The Kv2.2 C-terminus disrupts Kv2.1 clustering but not Kv2.1 gating.
The C-terminus is required for clustering but not for gating or modulation of Kv2.1.
Acknowledgements
The authors thank Shannon Guyot for expert technical assistance, Dr. Michael Tamkun for the gift of the Kv2.1 constructs and Dr. Steven Tavalin for NMDA receptor plasmids. This work was supported by National Institutes of Health Grant R00HL087591 to K.M.S.O.
Abbreviations
- NMDA
N-methyl-D-aspartate
- Kv
Voltage-gated K+
- HEK
Human Embryonic Kidney
- IK
Somatic K+ current
- GFP
Green Fluorescent Protein
- AP5
D,L-2-amino-5-phosphonopentanoic acid
- 7-CK
7-chlorokynurenic acid
- QD
Quantum Dot
- CIAP
calf intestinal alkaline phosphatase
- rHN
rat hippocampal neurons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. doi: 10.1016/s0306-4522(01)00476-6. [DOI] [PubMed] [Google Scholar]
- Bentley GN, Brooks MA, O'Neill CA, Findlay JB. Determinants of potassium channel assembly localised within the cytoplasmic C-terminal domain of Kv2.1. Biochim Biophys Acta. 1999;1418:176–184. doi: 10.1016/s0005-2736(99)00021-8. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997;71:143–155. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- Dai XQ, Kolic J, Marchi P, Sipione S, Macdonald PE. SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J Cell Sci. 2009;122:775–779. doi: 10.1242/jcs.036632. [DOI] [PubMed] [Google Scholar]
- Du J, Haak LL, Phillips-Tansey E, Russell JT, McBain CJ. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J Physiol. 2000;522(Pt 1):19–31. doi: 10.1111/j.1469-7793.2000.t01-2-00019.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinshreiber L, Singer-Lahat D, Friedrich R, Matti U, Sheinin A, Yizhar O, Nachman R, Chikvashvili D, Rettig J, Ashery U, Lotan I. Non-conducting function of the Kv2.1 channel enables it to recruit vesicles for release in neuroendocrine and nerve cells. J Cell Sci. 2010;123:1940–1947. doi: 10.1242/jcs.063719. [DOI] [PubMed] [Google Scholar]
- Howarth M, Takao K, Hayashi Y, Ting AY. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc Natl Acad Sci U S A. 2005;102:7583–7588. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kihira Y, Hermanstyne TO, Misonou H. Formation of heteromeric Kv2 channels in mammalian brain neurons. J Biol Chem. 2010;285:15048–15055. doi: 10.1074/jbc.M109.074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Antonucci DE, Scannevin RH, Trimmer JS. A novel targeting signal for proximal clustering of the Kv2.1 K+ channel in hippocampal neurons. Neuron. 2000;25:385–397. doi: 10.1016/s0896-6273(00)80902-2. [DOI] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Delayed rectifier K+ currents, IK, are encoded by Kv2 alpha-subunits and regulate tonic firing in mammalian sympathetic neurons. J Neurosci. 2002;22:10094–10105. doi: 10.1523/JNEUROSCI.22-23-10094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005a;26:743–752. doi: 10.1016/j.neuro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Menegola M, Trimmer JS. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J Neurosci. 2005b;25:11184–11193. doi: 10.1523/JNEUROSCI.3370-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Mohapatra DP, Guy LK, Park KS, Trimmer JS. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J Neurosci. 2006;26:13505–13514. doi: 10.1523/JNEUROSCI.3970-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Trimmer JS. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci. 2006;26:685–695. doi: 10.1523/JNEUROSCI.4620-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Park KS, Trimmer JS. Dynamic regulation of the voltage-gated Kv2.1 potassium channel by multisite phosphorylation. Biochem Soc Trans. 2007;35:1064–1068. doi: 10.1042/BST0351064. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Siino DF, Trimmer JS. Interdomain cytoplasmic interactions govern the intracellular trafficking, gating, and modulation of the Kv2.1 channel. J Neurosci. 2008;28:4982–4994. doi: 10.1523/JNEUROSCI.0186-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Misonou H, Pan SJ, Held JE, Surmeier DJ, Trimmer JS. Regulation of intrinsic excitability in hippocampal neurons by activity-dependent modulation of the KV2.1 potassium channel. Channels (Austin) 2009;3:46–56. doi: 10.4161/chan.3.1.7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, Hearing MC, Becker HC, Woodward JJ, Chandler LJ. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2.1 potassium channels. J Neurosci. 2008;28:8801–8809. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Trimmer JS. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci. 1999;19:1728–1735. doi: 10.1523/JNEUROSCI.19-05-01728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol. 1997;52:821–828. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- O'Connell KM, Tamkun MM. Targeting of voltage-gated potassium channel isoforms to distinct cell surface microdomains. J Cell Sci. 2005;118:2155–2166. doi: 10.1242/jcs.02348. [DOI] [PubMed] [Google Scholar]
- O'Connell KM, Loftus R, Tamkun MM. Localization-dependent activity of the Kv2.1 delayed-rectifier K+ channel. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1003028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell KM, Rolig AS, Whitesell JD, Tamkun MM. Kv2.1 potassium channels are retained within dynamic cell surface microdomains that are defined by a perimeter fence. J Neurosci. 2006;26:9609–9618. doi: 10.1523/JNEUROSCI.1825-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Mohapatra DP, Trimmer JS. Proteomic analyses of K(v)2.1 channel phosphorylation sites determining cell background specific differences in function. Channels (Austin) 2007;1:59–61. doi: 10.4161/chan.4388. [DOI] [PubMed] [Google Scholar]
- Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- Plant LD, Dowdell EJ, Dementieva IS, Marks JD, Goldstein SA. SUMO modification of cell surface Kv2.1 potassium channels regulates the activity of rat hippocampal neurons. J Gen Physiol. 2011;137:441–454. doi: 10.1085/jgp.201110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiere PD, Weigle CM, Tamkun MM. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Kleinklaus AK, Marrion NV, Trimmer JS. Properties of Kv2.1 K+ channels expressed in transfected mammalian cells. J Biol Chem. 1994;269:23204–23211. [PubMed] [Google Scholar]
- Singer-Lahat D, Chikvashvili D, Lotan I. Direct interaction of endogenous Kv channels with syntaxin enhances exocytosis by neuroendocrine cells. PLoS One. 2008;3:e1381. doi: 10.1371/journal.pone.0001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Foehring R. A mechanism for homeostatic plasticity. Nat Neurosci. 2004;7:691–692. doi: 10.1038/nn0704-691. [DOI] [PubMed] [Google Scholar]
- Tamkun MM, O'Connell KM, Rolig AS. A cytoskeletal-based perimeter fence selectively corrals a sub-population of cell surface Kv2.1 channels. J Cell Sci. 2007;120:2413–2423. doi: 10.1242/jcs.007351. [DOI] [PubMed] [Google Scholar]
- VanDongen AM, Frech GC, Drewe JA, Joho RH, Brown AM. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990;5:433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]