Abstract
SYA013, a homopiperazine analogue of haloperidol, was further evaluated for antipsychotic potential using additional animal models. Previously, SYA013 was tested in mice with an antipsychotic screening model in which it inhibited apomorphine induced climbing behavior, indicating antagonism of the dopaminergic system and the potential for use in the treatment of schizophrenia. In this study, SYA013 was shown to inhibit both d-amphetamine-induced locomotor activity in rats and conditioned avoidance response (CAR) in rats in a dose dependent manner and in the case of CAR, without producing any escape failure responses (EFR), two tests predictive of antipsychotic action. The selective 5HT1A antagonist WAY100,635 was used to determine if binding of SYA013 to the 5HT1A receptor contributed to suppression of CAR. The results indicated that 0.63 mg/kg WAY100,635 did not have a significant effect on the inhibition of CAR by SYA013. Pharmacokinetic parameters in brain and plasma were determined for SYA013. A log brain/plasma concentration ratio at tmax of 1.48 suggests that SYA013 readily crosses the blood brain barrier (BBB).
The hypothesis that binding of SYA013 to the 5HT1A receptor contributed to the lack of significant catalepsy was investigated using the 5HT1A antagonist WAY100,635. The results of acute and semi-chronic tests suggest that binding to the 5HT1A receptor alone did not significantly account for the lack of catalepsy. Lack of catalepsy was preserved after the semi-chronic challenge with SYA013. These tests further indicate that SYA013 has a pharmacological profile with the potential for use in the treatment of neuropsychiatric diseases. In addition, the 5HT1A receptor does not appear to play a significant role in the pharmacological profile of SYA013.
Keywords: 5HT1A receptor, Catalepsy test chamber instrument, Conditioned avoidance response, Locomotor activity, Antipsychotic profile, SYA013
1. Introduction
Approximately 1% of adults in the United States are affected with the brain disorder schizophrenia (Bourdon et al., 1992). Positive symptoms including delusions, hallucinations, and disorganized behavior, and negative symptoms of social avoidance, emotional withdrawal, reduced speech, and lack of ability to experience pleasure characterize this debilitating condition (Guitart et al., 1998). Chlorpromazine was introduced in the 1950s as the first antipsychotic that could reduce hallucinations and delusions (Ellenbroek BA, 2011; Meltzer, 1995). Other antipsychotics followed including haloperidol. However, extrapyramidal side effects (EPS), including rigidity, dystonia and akathisia, along with tardive dyskinesia, a chronic motor side effect, accompanied the use of these antipsychotics. Clozapine was introduced in the 1960s and produced few if any EPS (Geyer and Ellenbroek, 2003). Unfortunately, it is associated with agranulocytosis which can be fatal (Birch et al., 1999). More recently, atypical antipsychotics without agranulocytosis have been introduced and have found widespread utility. However, atypical antipsychotics in general predispose patients to metabolic disorders and weight gain (Arterburn, et al., 2011). These and other reasons provide the impetus for continuing the search for new drugs to treat schizophrenia and related neuropsychiatric illnesses.
Inhibition of the dopamine D2 receptor remains a necessary condition for antipsychotic efficacy, even after 50 years of drug developments (Kapur and Mamo, 2003). Clozapine binds with lower affinity to the D2 receptor (Birch et al., 1999) than haloperidol does which is thought to account for the higher incidence of EPS with haloperidol. Clozapine is also a 5HT1A partial agonist, which may contribute to its low EPS profile (Kleven et al., 2005; Newman-Tancredi et al., 1996). The contribution of 5HT1A receptor binding in reducing cataleptic response in rats is well documented. Some compounds that did not exhibit strong catalepsy alone (SLV313, bifeprunox, and sarizotan) produced marked catalepsy after rats were pretreated with the 5HT1A receptor antagonist WAY100,635. However, other compounds that did not exhibit strong catalepsy alone (aripiprazole and SSR181507) produced only modest catalepsy after antagonism of 5HT1A by WAY100,635 (Kleven et al., 2005). It is therefore thought that other mechanisms, such as partial agonism at D2 receptors or antagonism of the 5HT2A receptor, may contribute to the low EPS profile of an antipsychotic (Kleven et al., 2005; Meltzer, 1999; Meltzer et al., 1989, 2011; Natesan et al., 2008).
SYA013, a homopiperazine analogue of haloperidol, has been identified in our laboratories as a potential atypical antipsychotic agent. SYA013 has moderate affinity for the D2 and 5HT1A receptors, higher affinity for the 5HT2A receptor than the D2 receptor, inhibits apomorphine-induced climbing behavior in mice, and exhibited no significant cataleptic behavior in the bar test with rats at up to 5 × ED50 (Ablordeppey et al., 2008). This work further investigates the efficacy of SYA013 as a potential antipsychotic agent using additional animal models, including the cataleptogenic potential using the new Catalepsy Test Chamber (Med Associates, Inc., St. Albans, VT) to assist in performing the classic catalepsy bar test with rats, the role of the 5HT1A receptor in its pharmacological profile and its pharmacokinetic profile.
A strong increase in the locomotor activity of rats injected with d-amphetamine is due to increased dopaminergic activity in the mesolimbic system, especially the nucleus accumbens, and its reversal has been used to predict antipsychotic activity for many years (Depoortere et al., 2007; Ellenbroek, 1993; Geyer et al., 2003; Struyker-Boudier and Cools, 1984). In our previous reports, we concentrated on the reversal of apomorphine-induced stereotypy to predict the same. In this report, we have used both reversal of d-amphetamine-induced locomotor hyperactivity and the classic conditioned-avoidance response (CAR) test, to investigate and validate the antipsychotic properties of SYA013 (Wadenburg, 2010; Depoortere et al., 2007; Arnt, 1982). To probe the involvement of 5HT1A interaction in the behavioral profile of SYA013, we used the conditioned avoidance response paradigm to test SYA013, both with and without pre-treatment with the 5HT1A receptor antagonist WAY100,635.
The undesirable extrapyramidal side effects in humans of many antipsychotics can be predicted by the occurrence of catalepsy in rats (Jones and McCreary, 2008, Kleven et al., 2005). Different methods have been used to test for catalepsy in rats including the paw test, inclined grid, flat bar, round bar, and crossedlegs position test (Ellenbroek and Cools, 1988; Ahlenius and Hillegaart, 1986; Geffen et al., 2009; Siuciak et al., 2007; Sanberg et al. 1988). We have chosen to use the classic round bar test using a new Catalepsy Test Chamber (Med Associates, Inc., St. Albans, VT), a commercially available instrument that measures the time of contact between a rat's paw(s) on the bar and the floor of the chamber, eliminating many of the problems associated with human response time and interpretations. To our knowledge, this is the first time the use of this instrument has been reported in a peer-reviewed journal. In addition, we have used a second test of catalepsy that is reported to be sensitive to the anticataleptic actions of 5HT1A receptor agonists, the crossed-legs position (CLP) test (Depoortere et al., 2007; Kleven et al., 2005). Immediately following the two catalepsy tests, a righting test was performed to test for sedation or other effects (McCreary et al. 2007; Reeve et al., 1992).
To investigate if binding of SYA013 to the 5HT1A receptor reduces the catalepsy in rats observed in our earlier report (Ablordeppey et al., 2008), WAY100,635 was administered (Depoortere et al., 2007; Kleven et al., 2005; Prinssen et al., 1998) to antagonize the 5HT1A receptor prior to experimental observations. In vivo time/concentration curves in rats from iv and po doses of SYA013 were acquired to determine brain/plasma concentration ratios and pharmacokinetic parameters.
2. Materials and Methods
2.1. Animals
All experiments were carried out on male Sprague-Dawley rats (100–250 g), (~5–8 weeks old) from Harlan Laboratories, Inc. Animals were housed in the Florida A & M University Animal Care Facility which is fully AAALAC accredited, and operates with a 12 h light/dark cycle and controlled temperature (24 ± 2 °C). The rats were given free access to food and water and at least 5 days to adjust before the start of each experiment. Rats were then fasted the night before each experiment. All experimental procedures were performed in accordance with protocols approved by the Florida A & M University Institutional Animal Care and Use Committee.
2.2. Drugs and chemicals
SYA013 (Clog P = 5.15) was synthesized at Florida A & M University (Ablordeppey et al., 2008), with a purity of 99.8% as determined by CHN analysis. SYA013 (dihydrochloride salt) was dissolved in filtered (0.22μ) 1% lactic acid vehicle for all the animal studies. Doses were given in a volume of 11.4 mL/kg for intraperitoneal (ip) and intravenous (iv) injections, and 21 mL/kg for gavage (po) dosings, based on solubility in vehicle.
The HPLC internal standard DS-49 (Clog P = 5.17) used for calibration was also synthesized at Florida A & M University (Sikazwe et al., 2004, Chart 1) with CHN values within 0.4% of theoretical values as determined by CHN analysis.
Chart 1.
Lactic acid was ACS reagent grade (ACROS), phosphate buffered saline (PBS) and NaCl were from Fisher, ACS grade, d-amphetamine hemisulfate salt and WAY100,635 maleate salt were from Sigma-Aldrich, and the water used to make solutions was HPLC grade. Diethyl ether was from Fluka, residue analysis grade. The acetic acid, water, methanol, and acetonitrile were HPLC grade. The sodium sulfate was analytical reagent grade.
The Clog P values reported in this manuscript were calculated using ChemDraw Ultra, version 11.0.1 obtained from CambridgeSoft.
2.3. Pharmacokinetic analyses
2.3.1. Sample preparation for HPLC-PDA
2.3.1.1. Plasma
0.5 mL of rat plasma was mixed with 20 µL of DS-49 (250 µg/mL), (internal standard) in an 8 mL glass vial with a Teflon-lined cap. 100 µL of 2M NaOH was added and mixed, followed by diethyl ether (5 mL) and the tubes shaken for 10 min. The sample was then centrifuged for 10 min at 3000 rpm. The ether layer was transferred to a 5 mL glass vial and the ether evaporated under nitrogen at 40 °C until dryness. The sample was then reconstituted in 200 µL of mobile phase. The supernatant was transferred to a 0.6 mL high speed plastic centrifuge tube and centrifuged at 10,000 g for 5 min. 100 µL of supernatant was injected for HPLC analysis.
2.3.1.2. Brain tissue
Rat brains were frozen at −20 °C until needed. Each brain was weighed, and then macerated with a spatula, followed by the addition of 2 parts PBS and homogenization with a Tissue Tearor at a maximum speed setting of 30 for 2 min. This was followed by sonification for 20 seconds (3 times) with mixing in between using a Branson digital sonifier model 450, with a microtip, set at 50% amplitude. Homogenates were kept on ice between steps. One mL of homogenate (~1g) was mixed with 20 µL of 250 µg/mL DS-49 (internal standard, Clog P = 5.17) in a 5 mL glass vial with a Teflon-lined cap. This was mixed with 3 mL of acetonitrile and shaken for 5 min. After centrifugation at 3000 rpm for 10 min, the supernatant was transferred to an 8 mL glass vial with a Teflon-lined cap, and evaporated under N2 at 40 °C until near dryness. This was mixed with H2O (0.5 mL) and 2 M NaOH (100 µL)followed by extraction into 5 mL of ether and further cleanup and analysis as described for plasma. (Shimokawa et al. 2005)
2.3.2. HPLC conditions
A Waters 2695 Alliance HPLC system was used, equipped with an autosampler, and PDA detector. The column was an Alltima HP, C18, 5μ, 4.6 mm × 150 mm, protected by a guard cartridge with the same packing, which was changed when peak shape eventually degraded, restoring peak shape back to normal. The flow rate was 1 mL/min, and an analytical wavelength of 254 nm was used. The isocratic mobile phase was generated from 55%A and 45%C, with 100% methanol in the A reservoir, and an aqueous solution of 0.01M sodium sulfate in 4% v/v acetic acid in the C reservoir. Run time was 15 min. Retention time and PDA spectra were used to identify analytes.
2.3.3. Determination of SYA013 levels in plasma and brain samples
A single bolus dose (equal to the ID50 values obtained by iv or ip injections respectively) of SYA013, was given by iv (2.09 or 12.5 mg/kg) or gavage (48 mg/kg) to an average of 4 rats per time point group, and blood and brains collected at 5 min, 10 min, 15 min, 30 min, 1 hr, 2 hr, 3 hr, 6 hr, 9 hr, and 24 hr. For control purposes, vehicle was injected into 4 rats as well. Plasma and whole brains were stored at −20 °C until needed. The internal standard method was used to correct for % recoveries. Calibration lines were calculated using linear regression from 7–8 points, and had coefficients of determination of 0.994 or better.
2.4. Behavioral experiments
All behavioral experiments were conducted under standard temperatures (68–79 °F) and humidity (30–70%) in the animal care facility.
2.4.1. d-Amphetamine-induced locomotor hyperactivity
The method of Depoortere et al., 2007, was used to observe the effect of SYA013 on hyperlocomotion produced by d-amphetamine in rats. Male Sprague-Dawley rats were first injected ip or iv with SYA013, and then injected sc with d-amphetamine (0.63 mg/kg as free base) at 45 min post injection with SYA013. Spontaneous locomotor activity was determined by injecting sc phosphate buffered saline (PBS) instead of d-amphetamine for comparison. The home cage was then placed in an automated animal activity monitor (Opto-M3, or Opto-Varimex Mini, Columbus Instruments, Columbus, OH, USA), and 15 min after the second injection, the ambulatory infrared light beam interruptions of the X axis counted for 1 hr.
2.4.2. Conditioned avoidance response (CAR)
CAR was determined using the method of Depoortere et al., 2007. A shuttle box system (Medical Associates, St. Albans, VT, USA) with an infrared photobeam detection system, two chambers, each with a cue light and electric foot shock (0.8 mA, 30Hz) grid floor, one manual central door, and computer software to control the chambers and collect data, was used to train rats to avoid the shock in response to a cue light by moving to the other chamber before 10 seconds passed. The system was programmed for each trial to turn on the light in the chamber the rat was in for 10 seconds, and then deliver the shock to the grid floor for 10 seconds while still keeping the light on until the rat left the original chamber, with both light and shock terminating together after a total of 20 seconds. The ITI interval (time between start of each trial) was programmed to 40 seconds. Rats were trained at one session a day, with each session consisting of 30 consecutive trials. Each rat required 5 days to 2 weeks to train, and about 40–60% were found to be untrainable, due mainly to poor avoidance responding. This has also been observed by Sun et al., 2010, with 59% of rats reaching training criteria after 2 weeks. A conditioned avoidance response (CAR) was recorded when an escape occurred during the first 10 seconds of a trial, an escape recorded if the rat left during the second 10 seconds while being shocked, and an escape failure response (EFR) recorded if the rat stayed in the chamber for the whole 20 seconds.
During training, it was observed that occasionally, a small number of rats were able to take advantage of rat location detection problems (tail flicks in the opposite chamber, vertical stance to avoid beams, or center positioning) and positioning to avoid shock (contact with only one bar in one chamber, or one bar in both chambers, or clinging to the light fixture near the ceiling) to defeat the system causing EFR without getting shocked. This encouraged the rat to learn how to position itself to avoid shock from the cage floor bars or avoid detection by the photobeam system leading to the rat learning how to avoid shock without exiting the chamber in which shock was being applied. These rats were eliminated from training after 2–4 weeks if the learned EFR behavior was not extinguished. Improvements in the software have eliminated most of the photobeam location detection problems.
Acceptance criteria for rats used in the experiments were developed and included a minimum of 5 days of training, with 3 consecutive days and the day before the experiment at > 75% CAR, and no EFRs. Results are reported relative to the % CAR value determined the day before the test. These criteria fall within the range of procedures developed by other authors (Bundgaard et al. 2009, Schlumberger et al. 2010, Sun et al. 2010, Wadenberg and Hicks 1999). There were no escape failure responses for vehicle or SYA013 under these conditions. Similarly, Wadenberg et al., 2001 also record no EFR for haloperidol, risperidone, olanzapine, and quetiapine using a tilting grid floor apparatus with microswitch detection.
2.4.2.1. No pre-treatment
On the day of the experiment, trained rats were injected ip once with either SYA013 or vehicle (1% lactic acid) 60 min before the start of the session. Five rats were used for each dose.
2.4.2.2. Pre-treatment with saline or WAY100,635
On the day of the experiment, trained rats were injected twice, first with either saline or WAY100,635 (0.63 mg/kg as free base) (Depoortere et al., 2007) in saline sc, then 15 min later with SYA013 ip. The CAR session began 60 min after the second injection. Five to six rats were used for each dose. The dose of WAY100,635 (0.63 mg/kg) was used in our study based on the work reported by Depoortere et al., 2007. This dose should be effective in our study for two other reasons. First, SYA013 is less potent at the 5HT1A (Ki = 117 nM) receptor than F15063 (Ki = 4.27 nM) used by Depoortere. Secondly, the dose of SYA013 used by us (0.08 mmole/kg) is smaller than that used by Depoortere (0.1 mmole/kg) for F15063. Indeed, others have used smaller doses (0.16 mg/kg) of WAY100,635 to reverse 8-OH-DPAT effects (Assie and Koek, 1996).
2.4.3. Catalepsy bar test, new instrument
A new instrument, the Catalepsy Test Chamber (Med Associates, Inc., St. Albans, VT), was used to assist in performing the classic catalepsy bar test with rats. Forelimbs were place on a 1.3 cm diameter (4 diameters available) horizontal cylindrical metal bar at a comfortable standing height of 10 cm (4 heights available) and hind limbs on the stainless steel floor. The instrument measures the time that contact is maintained between the floor and the bar using computer software, and eliminates many human interpretations and response time. Contact time was recorded up to a maximum of 30 seconds (programmable). Visual data were also collected for comparison. Four chambers were used. Rats were pretreated by sc injection with saline or WAY100,635 (0.63 mg/kg as free base) in saline 15 min before injection (ip) with SYA013 or haloperidol. Sixty min after injection with SYA013 or haloperidol, rats were tested in the CLP test (see 2.4.4.) for 30 seconds, followed by the bar test for 30 seconds, followed by the righting test (see 2.4.5.) then returned to their home cage. This was repeated at 3 and 6 min from the start of the first trial. Five rats were used for each dose. The dose of 0.63 mg/kg WAY100,635 given sc has been used in studies to antagonize 5HT1A receptors to unmask the cataleptogenic properties of potential antipsychotics which demonstrate reduced catalepsy potential in the bar and or CLP tests (Depoortere et al., 2007; Kleven et al., 2005; Prinssen et al., 1998).
2.4.3.1. Haloperidol
To validate the newly acquired Catalepsy Test Chamber (Med Associates, Inc., St. Albans, VT) the induction of catalepsy was estimated for haloperidol and a catalepsy ED50 of 0.61 mg/kg was calculated.
2.4.4. Crossed-legs position (CLP) test
Rats were placed on the stainless steel floor of the catalepsy test chamber, abdomen towards the floor, and the hind paws brought forward and the front paws backwards so that the ipsilateral hind paws could hold onto the top of the front paws, and the time the rat stayed in this position recorded up to 30 seconds (Depoortere et al., 2007, Kleven et al., 1996).
2.4.4.1. Haloperidol
A catalepsy ED50 of 0.5 mg/kg was obtained for haloperidol with the CLP test.
2.4.5. Righting test
Rats were placed gently on their back and observed immediately following the bar and CLP tests. If the rat did not stay in this position, and flipped over without assistance it was scored as "righted" (McCreary et al. 2007; Reeve et al., 1992).
2.4.6. Semichronic challenge, comparison of saline and WAY100,635 pretreatments
Rats were injected ip with 25 mg/kg SYA013 for four days. On the fifth day, rats were pre-injected sc with saline or WAY100,635 (0.63 mg/kg as free base) (Depoortere et al., 2007), then injected 15 min later ip with 25 mg/kg SYA013. At 60 min post ip injection, rats were tested for catalepsy.
2.5. Statistical analyses
All statistical analyses were performed using Prism 5.03, GraphPad Software Inc. The differences between groups with p<0.001, 0.01, or 0.05 were considered significant. Statistical analysis of CAR versus pre-treatment group was achieved by two-way analysis of variance (ANOVA) with pre-treatment (WAY100,635 or vehicle) and the dose of SYA013 as the between subjects factor.
Pharmacokinetic parameters were calculated by non-compartmental analysis using PK Solutions, Summit Research Services.
All error bars are standard error of the mean (s.e.m.).
3. Results
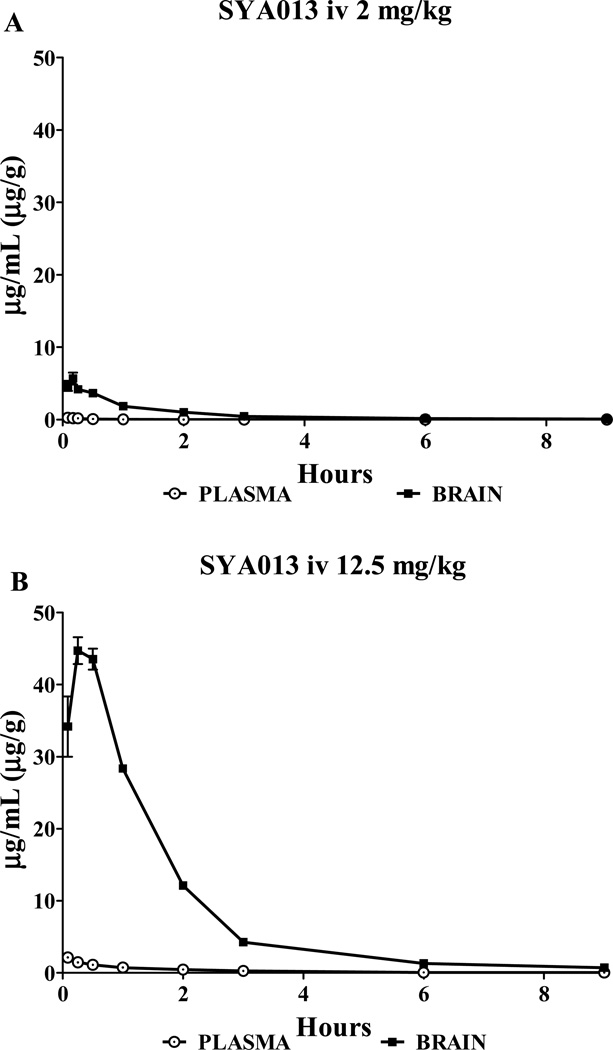
3.1. Pharmacokinetics of SYA013 in plasma and brain tissue, iv (Fig. 1)
Fig. 1.
Time profile of plasma and brain concentrations of SYA013. Blood plasma and brain tissue levels as SYA013 after 2.09 mg/kg iv (A) and 12.5 mg/kg iv (B).
The mean concentration values for plasma and brain (n=4–5) are presented in Fig. 1, separately for one and six times the locomotor activity test iv ID50 dose. Data through timepoint 9 hr are shown for clarity, although 24 hr was collected and used in calculations if greater than zero. The concentration maximum for brain occurred at a later time than plasma for both doses. Pharmacokinetic data are presented in Table 1. AUC was calculated through the time of the last observable concentration, t. The SYA013 brain levels significantly exceeded those in plasma by a factor of 21 or more, indicating a high degree of drug penetration into the brain.
Table 1.
IV pharmacokinetic data in rats for SYA013 at 2.09 and 12.5 mg/kg
| Tissue | Dose, mg/kg |
Cmax obsvd µg/g |
tmax obsvd hr |
AUC 0-t µg·hr/(mL or g) |
t1/2, hr Elimination |
|---|---|---|---|---|---|
| Brain | 2.09 | 5.69 | 0.17 | 6.72 | 1.6 |
| Plasma | 2.09 | 0.25 | 0.18 | 1.5 | |
| Brain | 12.5 | 44.7 | 0.25 | 82.5 | 2.6 |
| Plasma | 12.5 | 2.14 | 2.83 | 4.0 |
3.2. Pharmacokinetics of SYA013 in plasma and brain tissue, oral
The mean concentration values for plasma and brain (n=3–5) were determined and AUC0-t calculated (Table 2). The SYA013 brain levels significantly exceeded those in plasma by a factor of 21, indicating a high degree of drug penetration into the brain. The highest oral dose possible was 48 mg/kg due to solubility limits of SYA013 in the vehicle, and was chosen to maximize response during HPLC analysis.
Table 2.
PO pharmacokinetic data in rats for SYA013 at 48 mg/kg
| Tissue | Dose, mg/kg |
Cmax obsvd µg/g |
tmax obsvd hr |
AUC 0-t µg·hr/(mL or g) |
t1/2, hr Elimination |
|---|---|---|---|---|---|
| Brain | 48 | 2.50 | 1 | 5.25 | 1.4 |
| Plasma | 48 | 0.12 | 1 | 0.22 | 0.83 |
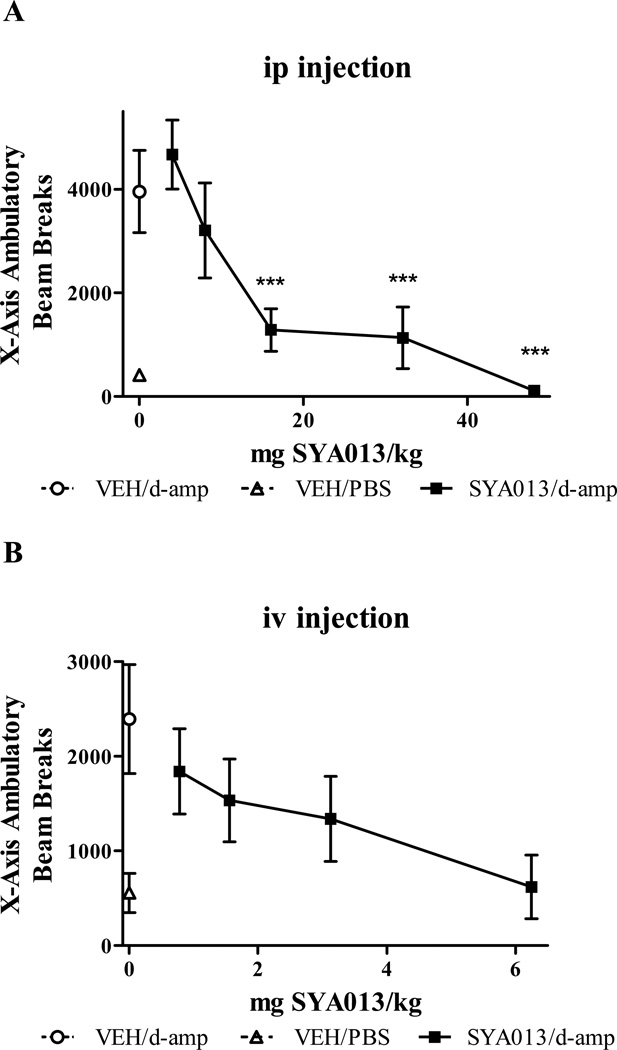
3.3. Effects of SYA013 on d-amphetamine-induced locomotor hyperactivity (Fig. 2)
Fig. 2.
Locomotor effects of d-amphetamine after pre-injection by ip (A) or iv (B) with vehicle or SYA013. ***Indicates p<0.001 compared with the vehicle/d-amphetamine group, (one-way ANOVA with post hoc Bonferroni tests). n= 4–8 rats/group.
The mean ambulatory beam breaks for the X-axis (n=4–8) are presented in Fig. 2 (A) ip and (B) iv (Opto-Varimex Mini and Opto-M3 respectively). The ID50 for SYA013 was 12.5 mg/kg for ip injections, and 2.09 mg/kg for iv injections. Spontaneous locomotor activity for 4–48 mg/kg ip SYA013 was not significantly different from vehicle treated rats. This suggests that antagonism of d-amphetamine effects was distinct from nonspecific motor and/or sedating effects.
3.4. Effects on conditioned avoidance response in rats
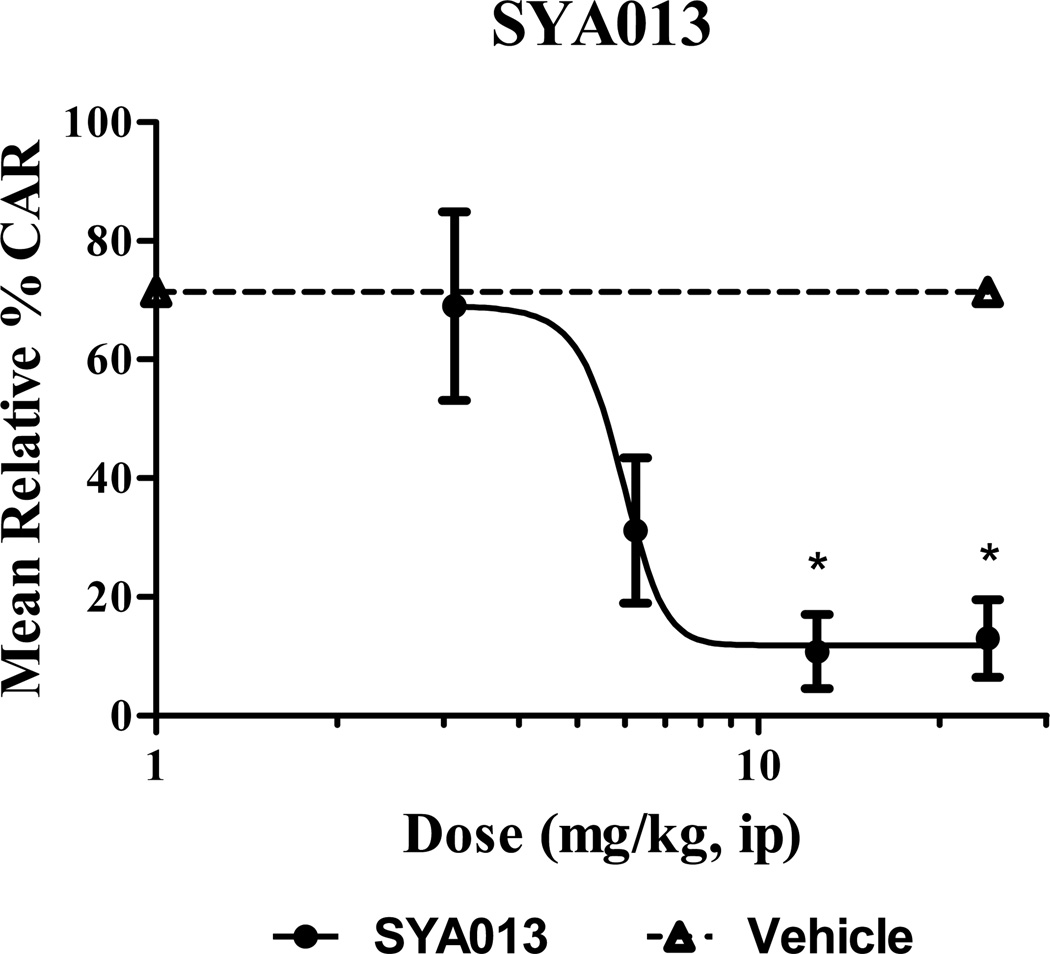
3.4.1. No pre-injection (Fig. 3)
Fig. 3.
Reduction by SYA013 of two-way active avoidance behavior in rats. * indicates p<0.05 compared with the vehicle treated group, (Kruskall-Wallis with Dunn's post test). n= 5 rats/group.
% CAR for each rat was calculated as (number of avoids / 30) × 100. The relative % CAR for each rat was calculated by dividing the % CAR for the day before by the % CAR for the day of the test, then multiplying by 100. The mean relative % CAR for 5 rats per dose was then plotted in Fig. 3. SYA013 reduced the mean relative % CAR in a dose-dependent manner as shown in Fig. 3, indicating antipsychotic properties in this classic animal model. ID50 for the relative % CAR was 5.6 mg/kg, ip. No EFRs were noted.
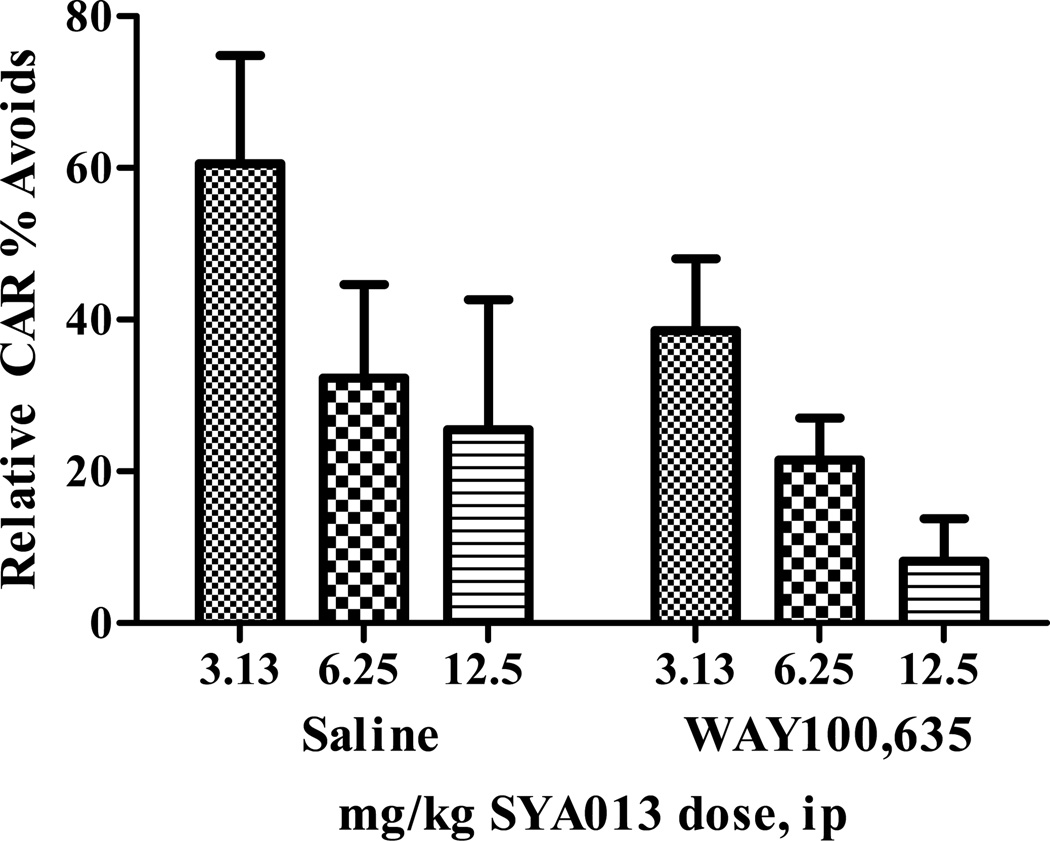
3.4.2. Pre-injection with saline or WAY100,635 (Fig. 4)
Fig. 4.
Conditioned avoidance behavior in rats was significantly influenced by the dose of SYA013 (p<0.05), but not by pretreatment with WAY100,635. Interaction factors were also not significant. (two-way ANOVA). n= 5–6 rats/group.
Pre-treatment with the 5-HT1A antagonist WAY100,635 was compared to pre-treatment with saline to investigate the role of the 5HT1A receptor in the behavioral profile of SYA013. Statistical analysis indicated that pretreatment with WAY100,635 did not influence the potency of SYA013 to inhibit CAR. Data are shown in Fig. 4.
3.5. Catalepsy Bar Test
The mean seconds for 3 trials for each rat was calculated. Then the mean of the 3 trial means for the number of rats was plotted in Fig 5 and Fig 6. A rat was deemed cataleptic if it reached a mean of 30 seconds without the loss of the righting reflex (McCreary et al., 2007).
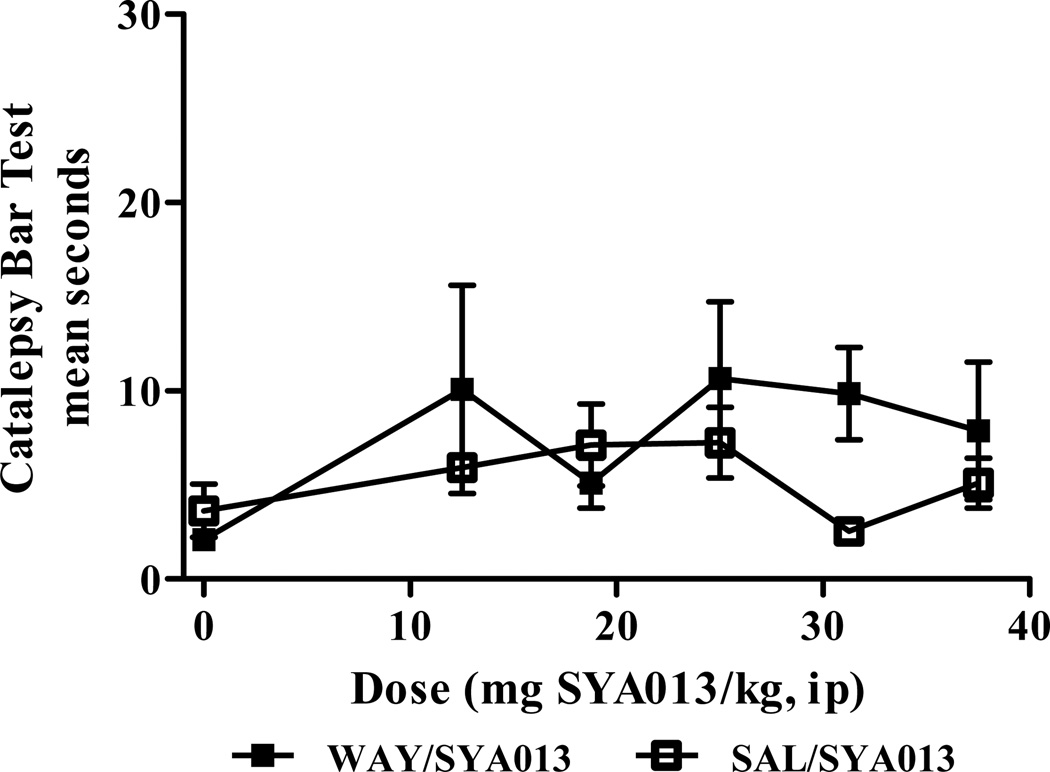
Fig. 5.
Pre-treatment of rats with the 5HT1A receptor antagonist WAY100,635 did not increase catalepsy when compared to saline pre-treatment. No significant effects of treatment, pretreatment, or interaction were found (two-way ANOVA). n=5 rats per group.
3.5.1. Acute, comparison of saline and WAY100,635 pretreatments (Fig. 5)
None of the rats pre-treated with saline, then injected with SYA013 demonstrated notable (mean >20 sec) catalepsy in the bar test. When the rats were pre-treated with the 5HT1A receptor antagonist WAY100,635, then injected with SYA013, a 2-way ANOVA with Bonferroni post hoc tests found no significant effects of treatment (dose of SYA013), pretreatment (WAY100,635 or saline), or interaction. These data are shown in Fig 5.
3.5.2. Semi-chronic, comparison of saline and WAY100,635 pretreatments
None of the rats injected with SYA013 for 4 days followed by injection on the 5th day with saline pre-treatment followed by SYA013 demonstrated notable catalepsy. When pre-treated with WAY100,635 (after 4 days of SYA013 semi-chronic dosing), followed by SYA013 injection, 3 rats out of 10 had a mean of 20 seconds or more. A one-tailed t-test of the 2 means found no significant difference (n=10 rats per group).
3.6. Catalepsy CLP Test
3.6.1. Acute, comparison of saline and WAY100,635 pretreatments
None of the rats pre-treated with saline or WAY100,635 followed by SYA013 injection demonstrated catalepsy in the CLP test.
3.6.2. Semi-chronic, comparison of saline and WAY100,635 pretreatments
None of the rats demonstrated catalepsy in the CLP position.
3.7. Righting Test
All rats righted themselves following the bar and CLP tests on all trials.
4. Discussion
The ratio of brain concentrations to plasma concentrations is a predictor of the degree of drug incorporation into the brain (Palenicek et al., 2011). For equilibrium concentrations, log BB, defined as logarithm of the ratio of the concentration of a drug in the brain and the blood, is an index of blood brain barrier (BBB) permeability, and can be determined in vivo in rats. It has been observed that molecules with activity on the central nervous system (CNS) have a log BB > 0.3 and cross the BBB readily, while drugs which lack action on the CNS have a log BB < −1 (Vilar et al., 2010). The log of the brain/plasma concentration ratio for SYA013 (Clog P = 5.15) at the tmax for brain for the 12.5 mg/kg dose is 1.48. Haloperidol (Clog P = 3.85), clozapine (Clog P = 3.71), and risperidone (Clog P = 2.71) were reported to have log BB of 1.34, 1.38 and −0.66 respectively (Olsen et al., 2008). Thus, log BB of 1.48, predicts SYA013 to cross the BBB readily.
The plasma elimination half life of SYA013 in fasted rats was estimated to be 1.5 – 4 hr for the intravenous doses tested, and 0.83 hr for the oral dose. Clozapine has been reported to have a terminal half life of 1.36 hr in the rat (Sun and Lau, 2000), and 8 hours in man (FDA Reference ID: 3031346). It has been noted that the half life of an antipsychotic in rats is relatively shorter than in humans (Olsen et al., 2008), and this must be considered when comparing data between different species.
SYA013 is active in animal models that predict activity against the positive symptoms of schizophrenia. It antagonized d-amphetamine-induced locomotor hyperactivity in rats in a dose dependent manner with an ID50,ip of 12.5 mg/kg and an ID50,iv of 2.09 mg/kg. This suggests dopamine receptor antagonism. It caused suppression of conditioned-avoidance response behavior in rats in a dose dependent manner with an ID50,ip of 5.6 mg/kg. These tests compliment earlier work in which SYA013 inhibited apomorphine-induced climbing activity in mice, also in a dose dependent manner (Ablordeppey et al., 2008), providing further evidence of potential antipsychotic drug activity.
The possible influence of SYA013 on CAR due to its moderate binding affinity to the 5HT1A receptor was tested by antagonizing the 5HT1A receptor with WAY100,635 and observing CAR at different doses. The results were not significant leading to the suggestion that SYA013 did not significantly inhibit CAR due to its binding to the 5HT1A receptor.
The newly released Catalepsy Test Chamber from MED Associates, Inc. for measuring catalepsy with the bar test performed well. The instrument was tested with the antipsychotic haloperidol which is known to produce catalepsy in rats in the bar test (Hoffman et al., 1995), which is predictive of EPS in humans (McElroy, 1993). A dose-response curve was obtained with a catalepsy ED50 for haloperidol, ip, of 0.61 mg/kg, in the range of values found by other researchers (0.4–0.6 mg/kg) (Campbell et al., 1988; Needham et al., 1996; Jones and McCreary, 2008).
In an experiment designed to investigate if binding of SYA013 to the serotonin 5HT1A receptor contributes to the lack of significant catalepsy observed, rats were pre-treated with saline or WAY100,635 before testing with SYA013. No significant difference was noted in the bar or CLP tests, suggesting that binding to the 5HT1A receptor does not play a significant role.
Semichronic treatment of rats with SYA013 for 4 days, followed by pre-treatment with saline and then the same dose of SYA013 on the fifth day did not result in notable catalepsy. When compared to rats treated semichronically with SYA013 followed by pre-treatment with WAY100,635 on day 5, no significant difference was observed. This also suggests that binding of SYA013 to the 5HT1A receptor alone does not account for the lack of catalepsy. Other factors, such as moderate binding to the D2 receptor may explain its lack of cataleptogenic properties. Alternatively, SYA013 has a 5HT2A affinity that is higher than its D2 affinity, which has also been observed for other atypical antipsychotics (Meltzer et al., 1989).
In summary, SYA013 was found to be efficacious in animal models predictive of anti-psychotic action, and is predicted to cross the blood brain barrier based on pharmacokinetic data. A new instrument for catalepsy testing in rats was evaluated and used to examine the cataleptogenic potential of SYA013. It was found that while SYA013 binds to the 5HT1A receptor with moderate affinity, this was not a significant reason for its potency or lack of catalepsy.
Unlike typical antipsychotics, SYA013 continues to demonstrate a lack of significant catalepsy at doses that are predicted to be efficacious in humans, based on the d-amphetamine-induced locomotor test and conditioned avoidance response test. This property was maintained after semi-chronic treatment of rats with SYA013.
Several lines of evidence support the observation that binding affinity at the H1 receptor correlates with a drug’s propensity to induce weight gain. For example, Coccurello and Moles, 2010, rank the liability for weight gain for several atypical antipsychotics as follows: clozapine (H1Ki = 1.8 nM) = olanzapine (H1Ki = 2.8 nM) >quetiapine (H1Ki = 8.7 nM) ≥ risperidone (H1Ki = 19 nM) >aripiprazole (H1Ki = 28 nM) >ziprasidone (H1Ki = 47 nM), with clozapine and olanzapine having the highest propensity to induce weight gain, and aripiprazole and ziprasidone the least or no weight gain (Starrenburg and Bogers, 2009; Ablordeppey et al., 2008). Given the Kis for these atypical antipsychotics, it is reasonable to suggest that since SYA013 binds to the H1 receptor with Ki = 186 nM the drug should have a much attenuated capacity to induce weight gain if at all.
In addition, SYA013 fares very well with another proposed indicator for a drug’s propensity to induce Type II diabetes, i.e., binding to the M3 muscarinic receptor (Seeger et al., 1995). Affinity for the M3 muscarinic receptor is considered to be one of the best predictors of anti-psychotic-induced Type II diabetes (Starrenburg and Bogers, 2009). Clozapine or olanzapine usage has the highest risk of developing Type II diabetes, while the use of aripiprazole or ziprasidone results in a lower glucose level in the blood. This correlates with high binding affinity to the M3 receptor (clozapine Ki = 26 nM; olanzapine Ki = 44 nM) for increased risk of Type II diabetes, and low binding affinity to the M3 receptor (aripiprazole Ki = 4677 nM; ziprasidone Ki= 5623 nM) for decreased risk. SYA013 has an M3 Ki of 3000 nM, and so would be predicted again to have a decreased risk of Type II diabetes.
These observations are significant and suggest that SYA013 may provide a new treatment option for schizophrenia with reduced EPS, reduced risk for weight gain and may not be associated with antipsychotic treatment-emergent Type II diabetes.
Research Highlights.
SYA 013 was shown to inhibit both d-amphetamine induced locomotor activity and conditioned avoidance response (CAR) in rats in a dose dependent manner and in the case of CAR, without producing any escape failure responses (EFR).
SYA 013 has a log brain/plasma concentration ratio at tmax of 1.48 suggesting that SYA 013 readily crosses the blood brain barrier (BBB).
WAY100,635 did not have a significant effect on the lack of catalepsy and the inhibition of CAR by SYA 013 and thus suggest 5HT1A receptor does not appear to play a significant role in the pharmacological profile of SYA 013.
Acknowledgements
The authors gratefully acknowledge the financial support of the National Institute of General Medical Studies (NIGMS) for MBRS Grant No. 1SC1GM088451-01 and a Title III Grant to Florida A&M University. This work was also supported in part by the Pharmaceutical Research Center NIH/NCRR 1 C06-RR12512-01 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Kumar EVKS, Jackson T, Khan A, Roth BL. Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-Chlorophenyl)-1,4-diazepan-1yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008;16:7291–7301. doi: 10.1016/j.bmc.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenius S, Hillegaart V. Involvement of extrapyramidal motor mechanisms in the suppression of locomotor activity by antipsychotic drugs: a comparison between the effects produced by pre- and postsynaptic inhibition of dopaminergic neurotransmission. Pharmacol Biochem Behav. 1986;24:1409–1415. doi: 10.1016/0091-3057(86)90203-0. [DOI] [PubMed] [Google Scholar]
- Arnt J. Pharmacological specificity of conditioned avoidance response inhibition in rats. Inhibition by neuroleptics and correlation to dopamine receptor blockade. Acta Pharmacol Toxicol. 1982;51:321–329. doi: 10.1111/j.1600-0773.1982.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Arterburn D, Rukstalis M, Wood C, Westbrook E, Theis K, Daar Z, Still C, Gerhard G. C-a3-02: identification of extreme weight gain among users of atypical antipsychotic medications. Clin Med Res. 2011;9(3–4):160. [Google Scholar]
- Assie M-B, Koek W. Effects of 5-HT1A receptor antagonists on hippocampal 5-hydroxytryptamine levels: (S)-WAY100135, but not WAY100635, has partial agonist properties. Eur J Pharmacol. 1996;304:15–21. doi: 10.1016/0014-2999(96)00086-6. [DOI] [PubMed] [Google Scholar]
- Birch AM, Bradley PA, Gill JC, Kerrigan F, Needham PL. N-Substituted (2,3-Dihydro-1,4-benzodioxin-2yl)methylamine Derivatives as D2 Antagonists/5-HT1A Partial Agonists with Potential as Atypical Antipsychotic Agents. J Med Chem. 1999;42:3341–3355. doi: 10.1021/jm9910122. [DOI] [PubMed] [Google Scholar]
- Bourdon KH, Rae DS, Locke BZ, Narrow WE, Regier DA. Estimating the Prevalence of Mental Disorders in U.S. Adults from the Epidemiologic Catchment Area Survey. Public Health Rep. 1992;107:663–8. [PMC free article] [PubMed] [Google Scholar]
- Bundgaard C, Larsen F, Kreilgaard M, Brennum LT, Olsen CK. Pharmacokinetics of Sertindole and its Metabolite Dehydrosertindole in Rats and Characterization of their Comparative Pharmacodynamics based on In Vivo D2 Receptor Occupancy and Behavioural Conditioned Avoidance Response. Biopharm Drug Dispos. 2009;30:209–220. doi: 10.1002/bdd.656. [DOI] [PubMed] [Google Scholar]
- Campbell A, Baldessarini RJ, Cremens MC. Dose-catalepsy response to haloperidol in rat: effects of strain and sex. Neuropharmacology. 1988;27:1197–1199. doi: 10.1016/0028-3908(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Coccurello R, Moles A. Potential mechanisms of atypical antipsychotic-induced metabolic derangement: Clues for understanding obesity and novel drug design. Pharmacol Therapeutics. 2010;127:210–251. doi: 10.1016/j.pharmthera.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Bardin L, Auclair AL, Kleven MS, Prinssen E, Colpaert F, Vacher B, Newman-Tancredi A. F15063, a compound with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (II) Activity in models of positive symptoms of schizophrenia. Br J Pharmacol. 2007;151:253–265. doi: 10.1038/sj.bjp.0707159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA. Treatment of schizophrenia: a clinical and preclinical evaluation of neuroleptic drugs. Pharmacol Ther. 1993;57:1–78. doi: 10.1016/0163-7258(93)90036-d. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA. Psychopharmacological treatment of schizophrenia: What do we have, and what could we get? Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.03.013. (in press). [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. The paw test: An animal model for neuroleptic drugs which fulfils the criteria for pharmacological isomorphism. Life Sci. 1988;42:1205–1213. doi: 10.1016/0024-3205(88)90551-6. [DOI] [PubMed] [Google Scholar]
- FDA Reference ID: 3031346. Label information. :4. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019758s063lbl.pdf.
- Geffen Y, Nudelman A, Gil-Ad I, Rephaeli A, Huang M, Savitsky K, Klapper L, Winkler I, Meltzer HY, Weizman A. BL-1020: A novel antipsychotic drug with GABAergic activity and low catalepsy, is efficacious in a rat model of schizophrenia. European Neuropsychopharmacology. 2009;19:1–13. doi: 10.1016/j.euroneuro.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Geyer M, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Progress in Neuro-Psychopharmacol and Biological Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Ballarin M, Dordal A, Farre AJ. E-5842: A New Potent and Preferential Sigma Ligand. Preclinical Pharmacological Profile. CNS Drug Reviews. 1998;4:201–224. [Google Scholar]
- Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology. 1995;120:128–133. doi: 10.1007/BF02246184. [DOI] [PubMed] [Google Scholar]
- Jones CA, McCreary AC. Serotonergic approaches in the development of novel antipsychotics. Neuropharmacology. 2008;55:1056–1065. doi: 10.1016/j.neuropharm.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Progress in Neuro-Psychopharmacol and Biological Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Barret-Grevoz C, Slot LB, Newman-Tancredi A. Novel antipsychotic agents with 5-HT1A agonist properties: Role of 5-HT1A receptor activation in attenuation of catalepsy induction in rats. Neuropharmacology. 2005;49:135–143. doi: 10.1016/j.neuropharm.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kleven M, Prinssen EPM, Koek W. Role of 5-HT1A receptors in the ability of mixed 5-HT1A receptor agonist/dopamine D2 receptor antagonists to inhibit methylphenidate-induced behaviors in rats. Eur J Pharmacol. 1996;313:25–34. doi: 10.1016/0014-2999(96)00498-0. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Glennon JC, Ashby CR, Jr, Meltzer HY, Li Z, Reinders JH, Hesselink MB, Long SK, Herremans AH, Stuivenberg HV, Feenstra RW, Kruse CG. SLV313 (1-(2,3-DihydroBenzo[1,4]-Dioxin-5-yl)-4-[5-(4-Fluoro-Phenyl)-Pyridin-3-ylmethyl]-PiperazineMonohydrochloride): A Novel Dopamine D2 Receptor Antagonist and 5-HT1A Receptor Agonist Potential Antipsychotic Drug. Neuropsychopharmacology. 2007;32:78–94. doi: 10.1038/sj.npp.1301098. [DOI] [PubMed] [Google Scholar]
- McElroy JF. Inhibition of haloperidol- and chlorpromazine-induced catalepsy by XJ448, a sigma-selective receptor antagonist. Schizophrenia Research. 1993;9:224. [Google Scholar]
- Meltzer HY. Atypical antipsychotic drugs. In: Bloom FE, Kupfer D, editors. Psychopharmacology: Fourth Generation of Progress. New York: Raven Press; 1995. pp. 1277–1286. [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106–115. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Massey BW. The role of serotonin receptors in the action of atypical antipsychotic drugs. Current Opinion in Pharmacology. 2011;11:59–67. doi: 10.1016/j.coph.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- Natesan S, Reckless GE, Barlow KBL, Nobrega JN, Kapur S. Amisulpride the ‘atypical’atypical antipsychotic- Comparison to haloperidol, risperidone and clozapine. Schizophrenia Res. 2008;105:224–235. doi: 10.1016/j.schres.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Needham PL, Atkinson J, Skill MJ, Heal DJ. Zotepine: Preclinical Tests Predict Antipsychotic Efficacy and an Atypical Profile. Psychopharmacol Bull. 1996;32:123–128. [PubMed] [Google Scholar]
- Newman-Tancredi A, Chaput C, Verriele L, Millan MJ. Clozapine is a partial agonist at cloned, human serotonin 5-HT1A receptors. Neuropharmacology. 1996;35:119–121. doi: 10.1016/0028-3908(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Olsen CK, Brennum LT, Kreilgaard M. Using pharmacokinetic-pharmacodynamic modelling as a tool for prediction of therapeutic effective plasma levels of antipsychotics. Eur J Pharmacol. 2008;584:318–327. doi: 10.1016/j.ejphar.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Palenicek T, Balikova M, Rohanova M, Novak T, Horacek J, Fujakova M, Hoschl C. Behavioral, hyperthermic and pharmacokinetic profile of para-methoxymethamphetamine (PMMA) in rats. Pharmacol Biochem Behav. 2011;98:130–139. doi: 10.1016/j.pbb.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Prinssen EPM, Kleven MS, Koek W. The cataleptogenic effects of the neuroleptic nemonapride are attenuated by its 5-HT1A receptor agonist properties. Eur J Pharmacol. 1998;356:189–192. doi: 10.1016/s0014-2999(98)00536-6. [DOI] [PubMed] [Google Scholar]
- Reeve B, Dingwall B, Darlington CL, Scott SJ, Sansom AJ, Smith PF. Simple Device for Quantifying Drug Effects on the Righting Reflex. Pharmacol Biochem Behav. 1992;42:183–185. doi: 10.1016/0091-3057(92)90464-q. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Bunsey MD, Giordano M, Norman AB. The Catalepsy Test: Its Ups and Downs. Behav Neurosci. 1988;102:748–759. doi: 10.1037//0735-7044.102.5.748. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Pietraszek M, Gravius A, Danysz W. Effects of a positive allosteric modulator of mGluR5 ADX47273 on conditioned avoidance response and PCP-induced hyperlocomotion in the rat as models for schizophrenia. Pharmacol Biochem Behav. 2010;95:23–30. doi: 10.1016/j.pbb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Seeger TF, Seymour PA, Schmidt AW, Zorn SH, Schulz DW, Lebel LA, McLean S, Guanowsky V, Howard HR, Lowe JA, Heym JJ. Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. Pharmacol Exp Ther. 1995;275:101–113. [PubMed] [Google Scholar]
- Shimokawa Y, Akiyama H, Kashiyama E, Koga T, Miyamoto E. High performance liquid chromatographic methods for the determination of aripiprazole with ultraviolet detection in rat plasma and brain: Application to the pharmacokinetic study. J Chromatogr B. 2005;821:8–14. doi: 10.1016/j.jchromb.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Sikazwe DMN, Li S, Mardenborough L, Cody V, Roth BL, Ablordeppey SY. Haloperidol: towards further understanding of the structural contributions of its pharmacophoric elements at D2-like receptors. Bioorg Med Chem Lett. 2004;14:5739–5742. doi: 10.1016/j.bmcl.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown JC, Phoebe Marala R, Patterson T, Seymour PA, Swick A, Iredale PA. CP-809,101, a selective 5-HT2C, agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology. 2007;52:279–290. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Starrenburg FCJ, Bogers JPAM. How can antipsychotics cause diabetes mellitus? Insights based on receptor-binding profiles, humoral factors and transporter proteins. Eur Psychiatry. 2009;24:164–170. doi: 10.1016/j.eurpsy.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Struyker-Boudier HA, Cools AR. (3,4-Dihydroxyphenylimino)-2-imidazoline (DPI): a stimulant of alpha-adrenoceptors and dopamine receptors. J Pharm Pharmacol. 1984;36:859–860. doi: 10.1111/j.2042-7158.1984.tb04897.x. [DOI] [PubMed] [Google Scholar]
- Sun L, Lau CE. Intravenous and oral clozapine pharmacokinetics, pharmacodynamics, and concentration-effect relations: acute tolerance. Eur J Pharmacol. 2000;398:225–238. doi: 10.1016/s0014-2999(00)00277-6. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhao C, Hu G, Li M. Iptakalim: A potential antipsychotic drug with novel mechanisms? Eur J Pharmacol. 2010;634:68–76. doi: 10.1016/j.ejphar.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Vilar S, Chakrabarti M, Costanzi S. Prediction of passive blood-brain partitioning: Straightforward and effective classification models based on in silico derived physicochemical descriptors. J Molecular Graphics Model. 2010;28:899–903. doi: 10.1016/j.jmgm.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg MLG. Dopamine D2 Receptor Occupancy Is a Common Mechanism Underlying Animal Models of Antipsychotics and Their Clinical Effects. Neuropsychopharmacology. 2001;25:633–641. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- Wadenberg MLG. Conditioned Avoidance Response in the Development of New Antipsychotics. Current Pharmaceutical Design. 2010;16:358–370. doi: 10.2174/138161210790170085. [DOI] [PubMed] [Google Scholar]
- Wadenberg MLG, Hicks PB. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev. 1999;23:851–862. doi: 10.1016/s0149-7634(99)00037-8. [DOI] [PubMed] [Google Scholar]