Abstract
Apicomplexan parasites utilize a peripheral membrane system called the inner membrane complex (IMC) to facilitate host cell invasion and parasite replication. We recently identified a novel family of Toxoplasma IMC Sub-compartment Proteins (ISP1/2/3) that localize to sub-domains of the IMC using a targeting mechanism that is dependent on coordinated myristoylation and palmitoylation of a series of residues in the N-terminus of the protein. While the precise functions of the ISPs are unknown, deletion of ISP2 results in replication defects, suggesting that this family of proteins plays a role in daughter cell formation. Here we have characterized a fourth ISP family member (ISP4) and discovered that this protein localizes to the central IMC sub-compartment, similar to ISP2. Like ISP1/3, ISP4 is dispensable for the tachyzoite lytic cycle as the disruption of ISP4 does not produce any gross replication or growth defects. Surprisingly, targeting of ISP4 to the IMC membranes is dependent on residues predicted for palmitoylation but not myristoylation, setting its trafficking apart from the other ISP proteins and demonstrating distinct mechanisms of protein localization to the IMC membranes, even within a family of highly-related proteins.
Keywords: Toxoplasma, Inner Membrane Complex, ISP, palmitoylation, myristoylation, endodyogeny
1. Introduction
The phylum Apicomplexa consists of approximately five thousand known species of obligate intracellular parasites, many of which cause serious diseases in humans and animals worldwide. Apicomplexans of major importance to human health include the malaria parasite Plasmodium falciparum, which accounts for approximately one million deaths annually [1], and Toxoplasma gondii, a pathogen that chronically infects about one-third of the human population [2]. Although infected individuals with an intact immune system are typically unharmed, Toxoplasma is capable of causing severe neurological disorders and even death in immunocompromised patients [3]. In addition, infants that become infected with Toxoplasma congenitally can suffer from severe birth defects, ranging from ocular disorders to hydrocephalus [4].
Apicomplexans possess a number of unique cellular structures that compartmentalize parasite-specific functions and also represent new potential therapeutic targets. One of these is a peripheral membrane system called the inner membrane complex (IMC). The IMC is a double membrane structure composed of flattened vesicles called alveoli that underlie the plasma membrane and are linked to a supporting cytoskeletal meshwork which faces the cytoplasm [5, 6]. Freeze-fracture studies of the IMC reveal that the flattened alveoli are organized into a patchwork of tightly sutured rectangular plates. In the cyst-forming coccidal sub-group of apicomplexans, this structure is capped by a single cone-shaped plate at the apical end of the cell [7]. An actin-myosin motor embedded in the IMC produces a form of gliding motility critical for extracellular transit, host cell invasion, and egress [8, 9]. In addition to supporting parasite motility, the IMC serves as a scaffold for the assembly of new daughter buds during parasite replication via an internal buddi ng process known as endodyogeny [10]. While a number of IMC protein constituents have been identified, these probably represent only a small fraction of the total proteins and corresponding activities that are present in this organelle.
Recently, we identified a family of IMC Sub-compartment Proteins (ISP1/2/3) in Toxoplasma gondii and showed that it plays a role in coordinating cell division. While the ISP family is conserved across Apicomplexa, different species maintain varying numbers of ISP proteins (for example, Plasmodium species appear to possess only two family members) [11]. The ISPs contain no identifiable domains and appear to be restricted to this phylum, likely representing specialized apicomplexan functions as indicated by the dysregulation of endodyogeny upon disruption of ISP2 [11]. While ultrastructural observations showed that the IMC is non-contiguous, a higher degree of compartmentalization was appreciated by the discovery that ISP1/2/3 localize to distinct membrane plates or groups of plates within the IMC. ISP1 targets to the cone-shaped apical cap, while ISP2 localizes to a central region of the IMC which begins at the base of the apical cap and extends about two-thirds the length of the cell. ISP3 is found in the central IMC region but also extends to the basal end of the parasite [11]. Coordinated acylations are responsible for IMC membrane targeting, suggesting a “kinetic trapping” model in which the ISP proteins are first myristoylated in the cytosol to enable transient sampling of membranes, followed by palmitoylation at the IMC, which locks the proteins into the appropriate membrane compartment. Disruption of either myristoylation or palmitoylation signals in ISP1/2/3 completely ablates IMC targeting and results in a cytoplasmic localization. In addition, ISP1 performs a gate-keeping function that excludes ISP2 and ISP3 from the apical cap region, revealing a hierarchical targeting within this membrane system that is just beginning to be understood [11].
BLAST analysis of the T. gondii genome using the ISP1–3 sequences identified a potential fourth ISP family member (TGGT1_063420, www.toxodb.org), but this protein was not previously characterized due to poor expression levels and an uncertain gene model [11]. Here, we demonstrate that TGGT1_063420 (denoted ISP4) localizes to the central IMC sub-compartment, similar to ISP2. Disruption of ISP4 did not result in any apparent replication or growth defects, suggesting that other family members may substitute in its absence. Finally, we show that ISP4 targets to the IMC by a mechanism distinct from the other three family members. While trafficking of ISP1/2/3 to the IMC is dependent on both myristoylation and palmitoylation [11], ISP4 targeting is only contingent upon residues predicted for palmitoylation. Together, these experiments provide new molecular insight into the organization and construction of the IMC, a unique membrane structure critical to Toxoplasma pathogenesis.
2. Materials and Methods
2.1. Toxoplasma and host cell culture
T. gondii RHΔhpt (parental) strain and modified strains were grown on confluent monolayers of human foreskin fibroblast (HFF) host cells in DMEM supplemented with 10% fetal bovine serum, as previously described [12].
2.2 Transcriptional profile analysis of ISP4
Expression data for ISP4 and other indicated IMC proteins was acquired from a previously described genome-wide microarray expression dataset [13].
2.3. Antibodies
The following previously described primary antibodies were used in immunofluorescence (IFA) or Western blot assays: rabbit polyclonal anti-tubulin [14], anti-IMC1 mAb 45.15 [15], anti-ISP1 mAb 7E8 [11], mouse polyclonal anti-ISP2 [11], and anti-ROP1 mAb TG49 [16]. The hemagglutinin (HA) epitope was detected with mouse mAb HA.11 (Covance), rabbit anti-HA (Invitrogen), or rat mAb 3F10 (Roche).
For production of polyclonal mouse anti-ISP4 (TGGT1_063420), the coding sequence for residues 60–181 of ISP4 was PCR-amplified from a Toxoplasma cDNA library (primers P1/P2) and cloned into pET101/D-TOPO (Invitrogen). Constructs were transformed into E. coli BL-21 and protein expression was induced with 0.5 mM IPTG. ISP460–181 was purified over Ni-NTA agarose (Qiagen) and injected into a BALB/c mouse (~200 µg per immunization). Sera was collected from the mouse following each boost and screened by IFA and Western blot.
For production of rabbit anti-TgCentrin1 antibody, the full length ORF of TgCentrin1 was PCR amplified from cDNA (primers P3/P4), inserted by ligation independent cloning into plasmid pAVA0421 [17] and purified as described previously [18] to generate a His6-tagged N-terminal fusion protein. Briefly, recombinant protein was expressed in E. coli BL21 STAR (DE3)pLys (Invitrogen) by induction with 0.5 mM IPTG and purified over TALON Metal Affinity Resin (Clontech). Polyclonal antiserum was generated by rabbit immunizations (Covance, Denver, PA) and affinity purified against the recombinant protein cross-linked to cyanogen bromide Sepharose 4B (Sigma).
2.4. Immunofluorescence assays (IFA) and Western blot
For IFA, HFFs were grown to confluency on coverslips and infected with Toxoplasma gondii. After 18–24 hours, the coverslips were fixed and processed for indirect immunofluorescence as previously described [19]. The coverslips were mounted in vectashield (Vector Labs) and viewed with an Axio Imager.Z1 fluorescent microscope (Zeiss) as previously described [11].
For Western blot, parasite lysates were separated by SDS-PAGE and transferred overnight onto nitrocellulose filter paper. Target proteins were detected with the indicated primary antibodies followed by secondary antibodies conjugated to horse radish peroxidase as previously described [20].
2.5. Endogenous Tagging and Second-Copy Expression of ISP4
For endogenous tagging of ISP4, we first replaced the DHFR-TSc3 selectable marker with the HPT selectable marker in the plasmid p3xHA-LIC-DHFR [21] to generate the plasmid p3xHA-LIC-HPT. A 3’ portion of the ISP4 gene was PCR-amplified (P5/P6) and inserted into p3xHA-LIC-HPT using a ligation-independent cloning approach to generate a triple HA-epitope tag fusion just before the stop codon [22]. 25 µg of the construct was linearized with EcoRV and transfected into Δku80Δhpt parasites [11]. The parasites were selected in MX media (50 µg/ml mycophenolic acid and 50 µg/ml xanthine), cloned by limiting dilution, and screened by Western blot and IFA against the HA tag. A clone that had undergone the intended recombination event was selected and designated ISP4–3xHA.
To generate an ISP4 expression vector, the ISP4 gene was PCR-amplified (primers P7/P8) from a Toxoplasma cDNA library with a REV primer designed to create an in-frame HA tag fusion at the C-terminus. This PCR product was cloned into the vector pTub-YFP,YFP [23] between BglII/AscI, resulting in the plasmid pTubISP4HA.
2.6. Detergent extraction of ISP4
For detergent extraction experiments, 3×107 ISP4–3xHA parasites were washed in PBS, pelleted and lysed in 1 mL TBS (50mM Tris-HCl [pH 7.4], 150mM NaCl) containing 0.5% NP-40 and Complete Protease Inhibitor Cocktail (Roche) for 15 min at 4°C. Lysates were centrifuged for 15 min at 14,000 × g. Equivalent amounts of total, supernatant, and pellet fractions were separated by SDS-PAGE and analyzed by Western blot.
2.7. Disruption of ISP4
5’ and 3’ flanking regions of the ISP4 gene were PCR- amplified (primers P9/P10 and P11/P12, respectively) from wild-type genomic DNA and inserted into the plasmid pMiniGFP.ht-DHFR [11] at NotI and ApaI, respectively. 50 µg of the knockout vector was linearized with EcoRV and transfected into ISP4–3xHA parasites. The parasites were selected with 1 µM pyrimethamine for three passages and cloned by limiting dilution. Plaques were screened for GFP fluorescence, GFP nulls were selected, and loss of ISP4 was confirmed by IFA and Western blot. A knockout clone was isolated and designated Δisp4.
2.8. Determination of the correct ISP4 start methionine
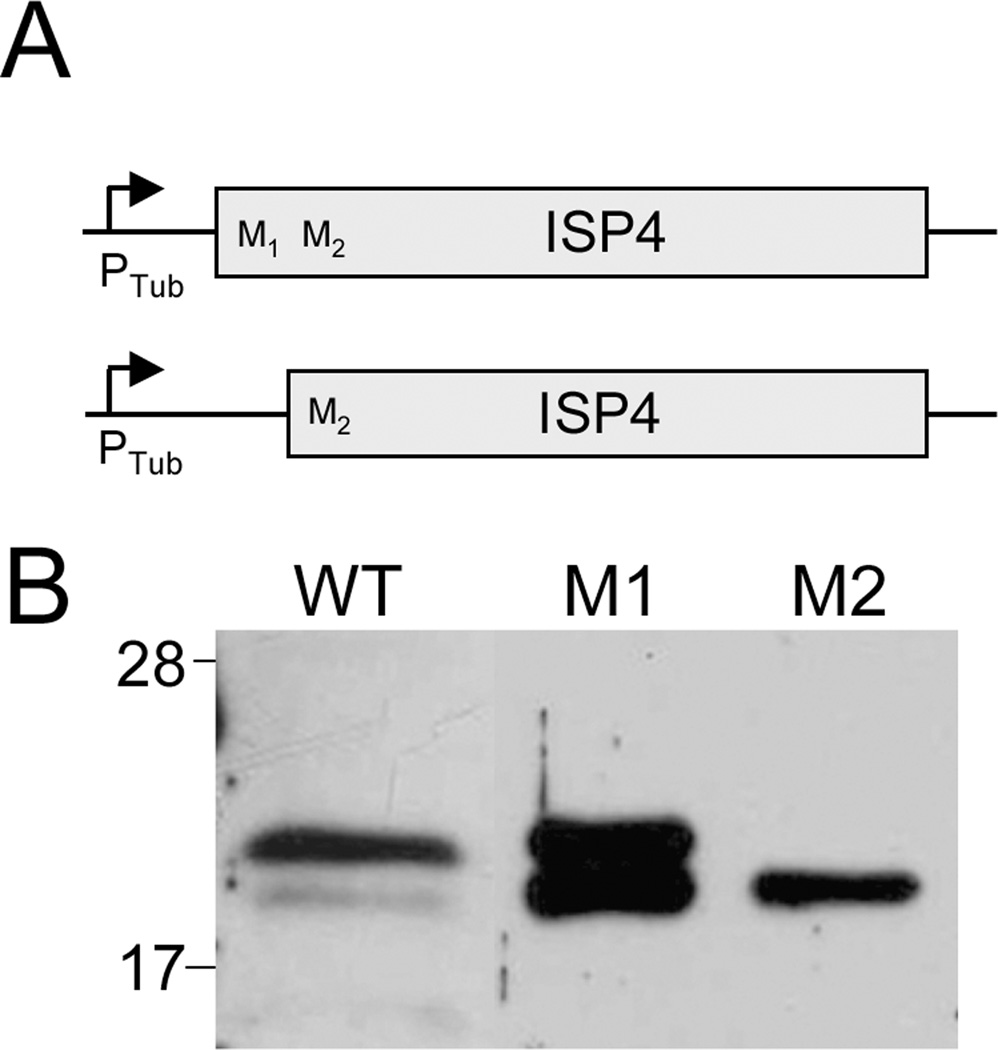
ISP4 cDNA sequences were PCR-amplified from a Toxoplasma cDNA library either beginning from the position one methionine codon (primers P7/P13, designated ISP4 M1) or truncating the first 10 codons and beginning at the position eleven methionine codon (primers P14/P13, designated ISP4 M2). These fragments were inserted into the plasmid pTub-YFP,YFP between BglII/AscI [23]. 50 µg of each vector was linearized with PmeI and transfected into Δisp4 parasites and selected with MX media. These transfected populations (designated ISP4 M1 and ISP4 M2) were screened by Western blot using our mouse ISP4 anti-sera.
2.9. Site-directed mutagenesis
PCR-based mutagenesis of ISP4 was carried out in the pTubISP4HA construct using the following primers (forward primer given, reverse complement was also used): C26,27S (P15), C72,73S (P16), and G15A (P17). Mutations were sequenced-verified and 25 µg of the wild-type and each mutagenized vector were linearized with PmeI and transfected into RHΔhpt parasites. The transfected populations were selected with MX media and screened by IFA against the HA tag.
3. Results
3.1. Identification of ISP4
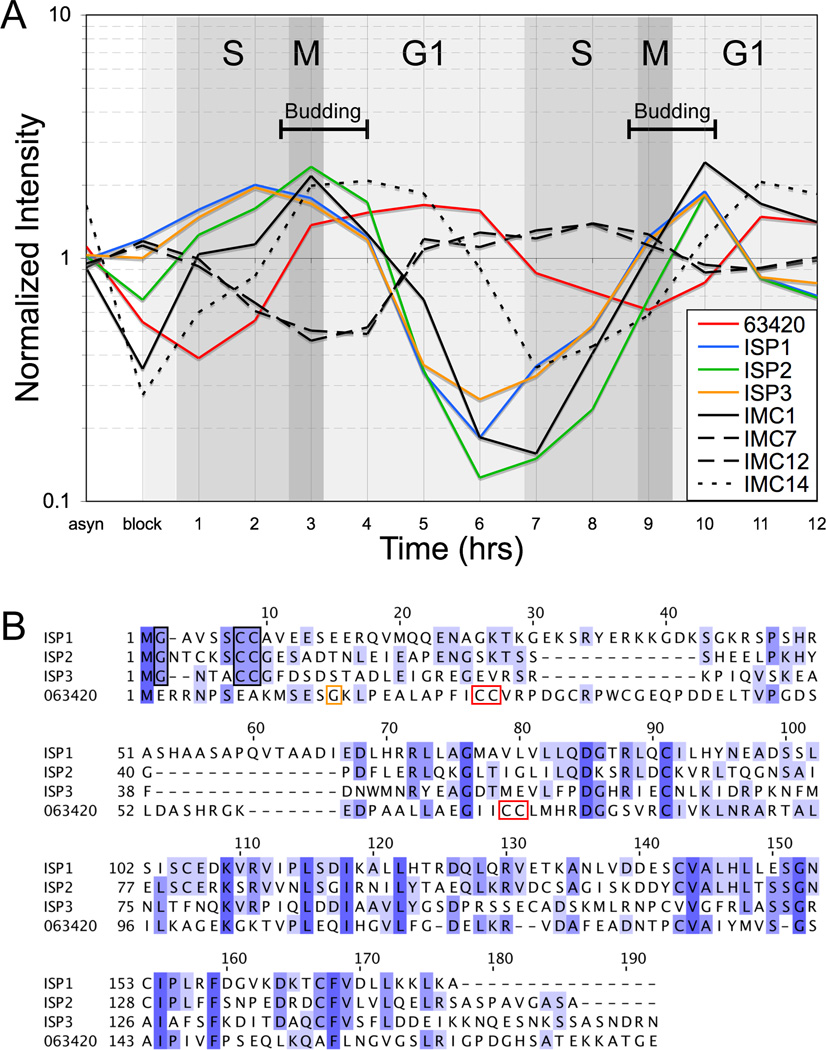
We previously identified a fourth putative ISP family member (TGGT1_063420) in the Toxoplasma genome. However this protein was not examined further due to an uncertain gene model and its low expression level relative to the other ISPs [11]. Analysis of expression timing data for TGGT1_063420 reveals a periodic pattern with peak transcription levels consistent with a cell-cycle regulated protein [13] (Figure 1A). Interestingly, a comparison with ISP1–3 as well as other characterized IMC proteins shows that peak expression of TGGT1_063420 lags behind ISP1–3 and most of the known components of the IMC protein meshwork (IMC1, 3–6, 8–11, 13 and 15) by 1 hour, similar to the IMC meshwork protein IMC14 [24]. Taken together with the importance of ISP2 in parasite division, these observations provided an impetus for further characterization of TGGT1_063420.
Figure 1. Toxoplasma TGGT1_063420 is a fourth member of the ISP family.
A. The expression profile of TGGT1_063420 throughout the Toxoplasma cell cycle was compared to the profiles of ISP1–3 and the intermediate filament-like IMC proteins of protein meshwork. Three general classes of periodic transcription profiles are observed for IMC proteins with peaks early in budding (ISP1–3, IMC1), late in budding (IMC14), or outside of budding during late G1/S (IMC7 & 12). TGGT1_063420 expression also adopts a periodic pattern indicating is it regulated in a cell-cycle dependent manner with peak expression lagging behind ISP1–3 by one hour, similar to IMC14.
B. A sequence alignment of TGGT1_063420 with ISP1–3 shows the highest degree of homology within the C-terminal two-thirds of the proteins, while the N-terminal regions are more divergent. Notably, ISP1/2/3 each contain a conserved glycine at position two, which is myristoylated, and a pair of cysteines within the first ten residues, which are palmitoylated (black boxes). However, these conserved residues, which are essential for IMC targeting, are not present within the TGGT1_063420 protein sequence.
TGGT1_063420 does contain two pairs of cysteines at positions 26,27 and 72,73, which are predicted to be palmitoylated (red boxes). A single glycine residue is present upstream of the first cysteine pair at position 15 (yellow box). ISP1–3 sequences are available in GenBank under the accession numbers HQ012577-HQ012579. The TGGT1_063420 (ISP4) sequence is available under the accession number JX082399.
The gene model for TGGT1_063420 indicates the protein lacks the conserved myristoylation and palmitoylation signals in other ISP family members. However, only a single EST is available for TGGT1_063420 which covers the C-terminal two-thirds of the protein, leaving uncertainty about the N-terminus of the gene model. To determine the correct coding sequence, we cloned and sequenced cDNAs for this gene. This showed that the predicted first exon of the gene model is inaccurate and revealed the correct first exon, which agrees with a recently generated RNAseq data set [25]. Surprisingly, the corrected protein sequence also lacks the conserved myristoylation signal required for targeting other ISP family members to the IMC membranes, although two cysteine pairs predicted for palmitoylation are present further into the sequence (Figure 1B). Despite these differences, sequence similarity to ISP1–3 suggests that TGGT1_063420 is an ISP family member.
3.2 Characterization of ISP4
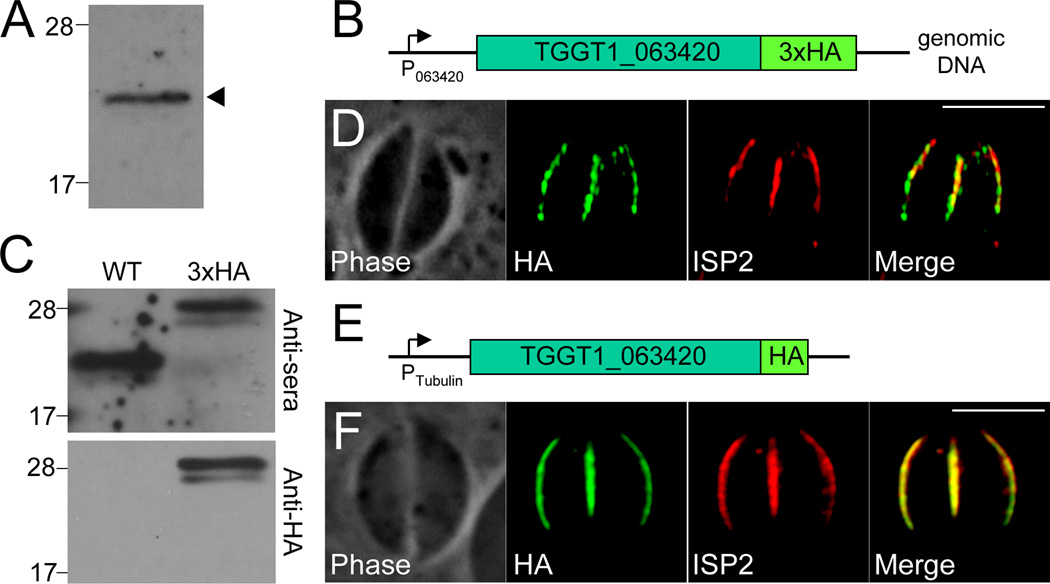
To determine whether TGGT1_063420 localizes to the IMC, we raised an antibody against a recombinant portion of the protein. The resulting anti-sera detected a band at ~22 kD by Western blot of Toxoplasma lysates, the expected size for the protein (Figure 2A). However, the anti-sera did not give any signal by immunofluorescence assay (IFA) under a variety of fixation conditions (data not shown). To resolve the localization of TGGT1_063420, we integrated a 3xHA endogenous tag at the C-terminus of the protein (Figure 2B). Western blot analysis with our anti-sera shows an ~5 kD up-shift in the target band corresponding to the triple HA fusion, validating the specificity of the antibody (Figure 2C). A band of the same size is also seen with anti-HA antibodies in the tagged strain but not the parent (Figure 2C), confirming that this clone contains an endogenous tag of the gene. Although signal strength was poor (likely due to low expression levels), IFA using an anti-HA antibody shows that this protein targets to the central region of the IMC and is not found in the apical cap or basal IMC sub-compartments, similar to the localization of ISP2 (Figure 2D). To more clearly evaluate localization, we expressed a second copy of the gene from the stronger tubulin promoter and observed clear co-localization with ISP2 (Figure 2E–F). Thus, we named this protein ISP4.
Figure 2. TGGT1_063420 (ISP4) localizes to the central sub-compartment of the IMC.
A. Western blot analysis of Toxoplasma lysates with anti-TGGT1_063420 detects a single band at ~20 kD, which is the predicted size of the protein.
B. Schematic showing integration of a 3xHA epitope tag at the C-terminus of the endogenous TGGT1_063420 locus.
C. Western blot analysis of parental and TGGT1_063420-3xHA lysates. Anti-TGGT1_063420 detects a band at ~20 kD in the parental strain and a band with a ~5 kD upshift (corresponding to the addition of the 3xHA tag) in TGGT1_063420-3xHA parasites (upper panel). Anti-HA detects a band with the same upshift in TGGT1_063420-3xHA parasites and nothing in the wild-type lane (lower panel), confirming that the TGGT1_063420-3xHA strain contains the expected endogenous tag of TGGT1_063420.
D. TGGT1_063420-3xHA signal co-localizes with ISP2 in the central IMC sub-compartment and thus the protein was named ISP4. Like ISP2, ISP4 signal is not detected in the apical cap or basal sub-compartments of the IMC. Green: anti-HA antibody detected by Alexa488-anti-rabbit IgG. Red: anti-ISP2 antibody detected by Alexa594-anti-mouse IgG.
E. Schematic showing the expression of a 1xHA-tagged, second copy of ISP4 under the control of the tubulin promoter.
F. Expression of ISP4-HA under the control of the tubulin promoter results in higher protein levels and better signal detection. Clear co-localization is observed between ISP4 and ISP2. Green: anti-HA antibody detected by Alexa488-anti-rat IgG. Red: anti-ISP2 antibody detected by Alexa594-anti-mouse IgG. All scale bars = 5µm.
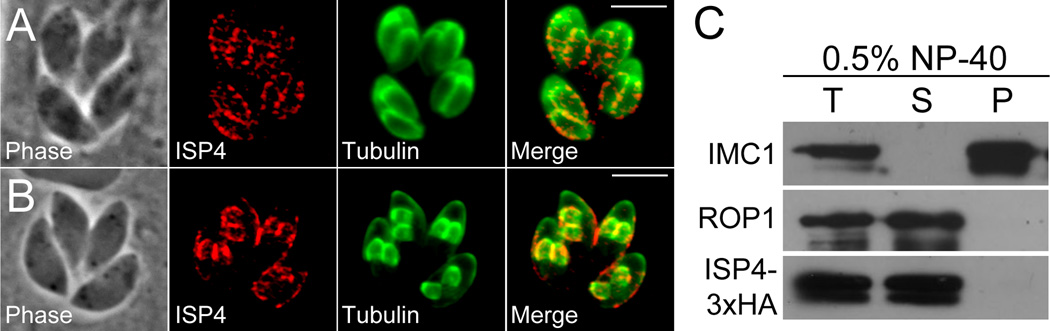
While co-localization with ISP2 suggests that ISP4 is associated with the IMC membranes, this data does not rule out the possibility of an association with the plasma membrane or cytoskeletal meshwork of the IMC. Because localization to the forming daughter buds precludes a plasma membrane association, since buds at this stage of division have not yet adopted the maternal plasma membrane, we examined whether ISP4 could be detected in dividing parasites similar to other ISP family members. We were able to detect the protein in forming daughter parasites in both the endogenous tagged strain (Figure 3A) and in parasites expressing a second copy of ISP4 under the control of the tubulin promoter (Figure 3B). This data indicates ISP4 is a component of the IMC and not the plasma membrane.
Figure 3. ISP4 localizes to the IMC in forming daughter parasites and is not imbedded in the underlying protein meshwork.
A. IFA of parasites late in endodyogeny. ISP4 is detected in forming daughter parasites, indicating an association with the IMC and not the plasma membrane. Red: anti-HA antibody detected by Alexa594-anti-mouse IgG. Green: anti-tubulin antibody detected by Alexa488-anti-rabbit IgG.
B. IFA of tubulin promoter-driven ISP4-HA parasites at the midpoint of endodyogeny. ISP4 signal is seen in both daughters and in the central IMC sub-compartment of mature parasites. Red: anti-HA antibody detected by Alexa594-anti-mouse IgG. Green: anti-tubulin antibody detected by Alexa488-anti-rabbit IgG. Scale bars = 5µm.
C. ISP4 is not embedded in the IMC protein meshwork. ISP4–3xHA parasites were extracted with 0.5% NP-40 and separated into total (T), soluble (S), and pellet (P) fractions. While IMC1, which is part of the detergent-resistant IMC protein meshwork, remains in the pellet fraction, ISP4 and the soluble control protein ROP1 are found in the soluble fraction.
To address whether ISP4 associates with the cytoskeletal meshwork, we conducted a detergent extraction experiment with ISP4–3xHA strain parasites. In this experiment, ISP4 is completely solubilized by detergent extraction, similar to the soluble control protein ROP1, and does not fractionate with IMC1, a component of the insoluble IMC network (Figure 3C). These results indicate that ISP4 is not embedded in the cytoskeletal meshwork of the IMC, similar to ISP1–3.
Previously, we described a hierarchical membrane targeting system within the ISP family whereby ISP2/3 are excluded from the IMC apical cap compartment by an ISP1-dependent mechanism [11]. To assess whether this gate-keeping function of ISP1 is also responsible for exclusion of ISP4 from the apical cap, we expressed HA-tagged ISP4 under the control of the tubulin promoter in wild-type and Δisp1 parasites. In wild-type parasites, the central IMC sub-compartment localization of ISP4 terminates at a ring of TgCentrin2 annuli known to delineate the boundary between the ISP1 apical cap and the remainder of the IMC [11, 26] (Figure 4). In Δisp1 parasites, however, ISP4 is no longer excluded from the IMC apical cap but instead relocalizes into the cap region that is normally occupied by ISP1 (Figure 4). These results confirm that ISP4 is also subject to the ISP1-dependent hierarchical targeting system that acts on ISP2/3.
Figure 4. ISP4 is relocalized to the apical cap in the absence of ISP1.
ISP4 is subject to ISP1-dependent exclusion from the apical cap. To detect the borders of the apical cap IMC sub-compartment occupied by ISP1, we utilized a pan-centrin antibody that recognizes TgCentrin1 in the centriole as well as TgCentrin2 annuli (arrowheads), which delimit the boundary between the apical cap and the rest of the IMC. In wild-type parasites (WT), ISP4 localization ends sharply at the base of the apical cap. However, in Δisp1 parasites, ISP4 is relocalized anterior to the centrin annuli into the apical cap. Red: anti-HA antibody detected by Alexa594-anti-mouse IgG. Green: anti-TgCentrin1 antibody detected by Alexa488-anti-rabbit IgG. Scale bars = 5µm.
3.3. ISP4 is dispensable for the tachyzoite lytic cycle and endodyogeny
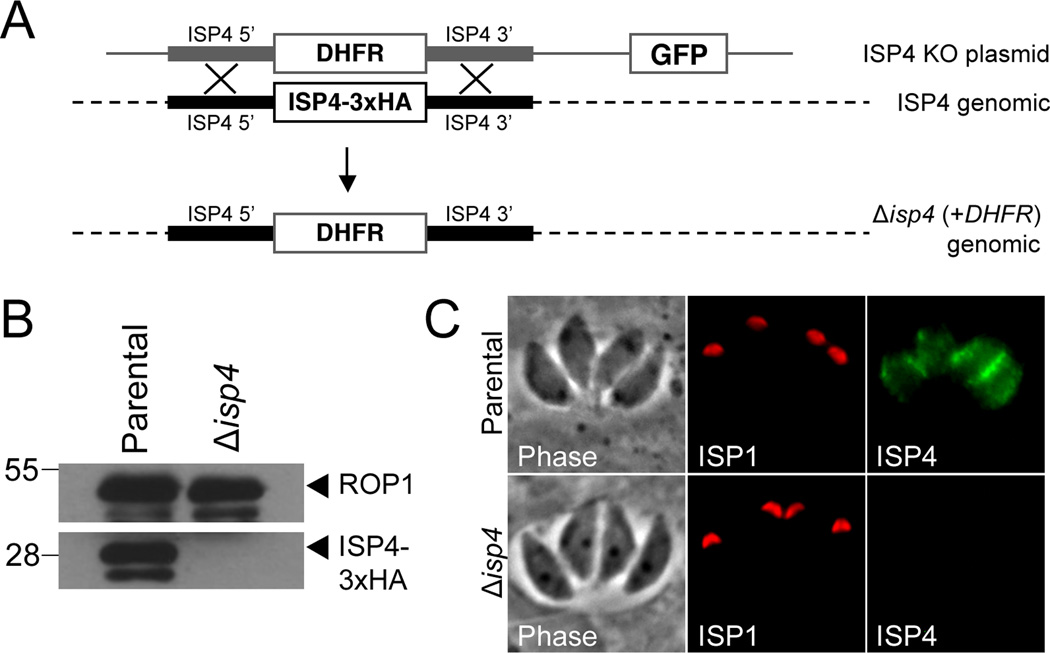
We have previously shown that ISPs 1–3 are not individually essential in T. gondii, but that parasites lacking ISP2 are substantially less fit and frequently switch from endodyogeny to an endopolygeny-like replicative mode [11]. To assess the consequence of the ablation of ISP4, we carried out gene disruption by homologous recombination in the ISP4–3xHA strain (Figure 5A). Disruption of ISP4 was confirmed by loss of HA staining by Western blot and IFA in a clonal Δisp4 line (Figure 5B–C). Using competitive growth assays and IFA, we examined the Δisp4 parasite strain for changes in fitness and daughter cell formation but found no apparent defects compared to the parental line (data not shown). These results demonstrate that ISP4 is not essential and suggest a possible functional redundancy among certain members of the ISP family. Additionally, the knockout provides a background lacking ISP4 to study the biosynthesis and trafficking of the protein to the IMC.
Figure 5. Disruption of the ISP4 gene.
A. ISP4 knockout schematic. The 5’ and 3’ genomic flanks of ISP4 were amplified and cloned into a knock-out construct containing the selectable marker DHFR-TSC3 and a downstream GFP cassette. Transfection of the knockout vector into ISP4–3xHA parasites allows for the replacement of ISP4–3xHA with the selectable marker DHFR-TSC3 via homologous recombination. The GFP cassette provides a convenient method for identification of non-homologous recombinants.
B. Western blot probed with mouse anti-HA. ISP4–3xHA was detected in the parental strain but not in Δisp4 parasites. ROP1 serves as a loading control.
C. IFA of parental and Δisp4 parasites. ISP4–3xHA is no longer detected in Δisp4 parasites. Red: anti-ISP1 antibody (mAb 7E8) detected by Alexa594-anti-mouse IgG. Green: anti-HA antibody detected by Alexa488-anti-rabbit IgG.
3.4. Determination of the correct ISP4 start methionine
The first exon of ISP4 contains two potential start methionines within the first eleven amino acid residues. As we could not determine which of these residues constitutes the correct start codon from the size of the protein on a gel, we engineered two untagged ISP4 cDNA expression vectors, one which contains both and the other which only contains the second methionine (designated M1 and M2, respectively, Figure 6A). Because detection of ISP4 expressed from its endogenous promoter is difficult, we drove expression of these constructs from the more robust tubulin promoter. We expressed each construct in Δisp4 parasites and compared their SDS-PAGE migration to the endogenous protein in wild-type parasites. Expression of ISP4 from the M1 construct resulted in a doublet with the top band corresponding with the size of endogenous ISP4. Expression of the M2 construct resulted in a single band that migrates at the same position as the lower band of the M1 doublet (Figure 6B). Together, these data indicate that endogenous ISP4 is primarily translated from the M1 start site and that the second start site is likely also used when driven from the tubulin promoter. A faint band at the M2 size is also detected for endogenous ISP4, suggesting that M2 may serve as a less frequent translation start site in the endogenous context, although we cannot exclude the possibility that this band is the result of degradation.
Figure 6. Determination of the correct ISP4 start methionine.
A. Schematic of M1 and M2 ISP4 expression constructs under the control of the tubulin promoter.
B. M1 and M2 constructs were expressed in Δisp4 parasites and compared to WT parasites by Western blot using anti-ISP4 antibody. Expression of the M1 version of ISP4 results in a doublet with the upper band running at the same size as the endogenous ISP4 band in WT parasites. Expression of M2 produces a single band which runs at the same size as the lower M1 band, indicating the M1 methionine is the correct ISP4 start codon. A faint lower band is also seen in WT parasites that corresponds with the M2 band and lower band of M1 doublet. These lower bands suggest that the M2 methionine can serve as an alternate start codon under the control of the tubulin promoter and less frequently in the endogenous context, but does not exclude the possibility that the lower band in WT parasites results from degradation.
3.5. Palmitoylation, but not myristoylation, is critical for ISP4 trafficking
Both myristoylation and palmitoylation are required to anchor the ISP1–3 proteins to the IMC membranes. This suggests a model wherein the second position glycine is myristoylated co-translationally by an N-myristoyltransferase, allowing ISP1–3 to transiently associate with the IMC. At the IMC membranes, the proteins then encounter a palmitoyl acyl transferase (PAT) which palmitoylates the conserved cysteine pair to strengthen and stabilize the association with the IMC [11].
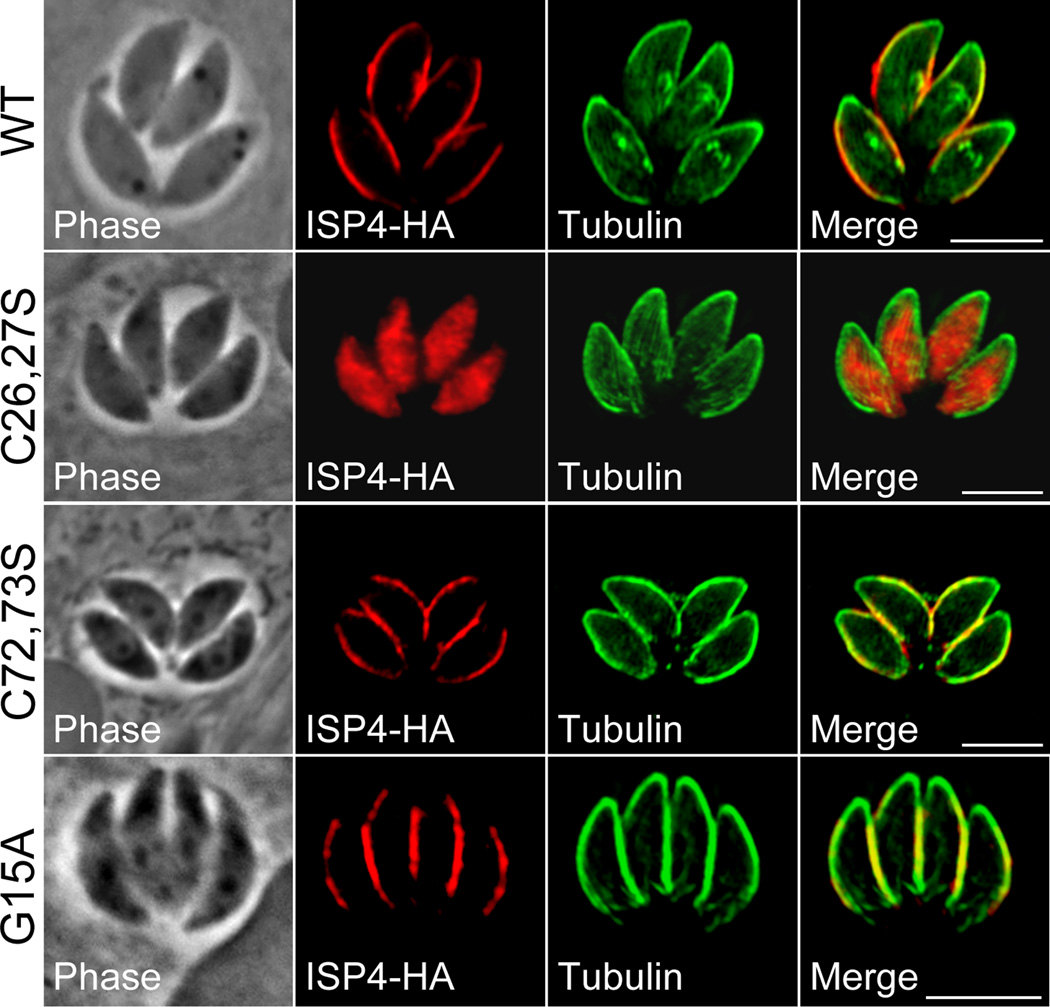
The absence of a position two glycine in ISP4 suggests that membrane association occurs via a mechanism distinct from the other ISPs. ISP4 does however contain two cysteine pairs that are predicted to be palmitoylated (Figure 1B, residues 26,27 and 72,73, red boxes). To assess their role in ISP4 trafficking, we mutated these cysteines pairwise into serines in our ISP4-HA expression construct. Mutation of the cysteine pair at position 26,27 abrogates IMC targeting, resulting in ISP4 localized throughout the parasite cytosol. In contrast, mutation of the cysteine pair at position 72,73 had no gross effect on the localization of ISP4 (Figure 7).
Figure 7. IMC targeting of ISP4 is dependent on residues predicted for palmitoylation but not myristoylation.
Mutations of indicated residues predicted for palmitoylation were generated in an HA epitope-tagged copy of ISP4 and expressed in parasites under the control of the tubulin promoter. Wild-type (WT) ISP4-HA targets to the central sub-compartment of the IMC, similar to the endogenous ISP4 protein. Mutations of the cysteine pair at position 26,27 into serines (C26,27S) results in gross mistargeting of ISP4 throughout the cytosol of the cell, while mutation of the cysteine pair at position 72,73 (C72,73S) produces no significant effect on targeting. Mutation of the N-terminal glycine at position 15 (G15A) into an alanine also produces no significant effect on ISP4-HA targeting.
Some proteins lacking a second position glycine are known to undergo N-myristoylation post-translationally following a protein cleavage event that exposes an internal glycine residue at the N-terminus of the processed protein [27]. Thus, while ISP4 does not contain a position two glycine, any glycine residue upstream of the critical cysteine pair at 26,27 could facilitate N-myristoylation following proteolysis. A single glycine present at position 15 fits these qualifications (Figure 1B, yellow box). To rule out the possibility of myristoylation in ISP4 targeting, we mutated this residue to alanine and found no targeting defects in this mutant (Figure 7). Together, these results indicate that ISP4 targeting is dependent upon cysteine residues 26,27, which are predicted for palmitoylation, but not on myristoylation, demonstrating a new means of targeting for this protein family.
4. Discussion
4.1. ISP4 is a additional member of the Toxoplasma ISP family
The Toxoplasma IMC is a critical organelle for processes of cell division and parasite motility. The ISPs are a family of IMC membrane-anchored proteins conserved throughout Apicomplexa but not present in other eukaryotes. Here, we have shown that the Toxoplasma ISP family contains a fourth member that targets to the central sub-compartment of the IMC and displays features common to other family members, including lipid-based anchoring to the IMC membranes and ISP1-dependent exclusion from the apical cap. Expression timing of IMC proteins generally fits into three categories which peak early in budding, late in budding, or outside of budding during late G1 (Figure 1A). The periodic expression timing observed for ISP4 fits the second category, lagging behind ISP1–3 by about one hour, similar to IMC14. Interestingly, while ISP4 is observed in forming buds (Figure 3A), IMC14 is only seen in mature parasites [24], suggesting that additional factors beyond timing of expression guide trafficking of these proteins to either the maternal or daughter IMC.
Similar to ISP1 and 3, ISP4 is dispensable and deletion of ISP4 produces no major changes in fitness or endodyogeny. In contrast, loss of ISP2, which also localizes to the central IMC sub-compartment, results in major fitness defects and replication errors. Interestingly, after serial passage for several months, Δisp2 parasites largely recover from replication and fitness [11], raising the possibility that changes in the regulation of ISP3 or ISP4 expression might compensate for the loss of ISP2 since they each localize to the central sub-compartment. Thus, it will be interesting to determine if coordinate disruption of multiple ISP genes will produce more severe defects in division. Considering the absence of identifiable domains in these apicomplexan-specific proteins, definition of the precise functional roles of the ISP family will benefit from combining these genetic approaches with future studies aimed at structural analysis.
4.2 ISP4 targeting is contingent on residues predicted for palmitoylation but not myristoylation
Eukaryotes often catalyze the addition of fatty acyl groups to proteins that lack transmembrane domains to mediate their association with lipid membranes. Targeting of ISP1/2/3 to the IMC appears to function via kinetic trapping [28] where initial myristoylation in the cytosol permits sampling of the IMC membranes, during which the proteins encounter a PAT that catalyzes their stable association with the IMC by palmitoylation [11]. As both modifications are strictly required for ISP1/2/3 targeting, we were surprised to find that ISP4 lacks the N-terminal myristoylation signal conserved in other family members. We further eliminated the possibility of a targeting-dependent myristoylation event at glycine 15. Similar to ISP1/2/3, targeting of ISP4 depends on a pair of cysteines (positions 26,27) predicted to be palmitoylated, although these resides are recessed further into the protein than the critical palmitoylation signals in ISP1/2/3. An additional pair of cysteines in ISP4 (positions 72,73) are predicted for palmitoylation but dispensable for targeting. A model of ISP protein sorting to the IMC membrane sub-compartments is presented in Figure 8.
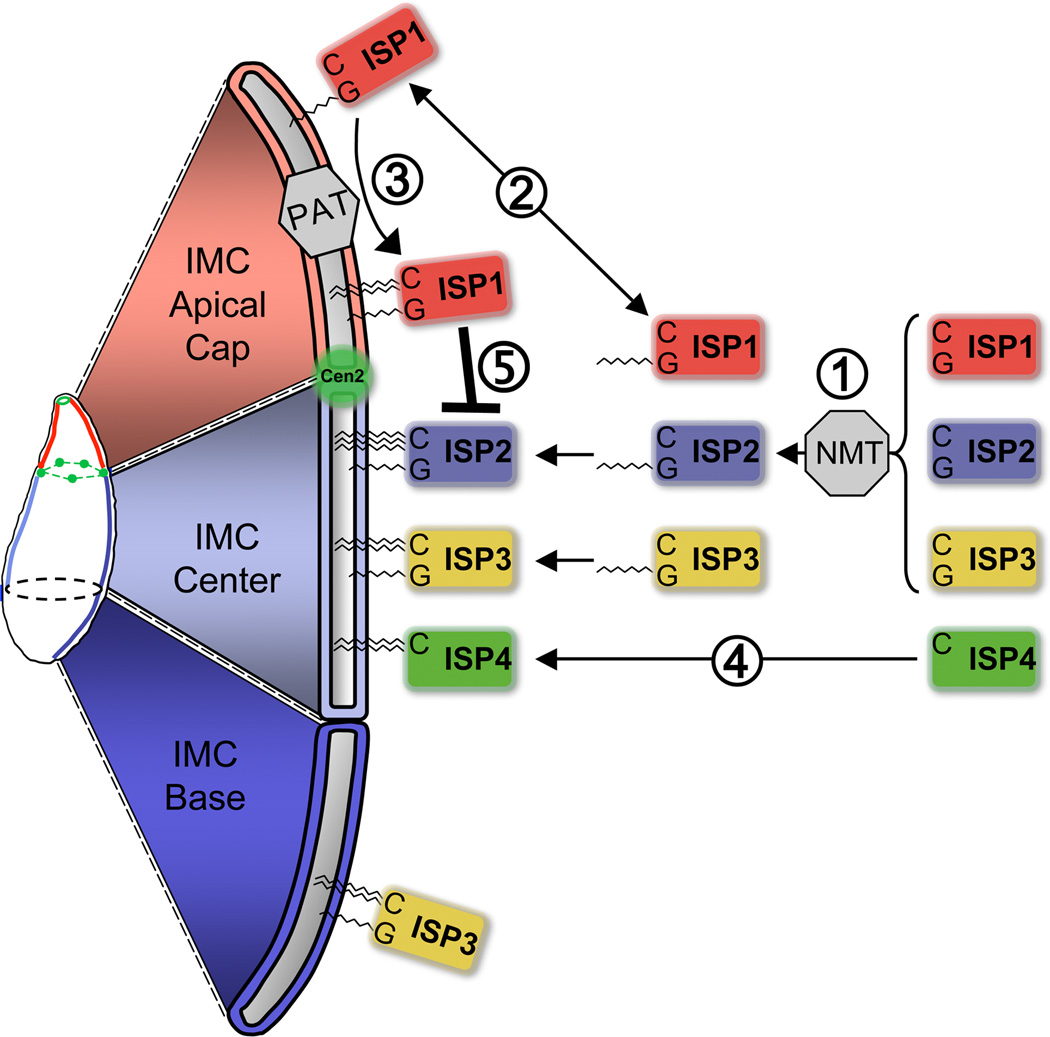
Figure 8. Model of ISP sorting within the Toxoplasma IMC.
Schematic showing the model for ISP sorting within the IMC. (1) ISP1/2/3 are myristoylated at a conserved position two glycine by an N-myristoyl transferase (NMT). (2) Myristoylation allows for ISP1/2/3 to transiently sample various membranes within the cell. (3) Once ISP1/2/3 associate with the IMC, different PATs or PAT activities located in the three IMC sub-domains recognize and palmitoylate their respective ISP substrates, locking ISP1/2/3 into specific sub-compartments. (4) ISP4 is targeted to the IMC central sub-compartment independent of a myristoylation event. (5) ISP2/3/4 are excluded from the apical cap in an ISP1-dependent manner. Modified from [11].
The finding that myristoylation does not play a role in ISP4 targeting is unexpected and in light of our kinetic trapping model, raises the question of how ISP4 is brought into close enough proximity to an IMC-resident PAT to become palmitoylated. It is possible that an accessory factor associates with ISP4 and delivers it to an IMC-resident PAT for acylation and future studies aimed at identification of ISP4 interacting partners will explore this possibility. However, many IMC proteins only contain palmitoylation signals (see below) and thus the necessity of myristoylation for ISP1/2/3 trafficking may represent an exception to a more general palmitoylation-only mechanism of membrane association. In addition, the fact that ISP4 is subject to the same ISP1-dependent apical cap exclusion as ISP2/3 indicates that myristoylation is not required for this hierarchical component of ISP sub-compartmentalization.
4.3 Palmitoylation is a critical lipid modification for IMC assembly and function
Palmitoylation is a unifying feature in the targeting of all four ISP family members and represents an emerging key activity for assembly and organization of the IMC. In addition to the ISPs, several other IMC proteins with diverse functions are known to undergo palmitoylation. The glideosome-associated protein GAP45 is localized throughout the IMC and tethered to the both the alveoli membranes and the cytosolic face of the plasma membrane. Plasma membrane association is accomplished by myristoylation and palmitoylation of the GAP45 N-terminus, while the C-terminus of this protein associates with the IMC, possibly via additional C-terminal palmitoylations [29, 30]. GAP70, a coccidian-specific homolog of GAP45, similarly bridges the plasma membrane and IMC via acylations, but localizes only to the apical cap. An additional member of the glidesome complex, myosin light chain 1, is predicted for palmitoylation at several N-terminal cysteines. Mutation of these cysteines simultaneously abolishes IMC association and GAP45 binding, suggesting that they are palmitoylated and important for IMC association, although this could not be separated from GAP45 binding. In addition to glideosome components, an isoform of the purine salvage enzyme hypoxanthine-xanthine-guanine phosphoribosyltransferase is localized to the IMC via palmitoylation but not myristoylation [31], showing that palmitoylation is also employed for recruiting metabolic activities to the IMC, although the purpose of targeting such an enzyme to the IMC remains unclear. Finally, several other IMC proteins contain residues that are predicted for palmitoylation but the importance of these residues for IMC targeting has not yet been directly tested. These include TgHSP20, which associates with the outer leaflet of the IMC membranes and plays a role in the regulation of gliding motility, the glidesome component myosin light chain 2, and several members of the intermediate filament-like IMC meshwork proteins, which may associate with the cytoplasmic face of the IMC membranes via this lipid modification [24, 32–34]. Considering the prevalence of IMC protein palmitoylation, identification of the enzyme(s) responsible for this modification will be an important step in unraveling the biology of this parasite organelle.
At present, no PATs have been reported on in the Apicomplexa. BLAST analysis of the T. gondii genome with known PAT sequences from S. cerevisiae identified eighteen putative PAT homologs, all of which contain the hallmark Asp-His-His-Cys-cysteine-rich domain (DHHC-CRD) [11, 35]. This relatively high number suggests that PATs may play an extensive role in the sorting of proteins to the unique and specialized membrane systems within Toxoplasma [36, 37]. The identification and localization of IMC PATs will provide a better understanding of ISP protein sorting within the IMC and open new avenues for biochemical analyses of the enzymatic activities that are critical to the organization of this unique membrane structure.
Table 1.
Primers used in this study as discussed in text. Restriction sites and mutated bases are shown in lowercase.
| P1 | CACCATGCCTGCTGTTTCCCAGGAGG |
| P2 | TTCTCCGGTTGCTTTCTTTTCTGTAGC |
| P3 | GGGTCCTGGTTCGATGCATAGTCGGAAAGG |
| P4 | CTTGTTCGTGCTGTTTACTAGAACAGATTCGTCTTTC |
| P5 | TACTTCCAATCCAATTTAGCAGTTGATTGTGCTTCTGTGC |
| P6 | TCCTCCACTTCCAATTTTAGCTTCTCCGGTTGCTTTCTTTTCTG |
| P7 | GCGCagatctATGGAAAGACGAAATCCATC |
| P8 | GTACggcgcgccTCATGCGTAGTCGGGGACGTCGTACGGGTATTCTCCGGTTGCTTTCTTTTCTGTAGC |
| P9 | GACTgcggccgcCAACTTCCACTGGGGTATAC |
| P10 | GACTgcggccgcGTTGCTCTCATCACAGCGAG |
| P11 | GACTgggcccGAGACGAGCGACCACCTC |
| P12 | GTCAgggcccGACGCTGGTTCAGTTTGCG |
| P13 | GTACggcgcgccCTATTCTCCGGTTGCTTTCTTTTC |
| P14 | GCGCagatctATGTCGGAAAGTGGCAAGC |
| P15 | CACTAGCGCCATTCATCTcgaGCGTCcGACCTGATGGTTGCCG |
| P16 | GCCGAAGGAATTATcTcgaGCcTAATGCACCGTGACGG |
| P17 | GGCGAAGATGTCGGAAAGTGcCAAGCTtCCTGAAGCACTAG |
Highlights.
ISP4 is a fourth member of the ISP family of Toxoplasma IMC proteins.
ISP4 localizes to the central IMC sub-compartment.
ISP4 is subject to ISP1-dependent apical cap exclusion, similar to ISP2/3.
In contrast to other ISPs, myristoylation does not play a role in ISP4 targeting.
ISP4 targeting is dependent upon cysteine residues predicted for palmitoylation.
Acknowledgements
This work was supported by the Microbial Pathogenesis Training Grant (T32-AI07323) to JRB, an NIH R01 (AI064616) to PJB and an NIH R01 (AI081924) to MJG. We thank members of the Bradley lab for helpful discussions.
Abbreviations
- IMC
inner membrane complex
- ISP
IMC Sub-compartment Protein
- PAT
palmitoyl acyl transferase
- NMT
N-myristoyl transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hay SI, Okiro EA, Gething PW, Patil AP, Tatem AJ, Guerra CA, et al. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000290. e1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill DE, Chirukandoth S, Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- 3.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin S. Congenital toxoplasmosis. Neonatal Netw. 2001;20:23–30. doi: 10.1891/0730-0832.20.4.23. [DOI] [PubMed] [Google Scholar]
- 5.D'Haese J, Mehlhorn H, Peters W. Comparative electron microscope study of pellicular structures in coccidia (Sarcocystis, Besnoitia and Eimeria) Int J Parasitol. 1977;7:505–518. doi: 10.1016/0020-7519(77)90014-5. [DOI] [PubMed] [Google Scholar]
- 6.Mann T, Beckers C. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol Biochem Parasitol. 2001;115:257–268. doi: 10.1016/s0166-6851(01)00289-4. [DOI] [PubMed] [Google Scholar]
- 7.Porchet E, Torpier G. Freeze fracture study of Toxoplasma and Sarcocystis infective stages (author's transl) Z Parasitenkd. 1977;54:101–124. doi: 10.1007/BF00380795. [DOI] [PubMed] [Google Scholar]
- 8.Hakansson S, Morisaki H, Heuser J, Sibley LD. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol Biol Cell. 1999;10:3539–3547. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeley A, Soldati D. The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. doi: 10.1016/j.tcb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Striepen B, Jordan CN, Reiff S, van Dooren GG. Building the perfect parasite: cell division in apicomplexa. PLoS Pathog. 2007;3:e78. doi: 10.1371/journal.ppat.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck JR, Rodriguez-Fernandez IA, Cruz de Leon J, Huynh MH, Carruthers VB, Morrissette NS, et al. A novel family of Toxoplasma IMC proteins displays a hierarchical organization and functions in coordinating parasite division. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001094. e1001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donald RG, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- 13.Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, Nawas J, et al. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012354. e12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrissette NS, Sibley LD. Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J Cell Sci. 2002;115:1017–1025. doi: 10.1242/jcs.115.5.1017. [DOI] [PubMed] [Google Scholar]
- 15.Wichroski MJ, Melton JA, Donahue CG, Tweten RK, Ward GE. Clostridium septicum alpha-toxin is active against the parasitic protozoan Toxoplasma gondii and targets members of the SAG family of glycosylphosphatidylinositol-anchored surface proteins. Infect Immun. 2002;70:4353–4361. doi: 10.1128/IAI.70.8.4353-4361.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartzman JD, Krug EC. Toxoplasma gondii: characterization of monoclonal antibodies that recognize rhoptries. Exp Parasitol. 1989;68:74–82. doi: 10.1016/0014-4894(89)90010-6. [DOI] [PubMed] [Google Scholar]
- 17.Alexandrov A, Vignali M, LaCount DJ, Quartley E, de Vries C, De Rosa D, et al. A facile method for high-throughput co-expression of protein pairs. Mol Cell Proteomics. 2004;3:934–938. doi: 10.1074/mcp.T400008-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Gubbels MJ, Vaishnava S, Boot N, Dubremetz JF, Striepen B. A MORN-repeat protein is a dynamic component of the Toxoplasma gondii cell division apparatus. J Cell Sci. 2006;119:2236–2245. doi: 10.1242/jcs.02949. [DOI] [PubMed] [Google Scholar]
- 19.Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem. 2005;280:34245–34258. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- 20.Bradley PJ, Hsieh CL, Boothroyd JC. Unprocessed Toxoplasma ROP1 is effectively targeted and secreted into the nascent parasitophorous vacuole. Mol Biochem Parasitol. 2002;125:189–193. doi: 10.1016/s0166-6851(02)00162-7. [DOI] [PubMed] [Google Scholar]
- 21.Konrad C, Wek RC, Sullivan WJ., Jr A GCN2-like eukaryotic initiation factor 2 kinase increases the viability of extracellular Toxoplasma gondii parasites. Eukaryot Cell. 10:1403–1412. doi: 10.1128/EC.05117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huynh MH, Carruthers VB. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot Cell. 2009;8:530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gubbels MJ, Li C, Striepen B. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob Agents Chemother. 2003;47:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson-White BR, Ivey FD, Cheng K, Szatanek T, Lorestani A, Beckers CJ, et al. A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii. Cell Microbiol. 2011;13:18–31. doi: 10.1111/j.1462-5822.2010.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid AJVS, Cotton JA, Harris D, Hill-Cawthorne GA, Könen-Waisman S, Latham SM, Mourier T, Norton R, Quail MA, Sanders M, Shanmugam D, Sohal A, Wasmuth JD, Brunk B, Grigg ME, Howard JC, Parkinson J, Roos DS, Trees AJ, Berriman M, Pain A, Wastling JM. Comparative Genomics of the Apicomplexan Parasites Toxoplasma gondii and Neospora caninum: Coccidia Differing in Host Range and Transmission Strategy. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002567. e1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu K, Johnson J, Florens L, Fraunholz M, Suravajjala S, DiLullo C, et al. Cytoskeletal components of an invasion machine--the apical complex of Toxoplasma gondii. PLoS Pathog. 2006;2:e13. doi: 10.1371/journal.ppat.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin DD, Beauchamp E, Berthiaume LG. Post-translational myristoylation: Fat matters in cellular life and death. Biochimie. 2011;93:18–31. doi: 10.1016/j.biochi.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol. 2006;2:584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- 29.Rees-Channer RR, Martin SR, Green JL, Bowyer PW, Grainger M, Molloy JE, et al. Dual acylation of the 45 kDa gliding-associated protein (GAP45) in Plasmodium falciparum merozoites. Mol Biochem Parasitol. 2006;149:113–116. doi: 10.1016/j.molbiopara.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe. 2010;8:343–357. doi: 10.1016/j.chom.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary K, Donald RG, Nishi M, Carter D, Ullman B, Roos DS. Differential localization of alternatively spliced hypoxanthine-xanthine-guanine phosphoribosyltransferase isoforms in Toxoplasma gondii. J Biol Chem. 2005;280:22053–22059. doi: 10.1074/jbc.M503178200. [DOI] [PubMed] [Google Scholar]
- 32.Polonais V, Javier Foth B, Chinthalapudi K, Marq JB, Manstein DJ, Soldati-Favre D, et al. Unusual anchor of a motor complex (MyoD-MLC2) to the plasma membrane of Toxoplasma gondii. Traffic. 2011;12:287–300. doi: 10.1111/j.1600-0854.2010.01148.x. [DOI] [PubMed] [Google Scholar]
- 33.de Miguel N, Lebrun M, Heaslip A, Hu K, Beckers CJ, Matrajt M, et al. Toxoplasma gondii Hsp20 is a stripe-arranged chaperone-like protein associated with the outer leaflet of the inner membrane complex. Biol Cell. 2008;100:479–489. doi: 10.1042/BC20080004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montagna GN, Buscaglia CA, Munter S, Goosmann C, Frischknecht F, Brinkmann V, et al. Critical role for heat shock protein 20 (HSP20) in migration of malarial sporozoites. J Biol Chem. 2012;287:2410–2422. doi: 10.1074/jbc.M111.302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 36.Saric M, Vahrmann A, Niebur D, Kluempers V, Hehl AB, Scholze H. Dual acylation accounts for the localization of {alpha}19-giardin in the ventral flagellum pair of Giardia lamblia. Eukaryot Cell. 2009;8:1567–1574. doi: 10.1128/EC.00136-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL, Engman DM. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J Cell Sci. 2009;122:867–874. doi: 10.1242/jcs.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]