Abstract
Background
Evaluating the integrity of white matter tracts with diffusion tensor imaging may differentiate primary lateral sclerosis from progressive supranuclear palsy.
Methods
Thirty-three prospectively recruited subjects had standardized evaluations and diffusion tensor imaging: 3 with primary lateral sclerosis who presented with features suggestive of progressive supranuclear palsy, 10 with probable or definite progressive supranuclear palsy, and 20 matched controls. We compared fractional anisotropy of the corticospinal tract, superior cerebellar peduncle and body of the corpus callosum between groups.
Results
Both the primary lateral sclerosis and progressive supranuclear palsy subjects showed reduced fractional anisotropy in superior cerebellar peduncles and body of the corpus callosum compared to controls, but only primary lateral sclerosis subjects showed reductions in the corticospinal tracts. A ratio of corticospinal tract/superior cerebellar peduncle best distinguished the disorders (p<0.02).
Conclusions
The corticospinal tract/superior cerebellar peduncle ratio is a marker to differentiate primary lateral sclerosis from progressive supranuclear palsy.
Keywords: Progressive supranuclear palsy, primary lateral sclerosis, motor neuron disease, diffusion tensor imaging
INTRODUCTION
Progressive supranuclear palsy (PSP) and primary lateral sclerosis (PLS) are neurodegenerative disorders that affect white matter tracts and may present with motor and bulbar symptoms1–3. PSP is a slowly progressive syndrome with axial rigidity, gait and postural instability with early falls, and vertical supranuclear gaze palsy2, 4. Often, early clinical features are subtle and may overlap with other neurodegenerative diseases1, 5. One such disease is PLS, a progressive disorder of upper motor neurons that typically manifests with spastic gait and bulbar weakness; eye movement abnormalities may be present2, 6, 7.
In PSP, white matter tracts of the brain stem and cerebellum are primarily affected8, 9. In PLS, the most common pathologic finding is degeneration of the corticospinal tract with cerebellar tracts affected to a lesser extent6, 10. Diffusion tensor imaging studies use fractional anisotropy to assess the integrity of white matter tracts and have demonstrated specific abnormalities in both PSP and PLS11–17. Therefore, the aim of this study was to determine whether DTI could differentiate PLS presenting like PSP, from PSP. In addition to clinical features, we compared fractional anisotropy of the superior cerebellar peduncle, corticospinal tract, and body of the corpus callosum in 3 subjects with PLS who presented with features suggestive of PSP to subjects with probable or definite PSP and controls.
METHODS
Participants
We assessed three patients that were referred for a second opinion regarding a prior diagnosis of possible PSP but after neurological and imaging evaluation were diagnosed as PLS1, 18, and 10 subjects who met clinical research criteria for probable or definite PSP4. All participants underwent a detailed neurological evaluation including assessment for motor neuron disease features (spastic dysarthria, limb spasticity or hyperreflexia with clonus) and pseudobulbar affect. Parkinsonian features were assessed with the Movement-Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (part III) (MDS-UPDRS)19. All 13 subjects had standardized behavioral and cognitive assessments including Mini-Mental State Examination (MMSE)20, Frontal Behavioral Inventory (FBI)21 and Frontal Assessment Battery22. Electromyography (EMG) had been performed in all 3 PLS subjects, 2 at our institution and 1 elsewhere. Twenty prospectively recruited healthy controls were age and gender matched to the PLS and PSP subjects. The study was approved by the Mayo IRB. Informed consent was obtained from all subjects.
Image acquisition and analysis
All participants underwent DTI at 3.0T. Details regarding DTI acquisition and processing have been previously published11. For this study, regions-of-interest were placed on selected white matter tracts on color-coded fractional anisotropy maps using Analyze software (Biomedical Imaging Resource, Mayo Clinic, Rochester, Minnesota) by one rater (J.L.W) blinded to clinical diagnosis. Regions-of-interest were placed on corticospinal tract at the level of the pons, in the superior cerebellar peduncle at the level of the decussation, and on the body of the corpus callosum using axial images, with coronal and sagittal images viewed simultaneously to guide placement. In order to validate the manual measurements, automated voxel-wise statistical analyses of fractional anisotropy were also performed comparing PLS to PSP using Tract-Based Spatial Statistics23, as previously described11.
Statistical Analysis
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 6.0.0; SAS Institute Inc, Cary, NC) with statistical significance set at p<0.05. Kruskal-Wallis analysis was used for continuous data across all three groups followed by Mann-Whitney U test to compare PLS and PSP, if significant. Fisher’s exact test was used to compare nominal data.
RESULTS
Brief clinical histories of PLS subjects
Patient 1: A 69 year-old woman sought a second opinion regarding a diagnosis of possible PSP by a movement disorders specialist elsewhere. She had a 3 year history of speech and gait difficulties with one fall. On examination she had a severe spastic dysarthria and pseudobulbar affect. Extraocular testing revealed eye movement abnormalities which were determined to be oculomotor impersistence. Vertical saccadic eye movements, when observed, were of normal velocity and amplitude (video). In the left upper extremity she was spastic with brisk reflexes and clonus. Head MRI revealed mild generalized cerebral atrophy and FDG-PET showed focally reduced uptake in motor cortices, greater on the right. One year after initial evaluation, she was anarthric. She began falling multiple times per week with pronounced dysphagia, spasticity and weakness in all limbs.
Patient 2: A 68 year old man developed progressive balance dysfunction, a shuffling gait and frequent falls at age 68. He was told elsewhere that he had a “parkinsonian variant” and tried on carbidopa/levodopa. At presentation to our clinic 18 months later, he reported stiff legs with difficulty rising from a chair and loss of fine motor movements. Neurologic examination revealed spastic dysarthria with pseudobulbar affect and loss of vertical optokinetic nystagmus but normal velocity and amplitude of saccadic eye movements. He had marked Gegenhaulten-type rigidity with spastic lower extremities, hyperreflexia and normal strength. Lower extremity apraxia, occasional myoclonic jerks and hyperekplexia was observed. Prior evaluation included multiple normal MRIs. A FDG-PET study showed generalized decrease in cerebral metabolic activity with focal decrease in the medial superior frontal regions. Over the ensuing 9 months, his spasticity and motor function worsened.
Patient 3: A 63 year-old man with a 4 year history of gait difficulties presented after receiving a diagnosis of progressive akinesia of gait freezing variant of PSP by a movement disorders specialist elsewhere. He was falling frequently; reporting 50 falls within 1 month. He noted slow movements, difficulty turning in bed, muscle stiffness and weakness. Neurological examination demonstrated hypomimia, spastic dysarthria and normal extraocular movements. He had mild axial and limb rigidity and slowed rapid alternating movements. He had difficulty rising from a chair and was stooped with a slow spastic gait and absent arm swing. At 1 year, he had worsening dysarthria, dysphagia and motor function.
Group Comparisons
Demographics, clinical test scores and DTI results are presented in Table 1. The PSP subjects scored worse on the FBI (p=0.07) and FAB (0.02) compared to PLS subjects, but no such difference was observed on MMSE or UPDRS III. PLS subjects were more likely to exhibit pseudobulbar affect (p=0.01) and features of motor neuron disease (p=0.01) compared to PSP subjects.
Table 1.
Demographic, Clinical and Imaging Characteristics of the PLS, PSP and Control Groups
| Median (range) | ||||
|---|---|---|---|---|
| Characteristic | Controls (n=20) | PLS (n=3) | PSP (n=10) | P value (PLS vs. PSP) |
| Demographics | ||||
| Female sex, No. (%) | 11 (55) | 1 (33) | 6 (60) | 0.60 |
| Age at disease onset, y | NA | 67 (60–68) | 65 (54–75) | 0.93 |
| Age at examination, y | 71.5 (51–78) | 70 (64–70) | 69 (58–79) | 0.93 |
| Disease duration, y | NA | 4 (2–4) | 4 (2–9) | 0.55 |
| Clinical features | ||||
| FBI (/72)a | NA | 0 (0–4) | 9 (0–28) | 0.07 |
| FAB (/18) a | NA | 17 (16–18) | 13 (10–16) | 0.02 |
| MMSE (/30) a | NA | 30 (29–30) | 29 (22–30) | 0.19 |
| UPDRS III (/132) a | NA | 51 (25–70) | 46 (18–59) | 0.80 |
| Pseudobulbar affect, No. (%) | NA | 3 (100) | 1 (10) | 0.01 |
| MND features | NA | 3 (100) | 2 (20) | 0.01 |
| Fractional anisotropy of white matter tracts | ||||
| CST | 0.63 (0.50–0.81) | 0.49 (0.43–0.55) | 0.60 (0.51–0.70) | 0.04 |
| SCP | 0.86 (0.72–0.91) | 0.75 (0.68–0.78) | 0.63 (0.53–0.80) | 0.09 |
| BCC | 0.72 (0.61–0.83) | 0.66 (0.56–0.73) | 0.60 (0.45–0.75) | 0.50 |
| CST/SCP ratio | 0.72 (0.58–0.94) | 0.66 (0.55–0.81) | 0.89 (0.76–1.17) | 0.02 |
Abbreviations: BCC, body of the corpus callosum; CST, corticospinal tract; FAB, frontal assessment battery; FBI, frontal behavioral inventory; MMSE, mini mental status examination; MND, motor neuron disease; NA, not applicable; PLS, primary lateral sclerosis; PSP, progressive supranuclear palsy; SCP, superior cerebellar peduncle; UPDRS III, United Parkinson’s disease rating scale.
Highest possible score on the scales represented as (/score).
None of the PLS or PSP subjects had a family history of neurodegenerative disease and laboratory examination of serum and cerebrospinal fluid was negative. PLS subjects 1 and 2 had normal EMGs. PLS subject 3 had 2 EMGs performed 11 months apart showing reduced recruitment of large motor unit potentials in extremity, cranial and paraspinal muscles with sparse fibrillation potentials.
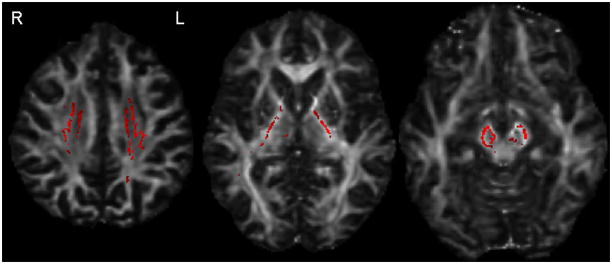
Fractional anisotropy was decreased in the corticospinal tract (p=0.02), superior cerebellar peduncle (p=0.02) and body of the corpus callosum (p=0.02) in the PLS group compared to controls. PSP subjects had decreased fractional anisotropy in the superior cerebellar peduncle (p<0.0001) and body of the corpus callosum (p=0.04), but not the corticospinal tract (p=0.40) compared to controls. Significantly lower fractional anisotropy in the corticospinal tract was observed in PLS compared to PSP (p=0.04). The ratio of corticospinal tract/superior cerebellar peduncle fractional anisotropy was lower in PLS compared to PSP (p<0.02). Tract-Based Spatial Statistics demonstrated reduced fractional anisotropy throughout the corticospinal tracts in PLS compared to PSP (Figure 1).
Figure 1.
Decreased fractional anisotropy (red) in the superior corona radiata, posterior limb of the internal capsule and cerebral peduncles in PLS versus PSP using Tract-Based Spatial Statistics (uncorrected for multiple comparisons, p<0.05). Results also survived correction using family-wise error at p<0.05.
DISCUSSION
We demonstrate clinical, neuropsychometric and DTI results that distinguish PLS when presenting like PSP, from PSP. Both diseases tend to occur during middle age or later7, 24 which was the case in this study. Initial bulbar symptoms of dysarthria and dysphagia are common in PLS and were present in our PLS subjects though these symptoms can also occur in PSP4, 7. Extraocular abnormalities, while classic in PSP, also can occur in PLS. Saccadic break down of smooth pursuit and progressive supranuclear paralysis is reported in PLS1, 6 which overlaps with slowing of vertical saccades that precedes supranuclear gaze palsy in PSP2, 25–27. While 2 PLS subjects in our study had an abnormal extraocular examination, neither had supranuclear gaze palsy. Therefore careful oculomotor examination is important in order to different PLS from PSP. Cognition is usually preserved in PLS28 while a majority of PSP patients develop mild executive dysfunction and some behavioral changes, early in disease25, 27, 29, 30 consistent with our cognitive and behavioral results.
Our DTI analysis confirmed involvement of the corticospinal tract in those diagnosed with PLS but also identified involvement of the superior cerebellar peduncle, not previously described in PLS17, 31–33. Involvement of the superior cerebellar peduncle is almost pathognomonic for PSP2, 9, 11, 34–37 and hence the superior cerebellar peduncle involvement in our PLS subjects may explain the overlap in features with PSP. It should be noted that early post mortem studies in PLS describe mild degeneration of the cerebellar tracts and fasciculus gracilis in addition to the corticospinal tract loss but involvement of the superior cerebellar peduncle has not been described7, 17, 38. Given the greater involvement of the corticospinal tract in our PLS subjects, and the greater involvement of the superior cerebellar peduncle in our PSP subjects, we calculated a corticospinal tract/superior cerebellar peduncle ratio and found this ratio to be very good discriminator between both groups suggesting that this may be a good marker to differentiate between PLS and PSP.
Supplementary Material
(Patient 1) Extraocular movement testing reveals difficulty with down gaze that is overcome with stimulus. On gait examination, there is decreased left arm swing but normal posture, stride length, heel strike and 180 degree turn. Rapid alternating movements of the left hand are slowed without amplitude dampening or arrest.
Acknowledgments
Study Funding: Supported by NIH grants R01-DC010367, R01-AG037491, and The Dana Foundation
Footnotes
Financial Disclosure/Conflict of Interest: None related to the manuscript.
AUTHOR CONTRIBUTIONS
- Research project: A. Conception, B. Organization, C. Execution;
- Statistical analysis: D. Design, E. Execution, F. Review and Critique
- Manuscript: G. Writing of the first draft, H. Review and Critique
Dr. Coon: B, C, E, G
Dr. Whitwell: C, F, H
Dr. Josephs: A, B, C, D, E, H
DISCLOSURES
Dr. Coon has no disclosures.
Dr. Whitwell is funded by R21-AG38736 (PI), R01-DC010367 (Co-I), R01-AG037491 (Co-I), and the Dana Foundation (Co-I).
Dr. Jack servesas a consultant for Elan Corporation; and receives researchsupport from Pfizer, Inc., the NIA [R01-AG11378 (PI), P50-AG16574(Co-I), R21-AG38736 (Co-I), R01-DC010367 (Co-I), R01-AG037491 (Co-I), and U01 AG024904-01 (Co-I)],and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation.
Dr. Josephs is funded by R01-DC010367 (PI), R01-AG037491 (PI), R21-AG38736 (Co-I), and the Dana Foundation (PI).
References
- 1.Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain : a journal of neurology. 1992;115 ( Pt 2):495–520. doi: 10.1093/brain/115.2.495. [DOI] [PubMed] [Google Scholar]
- 2.Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum with Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 3.Williams DR, Lees AJ. How do patients with parkinsonism present? A clinicopathological study. Intern Med J. 2009;39(1):7–12. doi: 10.1111/j.1445-5994.2008.01635.x. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Osaki Y, Ben-Shlomo Y, Lees AJ, et al. Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord. 2004;19(2):181–189. doi: 10.1002/mds.10680. [DOI] [PubMed] [Google Scholar]
- 6.Le Forestier N, Maisonobe T, Piquard A, et al. Does primary lateral sclerosis exist? A study of 20 patients and a review of the literature. Brain : a journal of neurology. 2001;124(Pt 10):1989–1999. doi: 10.1093/brain/124.10.1989. [DOI] [PubMed] [Google Scholar]
- 7.Singer MA, Statland JM, Wolfe GI, Barohn RJ. Primary lateral sclerosis. Muscle Nerve. 2007;35(3):291–302. doi: 10.1002/mus.20728. [DOI] [PubMed] [Google Scholar]
- 8.Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44(11):2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 9.Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW. Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2003;60(11):1766–1769. doi: 10.1212/01.wnl.0000068011.21396.f4. [DOI] [PubMed] [Google Scholar]
- 10.Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55(1):97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Whitwell JL, Master AV, Avula R, et al. Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch Neurol. 2011;68(6):753–760. doi: 10.1001/archneurol.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blain CR, Barker GJ, Jarosz JM, et al. Measuring brain stem and cerebellar damage in parkinsonian syndromes using diffusion tensor MRI. Neurology. 2006;67(12):2199–2205. doi: 10.1212/01.wnl.0000249307.59950.f8. [DOI] [PubMed] [Google Scholar]
- 13.Erbetta A, Mandelli ML, Savoiardo M, et al. Diffusion tensor imaging shows different topographic involvement of the thalamus in progressive supranuclear palsy and corticobasal degeneration. AJNR American journal of neuroradiology. 2009;30(8):1482–1487. doi: 10.3174/ajnr.A1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson C, Markenroth Bloch K, Brockstedt S, Latt J, Widner H, Larsson EM. Tracking the neurodegeneration of parkinsonian disorders--a pilot study. Neuroradiology. 2007;49(2):111–119. doi: 10.1007/s00234-006-0165-1. [DOI] [PubMed] [Google Scholar]
- 15.Ito S, Makino T, Shirai W, Hattori T. Diffusion tensor analysis of corpus callosum in progressive supranuclear palsy. Neuroradiology. 2008;50(11):981–985. doi: 10.1007/s00234-008-0447-x. [DOI] [PubMed] [Google Scholar]
- 16.Padovani A, Borroni B, Brambati SM, et al. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. Journal of neurology, neurosurgery, and psychiatry. 2006;77(4):457–463. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwata NK, Kwan JY, Danielian LE, et al. White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain : a journal of neurology. 2011;134(Pt 9):2642–2655. doi: 10.1093/brain/awr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon PH, Cheng B, Katz IB, et al. The natural history of primary lateral sclerosis. Neurology. 2006;66(5):647–653. doi: 10.1212/01.wnl.0000200962.94777.71. [DOI] [PubMed] [Google Scholar]
- 19.Goetz CG, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): Process, format, and clinimetric testing plan. Movement disorders : official journal of the Movement Disorder Society. 2007;22(1):41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Archives of general psychiatry. 1983;40(7):812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 21.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 1997;24(1):29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 22.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354(9192):1771–1775. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- 25.Donker Kaat L, Boon AJ, Kamphorst W, Ravid R, Duivenvoorden HJ, van Swieten JC. Frontal presentation in progressive supranuclear palsy. Neurology. 2007;69(8):723–729. doi: 10.1212/01.wnl.0000267643.24870.26. [DOI] [PubMed] [Google Scholar]
- 26.Rottach KG, Riley DE, DiScenna AO, Zivotofsky AZ, Leigh RJ. Dynamic properties of horizontal and vertical eye movements in parkinsonian syndromes. Ann Neurol. 1996;39(3):368–377. doi: 10.1002/ana.410390314. [DOI] [PubMed] [Google Scholar]
- 27.Williams DR, de Silva R, Paviour DC, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005;128(Pt 6):1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- 28.Caselli RJ, Smith BE, Osborne D. Primary lateral sclerosis: a neuropsychological study. Neurology. 1995;45(11):2005–2009. doi: 10.1212/wnl.45.11.2005. [DOI] [PubMed] [Google Scholar]
- 29.Nath U, Ben-Shlomo Y, Thomson RG, Lees AJ, Burn DJ. Clinical features and natural history of progressive supranuclear palsy: a clinical cohort study. Neurology. 2003;60(6):910–916. doi: 10.1212/01.wnl.0000052991.70149.68. [DOI] [PubMed] [Google Scholar]
- 30.Richardson JC, Steele J, Olszewski J. Supranuclear Ophthalmoplegia, Pseudobulbar Palsy, Nuchal Dystonia and Dementia. A Clinical Report on Eight Cases of "Heterogenous System Degeneration". Trans Am Neurol Assoc. 1963;88:25–29. [PubMed] [Google Scholar]
- 31.Ciccarelli O, Behrens TE, Johansen-Berg H, et al. Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp. 2009;30(2):615–624. doi: 10.1002/hbm.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suh SI, Song IC, Koh SB. Primary lateral sclerosis with MR diffusion tensor image and tract tracking. Am J Phys Med Rehabil. 2006;85(11):863–864. doi: 10.1097/01.phm.0000242651.30244.a4. [DOI] [PubMed] [Google Scholar]
- 33.Ulug AM, Grunewald T, Lin MT, et al. Diffusion tensor imaging in the diagnosis of primary lateral sclerosis. J Magn Reson Imaging. 2004;19(1):34–39. doi: 10.1002/jmri.10433. [DOI] [PubMed] [Google Scholar]
- 34.Behrman S, Carroll JD, Janota I, Matthews WB. Progressive supranuclear palsy. Clinico- pathological study of four cases. Brain. 1969;92(3):663–678. doi: 10.1093/brain/92.3.663. [DOI] [PubMed] [Google Scholar]
- 35.Ishizawa K, Lin WL, Tiseo P, Honer WG, Davies P, Dickson DW. A qualitative and quantitative study of grumose degeneration in progressive supranuclear palsy. J Neuropathol Exp Neurol. 2000;59(6):513–524. doi: 10.1093/jnen/59.6.513. [DOI] [PubMed] [Google Scholar]
- 36.Paviour DC, Price SL, Stevens JM, Lees AJ, Fox NC. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2005;64(4):675–679. doi: 10.1212/01.WNL.0000151854.85743.C7. [DOI] [PubMed] [Google Scholar]
- 37.Slowinski J, Imamura A, Uitti RJ, et al. MR imaging of brainstem atrophy in progressive supranuclear palsy. J Neurol. 2008;255(1):37–44. doi: 10.1007/s00415-007-0656-y. [DOI] [PubMed] [Google Scholar]
- 38.Erb W. Concerning Spastic and Syphilitic Spinal Paralysis. Br Med J. 1902;2(2180):1114–1119. doi: 10.1136/bmj.2.2180.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(Patient 1) Extraocular movement testing reveals difficulty with down gaze that is overcome with stimulus. On gait examination, there is decreased left arm swing but normal posture, stride length, heel strike and 180 degree turn. Rapid alternating movements of the left hand are slowed without amplitude dampening or arrest.