Introduction
Robot-assisted surgery in the field of head and neck has been gaining popularity due to appeal in using minimally invasive approaches, obtaining excellent visualization, and overall low risk. Reports among multiple different specialties have also shown reduction in surgery duration, recovery time in the intensive care unit, and overall time of hospitalization stays when compared to classic open procedures [1]. Techniques for resecting skull base cancers are traditionally performed through large incisions and require mobilization of large amounts of tissue. Transoral robotic surgery (TORS) may provide a more accurate means to treat head and neck and skull base cancer, while allowing for decreased levels of morbidity and decreased levels of adjuvant radiation and chemotherapy.
Reports on the development of TORS application to anterior skull base masses have previously described in small case descriptions in preclinical experimental canine and cadaver models using transcervical or suprahyoid ports [2–4]. Subsequently, TORS was applied to a small case series and feasible in 9 of 10 patients with parapharyngeal space tumors with no carotid encasement or bone erosion [5]. The objective of this study is to discuss feasible approaches using transoral robotic surgery (TORS) to access the anterior and lateral skull base and describe a novel approach for nodal dissection in the retropharyngeal space.
Materials and Methods
The da Vinci surgical robot (Intuitive Surgical, Inc., Sunnyvale, CA) was used on four patients for complete resection of skull base tumors. The da Vinci surgical robot has been FDA approved for resection of T1 and T2 oropharyngeal cancers. We applied robotic technology for off-label use to resect skull base tumors. The pathologic diagnosis was two pleomorphic adenomas in the parapharyngeal space, one pleomorphic adenoma in the infratemporal fossa, and one metastatic papillary thyroid cancer node in the high retropharyngeal node basin. Details on patients included in the study are shown in Table 1. A transpalatal approach was used to access the infratemporal fossa (n=1) and retropharynx (n=1). Lateral pharyngotomies were performed to access parapharyngeal spaces (n=2). All mucosal incisions were closed primarily.
Table 1.
Demographics and Pathology on Patients
| Patient | Age (years) |
Gender | Race | Site | Histology |
|---|---|---|---|---|---|
| 1 | 78 | M | Caucasian | R parapharyngeal space |
Pleomorphic adenoma |
| 2 | 40 | M | Caucasian | L parapharyngeal space |
Pleomorphic adenoma |
| 3 | 49 | M | Caucasian | R infratemporal fossa |
Pleomorphic adenoma |
| 4 | 36 | F | Caucasian | L retropharyngeal node basin |
Papillary thyroid ca |
Results
TORS was performed on 4 dentate patients (3 males, mean age = 51 years, age range =36–78 years old) who had an anterior and lateral skull base lesion (Table 1). All patients were place in the supine position with a shoulder roll and underwent general endotracheal anesthesia. The reinforced endotracheal tube was secured laterally in the oral cavity, depending on the site of mass. The operating table was rotated 180 degrees away from the anesthesiologist’s field. Prior to TORS, all patients were examined under direct rigid laryngoscopy using a McIvor mouth gag for adequate exposure. Once the site of mass was identified, the da Vinci surgical robot was introduced in the surgical field. The articulating robotic arms were positioned intraorally, along with the zero or 30-degree endoscope. With robotic control, the endoscope was manipulated to provide visualization of the oral cavity and pharynx.
In order to obtain adequate access to the anterior and lateral skull base spaces, either a bovie cautery or CO2 laser was used to dissect tissue in the posterior oral cavity or oropharynx. Two patients with pleomorphic adenomas in the lateral parapharyngeal space required lateral pharyngectomies. Partial pharyngectomies were performed with the aid of the robot. The patient with a pleomorphic adenoma in the infratemporal fossa and patient with a metastatic papillary thyroid cancer node in the retropharynx required a lateral transpalatal approach. Palate resection with dissection of the soft palate to the level of the lateral glossopharyngeal fold was accomplished with the aid of the robot, as well as manual aid from the bedside assistant. Adequate exposure was obtained throughout the case and the internal carotid arteries and cranial nerves were identified. Complete excision of the skull base mass was performed in en bloc (n=3) or piece-meal (n=1) fashion using the di Vinci surgical robot. The bedside assistant provided retraction and helped control bleeding. Hemostasis was adequately achieved using bipolar cautery or suction bovie cautery when away from the carotid artery. On completion of the surgery, all robotic instruments, endoscopes, and retractors were removed with no inadvertent trauma or injury to the patient. The surgical wounds were irrigated copiously with warm water and all mucosal incisions were closed primarily.
There were no intraoperative arterial injuries or other complications. Postoperatively, one patient experienced an episode of transient 1mm ptosis that resolved spontaneously prior to the first post-operative clinic visit. There were no cranial neuropathies and no cases with velopharyngeal insufficiency. All patients regained normal swallowing function within 5 days of surgery. Postoperative imaging (MRI in 1 patient, CT scan in 1 patient, and I-123 in 1 patient) confirmed complete resection (Figure 1). No cases of recurrence were found within short follow-up (8–14 months).
Figure 1.
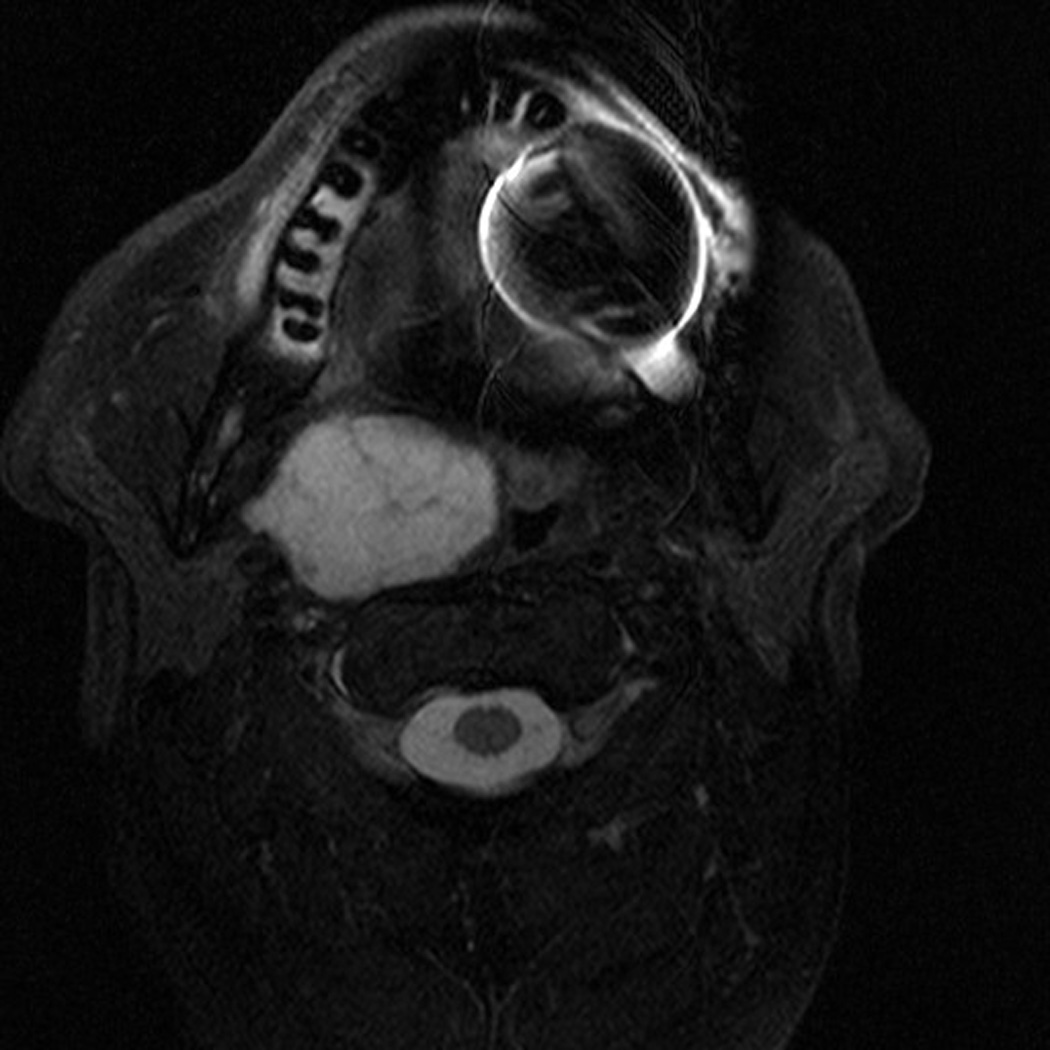
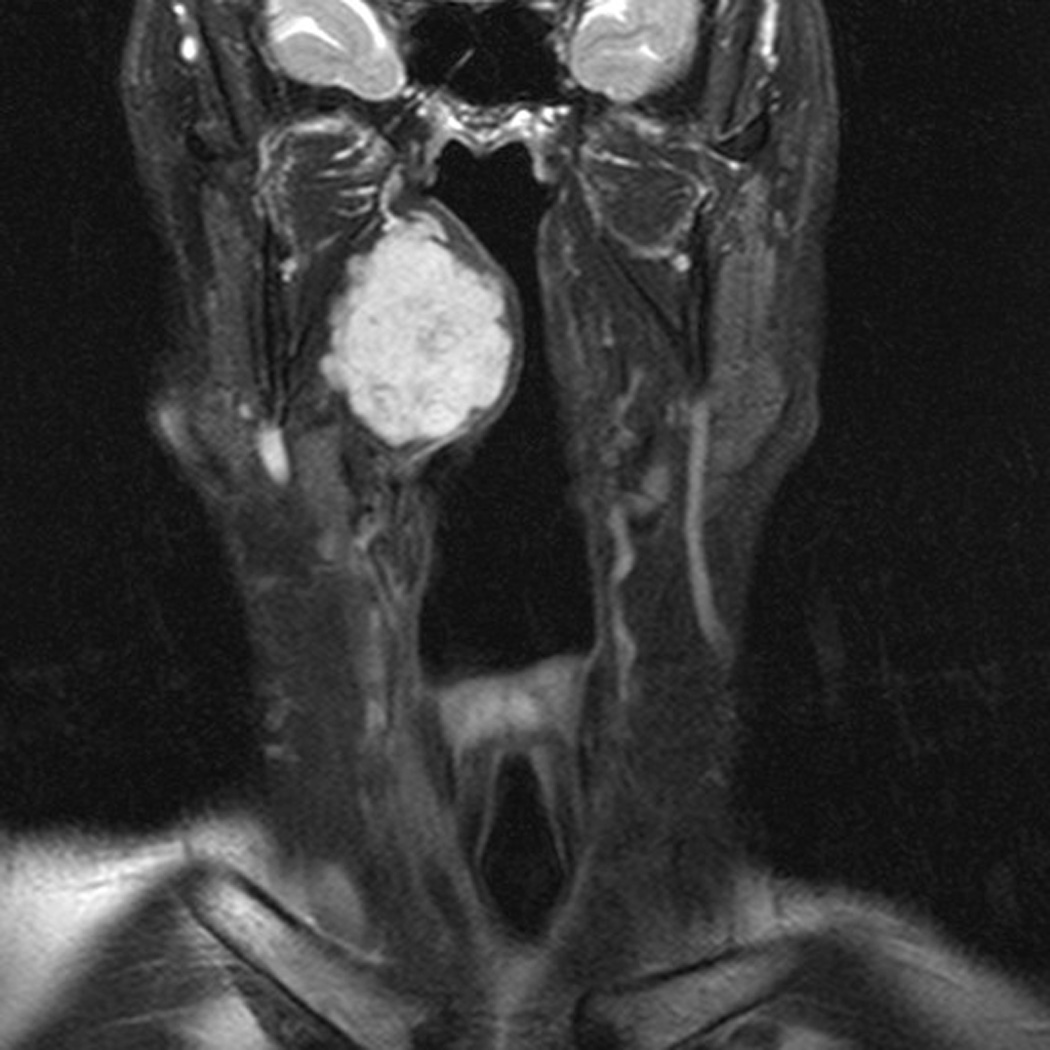
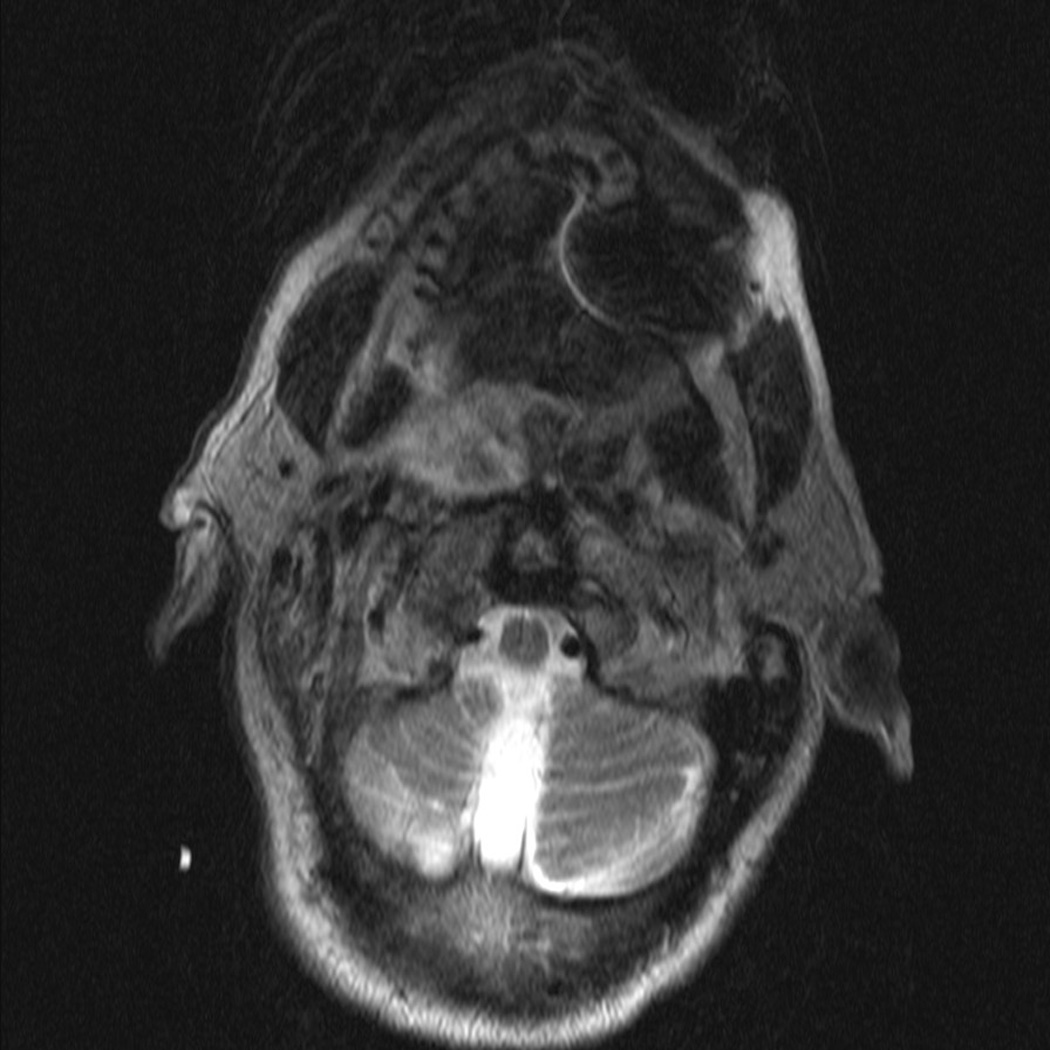
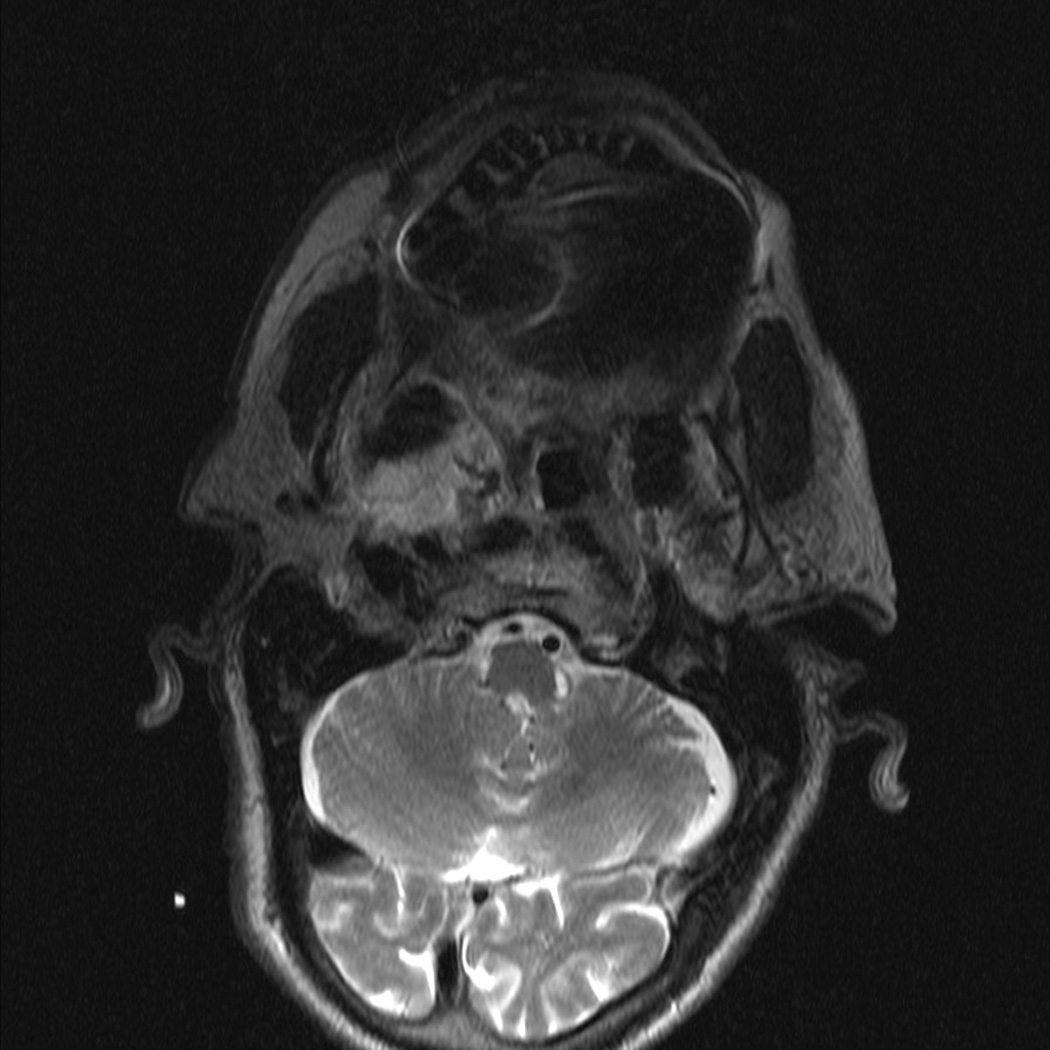
Pre- and Post-operative Imaging of Complete Resection. Patient with pleomorphic adenoma in the right parapharyngeal space. (A) pre-operative MRI scan, axial; (B) pre-operative MRI scan, coronal; (C) post-operative MRI scan, axial showing superior aspect of mass; (D) post-operative MRI scan, axial showing inferior aspect of mass.
Discussion
The application of the surgical robotic technology in skull base resections shows much promise. We have demonstrated in 4 adult patients that exposure with a McIvor mouth gag, visualization using an upward 30-degree endoscope, and manipulation of tissues with robotic assistance allowed for successful resection of anterior and lateral skull base lesions. Advantages of robotic-assisted surgery compared to conventional open procedures include small incision sites, three-dimensional vision, high degree of precision with increased freedom of instrument movement, and tremor filtration. Another advantage of TORS compared to non-robotic transoral approaches is the ability to have 4 working instruments to operate simultaneously (2 robotic arms and 2 from the bedside assistant). Despite one patient who had a transient episode of 1mm ptosis, we believe these patients had favorable clinical outcomes and improved functional abilities (e.g., swallowing) than expected by an invasive open procedure. We believe that the robot offers potential ability to reduce morbidity relative to conventional open procedures and decrease the side effects from reduced dosage of adjuvant chemoradiation therapy.
All of our patients underwent exclusive transoral robotic surgery, without the need for a cervical or suprahyoid port [2–4] (Figure 2). Although cervical-transoral robotic surgery (c-TORS) has demonstrated successful resection of anterior and midline skull base masses in canine and cadaver studies [2–3], we believe that the transpalatal or lateral pharyngeal approaches may provide better exposure of the infratemporal fossa and parapharyngeal space. To date, there is one small case series that reported 9 of 10 patients who underwent resection of parapharyngeal space tumors with no carotid encasement or bone erosion [5]. In our series, we had two patients with a mass that directly abutted the carotid artery (Figure 3) and blunt dissection was performed to free the mass without violation of the carotid sheath.
Figure 2.
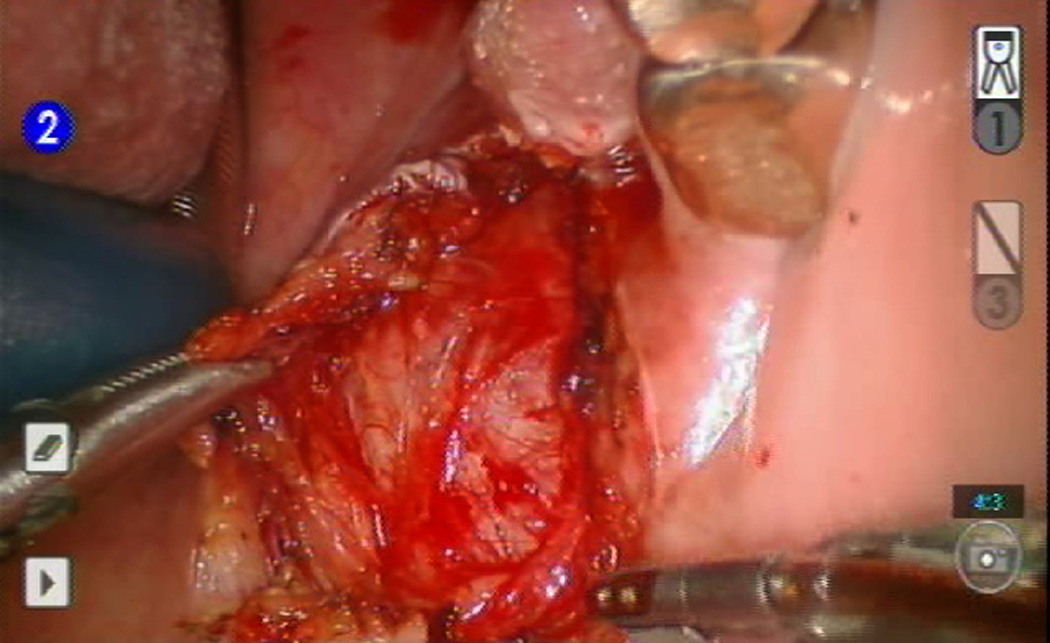
Resection of left parapharyngeal mass using 5mm robotic arm and OmniGuide laser dissector with robotic arm.
Figure 3.
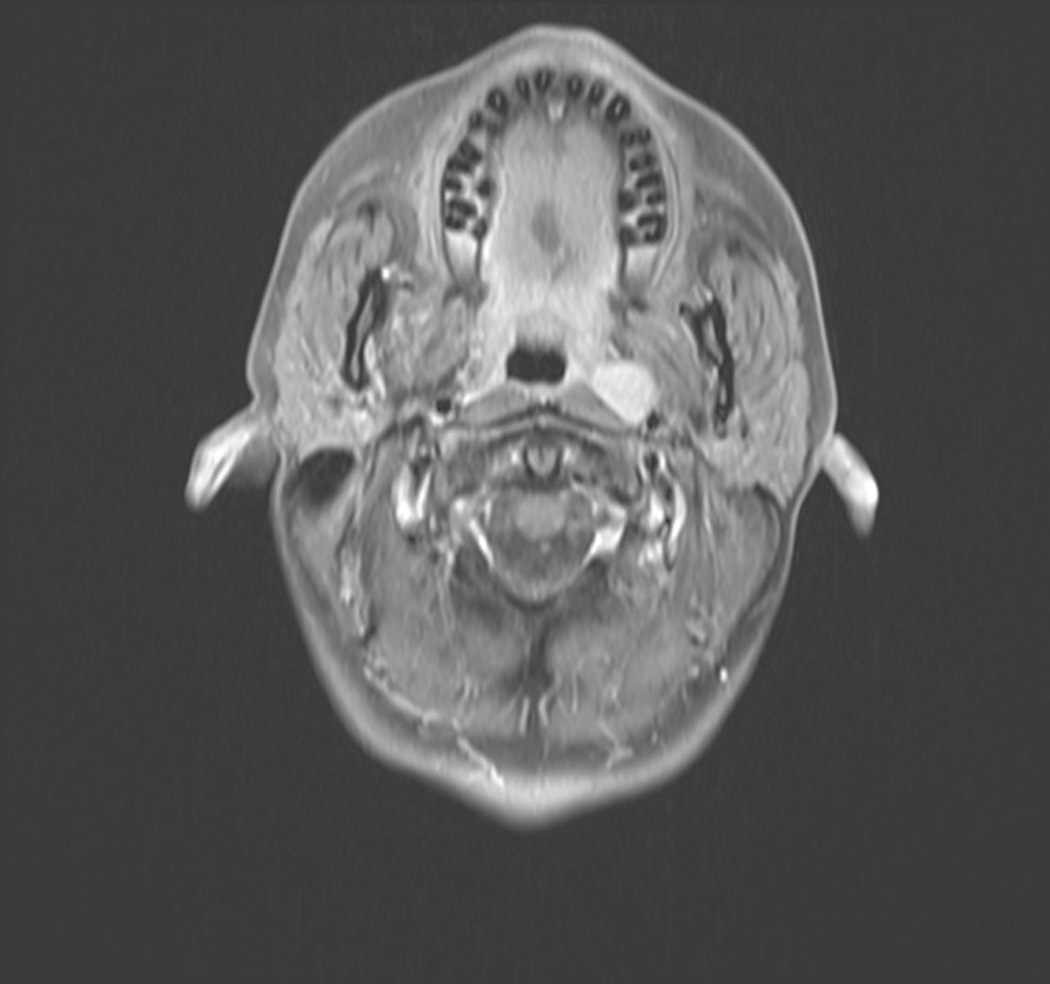
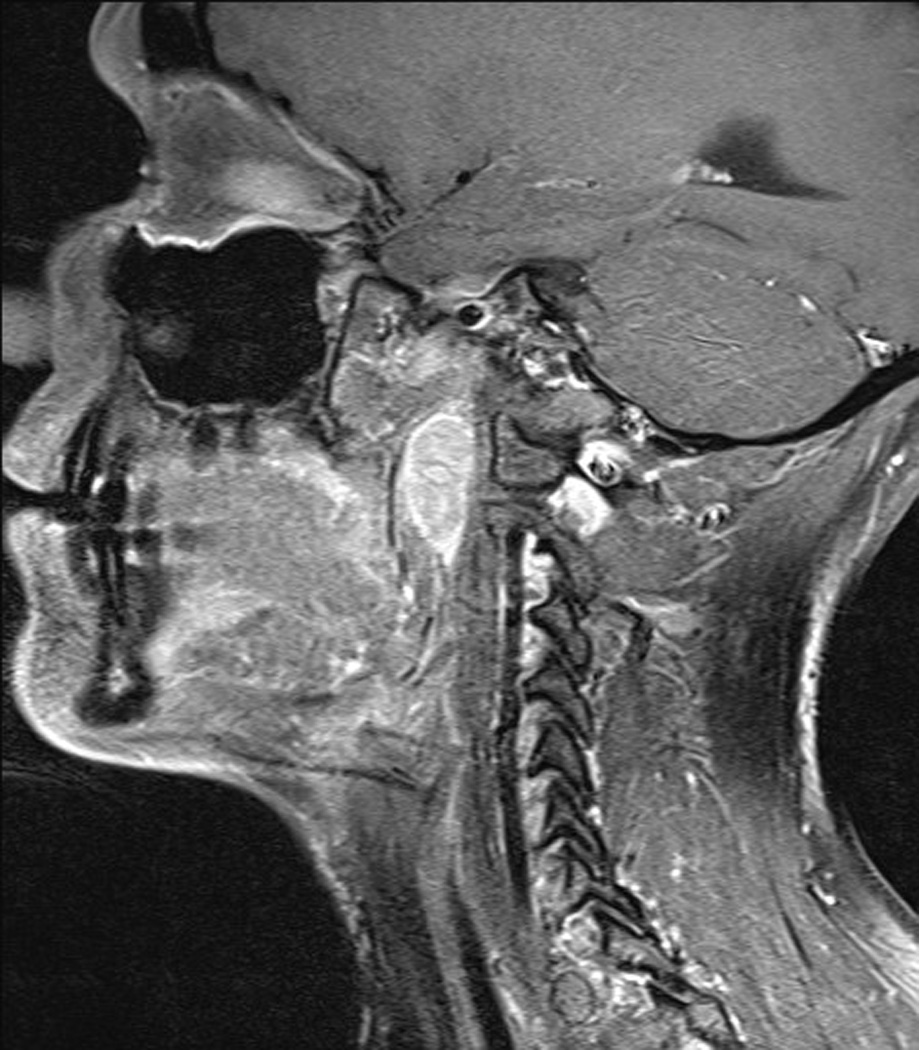
Patient with L retropharyngeal node from metastatic papillary thyroid cancer.(A) T1 with contrast, axial; (B) T1 with contrast, sagittal.
The limitation of tactile feedback when using the robot along the carotid is an issue. For the carotid dissection, the Attending Skull Base Surgeon performed the dissection at the bedside with handheld instruments while the assistant provided retraction using the robot. Anticipating possible carotid injury, the neck was prepped, the neck instrument tray was set up to be immediately available, and two active suctions were placed on the surgical field prior to surgery.
Discussions regarding cranial neuropathies, Horner’s syndrome, possibility to convert to open neck procedure, and swallowing dysfunction or velopharyngeal insufficiency must be had with the patient prior to surgery. This small series did not have any significant complications; however, these are real risks given the parapharyngeal and infratemporal fossa anatomy.
Future research into the application of robotic technology to the skull base is warranted. The initial challenges of anterior skull base exposure and introduction of robotic arms through the oral cavity and oropharynx to access the anterior skull base have been shown to be manageable [2–5]. In addition, we have demonstrated that access to the lateral skull base is feasible via transpalatal or lateral pharyngeal approaches. With the development of surgical robotic technology, smaller and more flexible instruments may provide greater opportunities to treat difficult to access skull base masses.
Conclusion
Novel approaches using transoral robotic surgery offers potential for safe and successful resection of skull base tumors. Future advances will include new technology and better understanding of skull base anatomy via the TORS approaches.
Acknowledgements
This work was supported by a grant for the National Institute on Deafness and other Communicative Disorders, T32DC005360 (GGK).
Footnotes
Presentation: Poster at the 2012 Triologic Combined Sections Meeting, Miami Beach, FL, USA, January 26–28, 2012
No financial disclosures
Conflict of Interest: None.
References
- 1.Donias HW, Karamanoukian HL, D’Ancona G, Hoover EL. Minimally invasive mitral valve surgery: from Port Access to fully robotic-assisted surgery. Angiology. 2003;54(1):93–101. doi: 10.1177/000331970305400112. [DOI] [PubMed] [Google Scholar]
- 2.O’Malley BW, Jr, Weinstein GS. Robotic anterior and midline skull base surgery: preclinical investigations. Int J Radiation Oncology Biol Phys. 2007;69(2 Suppl):S125–S128. doi: 10.1016/j.ijrobp.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 3.O’Malley BW, Jr, Weinstein GS. Robotic skull base surgery: preclinical investigations to human clinical applications. Arch Otolaryngol Head Neck Surg. 2007 Dec;133(12):1215–1219. doi: 10.1001/archotol.133.12.1215. [DOI] [PubMed] [Google Scholar]
- 4.McCool RR, Warren FM, Wiggins RH, III, Hunt JP. Robotic surgery of the infratemporal fossa utilizing novel suprahyoid port. Laryngoscope. 2010 Sep;120(9):1738–1743. doi: 10.1002/lary.21020. [DOI] [PubMed] [Google Scholar]
- 5.O’Malley BW, Jr, Quan H, Leonhardt FD, Chalian AA, Weinstein GS. Transoral robotic surgery for parapharyngeal space tumors. ORL J Otorhinolaryngol Relat Spec. 2010;72(6):332–336. doi: 10.1159/000320596. Epub 2010 Oct 1. [DOI] [PubMed] [Google Scholar]


