Abstract
Injury to the central nervous system (CNS) can result in lifelong loss of function due in part to the regenerative failure of CNS neurons. Inhibitory proteins derived from myelin and the astroglial scar are major barriers for the successful regeneration of injured CNS neurons. Previously, we described the identification of a novel compound, F05, which promotes neurite growth from neurons challenged with inhibitory substrates in vitro, and promotes axonal regeneration in vivo (Usher et al., 2010). To identify additional regeneration-promoting compounds, we used F05-induced gene expression profiles to query the Broad Institute Connectivity Map, a gene expression database of cells treated with >1,300 compounds. Despite no shared chemical similarity, F05-induced changes in gene expression were remarkably similar to those seen with a group of piperazine phenothiazine antipsychotics (PhAPs). In contrast to antipsychotics of other structural classes, PhAPs promoted neurite growth of CNS neurons challenged with two different glial derived inhibitory substrates. Our pharmacological studies suggest a mechanism whereby PhAPs promote growth through antagonism of calmodulin signaling, independent of dopamine receptor antagonism. These findings shed light on mechanisms underlying neurite-inhibitory signaling, and suggest that clinically approved antipsychotic compounds may be repurposed for use in CNS injured patients.
Keywords: piperazine, phenothiazine, antipsychotics, chondroitin sulfate proteoglycans, myelin, regeneration, trifluoperazine, prochlorperazine
Introduction
A striking feature of the adult mammalian central nervous system (CNS) is that severed axons often fail to regrow and reform lost connections after injury. As a consequence, spinal cord injury (SCI) typically results in a lifelong loss of sensory and motor function below the level of the lesion. Unfortunately, there are currently no proven clinical therapies that can stimulate long distance regeneration and improve functional recovery for SCI patients.
CNS regenerative failure results from the poor intrinsic growth capacity of mature CNS neurons (Goldberg et al., 2002), together with the inhibitory environment of CNS lesions (Yiu and He, 2003). Genetic perturbations that stimulate intrinsic growth programs can improve regeneration (Liu et al., 2010; Moore et al., 2009; Park et al., 2008); however, environmental obstacles, such as those found in myelin and the glial scar, still limit axon growth through the lesion site (Yiu and He, 2003). Inhibitory proteins in myelin include Nogo (Huber and Schwab, 2000), myelin-associated glycoprotein (MAG; McKerracher et al., 1994), and oligodendrocyte myelin glycoprotein (Wang et al., 2002). Manipulations designed to overcome these myelin-derived molecules have resulted in limited improvements in regeneration (Atwal et al., 2008; Cafferty et al., 2010; Kubo et al., 2008; Lee et al., 2010; Walmsley and Mir, 2007). Similarly, glial scar proteins such as chondroitin sulfate proteoglycans (CSPGs) inhibit growth in vitro (Monnier et al., 2003; Snow, et al., 1990; Ughrin et al., 2003), and their expression correlates with regenerative failure in vivo (Davies et al., 1999; McKeon et al., 1991). Reducing expression of these CSPGs (Bradbury et al., 2002) or targeting their downstream signaling pathways (Koprivica et al., 2005; Shen et al., 2009; Sivasankaran et al., 2004) has led to a modest degree of regeneration and functional recovery after injury. Despite these successes, the signaling pathways responsible for growth inhibition by myelin proteins and CSPGs are not fully understood and regeneration-promoting therapies have yet to reach clinical practice.
The translation of novel therapies from the lab to the clinic is rife with obstacles. Failure rates for new drugs can be as high as 95%, and Food and Drug Administration (FDA)-approved new drugs spend an average of 13 years in testing at a cost of around $1 billion (Collins, 2011). An increasingly attractive strategy to circumvent these problems is to discover new applications for drugs that are already clinically approved. One approach to achieve this “drug repurposing” is to compare the gene expression profiles of clinically approved compounds to profiles of perturbations that induce a phenotype of interest. Such strategies have been previously used to identify potentially novel therapies for hepatocellular carcinoma (Braconi et al., 2009; Chen et al., 2011), gastric cancer (Claerhout et al., 2011), ovarian cancer (Gullbo et al., 2011), lung cancer (Wang et al., 2011), colorectal cancer (Vilar et al., 2009), neuroblastoma (De Preter et al., 2009), and influenza (Josset et al., 2010). Here, we apply this comparative microarray approach to implicate novel strategies for improving regeneration after CNS injury.
Previously, we identified four novel compounds that promote CNS neurite outgrowth in the presence of an inhibitory myelin substrate in vitro. All four compounds also promoted growth on CSPG-derived substrates and on an in vitro model of the glial scar, but did not affect growth on permissive substrates (Usher et al., 2010). The signaling mechanisms through which these compounds act are not known, though they perturb growth cone microtubule dynamics. One compound, F05, promoted regeneration in vivo after acute transection of dorsal column sensory axons, as well as regrowth of retinal ganglion cell axons after optic nerve crush (Usher et al., 2010). These results suggest that the compounds can be exploited to identify convergent mechanisms of inhibitory environmental signaling and to develop treatment strategies for SCI.
In this study, we sought to uncover the signaling pathways affected by F05 and to identify clinically relevant regeneration-promoting compounds. Our approach makes use of the Broad Institute “Connectivity Map”, a repository of gene expression signatures for over 1,300 small molecules (Lamb et al., 2006). Importantly, the database derives signatures from both nondrug bioactive compounds as well as a variety of FDA-approved compounds, thereby allowing the opportunity to suggest novel uses for currently prescribed drugs. Our analysis identified a subclass of antipsychotics (piperazine phenothiazines) that induce remarkably similar changes in gene expression to those seen with F05. We found that this structural class of antipsychotics promotes neurite growth in different types of cultured CNS neurons challenged with either myelin proteins or CSPGs through a mechanism dependent on antagonism of calmodulin signaling.
Materials & Methods
Microarray
Microarray data from F05 and vehicle treated samples were obtained using methods similar to those employed by the Broad Institute to generate gene expression signatures for the Connectivity Map (Lamb et al., 2006). Human MCF7 breast adenocarcinoma cells (American Type Culture Collection) were grown to ~70% confluency over a period of 24 hours in DMEM media containing 10% fetal bovine serum and 1% penicillin-streptomycin-glutamine (Gibco). The cells were then treated for 6 hr with F05 (5 μM) or vehicle (DMSO, 0.05%) in the same media. Following treatment, total RNA was isolated using a standard TRIzol isolation procedure (Invitrogen), and purified using the RNeasy Kit (Qiagen, Valencia, CA). RNA quantity and quality (absorbance ratios 260/280>2; 260/230>1.8) were assessed using a NanoDrop® Spectrophotometer (ND-1000, Thermo Scientific). Three biological replicates were analyzed for each of the two conditions.
Total RNA was submitted to the Duke University Microarray Facility through the NINDS/NIMH Microarray Consortium. Samples were processed using Ambion MessageAmp Premier Target Labeling Service and hybridized to Affymetrix Human U133 2.0 Plus Arrays. Average probe set signal intensities (background subtracted, perfect match - mismatch) and detection calls for each array were calculated using MAS 5.0 software (Affymetrix). The data are publically available (GEO accession #GSE34331). Genes affected by F05 were identified using the following criteria: p<0.05 using a t-test between the average signal intensities across the chips for DMSO vs. F05 treated; fold change ratios of F05/DMSO >1.5 or <−1.5; and detection calls of “present” for all 6 chips (3 DMSO, 3 F05 treated). Probes meeting these criteria were used as “up tags” or “down tags” to query the Connectivity Map. The database automatically calculates a “Connectivity Score” using a non-parametric rank based pattern matching strategy (Gene Set Enrichment Analysis; Subramanian et al., 2005) that yields a value between +1 and −1 for each of the reference compounds. A value close to +1 indicates a high degree of similarity between the query and reference compound, whereas a value close to −1 essentially indicates a reversal of expression patterns.
Cell culture
Tissue culture dishes were prepared by coating with 100 μg/mL poly-D-lysine overnight followed by another overnight incubation with 5 μg/mL laminin (Cultrex®, Trevigen®) either alone or in combination with 1 μg/mL CSPG (Millipore CC117; Ernst et al., 1995; Monnier et al., 2003). For the MAG experiments, hippocampal neurons were grown on a confluent monolayer of MAG or control-transfected Chinese hamster ovarian (CHO) cells (generously donated by Roman Giger, University of Michigan) using methods previously described (Mukhopadhyay et al., 1994).
Hippocampal neurons were prepared from E18 rats as described (Banker and Cowan, 1977; Bradke and Dotti, 1997) and cultured for 48 hours at 37°C, 5% CO2 in a Neurobasal medium containing 100 units/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamax, 10 mM Hepes, and 1X Neurocult® SM1 Neuronal Supplement (Stemcell). Retinal ganglion cells (RGCs) were harvested from P6 Sprague Dawley rats and purified by sequential immunopanning (Barres et al., 1988; Meyer-Franke et al., 1995). RGCs were grown for 48 hours at 37°C, 10% CO2 in a defined serum-free medium (Meyer-Franke et al., 1995; Wang et al., 2007) containing 50 ng/mL BDNF, 10 ng/mL CNTF, 5 μg/mL insulin, and 5 μM forskolin.
Neurons were plated and allowed to adhere to the dish for 1 hour in 2/3 the final volume of media, prior to addition of compounds (Table 1) or vehicle controls at 3X concentration to make up the final volume of media. Unless otherwise indicated, all tissue culture reagents were from Invitrogen (Gibco). Antipsychotic compounds were obtained from Spectrum Chemicals (Ellisville, MO), Calp1 was from Tocris Bioscience (New Brunswick, NJ), and all other compounds were from Sigma (St. Louis, MO). Supplemental Table I shows all the compounds used, their abbreviations, sources, and concentrations used.
Table I.
A comparative microarray of MCF7 cells using the Broad Institute Connectivity Map identifies small molecules that induce changes in gene expression similar to those seen with a regeneration-promoting compound (F05). The top 15 compounds in the Connectivity Map database were ranked according to how similar their genetic signatures were to that of F05. The list is ranked in order of Connectivity Score, which is the degree of similarity between the signatures (1 = perfectly correlated, −1 = perfectly anticorrelated; see Lamb et al., 2006). The Table also shows significance values (p-values, p). The scores are averaged over n individual microarrays performed for each respective compound. Phenothiazine antipsychotic (PhAP) compounds (prochlorperazine, fluphenazine, and trifluoperazine) represented 2 of the top 3 and 3 of the top 14 hits.
| Rank | Perturbagen and Cell Line | Connectivity Score | p | n | Remarks |
|---|---|---|---|---|---|
|
| |||||
| 1 | astemizole - MCF7 | 0.997 | 0.00002 | 2 | antihistamine |
| 2 | prochlorperazine - MCF7 | 0.724 | 0.00002 | 9 | piperazine phenothiazine |
| 3 | fluphenazine - MCF7 | 0.705 | 0.00002 | 10 | piperazine phenothiazine |
| 4 | tetrandrine - MCF7 | 0.994 | 0.00004 | 2 | calcium channel blocker |
| 5 | clomipramine - MCF7 | 0.994 | 0.00004 | 2 | antidepressant |
| 6 | corticosterone - MCF7 | 0.992 | 0.00006 | 2 | glucocorticoid |
| 7 | terfenadine - MCF7 | 0.992 | 0.00006 | 2 | antihistamine |
| 8 | geldanamycin - MCF7 | 0.639 | 0.00016 | 10 | antibiotic |
| 9 | mometasone - MCF7 | 0.985 | 0.0004 | 2 | glucocorticoid |
| 10 | desipramine - MCF7 | 0.982 | 0.00062 | 2 | antidepressant |
| 11 | niclosamide - MCF7 | 0.978 | 0.00084 | 2 | antihelminthic |
| 12 | fendiline - MCF7 | 0.974 | 0.00111 | 2 | calcium channel blocker |
| 13 | dilazep - MCF7 | 0.973 | 0.00119 | 2 | vasodilator |
| 14 | trifluoperazine - HL60 | 0.83 | 0.00127 | 4 | piperazine phenothiazine |
| 15 | fluticasone - MCF7 | 0.963 | 0.00239 | 2 | glucocorticoid |
Immunocytochemistry
After two days in vitro (DIV), neurons were fixed with 4% paraformaldehyde/4% sucrose in PBS. The blocking and antibody buffers for the hippocampal neurons contained 0.2% fish gelatin and 0.03% Triton X-100 in PBS. For the RGC and MAG-CHO experiments, cells were blocked in 20% normal goat serum, 0.02% Triton X-100 in antibody buffer (150 mM NaCl, 50 mM Tris base, 1% bovine serum albumin, 100 mM L-Lysine, 0.04% sodium azide, pH 7.4). After blocking for 1 hour at RT, cells were incubated in primary antibody overnight at 4°C. Primary antibodies included mouse anti-β-tubulin (E7, Developmental Studies Hybridoma Bank, University of Iowa) to assess neurite outgrowth and an antibody against the N-terminal extracellular domain of the D2 receptor (Chemicon #AB1558). For the RGC and MAG-CHO experiments a neuronal specific rabbit anti-β-III tubulin antibody was used (Sigma #SAB4500088). Secondary antibodies (goat anti-mouse or rabbit AlexaFluor 488) and nuclear dye (Hoechst; Invitrogen) were incubated with cells for 1 hr at room temperature.
Image acquisition and analysis
Cells were imaged and analyzed in an automated fashion by the ArrayScan® VTI (Cellomics, Pittsburgh, PA). Neuronal processes were traced according to user-defined parameters using the Extended Neurite Outgrowth and/or Neuronal Profiling bioapplication software (Cellomics; Blackmore et al., 2010). The mean total neurite length per neuron (the sum of the lengths of all the processes for a single neuron), the length of the longest process, the number of branch points (the sum of the instances where a single process diverged), and the number of cells (valid neurons) were determined for cells with a minimum neurite length of 25 μm. For the MAG-CHO experiments, a blinded experimenter imaged randomly chosen fields (20X objective, Olympus IX81 inverted microscope). Total neurite length was assessed by manual tracing using Neurolucida (MicroBrightField). Each experiment was done using two separate, duplicate plates. Data were analyzed using either a t-test or a one-way ANOVA with the appropriate post-test (Dunnett’s, Tukey’s, or Bonferroni; GraphPad Prism). All data shown are mean ± SEM.
Results
The novel regeneration-promoting compound F05 induces changes in gene expression similar to those induced by piperazine phenothiazine antipsychotics (PhAPs)
The novel compound F05 can promote growth in a variety of in vitro assays in which neurons encounter glial inhibitory molecules, and it also promotes regeneration in vivo, but its mechanism of action is unclear (Usher et al., 2010). To learn more about mechanisms of growth inhibitory signaling, we took advantage of the Broad Institute Connectivity Map, which allows users to query a database containing gene expression signatures from human cells treated with FDA-approved drugs and other bioactive compounds (Lamb et al., 2006). We treated MCF7 breast cancer cells with F05, and identified gene expression changes compared to vehicle treatment after 6 hours, since this is the time point used to generate the Connectivity Map expression profiles. Only 21 genes were identified as changing significantly with F05 treatment under these conditions (Supplementary Table II). However, comparison of these changes with those in the Connectivity Map database revealed a number of compounds with gene expression signatures closely related to that of F05 (Table I). These “hits” (compounds whose signatures significantly and positively correlated with the F05 signature) included glucocorticoids, antihistamines, calcium channel blockers, antipsychotics, and antidepressants, among others (Tables I, II). Interestingly, 3 of the top 14 hits (prochlorperazine, fluphenazine, and trifluoperazine) were phenothiazine antipsychotic drugs (PhAPs; Table I). Indeed, this class was the number one-ranked group of signatures clustered according to the Anatomical Therapeutic Chemical [ATC] classifications (Table II). Since PhAPs are known to perturb a variety of cellular signaling processes (Mosnaim et al., 2006; Tajima et al., 2009), these results do not immediately suggest a mechanism of action that may be relevant to overcoming growth inhibition. However, they suggest that PhAPs may induce changes in cellular signaling like those produced by F05, and might, therefore, promote neurite outgrowth on inhibitory substrates.
Table II.
Results from the Connectivity Map analysis when reference compounds were grouped by the Anatomical Therapeutic Chemical (ATC) Code chemical classification system. In this analysis, PhAPs were the top hit.
| Rank | ATC Code | ATC Code Description | Place of Known Drug Action | Compound Names |
|---|---|---|---|---|
| 1 | N05AB | antipsychotics (phenothiazines with piperazine structure) | nervous system | perphenazine, thioproperazine, prochlorperazine, trifluoperazine, fluphenazine |
| 2 | D07XC | corticosteroids | dermatologicals | mometasone, betamethasone |
| 3 | R06AX | antihistamines for systemic use | respiratory system | cyproheptadine, antazoline, ketotifen, astemizole, terfenadine, deptropine, pimethixene, mebhydrolin, triprolidine |
| 4 | D07AD | corticosteroids, very potent | dermatologicals | halcinonide, clobetasol |
| 5 | C01DX | vasodilators used in cardiac diseases | cardiovascular system | heptaminol, molsidomine, prenylamine, trapidil, dilazep |
| 6 | C10AA | lipid modifying agents (hmg coa reductase inhibitors) | cardiovascular system | fluvastatin, simvastatin, lovastatin |
| 7 | C02AA | antihypertensives (antiadrenergic agents, centrally acting rauwolfia alkaloids) | cardiovascular system | reserpine, rescinnamine |
| 8 | C08EA | phenylalkylamine derivatives (non-selective calcium channel blockers) | cardiovascular system | fendiline, bepridil |
| 9 | N05AF | antipsychotics, psycholeptics (thioxanthene derivatives) | nervous system | flupentixol, zuclopenthixol, chlorprothixene |
| 10 | S01EA | ophthalmologicals (antiglaucoma preparations and miotics 1, sympathomimetics in glaucoma therapy) | sensory organs | dipivefrine, clonidine |
| 11 | P01BC | antiprotozoals, antimalarials (methanolquinolines) | antiparasitic products | mefloquine |
PhAPs, but not other structural classes of antipsychotics, promote neurite outgrowth in cultured neurons challenged with an inhibitory CSPG substrate
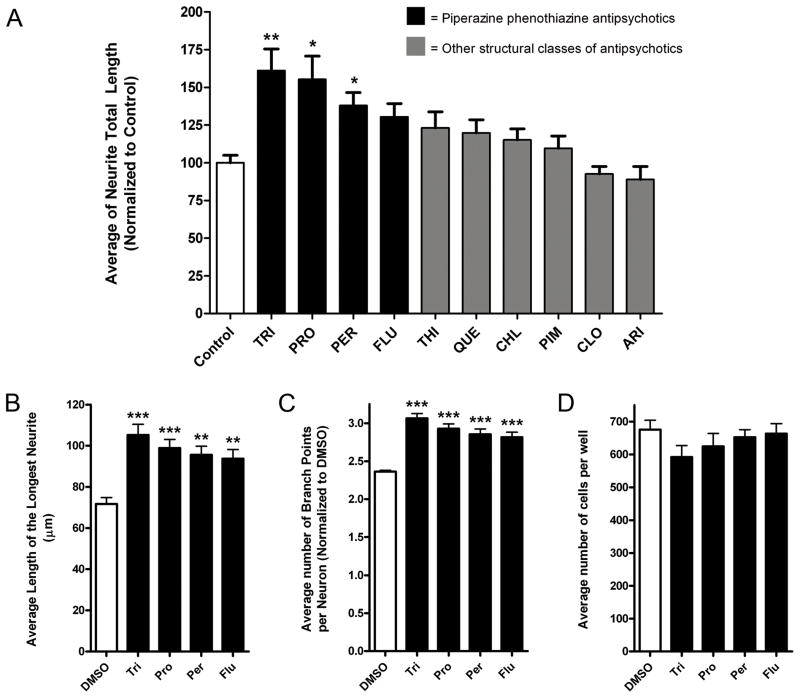
To test the hypothesis that PhAPs promote neurite outgrowth, we screened a number of PhAPs, together with other types of antipsychotics, for their ability to promote neurite growth of neurons challenged with inhibitory CSPGs. We used dissociated hippocampal neurons because their morphological development is well understood (Barnes and Polleux, 2009), and because their neurite growth can be strongly inhibited by a mixture of brain-derived CSPGs (Ernst et al., 1995; Usher et al., 2010). In these experiments, 3 of 4 PhAPs tested, but none of the other antipsychotics, significantly enhanced neurite growth on the CSPG substrate (Figure 1A). In subsequent experiments, we found that all 4 PhAPs tested (trifluoperazine, prochlorperazine, perphenazine, and fluphenazine) were able to increase overall neurite length (Figure 1A and Supplemental Figure 1), axon length (length of the longest process) and neurite branching (Figure 1B, C) in hippocampal neurons on CSPGs. PhAPs did not affect neuronal survival at this concentration (Figure 1D).
Figure 1.
Piperazine phenothiazine antipsychotics (PhAPs) promote neurite outgrowth in hippocampal neurons grown on inhibitory substrates. A) Neurons were treated with various antipsychotics at 1 μM (trifluoperazine, TRI; proclorperazine, PRO; perphenazine, PER; fluphenazine, FLU; thioridazine, THI; quetiapine, QUE; chlorprothixene, CHL; pimozide, PIM; clozapine, CLO; and aripiprazole, ARI) and cultured for 2 days on a mixture of inhibitory CSPGs before assessment of neurite outgrowth (N=3 independent experiments). B–E) PhAPs particularly increase the length of the longest process (B) as well as the total number of branch points of the processes (C) without significantly affecting neuronal cell numbers (E). Asterisks indicate a significant difference from vehicle control (white bars, mean ± SEM, *<0.05, **<0.01, ***<0.001).
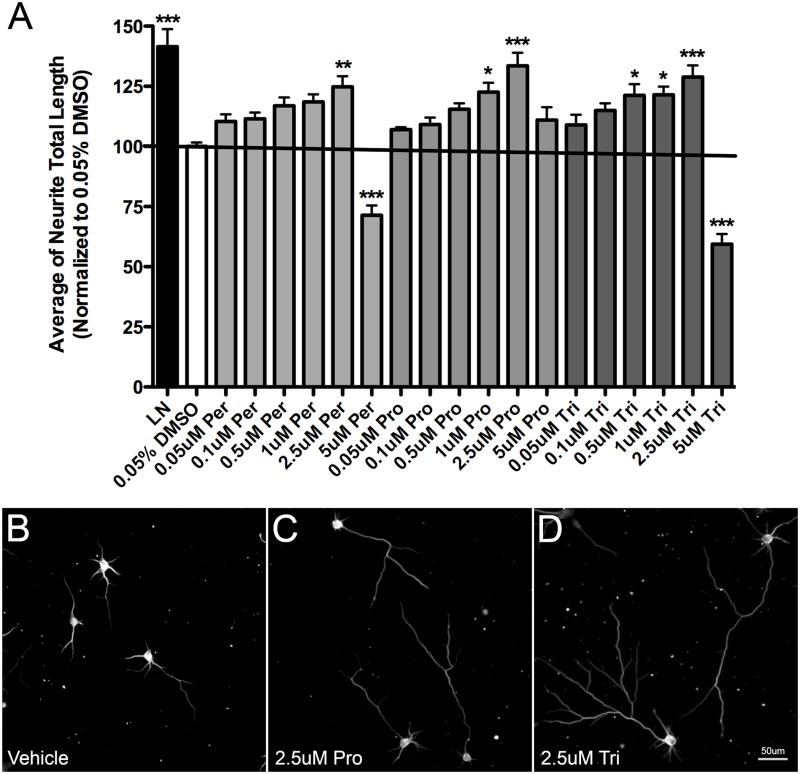
To determine the optimal concentrations of PhAPs for promoting neurite growth, we examined dose-response relationships for the PhAPs as well as several non-piperazine antipsychotics. For the 3 PhAPs tested, we obtained similar dose-response relationships, with maximal neurite promotion at 2.5 μM, and a failure to promote growth (prochlorperazine) or even growth inhibition (perphenazine and trifluoperazine) at 5 μM concentrations (Figure 2A and data not shown). Optimal (2.5 μM) PhAP concentrations robustly promoted neurite extension (Figure 2B–D). Curve fitting for the prochlorperazine data suggested an EC50 around 500 nM, and this range likely also applies to perphenazine and trifluoperazine. The reason for the U-shaped dose-response relationships is unknown, but these observations are consistent with the drugs’ having two mechanisms of action that are mutually antagonistic. In contrast to results with the PhAPs, the non-PhAP antipsychotics we tested, including thioridazine and pimozide, failed to promote growth at any concentration tested. These drugs, however, also inhibited neurite growth at higher concentrations (data not shown).
Figure 2.
Dose-response relationships for growth promotion by PhAPs. A) Hippocampal neurons grown on laminin (LN, black bar) or on CSPGs (white and gray shaded bars) were treated with various doses of three PhAPs (perphenazine, prochlorperazine, trifluoperazine). The optimal dose to promote growth was 2.5 μM, while at 5 μM the drugs became toxic to the cells (cell count data not shown). Asterisks indicate significant differences from CSPG vehicle control (white bar; *<0.05, **<0.01, ***<0.001; N=5). B–D) Representative images of cells on CSPGs treated with either vehicle or PhAPs shows the robust promotion of axonal outgrowth at 2DIV.
PhAPs promote neurite growth on a myelin-derived inhibitor but not on a permissive laminin substrate
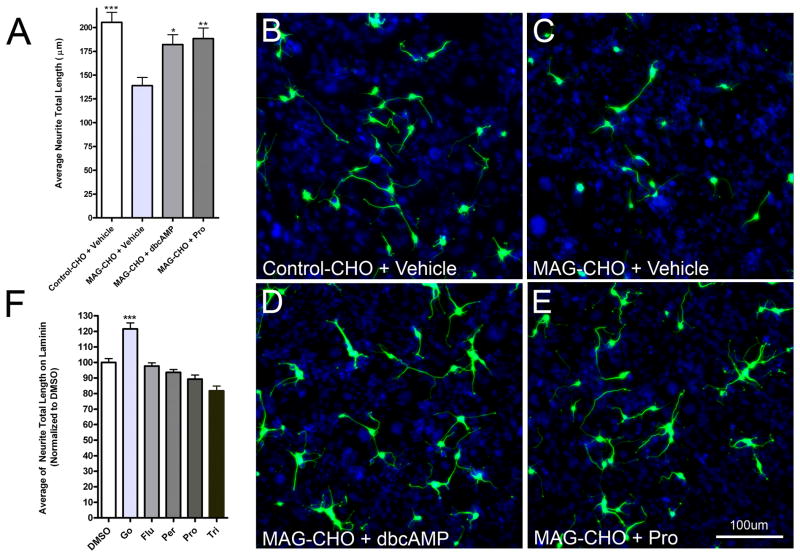
The novel compound F05 promotes neurite growth on a variety of inhibitory substrates, but does not affect growth on permissive substrates, suggesting that it selectively interferes with a general aspect of inhibitory signaling (Usher et al., 2010). To determine whether PhAPs might also promote growth on different inhibitory substrates, we tested prochlorperazine using neurons challenged with a protein derived from CNS myelin (myelin-associated glycoprotein, MAG; Filbin, 2003; McKerracher et al., 1994). Hippocampal neurons were cultured on a monolayer of MAG-CHO cells, which express MAG on their surfaces and cause growth inhibition in CNS neurons (Mukhopadhyay et al., 1994). We found that hippocampal neurite growth was inhibited by cell surface MAG (Figure 3A–C), and that prochlorperazine reversed this inhibition (Figure 3A, B, C, E). This reversal was comparable to that produced by dibutyryl-cyclic AMP (dbcAMP; Figure 3A, D, E), which is known to overcome inhibitory myelin signaling (Cai et al., 1999; Domeniconi and Filbin, 2005). Thus, at least one PhAP can overcome growth inhibition caused by multiple classes of regeneration-inhibitory proteins.
Figure 3.
PhAPs promote neurite growth an inhibitory protein from myelin (MAG), but not on a permissive laminin substrate. A) The average total neurite length per neuron on either control or MAG-transfected CHO cells was assessed at 2DIV after treatment with either vehicle (0.01% DMSO), 100 nM dbcAMP as a positive control, or 1 μM Pro. At least 100 neurons were analyzed per condition. Asterisks indicate significant differences from MAG-CHO vehicle control (*<0.05, **<0.01, ***<0.001). B–E) Representative images show neurons stained for neuronal-specific βIII tubulin (green) growing on the confluent monolayer of CHO cells (Hoechst nuclear stain, blue) under the various conditions. F) PhAPs (1 μM) do not promote neurite outgrowth on a permissive laminin substrate, in contrast to the PKC inhibitor Gő6976 (Go, 100 nM). Asterisks indicate a significant difference from DMSO vehicle control (**<0.01, N=5).
To test whether PhAPs, like F05, can promote neurite growth on inhibitory substrates but not on permissive substrates, we characterized hippocampal neurite growth on a permissive laminin (LN) substrate in the presence and absence of PhAPs. None of the 4 PhAPs tested was able to improve growth on a LN substrate, when used at the same concentrations that were effective on the CSPG and MAG substrates (Figure 3F). Because neurons grow long processes on LN substrates, which might make it difficult to increase growth rates above baseline, we tested whether it was possible to demonstrate growth promotion in this assay using other agents. Indeed, we found that LN-mediated growth could be significantly increased by treatment with the protein kinase C inhibitor Gő6976. Gő6976 has previously been shown to increase growth of cerebellar granule neurons on inhibitory substrates, but not on a permissive poly-D-lysine (PDL) substrate (Sivasankaran et al., 2004). In hippocampal neurons, Gő6976 increased growth both on inhibitory substrates and on permissive substrates such as LN and PDL (Figure 3F and unpublished results). Taken together, our results suggest that PhAPs, like F05, promote growth on inhibitory substrates by selectively reducing inhibitory signals in neurons.
PhAPs improve neurite growth in postnatal retinal ganglion cells on CSPG substrates
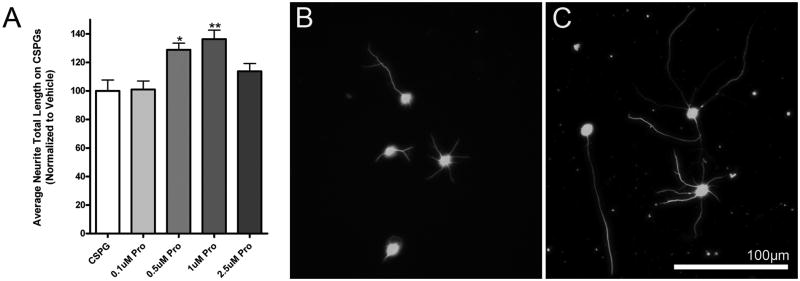
Adult CNS neurons exhibit lower growth potential than their embryonic counterparts (Blackmore et al., 2010; Bregman, 1998). In particular, there is a rapid developmental decline in the ability of rat retinal ganglion cells (RGCs) to grow axons during the first week after birth (Goldberg et al., 2002). To test whether PhAPs, like F05, can promote growth of postnatal RGCs on inhibitory substrates, we sought to determine whether they could increase neurite growth in postnatal day 6 RGCs cultured on CSPGs. For these experiments, we chose prochlorperazine, which was the least toxic in the dose response assay (Figure 2A and data not shown). Prochlorperazine significantly enhanced neurite growth on the CSPG substrate, despite the low intrinsic growth capacity of the RGCs (Figure 4). Thus the growth-promoting effects of PhAPs are not restricted to hippocampal or to embryonic neurons.
Figure 4.
PhAPs also promote growth of mature RGCs on CSPG substrates. A) Postnatal day 6 RGCs were cultured on a mixture of inhibitory CSPGs and treated with various concentrations of Pro. Neurite lengths were assessed at 2DIV. Asterisks indicate significant differences from CSPG vehicle control (white bar, *<0.05, **<0.01, N=4). B–C) Representative images show cells treated with vehicle (B) or 1 μM Pro (C).
Neurite growth promotion by PhAPs is dependent on calmodulin inhibition and not dopamine receptor antagonism
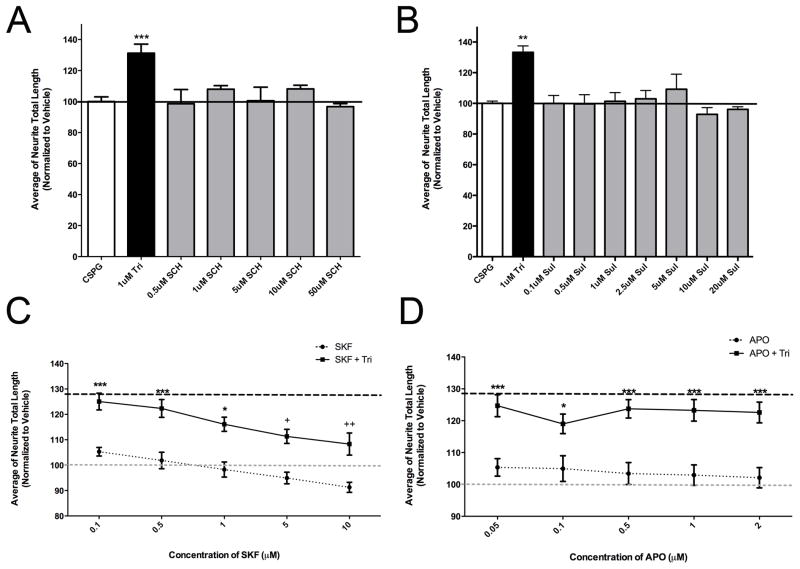
To gain insight into the mechanisms underlying the ability of PhAPs to promote neurite growth, we first tested whether the well-known ability of antipsychotics to antagonize dopamine receptors, particularly D2 receptors (Nord and Farde, 2011), was involved. Cultured hippocampal neurons express both D1 and D2 dopamine receptors (Chen et al., 2008), and we confirmed that they express D2 receptors on their surfaces in our culture conditions (Supplemental Figure 2). If dopamine receptor blockade is involved in growth promotion by PhAPs, we predicted 1) that other dopamine receptor antagonists would stimulate growth, and 2) that dopamine receptor agonists might interfere with the growth-promoting ability of PhAPs. However, we found that neither the D1 antagonist SCH-23390 nor the D2 antagonist sulpiride were able to promote neurite growth of hippocampal neurons on CSPG substrates (Figure 5A, B) despite using concentrations at and well above their known IC50 values (Iorio et al., 1983; Larson et al., 1995). The D1 agonist SKF- 38393 inhibited growth when provided alone (Figure 5C and Supplemental Figure 3), and in parallel, impaired the ability of trifluoperazine to promote growth. Because SKF-38393 inhibited trifluoperazine-induced growth only at concentrations ≥ 5uM (Figure 5C), more than 100X the known EC50 value (Van Vliet et al., 1990), this inhibition may not be due to D1 agonist activity. These results are further complicated by the fact that SKF-38393 elicited growth inhibitory effects when used alone. A D2 agonist, apomorphine, had no effects on neurite growth when used alone, and did not block growth promotion by trifluoperazine (Figure 5D), even at concentrations above its EC50 value (6.8 nM; Gardner et al., 1998). Overall, these results are inconsistent with the hypothesis that dopamine receptor antagonism is responsible for the growth-promoting effects of PhAPs.
Figure 5.
Antagonism of dopamine receptors is not responsible for PhAP-induced growth promotion. A) The D1 receptor antagonist SCH-23390 (SCH) does not mimic the growth-promoting effect of Tri. B) The D2 receptor antagonist sulpiride (Sul) also does not mimic trifluoperazine’s ability to overcome CSPG inhibition. C) The dopamine D1 receptor agonist SKF-38393 (SKF) significantly inhibits PhAP-induced growth promotion (1 μM trifluoperazine). However, SKF by itself elicited parallel inhibitory effects on neurite growth compared to CSPG vehicle control. D) Apomorphine (APO, a D2 receptor agonist) did not block growth promotion by trifluoperazine and did not have effects on growth when used alone. Asterisks indicate a significant difference from CSPG vehicle control (white bars in A and B and gray dashed lines in C and D; *<0.05, **<0.01, ***<0.001) and daggers denote a significant difference from 1 μM trifluoperazine (black bars in A and B and black dashed lines in C and D; †<0.05, ††<0.01).
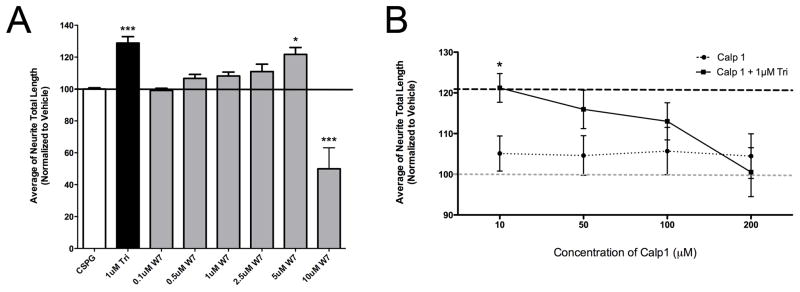
In addition to blockade of various neurotransmitter subtypes (Mosnaim et al., 2006; Tajima et al., 2009), some PhAPs bind to and inhibit signaling through calmodulin, a ubiquitously expressed calcium-binding protein (Weiss et al., 1980). In particular, trifluoperazine is a calmodulin antagonist (Vandonselaar et al., 1994), and is widely used for this purpose (Feldkamp et al., 2010; Tanokura and Yamada, 1986). To investigate whether calmodulin antagonism plays a role in the ability of PhAPs to promote growth on inhibitory substrates, we treated cells with agonists and antagonists of calmodulin, similar to our strategy for investigating the role of dopamine receptors. We found that a calmodulin antagonist, W7, significantly promoted neurite growth at a concentration of 5 μM (Figure 6A). Interestingly, the growth response profile was similar to that observed with increasing doses of PhAPs (compare the sharp drop-off in growth in Figures 2A and 6A). For a calmodulin agonist, we chose the calcium-mimetic peptide Calp1, which binds to and activates calmodulin with an EC50 in the 50 μM range (Manion et al., 2000; Villain et al., 2000). When hippocampal neurons were cultured on a CSPG substrate, 200 μM Calp1 was able to completely abolish trifluoperazine’s growth-promoting effect, but did not alter levels of neurite growth when used alone (Figure 6B). Taken together, these results suggest that the ability of trifluoperazine to promote growth on inhibitory substrates depends at least partly on calmodulin antagonism.
Figure 6.
PhAP induced neurite growth promotion involves calmodulin antagonism. A) The calmodulin antagonist W7 significantly enhances growth on CSPGs (N=3–6) in a dose-dependent manner, similar to PhAP effects. B) The calcium-like peptide Calp1 alone does not affect growth by itself but is able to completely negate trifluoperazine mediated growth promotion (N=4). Asterisks indicate significant differences from CSPG vehicle control. The black dashed line indicates treatment with 1 μM trifluoperazine alone, while the gray dashed line is the CSPG vehicle control. (*<0.05, ***<0.001).
Discussion
Using a comparative microarray analysis of compound-induced changes in gene expression, we have demonstrated an unexpected similarity between piperazine phenothiazine antipsychotics and F05, a novel regeneration-promoting compound. PhAPs, but not antipsychotics of other structural classes, shared F05’s ability to enhance CNS neuronal outgrowth in an assay in which growth was restricted by CSPGs. PhAPs were also able to overcome growth inhibition in response to the myelin-derived inhibitor MAG, but had no effect on neurite growth in the context of a permissive laminin substrate. The ability of PhAPs to promote growth over glial-inhibitory molecules was dependent on antagonism of calmodulin, and at least one other calmodulin antagonist, W7, is able to mimic the growth-promoting abilities of PhAPs.
These results highlight the dual utility of the comparative microarray approach. The Connectivity Map is a hypothesis-generating tool that can be used to identify signaling pathways affected by a compound of interest as well as to discover new properties of clinically prescribed drugs. The comparative microarray findings presented here led to the hypothesis that inhibition of calmodulin signaling might allow neurons to alleviate substrate-derived neurite growth restriction. These findings suggest that calmodulin could be exploited as a novel target for promoting CNS regeneration in the face of environmental barriers. In addition, our results suggest a previously unrecognized potential for piperazine phenothiazine antipsychotics to induce axonal regeneration when neurons are challenged with glial-derived inhibitory molecules. Thus, although antipsychotics are primarily prescribed to alleviate psychosis, our work suggests that they may be repurposed to improve regrowth after CNS injury.
The possibility of repurposing PhAPs for treatment of CNS injury raises questions concerning dosage and therapeutic index. In our in vitro assays, there was a narrow concentration window between efficacy (growth promotion) and toxicity (cell death), for the PhAPs tested. Presumably this reflects the existence of multiple targets for these drugs; future efforts could include structure-activity relationship studies to identify compounds that promote growth without killing cells. Alternatively, dose response studies in vivo could determine an optimal window for promotion of regeneration by existing PhAPs. Prochlorperazine is typically prescribed at doses between 20 and 150 mg per day (~0.3–2.1 mg/kg for a 70 kg adult), depending on the indication and symptom severity. In one study, patients chronically treated with 120 mg trifluoperazine/day showed brain levels of 1.0 μg/mL (1.65 μM; Karson et al., 1992). Thus, PhAP concentrations that we find to be effective for growth promotion in vitro can be achieved with dosing schemes currently used in therapy. While PhAPs produce serious side effects, including extrapyramidal disorder, these would need to be balanced against the possibility of functional recovery from CNS trauma.
Other notable hits from the Connectivity Map include several agents that act on aspects of calcium signaling, including tetrandrine and fendiline. This reinforces our findings that targeting downstream calcium signaling pathways allows cells to overcome growth-inhibitory signals. In addition to PhAPs, there were several other clinically prescribed classes of drugs that emerged from the comparative microarray analysis. Antihistamines, vasodilators, antidepressants, glucocorticoid agonists, and antibiotics could all be studied further in assays of neuronal growth and regeneration. Future studies using any of these Connectivity Map hits could elucidate novel mechanisms of regenerative failure in the CNS and also point towards potential therapies for CNS injury.
Our studies show that PhAPs promote neurite growth from cells growing on a mixture of inhibitory CSPGs. We chose this mixture, which likely consists mainly of neurocan, phosphacan, versican, and aggrecan (Ernst et al., 1995; Monnier et al., 2003), in order to mimic the CSPGs that accumulate at lesion sites after CNS injury (Asher et al., 2000; Levine 1994; McKeon et al., 1999; Monnier et al., 2003). Future studies could assess whether PhAPs are more active against some individual CSPGs as opposed to others, which might provide additional insight into the mechanism of growth promotion and the effects of shared domains among different CSPGs (e.g., immunoglobulin domains, hyaluronic acid-binding domains, chondroitin sulfate side chains).
Previous studies have suggested that antipsychotics could modulate neurite outgrowth, but none have implicated piperazine phenothiazines as a class. Antipsychotics such as olanzapine, quetiapine, and clozapine have been shown to enhance growth factor induced neurite outgrowth in PC12 cells, at concentrations of 10–40 μM (Lu and Dwyer, 2005). In neuroblastoma cells, high (100 μM) concentrations of haloperidol had the opposite effect, disrupting neuritic cytoskeletal organization (Benitez-King et al., 2010). Interestingly, clozapine, and to some extent fluphenazine, were able to increase axon lengths from mechanosensory neurons in C. elegans, when made available to larvae at high (160 μM) concentrations (Donohoe et al., 2008). These drugs also inhibited neuronal migration, and it is unclear to what extent the effects on axons were direct or reflected abnormal positioning. In rats, haloperidol reduced the density of dopaminergic axon terminals when administered immediately after a lesion to the substantia nigra, but improved dopamine terminal sprouting when administered after a delay (Tripanichkul et al., 2003), suggesting that the growth response of neurons to haloperidol can vary dramatically under slightly different circumstances. This parallels our observations that PhAPs can have either no effect or a robust growth-promoting effect when added to cells that are growing under permissive versus inhibitory conditions, respectively. The findings presented here are the first to show that a specific class of antipsychotics can improve growth of primary neurons in the face of inhibitory molecules that prevent CNS regeneration after injury, and do so at low micromolar concentrations.
All clinically effective antipsychotics inhibit dopamine receptors, primarily D2 type receptors (Nord and Farde, 2011). Dopamine receptor signaling has been linked to transcriptional regulation of axon guidance molecules (Jassen et al., 2006), suggesting that modulation of dopamine pathways could affect neurite growth. Indeed, treatment with the D1 agonist SKF-38393 increased neurite length and arborization of dissociated rat striatal cells cultured on polyornithine (Schmidt et al., 1996). The D2 agonist quinpirole can potentiate cortical neurite length and branching on a polylysine substrate (Todd, 1992), and dopamine itself has been shown to increase neurite growth in striatal neurons (Schmidt et al., 1998). Thus dopamine receptor agonism is generally associated with increases in neurite growth. Since antipsychotics act as dopamine antagonists, we hypothesized that their growth-promoting effects were independent of their effects on dopamine signaling. Accordingly, our results suggest that the growth-promoting effect of PhAPs did not depend on antagonism of dopamine receptors. Similarly, the ability of antipsychotics to cause excessive axon growth of mechanosensory axons in C. elegans appears to be independent of dopamine receptor antagonism (Donohoe et al., 2008). Overall, the results indicate that the ability of PhAPs to increase neurite growth is independent of their ability to antagonize dopamine receptors.
In addition to their effect on dopamine receptors, antipsychotics are known to target a variety of cell surface receptors and intracellular signaling cascades (Miyamoto et al., 2005). Some of these targets, including serotonin receptors (Dudok et al., 2009; Homma et al., 2006), histamine receptors (Munis et al., 1998), NMDA receptors (George et al., 2009; Kuo et al., 2010), adrenergic receptors (Kwon et al., 1996), and muscarinic receptors (VanDeMark et al., 2009), have been implicated in the modulation of neuronal growth and differentiation. We chose to investigate the calcium binding protein calmodulin, since phenothiazine-based compounds, and in particular the PhAP trifluoperazine, are known to bind to and inhibit calmodulin (Tanokura and Yamada, 1986; Vandonselaar et al., 1994).
Our results implicate calcium/calmodulin signaling in growth-inhibitory responses to extracellular cues after CNS injury. This is consistent with findings that both CSPGs and myelin proteins induce a local influx of calcium in growth cones that can affect turning behavior (Hasegawa et al., 2004; Henley et al., 2004; Snow et al., 1994), and with studies in invertebrates linking calmodulin to growth inhibition (Polak et al., 1991). However, since calcium influx can also potentiate neurite outgrowth (Homma et al., 2006; Kater and Mills, 1991), the relationship between calcium/calmodulin signaling and neurite growth is highly dynamic, and depends on the growth state of individual neurons. It is possible that the drop-off in neurite growth seen with high concentrations of both PhAPs and the calmodulin antagonist W7 reflects this dynamic relationship.
Since calmodulin appears to be a relevant target for the effects we observe, it is curious that pimozide, an antipsychotic known to inhibit calmodulin (Levin and Weiss, 1976), did not promote neurite outgrowth on CSPGs. It may be that pimozide affects additional signaling pathways that negate potential growth promotion resulting from calmodulin inhibition. Alternatively, pimozide and PhAPs could have differing effects on calmodulin’s interaction with downstream effector proteins. These distinct possibilities should be addressed in future mechanistic studies.
In addition to the relevance of these findings to CNS regeneration, our results also have implications for the mechanisms through which antipsychotics alleviate symptoms of psychosis. Schizophrenia is characterized by deficits in neuronal growth and connectivity (Lynall et al., 2010; Skudlarski et al., 2010; Zalesky et al., 2011), and genes associated with schizophrenia, such as Disrupted in Schizophrenia 1 (DISC1), are known to play significant roles in neurite growth (Hattori et al., 2010; Miyoshi et al., 2003; Ozeki et al., 2003). In particular, there is evidence that glial-derived growth inhibitors are associated with schizophrenia. For example, levels of both CSPGs and the myelin-derived inhibitor Nogo are increased in postmortem schizophrenic brains (Novak et al., 2002; Novak and Tallerico, 2006; Pantazopoulos et al., 2010). Therefore, the ability of antipsychotics to promote growth/sprouting in the presence of glial-derived inhibitory molecules may represent a mechanism for improving neuronal connectivity in schizophrenic patients. Similar considerations apply to other neurodevelopmental disorders for which antipsychotics are used, including autism and depression (McPheeters et al., 2011; Pae et al., 2011).
A major goal in both neuropsychiatric research and neuroregeneration studies is to understand and overcome the mechanisms that contribute to maladaptive pathology. Using a unique tool to compare gene expression profiles, we have uncovered a novel ability of PhAPs to improve neurite outgrowth from CNS neurons grown on glial-derived inhibitory molecules, and have identified calmodulin as a novel therapeutic target that could be manipulated to improve axonal regrowth. In addition, our results highlight an underappreciated link between CSPG and myelin inhibitory signaling and the developmental deficits associated with schizophrenia. Importantly, since piperazine antipsychotics are already clinically prescribed to alleviate psychosis, our work suggests that they could be repurposed to induce neuronal growth and restore connectivity after CNS injury.
Supplementary Material
All four piperazine phenothiazines significantly improve neurite growth on CSPGs. Neurons were treated with the antipsychotics (1 μM; trifluoperazine, Tri; proclorperazine, Pro; perphenazine, Per; fluphenazine, Flu; thioridazine) for 2 days before quantification of neurite outgrowth (N=4 independent experiments). Asterisks indicate a significant difference from vehicle control (DMSO, white bar, ***<0.001).
Cultured hippocampal neurons express the dopamine D2 receptor on their cell surfaces. Hippocampal neurons grown for 2DIV on CSPGs were fixed and stained under non-permeabilizing conditions using a primary antibody against the extracellular domain of the D2 receptor (left column). The cultures were then permeabilized and stained for tubulin (right column). There is no staining in the absence of primary antibody (C).
The D1 receptor agonist SKF-38393 (SKF) inhibits neurite growth at higher concentrations (A, >10μM), even before it induces toxicity at 50 μM (B). Data shown are from 3–5 experiments (mean ± SEM) or are values from one experiment (no error bars shown); both done in duplicate.
A complete list of the compounds used for the described studies, and the concentrations at which they were used.
Genes that were significantly increased or decreased in response to F05 treatment of MCF7 cells, as identified by microarray analysis (N=3). This gene list was used as a probe to query the Connectivity Map (“--” indicates a probe corresponding to an unannotated transcript).
Acknowledgments
We thank Dr. Stan Hoffman for generously donating CSPGs, and Dr. Roman Giger for the gift of MAG-expressing CHO cells. We also thank Dr. Justin Lamb (Broad Institute) for helping to determine the experimental conditions for optimizing the microarray experiments. We acknowledge the support of the NINDS/NIMH Microarray Consortium in performing the microarray experiments. We thank Tania Slepak, Dr. Anthony Oliva, Guerline Lambert, Dr. Hassan Al-Ali, Dr. Michael Steketee, and Ephraim Trakhtenberg for help with neuronal cultures. This work was supported by grants from the National Institutes of Health (R01NS059866, F31NS063593, R01HD057632, P30EY014801 and U01NS074490), from the Buoniconti Foundation, and from an unrestricted grant from Research to Prevent Blindness. VPL and JLG are supported by the Walter G. Ross Foundation.
Abbreviations
- CNS
Central Nervous System
- SCI
Spinal Cord Injury
- CSPGs
Chondroitin Sulfate Proteoglycans
- MAG
Myelin Associated Glycoprotein
- PhAPs
Phenothiazine Antipsychotics
- Tri
Trifluoperazine
- Pro
Prochlorperazine
- LN
Laminin
- PDL
Poly-D-Lysine
- RGC
Retinal Ganglion Cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Benitez-King G, Ortiz-Lopez L, Jimenez-Rubio G, Ramirez-Rodriguez G. Haloperidol causes cytoskeletal collapse in N1E-115 cells through tau hyperphosphorylation induced by oxidative stress: Implications for neurodevelopment. Eur J Pharmacol. 2010;644:24–31. doi: 10.1016/j.ejphar.2010.06.057. [DOI] [PubMed] [Google Scholar]
- Blackmore MG, Moore DL, Smith RP, Goldberg JL, Bixby JL, Lemmon VP. High content screening of cortical neurons identifies novel regulators of axon growth. Mol Cell Neurosci. 2010;44:43–54. doi: 10.1016/j.mcn.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braconi C, Meng F, Swenson E, Khrapenko L, Huang N, Patel T. Candidate therapeutic agents for hepatocellular cancer can be identified from phenotype-associated gene expression signatures. Cancer. 2009;115:3738–3748. doi: 10.1002/cncr.24417. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron. 1997;19:1175–1186. doi: 10.1016/s0896-6273(00)80410-9. [DOI] [PubMed] [Google Scholar]
- Bregman BS. Regeneration in the spinal cord. Curr Opin Neurobiol. 1998;8:800–807. doi: 10.1016/s0959-4388(98)80124-4. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Chen MH, Yang WL, Lin KT, Liu CH, Liu YW, Huang KW, Chang PM, Lai JM, Hsu CN, Chao KM, Kao CY, Huang CY. Gene expression-based chemical genomics identifies potential therapeutic drugs in hepatocellular carcinoma. PLoS One. 2011;6:e27186. doi: 10.1371/journal.pone.0027186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Owens GC, Edelman DB. Dopamine inhibits mitochondrial motility in hippocampal neurons. PLoS One. 2008;3:e2804. doi: 10.1371/journal.pone.0002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claerhout S, Lim JY, Choi W, Park YY, Kim K, Kim SB, Lee JS, Mills GB, Cho JY. Gene expression signature analysis identifies vorinostat as a candidate therapy for gastric cancer. PLoS One. 2011;6:e24662. doi: 10.1371/journal.pone.0024662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS. Mining for therapeutic gold Nat. Rev Drug Discov. 2011;10:397. doi: 10.1038/nrd3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Preter K, De Brouwer S, Van Maerken T, Pattyn F, Schramm A, Eggert A, Vandesompele J, Speleman F. Meta-mining of neuroblastoma and neuroblast gene expression profiles reveals candidate therapeutic compounds. Clin Cancer Res. 2009;15:3690–3696. doi: 10.1158/1078-0432.CCR-08-2699. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J Neurol Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Donohoe DR, Weeks K, Aamodt EJ, Dwyer DS. Antipsychotic drugs alter neuronal development including ALM neuroblast migration and PLM axonal outgrowth in Caenorhabditis elegans. Int J Dev Neurosci. 2008;26:371–380. doi: 10.1016/j.ijdevneu.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudok JJ, Groffen AJ, Witter MP, Voorn P, Verhage M. Chronic activation of the 5-HT(2) receptor reduces 5-HT neurite density as studied in organotypic slice cultures. Brain Res. 2009;1302:1–9. doi: 10.1016/j.brainres.2009.08.071. [DOI] [PubMed] [Google Scholar]
- Ernst H, Zanin MK, Everman D, Hoffman S. Receptor-mediated adhesive and anti-adhesive functions of chondroitin sulfate proteoglycan preparations from embryonic chicken brain. J Cell Sci. 1995;108 ( Pt 12):3807–3816. doi: 10.1242/jcs.108.12.3807. [DOI] [PubMed] [Google Scholar]
- Feldkamp MD, O’Donnell SE, Yu L, Shea MA. Allosteric effects of the antipsychotic drug trifluoperazine on the energetics of calcium binding by calmodulin. Proteins. 2010;78:2265–2282. doi: 10.1002/prot.22739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Gardner B, Strange PG. Agonist action at D2(long) dopamine receptors: ligand binding and functional assays. Br J Pharmacol. 1998;124:978–984. doi: 10.1038/sj.bjp.0701926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Dravid SM, Prakash A, Xie J, Peterson J, Jabba SV, Baden DG, Murray TF. Sodium channel activation augments NMDA receptor function and promotes neurite outgrowth in immature cerebrocortical neurons. J Neurosci. 2009;29:3288–3301. doi: 10.1523/JNEUROSCI.6104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- Gullbo J, Fryknas M, Rickardson L, Darcy P, Hagg M, Wickstrom M, Hassan S, Westman G, Brnjic S, Nygren P, Linder S, Larsson R. Phenotype-based drug screening in primary ovarian carcinoma cultures identifies intracellular iron depletion as a promising strategy for cancer treatment. Biochem Pharmacol. 2011;82:139–147. doi: 10.1016/j.bcp.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Fujitani M, Hata K, Tohyama M, Yamagishi S, Yamashita T. Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J Neurosci. 2004;24:6826–6832. doi: 10.1523/JNEUROSCI.1856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Shimizu S, Koyama Y, Yamada K, Kuwahara R, Kumamoto N, Matsuzaki S, Ito A, Katayama T, Tohyama M. DISC1 regulates cell-cell adhesion, cell-matrix adhesion and neurite outgrowth. Mol Psychiatry. 2010;15:778–798. 809. doi: 10.1038/mp.2010.60. [DOI] [PubMed] [Google Scholar]
- Hausott B, Rietzler A, Vallant N, Auer M, Haller I, Perkhofer S, Klimaschewski L. Inhibition of fibroblast growth factor receptor 1 endocytosis promotes axonal branching of adult sensory neurons. Neuroscience. 2011;188:13–22. doi: 10.1016/j.neuroscience.2011.04.064. [DOI] [PubMed] [Google Scholar]
- Henley JR, Huang KH, Wang D, Poo MM. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron. 2004;44:909–916. doi: 10.1016/j.neuron.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K, Kitamura Y, Ogawa H, Oka K. Serotonin induces the increase in intracellular Ca2+ that enhances neurite outgrowth in PC12 cells via activation of 5-HT3 receptors and voltage-gated calcium channels. J Neurosci Res. 2006;84:316–325. doi: 10.1002/jnr.20894. [DOI] [PubMed] [Google Scholar]
- Huber AB, Schwab ME. Nogo-A, a potent inhibitor of neurite outgrowth and regeneration. Biol Chem. 2000;381:407–419. doi: 10.1515/BC.2000.053. [DOI] [PubMed] [Google Scholar]
- Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983;226:462–468. [PubMed] [Google Scholar]
- Jassen AK, Yang H, Miller GM, Calder E, Madras BK. Receptor regulation of gene expression of axon guidance molecules: implications for adaptation. Mol Pharmacol. 2006;70:71–77. doi: 10.1124/mol.105.021998. [DOI] [PubMed] [Google Scholar]
- Josset L, Textoris J, Loriod B, Ferraris O, Moules V, Lina B, N’guyen C, Diaz JJ, Rosa-Calatrava M. Gene expression signature-based screening identifies new broadly effective influenza a antivirals. PLoS One. 2010;5:e13169. doi: 10.1371/journal.pone.0013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson CN, Newton JEO, Mohanakrishnan P, Sprigg J, Komoroski RA. Fluoxetine and trifluoperazine in human brain: A 19F-nuclear magnetic resonance spectroscopy study. Psychiatry Research: Neuroimaging. 1992;45:95–104. doi: 10.1016/0925-4927(92)90003-m. [DOI] [PubMed] [Google Scholar]
- Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- Kubo T, Yamaguchi A, Iwata N, Yamashita T. The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Ther Clin Risk Manag. 2008;4:605–615. doi: 10.2147/tcrm.s2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TY, Chen CY, Hsueh YP. Bcl11A/CTIP1 mediates the effect of the glutamate receptor on axon branching and dendrite outgrowth. J Neurochem. 2010;114:1381–1392. doi: 10.1111/j.1471-4159.2010.06852.x. [DOI] [PubMed] [Google Scholar]
- Kwon JH, Eves EM, Farrell S, Segovia J, Tobin AJ, Wainer BH, Downen M. Beta-adrenergic receptor activation promotes process outgrowth in an embryonic rat basal forebrain cell line and in primary neurons. Eur J Neurosci. 1996;8:2042–2055. doi: 10.1111/j.1460-9568.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, Wei G, Armstrong SA, Haggarty SJ, Clemons PA, Wei R, Carr SA, Lander ES, Golub TR. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- Larson BT, Samford MD, Camden JM, Piper EL, Kerley MS, Paterson JA, Turner JT. Ergovaline binding and activation of D2 dopamine receptors in GH4ZR7 cells. J Anim Sci. 1995;73:1396–1400. doi: 10.2527/1995.7351396x. [DOI] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RM, Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3′,5′-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976;12:581–589. [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XH, Dwyer DS. Second-generation antipsychotic drugs, olanzapine, quetiapine, and clozapine enhance neurite outgrowth in PC12 cells via PI3K/AKT, ERK, and pertussis toxin-sensitive pathways. J Mol Neurosci. 2005;27:43–64. doi: 10.1385/jmn:27:1:043. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manion MK, Villain M, Pan ZG, McDonald JM, Blalock JE. Cellular Uptake and in Situ Binding of a Peptide Agonist for Calmodulin. Biochem Biophys Res Commun. 2000;277:462–469. doi: 10.1006/bbrc.2000.3691. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127:e1312–21. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, Kuroda S, Katayama T, Tohyama M. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnaim AD, Ranade VV, Wolf ME, Puente J, Antonieta Valenzuela M. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. Am J Ther. 2006;13:261–273. doi: 10.1097/01.mjt.0000212897.20458.63. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Munis JR, Steiner JT, Ruat M, Snyder SH. Diamine oxidase induces neurite outgrowth in chick dorsal root ganglia by a nonenzymatic mechanism. J Neurochem. 1998;70:1323–1326. doi: 10.1046/j.1471-4159.1998.70031323.x. [DOI] [PubMed] [Google Scholar]
- Nord M, Farde L. Antipsychotic occupancy of dopamine receptors in schizophrenia. CNS Neurosci Ther. 2011;17:97–103. doi: 10.1111/j.1755-5949.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak G, Kim D, Seeman P, Tallerico T. Schizophrenia and Nogo: elevated mRNA in cortex, and high prevalence of a homozygous CAA insert. Brain Res Mol Brain Res. 2002;107:183–189. doi: 10.1016/s0169-328x(02)00492-8. [DOI] [PubMed] [Google Scholar]
- Novak G, Tallerico T. Nogo A, B and C expression in schizophrenia, depression and bipolar frontal cortex, and correlation of Nogo expression with CAA/TATC polymorphism in 3′-UTR. Brain Res. 2006;1120:161–171. doi: 10.1016/j.brainres.2006.08.071. [DOI] [PubMed] [Google Scholar]
- Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, Okawa M, Yamada N, Hatten ME, Snyder SH, Ross CA, Sawa A. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci U S A. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae CU, Forbes A, Patkar A. Aripiprazole as adjunctive therapy for patients with major depressive disorder: overview and implications of clinical trial data. CNS drugs. 2011;25:109–27. doi: 10.2165/11538980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry. 2010;67:155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak KA, Edelman AM, Wasley JW, Cohan CS. A novel calmodulin antagonist, CGS 9343B, modulates calcium-dependent changes in neurite outgrowth and growth cone movements. J Neurosci. 1991;11:534–542. doi: 10.1523/JNEUROSCI.11-02-00534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U, Beyer C, Oestreicher AB, Reisert I, Schilling K, Pilgrim C. Activation of dopaminergic D1 receptors promotes morphogenesis of developing striatal neurons. Neuroscience. 1996;74:453–460. doi: 10.1016/0306-4522(96)00201-1. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Pilgrim C, Beyer C. Differentiative effects of dopamine on striatal neurons involve stimulation of the cAMP/PKA pathway Mol. Cell Neurosci. 1998;11:9–18. doi: 10.1006/mcne.1998.0668. [DOI] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Atkinson PB, Hassinger TD, Letourneau PC, Kater SB. Chondroitin sulfate proteoglycan elevates cytoplasmic calcium in DRG neurons. Dev Biol. 1994;166:87–100. doi: 10.1006/dbio.1994.1298. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima K, Fernandez H, Lopez-Ibor JL, Carrasco JL, Diaz-Marsa M. Schizophrenia treatment. Critical review on the drugs and mechanisms of action of antipsychotics. Actas Esp Psiquiatr. 2009;37:330–342. [PubMed] [Google Scholar]
- Tanokura M, Yamada K. Effects of trifluoperazine on calcium binding by calmodulin. Heat capacity and entropy changes. J Biol Chem. 1986;261:10749–10752. [PubMed] [Google Scholar]
- Todd RD. Neural development is regulated by classical neurotransmitters: dopamine D2 receptor stimulation enhances neurite outgrowth. Biol Psychiatry. 1992;31:794–807. doi: 10.1016/0006-3223(92)90311-m. [DOI] [PubMed] [Google Scholar]
- Tripanichkul W, Stanic D, Drago J, Finkelstein DI, Horne MK. D2 Dopamine receptor blockade results in sprouting of DA axons in the intact animal but prevents sprouting following nigral lesions. Eur J Neurosci. 2003;17:1033–1045. doi: 10.1046/j.1460-9568.2003.02547.x. [DOI] [PubMed] [Google Scholar]
- Ughrin YM, Chen ZJ, Levine JM. Multiple regions of the NG2 proteoglycan inhibit neurite growth and induce growth cone collapse. J Neurosci. 2003;23:175–186. doi: 10.1523/JNEUROSCI.23-01-00175.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher LC, Johnstone A, Erturk A, Hu Y, Strikis D, Wanner IB, Moorman S, Lee JW, Min J, Ha HH, Duan Y, Hoffman S, Goldberg JL, Bradke F, Chang YT, Lemmon VP, Bixby JL. A chemical screen identifies novel compounds that overcome glial-mediated inhibition of neuronal regeneration. J Neurosci. 2010;30:4693–4706. doi: 10.1523/JNEUROSCI.0302-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDeMark KL, Guizzetti M, Giordano G, Costa LG. The activation of M1 muscarinic receptor signaling induces neuronal differentiation in pyramidal hippocampal neurons. J Pharmacol Exp Ther. 2009;329:532–542. doi: 10.1124/jpet.108.150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandonselaar M, Hickie RA, Quail JW, Delbaere LT. Trifluoperazine-induced conformational change in Ca(2+)-calmodulin. Nat Struct Biol. 1994;1:795–801. doi: 10.1038/nsb1194-795. [DOI] [PubMed] [Google Scholar]
- Vilar E, Mukherjee B, Kuick R, Raskin L, Misek DE, Taylor JM, Giordano TJ, Hanash SM, Fearon ER, Rennert G, Gruber SB. Gene expression patterns in mismatch repair-deficient colorectal cancers highlight the potential therapeutic role of inhibitors of the phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway. Clin Cancer Res. 2009;15:2829–2839. doi: 10.1158/1078-0432.CCR-08-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain M, Jackson PL, Manion MK, Dong WJ, Su Z, Fassina G, Johnson TM, Sakai TT, Krishna NR, Blalock JE. De novo design of peptides targeted to the EF hands of calmodulin. J Biol Chem. 2000;275:2676–2685. doi: 10.1074/jbc.275.4.2676. [DOI] [PubMed] [Google Scholar]
- Van Vliet BJ, Mulder AH, Schoffelmeer AN. Mu-opioid receptors mediate the inhibitory effect of opioids on dopamine-sensitive adenylate cyclase in primary cultures of rat neostriatal neurons. J Neurochem. 1990;55:1274–1280. doi: 10.1111/j.1471-4159.1990.tb03135.x. [DOI] [PubMed] [Google Scholar]
- Walmsley AR, Mir AK. Targeting the Nogo-A signalling pathway to promote recovery following acute CNS injury. Curr Pharm Des. 2007;13:2470–2484. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- Wang G, Ye Y, Yang X, Liao H, Zhao C, Liang S. Expression-based in silico screening of candidate therapeutic compounds for lung adenocarcinoma. PLoS One. 2011;6:e14573. doi: 10.1371/journal.pone.0014573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Kunzevitzky NJ, Dugas JC, Cameron M, Barres BA, Goldberg JL. Disease gene candidates revealed by expression profiling of retinal ganglion cell development. J Neurosci. 2007;27:8593–8603. doi: 10.1523/JNEUROSCI.4488-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Weiss B, Prozialeck W, Cimino M, Barnette MS, Wallace TL. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Signaling mechanisms of the myelin inhibitors of axon regeneration. Curr Opin Neurobiol. 2003;13:545–551. doi: 10.1016/j.conb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, Egan GF, Pantelis C. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All four piperazine phenothiazines significantly improve neurite growth on CSPGs. Neurons were treated with the antipsychotics (1 μM; trifluoperazine, Tri; proclorperazine, Pro; perphenazine, Per; fluphenazine, Flu; thioridazine) for 2 days before quantification of neurite outgrowth (N=4 independent experiments). Asterisks indicate a significant difference from vehicle control (DMSO, white bar, ***<0.001).
Cultured hippocampal neurons express the dopamine D2 receptor on their cell surfaces. Hippocampal neurons grown for 2DIV on CSPGs were fixed and stained under non-permeabilizing conditions using a primary antibody against the extracellular domain of the D2 receptor (left column). The cultures were then permeabilized and stained for tubulin (right column). There is no staining in the absence of primary antibody (C).
The D1 receptor agonist SKF-38393 (SKF) inhibits neurite growth at higher concentrations (A, >10μM), even before it induces toxicity at 50 μM (B). Data shown are from 3–5 experiments (mean ± SEM) or are values from one experiment (no error bars shown); both done in duplicate.
A complete list of the compounds used for the described studies, and the concentrations at which they were used.
Genes that were significantly increased or decreased in response to F05 treatment of MCF7 cells, as identified by microarray analysis (N=3). This gene list was used as a probe to query the Connectivity Map (“--” indicates a probe corresponding to an unannotated transcript).