Abstract
The bacterial cholesterol dependent cytolysins (CDCs) and membrane attack complex/perforin-like proteins (MACPF) represent two major branches of a large, exceptionally diverged superfamily. Most characterized CDC/MACPF proteins form large pores that function in immunity, venoms, and pathogenesis. Extensive structural, biochemical and biophysical studies have started to address some of the questions surrounding how the soluble, monomeric form of these remarkable molecules recognize diverse targets and assemble into oligomeric membrane embedded pores. This review explores mechanistic similarities and differences in how CDCs and MACPF proteins form pores.
Introduction and overview of mechanism
The membrane attack complex/perforin (MACPF) family of pore forming proteins was originally named because of a domain common to the complement membrane attack complex (MAC) proteins and perforin. Better characterized MACPF proteins include important mediators of immune defense [1], venoms [2], eukaryotic pathogenesis factors [3] and proteins that function in developmental and neurobiology (e.g. Tsl from Drosophila spp [4] and astrotactin [5] (reviewed in [6,7]). The ability of certain MACPF proteins (for example Complement C9 and perforin) to form supramolecular oligomeric pore complexes [8–10] has been known for many years. However, the detailed molecular features of the MACPF membrane pore have, until recently, remained almost completely obscure.
Several recent studies have presented the X-ray crystal structure of MACPF-domain containing proteins from both eukaryotic and prokaryotic sources [7,11–14]. These structures together present a significant advance in our understanding of MACPF pore structure and assembly. Most notably, these structural data revealed that the MACPF fold resembled the topology of the noncontiguous domains 1 and 3 of the cholesterol-dependent cytolysins (CDCs) [15–18] (Fig. 1A). This region is thus better defined as the CDC/MACPF domain.
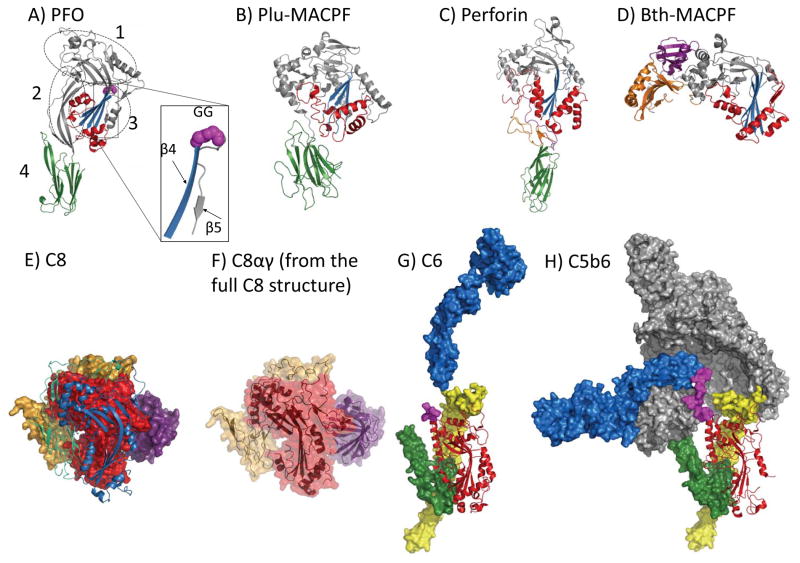
Figure 1. Key structures of CDC and MACPF proteins.
(A) Perfringolysin O (PFO) (PDB ID: 1PFO): the non-contiguous domains are circled and labeled. The CDC/MACPF fold is colored grey with the central β-sheet colored blue and both TMH regions colored red. Domain 4 is a C-terminal immunoglobulin-like fold (green). An inset box shows the β-strands, β4 (blue) and β5 (grey). Magenta spheres show the double glycine motif at the turn of the β-strands. (B) Plu-MACPF (PDB ID: 2QP2): the C-terminal β-prism domain is colored green. CDC/MACPF fold coloured as for A (PFO). (C) Perforin (PDB ID: 3NSJ): the C-terminal EGF-like domain (orange) and C2 domain (green) are highlighted as well as the C-terminal tail (purple) that lies across the surface of the MACPF and EGF-like domains. CDC/MACPF fold coloured as for A (PFO). (D) Bth-MACPF (PDB ID: 3KK7): C-terminal domains include a YegP-like fold (purple) and a second, uncharacterised fold (orange). CDC/MACPF fold coloured as for A (PFO). (E) C8 (PDB ID: 3OJY): The C8α and C8γ components of the C8 structure are shown in surface representation with the MACPF coloured red and the N and C-terminal auxiliary domains coloured yellow. C8γ is coloured purple. The C8β is shown in cartoon representation in front of C8αγ where the MACPF domain is coloured blue and the auxiliary domains are coloured green. (F) The C8αγ component of the whole C8 structure is shown in transparent surface with cartoon representation. Colours as for (E). (G) C6 (PDB ID: 3T5O): The MACPF domain is shown in red cartoon. The N-terminal auxiliary domains are shown as yellow surface. The C-terminal auxiliary domains, EGF and TSP3 domains, are shown in green surface, followed by a segment of the disordered flexible hinge region (magenta surface) that connects to the remaining C-terminal domains (coloured blue). (H) C5b6 (PDB ID: 4A5W): The MACPF domain is shown in red cartoon. The C5b molecule is shown as grey surface representation. The C6 auxiliary domains are colored as for G (C6).
CDCs are important toxins produced by members of at least seven genera of Gram-positive bacteria. Several of these molecules have been shown to play key roles in bacterial pathogenesis [19–22]. In contrast to the MACPF proteins, an extensive understanding of the CDC pore-forming mechanism has been obtained from analysis of the crystal structure of monomeric CDCs (and in particular PFO [16]), the EM structure of the pneumolysin pore [23] and biophysical studies [24,25]. The latter approaches have deployed site-specific cysteine substitution to investigate membrane insertion and conformational change (reviewed in [26,27]).
The CDC membrane pore is notable because it is extraordinarily large (on the order of 250–300 Å diameter) in comparison to other β-barrel pores formed by other families of pore forming toxins. Studies on the mechanism of pore assembly reveal that soluble monomers initially bind to membranes. Around 35–44 membrane bound CDC monomers [23,28] then assemble into a pre-pore form. Two β-hairpins from each monomer then insert into the membrane to form a large, amphipathic β-barrel. Interestingly, other β-pore forming toxins characterized to date, such as Staphylococcus aureus α-hemolysin, contribute only a single β-hairpin per monomer [29]. Of note, and also in contrast to most other pore forming proteins, the CDC monomers in the pre-pore assembly must undergo a significant secondary and tertiary structural change, particularly in the region defined as domain 3 (Fig 1A) [24,25,28,30,31](reviewed in [26,27]).
The CDC mechanism can be broken down into 3 major stages; membrane binding (Fig 2A), oligomerization (Fig 2B) and formation of the transmembrane β-barrel pore (Fig. 2C) [25,28,31–33]. Each stage involves several different structural transitions and allosteric interactions. It is currently unclear whether the mechanisms of pore forming MACPF proteins completely mirror that of the CDCs. Indeed, recent studies suggest some striking mechanistic variation. In this review, we therefore discuss, compare and contrast the current state of the field with respect to each of the three key events that lead to pore formation in CDCs and MACPF proteins.
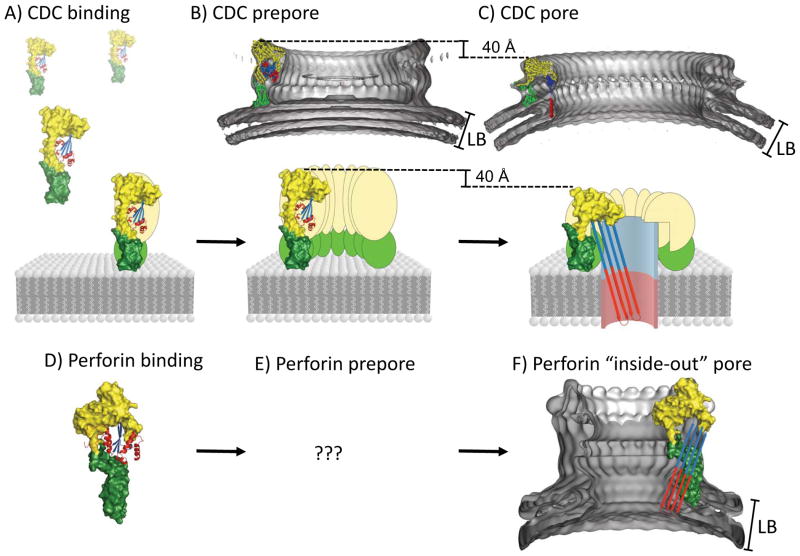
Figure 2. Major stages of CDC of pore formation and the perforin variation.
(A) Membrane recognition shows the initial recognition of the lipid bilayer (LB) membrane by domain 4 of a CDC coloured green. The X-ray crystal structure of PFO was used (PDB: 1PFO). Domains 1 and 2 of PFO are coloured yellow. Domain 3 is coloured blue at the β-sheet and red at the α-helices. (B) Oligomerization precedes pore formation in PFO by forming “prepores” [32,33] (C) Pore formation results in channel formed by an extended β-barrel where each molecule contributes 2 β-hairpins (4 β-strands) [25,31] by unraveling the α-helices (red). Shown above panels B and C are the electron density maps created from single particle cryo-electron microscopy of the pneumolysin prepore (EMDB ID: 1106, 28 Å resolution, PDB ID: 2BK2) and pore fitted with the PFO structure (EMDB ID: 1107, 28 Å resolution, PDB ID: 2BK2) [23] (D) The X-ray crystal structure of perforin (PDB: 3NSJ), orientated in the same way as PFO (part A). All C-terminal regions are coloured green, the MACPF domain is coloured yellow and the central β-sheet coloured blue with the bundles of α-helices coloured red. (E) It is unknown whether perforin is able to form a prepore intermediate as seen for CDC’s (F) The electron density map of the mouse perforin pore created from single particle cryo-electron microscopy [7] pore at 28.5 A resolution (EMDB ID: 1769). The putative “inside out” arrangement of the perforin TMHs is shown as an overlaid schematic. LB = Lipid bilayer.
Membrane and Cellular Recognition
The structure of the CDC PFO revealed a C-terminal Ig domain (Domain 4) that contains the initial determinants for interaction with the membrane. Most CDCs use cholesterol as their receptor, however, this molecule is also important for triggering conformational change. Indeed, initial interaction with the membrane is crucial for initiating structural changes in domain 3 that permit formation of the prepore oligomer and subsequent insertion of the β-barrel pore [24,34].
Interestingly, a small number of CDCs, such as intermedialysin, have been shown to use human CD59 as a receptor. However, these toxins still require cholesterol to complete the formation of the pore [35].
In all CDCs characterized to date (including CD59 binding CDCs), a conserved Thr-Leu pair represents the CDC cholesterol-recognition/binding motif (CRM), [36]. Once the CRM binds cholesterol, two other nearby short hydrophobic loops (L2, L3 of domain 4, Fig. 1A) insert into the bilayer and anchor the monomer perpendicular to the surface [37]. CD59-binding CDCs present an interesting variation on this mechanism in that these proteins disengage from CD59 during the prepore to pore transition [38]. Cholesterol is thus thought to present a second anchor point to maintain membrane contact [37].
In contrast to CDCs, MACPF proteins exhibit significant diversity in the C-terminal domain structures (Fig. 1) and specific membrane receptors for these molecules remain largely unknown. Certain MACPF proteins exhibit a C-terminal β-strand-rich structure that is analogous to the C-terminal domain 4 of the CDCs, whereas other MACPF proteins (such as the complement MAC proteins) appear to lack analogous structures. It is also currently unclear whether MACPF proteins display the CDC-like specificity [36,39] for protein or lipid components.
Of all the MACPF structures determined to date, perforin most closely resembles the overall shape of the CDCs. Of note, perforin contains a C-terminal lipid binding C2 domain that is distantly homologous to the C-terminal lipid binding Ig domain of CDCs. Unlike CDCs, however, and as a consequence of its Ca2+ binding C2 domain, perforin requires extracellular concentrations of Ca2+ to initially bind to target cell membranes [40]. Early studies also suggested that perforin may contain specificity for the phosphorylcholine headgroup present in sphingolipids [41]. However, later work suggested that the correct spacing of the outer membrane lipids, as presented by a cellular target rather than liposomes, outweighed lipid headgroup specificity [42]. Further, the conserved “Thr-Leu” CRM is absent in perforin; these data suggest that the cholesterol binding mechanism seen in CDCs is not conserved in perforin. Finally, it is unclear whether (as is the case for CDCs) lipid interactions with the C2 domain trigger allosteric structural changes elsewhere in the structure that relate to oligomerisation and/or pore formation.
Other MACPF proteins contain β-rich structures (for example the C-terminal β-prism domain of Plu-MACPF [13] that are associated with membrane binding in other proteins. For the majority of family members, however, the role and fold of the regions flanking the MACPF domain remains unknown. One exception is the two component fungal toxin, pleurotolysin. This system comprises two distinct components (pleurotolysin A and B) and is interesting from two perspectives. First, the “MACPF” pore forming component pleurotolysin-B can only bind to membranes when the small lipid binding protein component pleurotolysin-A is present. These studies further suggest that pleurotolysin-B assembles with pleurotolysin-A on the membrane surface. Secondly, pleurotolysin-A itself has been reported to have specificity for sphingomyelin-containing membranes [43,44]. Thus in a sense, this system provides an elegant illustration that MACPF domain containing proteins can both bind to protein receptors (i.e. pleurotolysin-B is suggested to bind to pleurotolysin-A) and that this system at least contains restricted specificity for lipids.
The terminal pathway of the complement the membrane attack complex (MAC) is comprised of a complex of proteins C5b, C6, C7, C8 (C5b8) bound within a ring of C9 molecules (where all molecules except C5b and C8γ contain the MACPF domain). These proteins completely lack an analog to the CDC domain 4 as demonstrated by the structure of C8 (a trimer of C8α, C8β and C8γ) [12] (Fig. 1E–F).
Formation of the membrane-bound MAC is initiated when C5b is produced by C5 convertase near a cell membrane. C6 is the first protein to bind to C5b. C7 then binds to the C5b6 complex (to form C5b7). It is suggested that C7 mediates initial lipophilic anchoring to the target cell membrane [45]. Full-membrane anchoring occurs through C8 recruitment to the C5b7 complex whereupon the C8α MACPF domain inserts into the membrane. Crucially, the C5b8 complex is able to recruit the major pore-forming component C9. Addition of further C9 molecules (through C9-mediated contacts) results in formation of a pore [46]. The small, GPI anchored host cell protein CD59 inhibits C8α and C9 membrane insertion, most likely through interacting with the second transmembrane spanning β-hairpin [47]. Overall, the mechanism of initial MAC membrane binding is very different to that observed for perforin and CDCs in that lipid anchoring appears to mediated through the MACPF membrane inserting regions rather than through a separate lipid or protein binding domain.
Assembly of CDC/MACPF proteins on the membrane surface
For CDCs, the interaction of the Ig domain with the membrane, together with intermolecular interaction of the bound monomers, triggers structural changes within the CDC domain 3 (Fig. 2A–C) that are required for oligomerization [24,48]. These changes include the disruption of the backbone hydrogen bonds between the domain 3 β-strands 4 (β4) and 5 (β5) (Fig. 1A), which then allows the free edge of β-strand 4 (β4) to pair with β-strand 1 (β1) of another membrane bound monomer and the formation of an intermolecular π-stacking interaction [24]. Two glycines at the turn between β4 and β5 are highly conserved in the CDCs and cannot be mutated without blocking the rotation of β5 away from β4 (Fig. 1A). For the CDCs studied to date, the end result of oligomerization on the membrane surface is formation of a distinct pre-pore structure [33].
CDCs and MACPF proteins share extremely low sequence identity. Despite this, structural studies reveal that the two glycine residues conserved in the CDCs (discussed above) are also conserved in MACPF proteins (Fig. 1B–H). The conservation of these residues suggests a general requirement for flexibility in this region for all CDCs and MACPFs. Perforin, C8 and Plu-MACPF, however, lack the analogous capping β5 strand suggesting a difference in how oligomerization is controlled.
Biochemical studies on perforin reveal that perforin oligomerization involves self-association events that are analogous to CDC oligomerisation. Charged residues on the two “flat faces” of the perforin MACPF domain are particularly important in these regards [49]. Interestingly both EM reconstructions of the perforin pore and EM labeling studies, suggest that the perforin monomers assemble into oligomers in the opposite orientation to CDCs. That is, the central β-sheet of the MACPF domain is orientated towards the outside of the pore, rather than towards the pore lumen (Fig 1F) [50]. Both MACPF and CDC proteins are extremely thin and flat, and, from a structural and evolutionary sense at least, it appears that the perforin molecule has evolved such that it can function “inside out” relative to CDCs. To date it is unclear whether, like CDCs, perforin forms a transient pre-pore form.
Excitingly, two very recent structures of C6, bound and unbound to C5b, have also begun to reveal high-resolution details of how the MAC assembles. In particular, these data reveal that interactions with C5b are primarily achieved through two interfaces of C6. One interface involves two N-terminal domains, the top of the second TSP domain and the LDLRA domain. The second interface involves a flexible hinge region and two CCP domains C-terminal to the MACPF domain (Figure 1H). In order for the C5b6 interaction to occur, the flexible hinge region permits the C6 CCP and FIMAC domains (C-terminal) to undergo a massive conformational change (Fig 1G–H) [51–53].
Hadders and colleagues were also able to use the structure of C5b6 to interpret a cryo-EM structure of the soluble C5b9 complex (sC5b9). These data reveal a low-resolution picture of the MACPF “arc”-like assembly that is clearly more consistent with a conventional CDC-like (rather than a perforin-like) orientation of MACPF monomers. In addition, these EM data, together with previous labeling studies of sC5b9 [54] allowed identification of MAC inhibitors such as vitronectin and clusterin. These proteins appear to form a surprisingly large interface with the base of the MACPF arc.
Membrane insertion and pore formation
The CDCs form a large β-barrel pore that is comprised of 35–44 monomers, depending on the CDC [23,28]. The two domain 3 α-helical bundles that flank the core β-sheet are converted to a pair of membrane spanning amphipathic β-hairpins (Fig 2A–C) [25,31]. These regions contribute to the formation of the large β-barrel pore, which for PFO means that approximately 144 amphipathic β-strands are organized into one of the largest known membrane spanning β-barrel structures. The CDCs monomers are anchored perpendicular to the membrane surface by domain 4, which positions domain 3 about 40 Å above the bilayer [28,30]. When extended, the β-hairpins are only long enough to reach the bilayer surface, yet both β-hairpins were shown to span the bilayer [25,31]. This conundrum was resolved in two studies [28,30] that showed the height of the pore complex was 40 Å less than the prepore complex and that domains 1 and 3 moved closer to the membrane, thus allowing the β-hairpins to span the bilayer. This collapse can be thought of as being akin to the “punch” of a molecular hole punch! Tilley and colleagues, using cryo-EM and image processing, showed that pneumolysin undergoes a similar vertical collapse [23] (Fig. 2A–C). Furthermore, comparison of the CDC pore form with the CDC monomer structure revealed a striking “straightening out” of the central β-sheet. The hinge point for this change is anticipated to be around the conserved glycine residues (Fig 1A). Finally, in CDC-like giant β-barrels, the β-strands appear to be oriented in a “close to vertical” orientation (Fig 2C). This arrangement contrasts the arrangement of β-strands currently seen in smaller transmembrane β-barrels structures which tend to be more sloped relative to the β-barrel axis [55].
Similar to CDCs, MACPF proteins are also suggested to form a β-barrel by unraveling two α-helical regions into membrane-spanning β-strands (Fig 2A–C) [7,11–14]. However, the EM structure of a MACPF pore suggests important differences in these mechanisms. Unlike the CDCs, there is no change in the height of the perforin structure upon formation of the pore complex [7]. Instead, to reach and span the membrane, the two transmembrane sequences in perforin are more than twice the length of the corresponding regions in the CDCs [50]. Interestingly, analysis of the structure of C6 and C8 alone has also revealed that the distorted central β-sheet of the MACPF domain may also possess the ability to “straighten” in a similar fashion to CDC’s [52].
The role of the pore–not just simple lysis
In addition to direct lytic function, both CDC and MACPF pores have been suggested to perform molecular transport roles. Indeed, the size of the CDC pore suggests that it can be used to translocate proteins across the membranes of eukaryotic cells and, accordingly, CDCs have been used as cell permabilization reagents to introduce other proteins for many years [56]. To date, only a single protein translocation function has been attributed to a CDC; the Streptococcus pyogenes streptolysin O (SLO) functions to mediate translocation of an NAD glycohydrolase (SPN) into keratinocytes [57]. Remarkably, this translocation function does not appear to require formation of a functional SLO pore [58]. The mechanism of this translocation system thus remains unknown. Whether other CDCs facilitate translocation of proteins also remains unknown.
At least one MACPF protein, perforin, clearly functions in vivo to achieve molecular transport. Indeed the role of perforin is to mediate the delivery of a cohort of cytotoxic proteases (including granzyme B) from the NK cell and cytotoxic T cells granules into virally infected or pre-cancerous target cells. These proteases, are small enough to be passively delivered through the perforin pore and into the target cell [50,59,60]. Granzyme mediated apoptosis then results in rapid cell death [40]. A current important area of debate is whether this delivery occurs at the plasma surface or via an endosomal uptake into the target cell and how endosomal uptake could occur [61]. Indeed, a recent model proposed suggests a two-step process whereby perforin pores induce endocytosis at the plasma membrane as well as delivering granzymes from the endosomes into the cytoplasm [62].
Although the MAC is generally proposed to result in direct cell lysis, experiments have shown the MAC may function to mediate delivery of other proteins. For example, early studies have shown that MAC mediated delivery of lysozyme into the periplasm of Gram negative cells enhances cell killing [63].
Conclusions
Structural, functional and limited sequence similarity between CDCs and MACPF proteins suggests that these proteins together form the most extensive superfamily of pore forming proteins identified to date. MACPF and CDC proteins appear in almost all forms of life and the better-characterized family members perform key roles in immunity, host defense, venom toxicity and pathogenicity. Bioinformatic and crystallographic studies reveal a remarkable divergence in the structure and sequence of the CDC/MACPF domain, as well as considerable variation in the overall domain composition of individual family members. These data are consistent with an extraordinary flexibility with respect to how different members of the superfamily recognize target membranes and assemble into higher order structures. For the CDC/MACPF family, however, “all roads lead to Rome”, and the common result, at least for the molecular systems studied to date, appears to be giant, membrane inserted β-barrel-lined pores that are unparalleled with respect to their size and architecture. It remains to be understood, however, as to whether all MACPF/CDC systems form pores. Of particular interest, with respect to the latter point, is whether developmental or neural MACPF systems are true pore formers or whether more functional surprises will be apparent in these regards.
Highlights.
Structural features of the membrane attack complex/perforin (MACPF) and cholesterol-dependent cytolysin (CDCS) protein families suggest common structural and evolutionary relationships
Similar to the CDCs the MACPF protein monomers may form a β-barrel pore
Studies suggest a divergent assembly pathway for the MACPF pore
Acknowledgments
M.A.D. is supported by an NHMRC CDA fellowship and an ARC Discovery project (DP0986811). R.K.T. is supported by a grant from the National Institutes of Health NIAID (5R01AI037657–16).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle A. Dunstone, Email: michelle.dunstone@monash.edu.
Rodney K. Tweten, Email: Rodney-Tweten@ouhsc.edu.
References
- 1.Tschopp J, Masson D, Stanley KK. Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. Nature. 1986;322:831–834. doi: 10.1038/322831a0. [DOI] [PubMed] [Google Scholar]
- 2.Nagai H, Oshiro N, Takuwa-Kuroda K, Iwanaga S, Nozaki M, Nakajima T. Novel proteinaceous toxins from the nematocyst venom of the Okinawan sea anemone Phyllodiscus semoni Kwietniewski. Biochem Biophys Res Commun. 2002;294:760–763. doi: 10.1016/S0006-291X(02)00547-8. [DOI] [PubMed] [Google Scholar]
- 3.Kadota K, Ishino T, Matsuyama T, Chinzei Y, Yuda M. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc Natl Acad Sci U S A. 2004;101:16310–16315. doi: 10.1073/pnas.0406187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JR, Raibaud A, Ollo R. Terminal pattern elements in Drosophila embryo induced by the torso-like protein. Nature. 1994;367:741–745. doi: 10.1038/367741a0. [DOI] [PubMed] [Google Scholar]
- 5.Zheng C, Heintz N, Hatten ME. CNS gene encoding astrotactin, which supports neuronal migration along glial fibers. Science. 1996;272:417–419. doi: 10.1126/science.272.5260.417. [DOI] [PubMed] [Google Scholar]
- 6.Rosado CJ, Kondos S, Bull TE, Kuiper MJ, Law RH, Buckle AM, Voskoboinik I, Bird PI, Trapani JA, Whisstock JC, et al. The MACPF/CDC family of pore-forming toxins. Cell Microbiol. 2008;10:1765–1774. doi: 10.1111/j.1462-5822.2008.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondos SC, Hatfaludi T, Voskoboinik I, Trapani JA, Law RH, Whisstock JC, Dunstone MA. The structure and function of mammalian membrane-attack complex/perforin-like proteins. Tissue Antigens. 2010;76:341–351. doi: 10.1111/j.1399-0039.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- 8.Dourmashkin RR. The structural events associated with the attachment of complement components to cell membranes in reactive lysis. Immunology. 1978;35:205–212. [PMC free article] [PubMed] [Google Scholar]
- 9.Tschopp J, Muller-Eberhard HJ, Podack ER. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982;298:534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- 10.Young JD, Nathan CF, Podack ER, Palladino MA, Cohn ZA. Functional channel formation associated with cytotoxic T-cell granules. Proc Natl Acad Sci U S A. 1986;83:150–154. doi: 10.1073/pnas.83.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadders MA, Beringer DX, Gros P. Structure of C8alpha-MACPF reveals mechanism of membrane attack in complement immune defense. Science. 2007;317:1552–1554. doi: 10.1126/science.1147103. [DOI] [PubMed] [Google Scholar]
- **12.Lovelace LL, Cooper CL, Sodetz JM, Lebioda L. Structure of human C8 protein provides mechanistic insight into membrane pore formation by complement. J Biol Chem. 2011;286:17585–17592. doi: 10.1074/jbc.M111.219766. The first structure of a complete MAC component, C8, that gives insight into the oligomer interface of the MAC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosado CJ, Buckle AM, Law RH, Butcher RE, Kan WT, Bird CH, Ung K, Browne KA, Baran K, Bashtannyk-Puhalovich TA, et al. A common fold mediates vertebrate defense and bacterial attack. Science. 2007;317:1548–1551. doi: 10.1126/science.1144706. [DOI] [PubMed] [Google Scholar]
- 14.Slade DJ, Lovelace LL, Chruszcz M, Minor W, Lebioda L, Sodetz JM. Crystal structure of the MACPF domain of human complement protein C8 alpha in complex with the C8 gamma subunit. J Mol Biol. 2008;379:331–342. doi: 10.1016/j.jmb.2008.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polekhina G, Giddings KS, Tweten RK, Parker MW. Insights into the action of the superfamily of cholesterol-dependent cytolysins from studies of intermedilysin. Proc Natl Acad Sci. 2005;102:600–605. doi: 10.1073/pnas.0403229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. Structure of a cholesterol-binding thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89:685–692. doi: 10.1016/s0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Abdubek P, Astakhova T, Axelrod HL, Bakolitsa C, Cai X, Carlton D, Chen C, Chiu HJ, Clayton T, et al. Structure of a membrane-attack complex/perforin (MACPF) family protein from the human gut symbiont Bacteroides thetaiotaomicron. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:1297–1305. doi: 10.1107/S1744309110023055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourdeau RW, Malito E, Chenal A, Bishop BL, Musch MW, Villereal ML, Chang EB, Mosser EM, Rest RF, Tang WJ. Cellular functions and X-ray structure of anthrolysin O, a cholesterol-dependent cytolysin secreted by Bacillus anthracis. J Biol Chem. 2009;284:14645–14656. doi: 10.1074/jbc.M807631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awad MM, Ellemor DM, Boyd RL, Emmins JJ, Rood JI. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect Immun. 2001;69:7904–7910. doi: 10.1128/IAI.69.12.7904-7910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellemor DM, Baird RN, Awad MM, Boyd RL, Rood JI, Emmins JJ. Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene. Infect Immun. 1999;67:4902–4907. doi: 10.1128/iai.67.9.4902-4907.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portnoy D, Jacks PS, Hinrichs D. The role of hemolysin for intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med. 2008;8:497–509. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 23.Tilley SJ, Orlova EV, Gilbert RJ, Andrew PW, Saibil HR. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell. 2005;121:247–256. doi: 10.1016/j.cell.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran R, Tweten RK, Johnson AE. Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit β-strand alignment. Nat Struct Mol Biol. 2004;11:697–705. doi: 10.1038/nsmb793. [DOI] [PubMed] [Google Scholar]
- 25.Shatursky O, Heuck AP, Shepard LA, Rossjohn J, Parker MW, Johnson AE, Tweten RK. The mechanism of membrane insertion for a cholesterol dependent cytolysin: A novel paradigm for pore-forming toxins. Cell. 1999;99:293–299. doi: 10.1016/s0092-8674(00)81660-8. [DOI] [PubMed] [Google Scholar]
- 26.Hotze EM, Tweten RK. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochim Biophys Acta. 2012;1818:1028–1038. doi: 10.1016/j.bbamem.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tweten RK. The cholesterol-dependent cytolysins; a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czajkowsky DM, Hotze EM, Shao Z, Tweten RK. Vertical collapse of a cytolysin prepore moves its transmembrane β-hairpins to the membrane. EMBO J. 2004;23:3206–3215. doi: 10.1038/sj.emboj.7600350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song LZ, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 30.Ramachandran R, Tweten RK, Johnson AE. The domains of a cholesterol-dependent cytolysin undergo a major FRET-detected rearrangement during pore formation. Proc Natl Acad Sci. 2005;102:7139–7144. doi: 10.1073/pnas.0500556102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepard LA, Heuck AP, Hamman BD, Rossjohn J, Parker MW, Ryan KR, Johnson AE, Tweten RK. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an α-helical to β-sheet transition identified by fluorescence spectroscopy. Biochemistry. 1998;37:14563–14574. doi: 10.1021/bi981452f. [DOI] [PubMed] [Google Scholar]
- 32.Hotze EM, Wilson-Kubalek EM, Rossjohn J, Parker MW, Johnson AE, Tweten RK. Arresting pore formation of a cholesterol-dependent cytolysin by disulfide trapping synchronizes the insertion of the transmembrane beta-sheet from a prepore intermediate. J Biol Chem. 2001;276:8261–8268. doi: 10.1074/jbc.M009865200. [DOI] [PubMed] [Google Scholar]
- 33.Shepard LA, Shatursky O, Johnson AE, Tweten RK. The mechanism of assembly and insertion of the membrane complex of the cholesterol-dependent cytolysin perfringolysin O: Formation of a large prepore complex. Biochemistry. 2000;39:10284–10293. doi: 10.1021/bi000436r. [DOI] [PubMed] [Google Scholar]
- 34.Soltani CE, Hotze EM, Johnson AE, Tweten RK. Specific protein-membrane contacts are required for prepore and pore assembly by a cholesterol-dependent cytolysin. J Biol Chem. 2007;282:15709–15716. doi: 10.1074/jbc.M701173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giddings KS, Zhao J, Sims PJ, Tweten RK. Human CD59 is a receptor for the cholesterol dependent cytolysin intermedilysin. Nat Struct Mol Biol. 2004;12:1173–1178. doi: 10.1038/nsmb862. [DOI] [PubMed] [Google Scholar]
- *36.Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc Natl Acad Sci U S A. 2010;107:4341–4346. doi: 10.1073/pnas.0911581107. Identification of a two amino acid cholesterol binding motif for CDCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramachandran R, Heuck AP, Tweten RK, Johnson AE. Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin. Nat Struct Biol. 2002;9:823–827. doi: 10.1038/nsb855. [DOI] [PubMed] [Google Scholar]
- 38.LaChapelle S, Tweten RK, Hotze EM. Intermedilysin-receptor interactions during assembly of the pore complex: assembly intermediates increase host cell susceptibility to complement-mediated lysis. J Biol Chem. 2009;284:12719–12726. doi: 10.1074/jbc.M900772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Wickham SE, Hotze EM, Farrand AJ, Polekhina G, Nero TL, Tomlinson S, Parker MW, Tweten RK. Mapping the intermedilysin-human CD59 receptor interface reveals a deep correspondence with the binding site on CD59 for complement binding proteins C8alpha and C9. J Biol Chem. 2011;286:20952–20962. doi: 10.1074/jbc.M111.237446. Investigation of the binding of C8α, C9 and CDCs to CD59 reveal a common binding site on CD59 despite no detectable conservation of the sites in the C8α, C9 and CDC molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voskoboinik I, Thia MC, Fletcher J, Ciccone A, Browne K, Smyth MJ, Trapani JA. Calcium-dependent plasma membrane binding and cell lysis by perforin are mediated through its C2 domain: A critical role for aspartate residues 429, 435, 483, and 485 but not 491. J Biol Chem. 2005;280:8426–8434. doi: 10.1074/jbc.M413303200. [DOI] [PubMed] [Google Scholar]
- 41.Tschopp J, Schafer S, Masson D, Peitsch MC, Heusser C. Phosphorylcholine acts as a Ca2+-dependent receptor molecule for lymphocyte perforin. Nature. 1989;337:272–274. doi: 10.1038/337272a0. [DOI] [PubMed] [Google Scholar]
- 42.Antia R, Schlegel RA, Williamson P. Binding of perforin to membranes is sensitive to lipid spacing and not headgroup. Immunol Lett. 1992;32:153–157. doi: 10.1016/0165-2478(92)90108-z. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai N, Kaneko J, Kamio Y, Tomita T. Cloning, expression, and pore-forming properties of mature and precursor forms of pleurotolysin, a sphingomyelin-specific two-component cytolysin from the edible mushroom Pleurotus ostreatus. Biochim Biophys Acta. 2004;1679:65–73. doi: 10.1016/j.bbaexp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Tomita T, Noguchi K, Mimuro H, Ukaji F, Ito K, Sugawara-Tomita N, Hashimoto Y. Pleurotolysin, a novel sphingomyelin-specific two-component cytolysin from the edible mushroom Pleurotus ostreatus, assembles into a transmembrane pore complex. J Biol Chem. 2004;279:26975–26982. doi: 10.1074/jbc.M402676200. [DOI] [PubMed] [Google Scholar]
- 45.Preissner KT, Podack ER, Muller-Eberhard HJ. The membrane attack complex of complement: relation of C7 to the metastable membrane binding site of the intermediate complex C5b-7. J Immunol. 1985;135:445–451. [PubMed] [Google Scholar]
- 46.Steckel EW, Welbaum BE, Sodetz JM. Evidence of direct insertion of terminal complement proteins into cell membrane bilayers during cytolysis. Labeling by a photosensitive membrane probe reveals a major role for the eighth and ninth components. J Biol Chem. 1983;258:4318–4324. [PubMed] [Google Scholar]
- 47.Huang Y, Qiao F, Abagyan R, Hazard S, Tomlinson S. Defining the CD59-C9 binding interaction. J Biol Chem. 2006;281:27398–27404. doi: 10.1074/jbc.M603690200. [DOI] [PubMed] [Google Scholar]
- 48.Hotze EM, Heuck AP, Czajkowsky DM, Shao Z, Johnson AE, Tweten RK. Monomer-monomer interactions drive the prepore to pore conversion of a beta-barrel-forming cholesterol-dependent cytolysin. J Biol Chem. 2002;277:11597–11605. doi: 10.1074/jbc.M111039200. [DOI] [PubMed] [Google Scholar]
- *49.Baran K, Dunstone M, Chia J, Ciccone A, Browne KA, Clarke CJ, Lukoyanova N, Saibil H, Whisstock JC, Voskoboinik I, et al. The molecular basis for perforin oligomerization and transmembrane pore assembly. Immunity. 2009;30:684–695. doi: 10.1016/j.immuni.2009.03.016. Demonstration that the oligomer interface of perforin pores is facilitiated by salt bridge formation. [DOI] [PubMed] [Google Scholar]
- **50.Law RH, Lukoyanova N, Voskoboinik I, Caradoc-Davies TT, Baran K, Dunstone MA, D’Angelo ME, Orlova EV, Coulibaly F, Verschoor S, et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature. 2010;468:447–451. doi: 10.1038/nature09518. The authors demonstrate that perforin pores can assemble in an inside-out conformation compared to the pore structure of pneumolysin. [DOI] [PubMed] [Google Scholar]
- **51.Hadders MA, Bubeck D, Roversi P, Hakobyan S, Forneris F, Morgan BP, Pangburn MK, Llorca O, Lea SM, Gros P. Assembly and Regulation of the Membrane Attack Complex Based on Structures of C5b6 and sC5b9. Cell Rep. 2012;1:1–8. doi: 10.1016/j.celrep.2012.02.003. The structure of complement C6 bound to C5b that shows how the MAC is initiated and the cryo-EM data that shows the MAC uses a CDC-like mechanism of pore formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **52.Aleshin AE, Schraufstatter IU, Stec B, Bankston LA, Liddington RC, Discipio RG. Structure of Complement C6 Suggests a Mechanism for Initiation and Unidirectional, Sequential Assembly of Membrane Attack Complex (MAC) J Biol Chem. 2012;287:10210–10222. doi: 10.1074/jbc.M111.327809. The first structure of the protein C6 which gives insight into the MAC formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *53.Aleshin AE, Discipio RG, Stec B, Liddington RC. Crystal structure of C5b-6 suggests a structural basis for priming the assembly of the Membrane Attack Complex (MAC) J Biol Chem. 2012 doi: 10.1074/jbc.M112.361121. The structure of complement C6 bound to C5b that shows how the labile C5b is trapped and how the MAC is initiated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preissner KP, Podack ER, Muller-Eberhard HJ. SC5b-7, SC5b-8 and SC5b-9 complexes of complement: ultrastructure and localization of the S-protein (vitronectin) within the macromolecules. Eur J Immunol. 1989;19:69–75. doi: 10.1002/eji.1830190112. [DOI] [PubMed] [Google Scholar]
- *55.Reboul CF, Mahmood K, Whisstock JC, Dunstone MA. Predicting giant transmembrane beta-barrel architecture. Bioinformatics. 2012 doi: 10.1093/bioinformatics/bts152. (in press). A new model of pore formation of the CDC giant β-barrel shows that CDCs are able to have either straight or slightly slanted strands that form the giant β-barrel compared to the slanted strands observed for small barrels. [DOI] [PubMed] [Google Scholar]
- 56.Bhakdi S, Weller U, Walev I, Martin E, Jonas D, Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol (Berl) 1993;182:167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- **57.Madden JC, Ruiz N, Caparon M. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell. 2001;104:143–152. doi: 10.1016/s0092-8674(01)00198-2. Demonstration that SLO can mediate the translocation of nicotinamide adeninedinucleotide-glycohydrolase into cells without the requirement for pore formation and independantly of clathrin-mediated endocytosis. [DOI] [PubMed] [Google Scholar]
- 58.Magassa N, Chandrasekaran S, Caparon MG. Streptococcus pyogenes cytolysin-mediated translocation does not require pore formation by streptolysin O. EMBO Rep. 2010;5:400–405. doi: 10.1038/embor.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakajima H, Park HL, Henkart PA. Synergistic roles of granzymes A and B in mediating target cell death by rat basophilic leukemia mast cell tumors also expressing cytolysin/perforin. J Exp Med. 1995;181:1037–1046. doi: 10.1084/jem.181.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi L, Mai S, Israels S, Browne K, Trapani JA, Greenberg AH. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J Exp Med. 1997;185:855–866. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Froelich CJ, Orth K, Turbov J, Seth P, Gottlieb R, Babior B, Shah GM, Bleackley RC, Dixit VM, Hanna W. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J Biol Chem. 1996;271:29073–29079. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- **62.Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, Goping IS, Bleackley RC, Kirchhausen T, Lieberman J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12:770–777. doi: 10.1038/ni.2050. A new hypothesis for perforin activity that suggests that perforin acts at the plasma surface followed by forming pores in the endosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez RJ, Carroll SF. Sequential metabolic expressions of the lethal process in human serum-treated Escherichia coli: role of lysozyme. Infect Immun. 1980;28:735–745. doi: 10.1128/iai.28.3.735-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]