Abstract
Background
Huntington’s Disease (HD) is a neurodegenerative disorder characterized by early cognitive decline, which progresses at later stages to dementia and severe movement disorder. HD is caused by a cytosine-adenine-guanine triplet-repeat expansion mutation in the Huntingtin gene, allowing early diagnosis by genetic testing. This study aims to identify the relationship of N-acetylaspartate and other brain metabolites to cognitive function in HD-mutation carriers by using high field strength magnetic-resonance-spectroscopy at 7-Tesla.
Methods
Twelve individuals with the HD-mutation in premanifest or early stage of disease versus twelve healthy controls underwent 1H magnetic-resonance-spectroscopy (7.2ml voxel in the posterior cingulate cortex) at 7-Tesla, and also T1-weighted structural magnetic-resonance-imaging. All participants received standardized tests of cognitive functioning including the Montreal Cognitive Assessment and standardized quantified neurological examination within an hour before scanning.
Results
Individuals with the HD mutation had significantly lower posterior cingulate cortex N-acetylaspartate (−9.6%, p=0.02) and glutamate levels (−10.1%, p=0.02) than controls. By contrast, in this small group, measures of brain morphology including striatal and ventricle volumes did not differ significantly. Linear regression with Montreal Cognitive Assessment scores revealed significant correlations with N-acetylaspartate (r2=0.50, p=0.01) and glutamate (r2=0.64, p=0.002) in HD subjects.
Conclusions
Our data suggest a relationship between reduced N-acetylaspartate and glutamate levels in the posterior cingulate cortex with cognitive decline in early stages of HD. N-acetylaspartate and glutamate magnetic-resonance-spectroscopy signals of the posterior cingulate cortex region may serve as potential biomarkers of disease progression or treatment outcome in HD and other neurodegenerative disorders with early cognitive dysfunction, when structural brain changes are still minor.
Keywords: MRS, NAA, glutamate, cognition, neurodegeneration, biomarker
Introduction
Huntington’s Disease (HD) is a neurodegenerative disorder, which is characterized by abnormal movements and early cognitive decline progressing to dementia 1–6. HD is caused by a cytosine-adenine-guanine (CAG) -repeat length expansion of the Huntingtin gene, resulting in a toxic effect of the mutant Huntingtin protein in neuronal populations of the central nervous system 7, 8. Subjects at risk for HD can be identified by predictive genetic testing when they still are in the “premanifest” or “prodromal” phase, and do not have movement disorder sufficient for clinical diagnosis 9, 10. HD therefore has been referred to as a model for other more common neurodegenerative disorders such as Alzheimer’s or Parkinson’s disease 7, 8. In the present study, the “premanifest” period will be referred to as any time before the presence of diagnosable motor signs and symptoms of HD, and the “prodromal” phase as the period closer to diagnosable onset, when there may be subtle motor, cognitive or emotional changes not yet sufficient to warrant a diagnosis of clinical HD 7, 8.
While individuals with the HD gene mutation develop characteristical morphological brain changes in the prodromal phase 4, 11–13, including cortical volume loss correlating with cognitive deficits 14–16, metabolic alterations may precede structural neuronal damage 17. There is considerable evidence for low N-acetyl aspartate (NAA) as a reflection of metabolic disturbances, and possibly neuronal loss, in subjects with the mutation responsible for HD 18–23. Recent magnetic-resonance-spectroscopy (MRS) studies additionally suggest elevated myo-inositol (mI) and reduced glutamate as possible biomarkers of HD-manifestation and progression 24, 25. However, at this point there are still relatively few studies of metabolic biomarkers in HD before significant atrophy of brain tissue, which would be a particularly promising time-point for therapeutic interventions, as brain alterations still might be reversible 26–28.
Dementia is a central clinical finding in HD, and first signs of cognitive dysfunction are observable in the prodromal phase, significantly preceding motor symptoms 2, 3, 5, 6, 26, 29. It therefore is the aim of this study to investigate NAA and other detectable brain metabolites in relation to cognitive decline in a sample of subjects with the HD mutation (most still clinically not diagnosable and thus “premanifest” or “prodromal”) versus unaffected controls.
To maximize sensitivity of MRS detection and the specificity of resonance assignment, this study was performed at the high field strength of 7 Tesla (7T). This has been reported to achieve increased signal to noise ratio (SNR) and spectral resolution compared to lower field strength 30. While most MRS-studies on HD so far have focused on the striatal area 19, 21, 24, 25 we focused on the posterior cingulate cortex (PCC), as distinct alterations related to cognitive deficits have been reported both for early HD 16 and also other neurodegenerative disorders 31–35. Based on previous studies, we hypothesized that PCC levels of NAA and possibly other brain metabolites would be reduced in subjects with the mutation responsible for HD, that metabolite levels would be related to impaired cognitive performance in premanifest and early HD, and that detection of decreased metabolite levels could be achieved even in the absence of significant volume loss of brain tissue.
Methods
Study population
Twelve subjects positive for the CAG-expansion in Huntingtin were recruited through the Baltimore Huntington’s Disease Center at Johns Hopkins University School of Medicine. Estimated time to onset of motor symptoms was calculated based on CAG-repeat length of the mutated Huntingtin allele and age 36. Disease burden score (DBS) was calculated as [(CAG-repeat length −35.5) * age]37. All gene-positive subjects received standardized neurological examination 38, two subjects had diagnosable HD based on movement disorder, the ten other participants where in the premanifest or prodromal period. Additionally twelve age-, sex- and education level-matched control subjects were recruited through Johns Hopkins University. None of the 24 participants had a history of diagnosed mood-, obsessive compulsive-, or psychotic disorder or substance abuse. Consent was obtained according to the Declaration of Helsinki 39 and approved by the Johns Hopkins University Institutional Review Board with respect to the United States Health Insurance Portability and Accountability Act of 1996 (“HIPAA”). Clinical personnel, trained in neuropsychological subject evaluation, performed the following interviews and tests on the day of scanning: Beck Depression Inventory (BDI) 40, Hamilton Depression Rating Scale (HDRS) 41, Mini Mental State Exam (MMS) to screen for dementia 42, the MOntreal Cognitive Assessment (MOCA) to screen for mild cognitive dysfunction 43, and the National Adult Reading Test (NART) as an estimate of premorbid intelligence (Full Scale Intelligence Quotient – FSIQ, Verbal Intelligence Quotient – VIQ) 44. A subgroup of early HD subjects with mild cognitive dysfunction as defined by MOCA-scores < 26 was formed 43. MOCA scores were lower in the HD subjects (Table 1); there were no significant differences in the other parameters assessed.
Table 1.
Demographic data and clinical assessment scores for controls and HD-subjects. Data are presented as mean (standard deviation).
| Controls | Early HD | T-test (p) | |
|---|---|---|---|
|
|
|||
| N | 12 | 12 | - |
| Females | 50% | 50% | 1 |
| Age | 43.2 (15) | 46.3 (7.9) | 0.53 |
| CAG repeat length | - | 43.8 (2.8) | - |
| Disease burden score (DBS) | - | 370 (97) | - |
| Estd. years to onset (YTO) | - | 7.7 (3.1) | - |
| QNE total | - | 9.8 (14) | - |
| QNE chorea | - | 2 (3.1) | - |
| Years of education | 18.5 (4.3) | 16 (3.2) | 0.12 |
| MMSE | 29.4 (1.7) | 28.4 (1.9) | 0.2 |
| MOCA | 28.5 (2.2) | 25 (4.7) | * 0.03 |
| NART-Verbal IQ | 113 (9.6) | 111 (10) | 0.58 |
| NART-Full Scale IQ | 114 (8.9) | 112 (9.0) | 0.65 |
| HDRS | 1.0 (1.9) | 2.7 (4.4) | 0.24 |
| BDI | 1.3 (1.5) | 1.9 (2.5) | 0.5 |
MRI and MRS acquisition
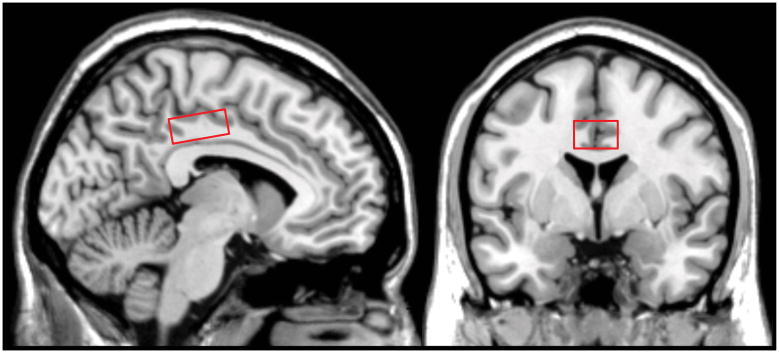
Structural magnetic resonance imaging (MRI) and MRS were performed at the F.M. Kirby Center for Functional Brain Imaging at the Kennedy Krieger Institute using a Philips 7-Tesla Achieva whole-body scanner (Philips Healthcare, Best, The Netherlands) equipped with a Nova Medical quadrature transmit head coil and 32-channel receive coil array. A high quality T1-weighted MPRAGE structural brain image (TE/TR=2.1ms/4.8ms; resolution: 0.6*0.6*0.6mm3; total scan time: 6min 32sec) was acquired for planning of the MRS voxel position and tissue segmentation. Localized proton spectra were acquired using a Stimulated Echo Acquisition Mode (STEAM) sequence 45, 46 with the following parameters: TE/TR/NS 13ms/3000ms/112. The 20x30x12mm3 voxel was placed bilaterally on the posterior cingulate cortex aligned tangential to the corpus callosum in a sagittal plane, centered on the midline, positioned a-p with the posterior edge centered to the splenium. (PCC, Figure 1). An unsuppressed water reference scan was acquired for quantification (TE/TR/NS 13ms/3000ms/112).
Figure 1.
Location of the voxel used for MRS within the posterior cingulate cortex region.
Post acquisition image analysis and MRS quantification
Analysis of T1 MPRAGE images was performed using TOpology-preserving, Anatomy-Driven Segmentation (TOADS) with desired topology and relationships as given by a template (http://medic.rad.jhmi.edu/download/public/index.shtml) 47. Resulting volumes of main cerebral structures were used for subsequent group comparisons of regional brain morphology.
To test for partial volume bias, the coordinate information for the volume of interest used for MRS was transformed into the coordinate space of the 7T MR acquisition. By segmenting the MR volume 47, each voxel was given a discrete classification of either cerebrospinal fluid (CSF), gray matter (GM), and white matter (WM) based on a fuzzy topological consistent classification. A simple ratio comparison was done in the transformed regions, comparing the mean ratios of each populations to the other and also to the mean of the entire population with unpaired t-tests showing no significant difference. Additional segmentation of the PCC-region resulting in subvolumes of the cingulate gyrus (anterior cingulate, anterior-middle cingulate, posterior-middle cingulate, dorsal posterior cingulate and ventral posterior cingulate) was performed with the Freesurfer image analysis suite, using standard operations for construction of cortical models and further data analysis (http://surfer.nmr.mgh.harvard.edu/). Quantification of MRS spectra was performed using the LCModel 48, 49 with unsuppressed water as internal reference (Figure 2). The basis set included a total of 19 metabolites: N-acetylaspartate (NAA), γ-aminobutyric acid (GABA), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (Ins), N-acetylaspartylglutamate (NAAG), phosphoethanolamine (PE), taurine (Tau), total Choline (glycerophosphocholine and phosphocholine), total creatine (phosphocreatine and creatine), alanine (Ala), ascorbate (Asc), aspartate (Asp), glucose (Glc), glycine (Gly), guanidinoacetate (Gua), lactate (Lac), scyllo-inositol (scyllo-Ins). Metabolites included in the basis-set that yielded values with Cramér-Rao lower bound (CRLB) above 20% in this study were excluded. Also metabolites with CRLB <=20% in fewer than 20 study participants were also excluded from the group analysis. There were no quality differences observable between controls and HD-subjects regarding MRS measures. The metabolites included in the current study had CRLB below 20% for all 24 participants (Table 3).
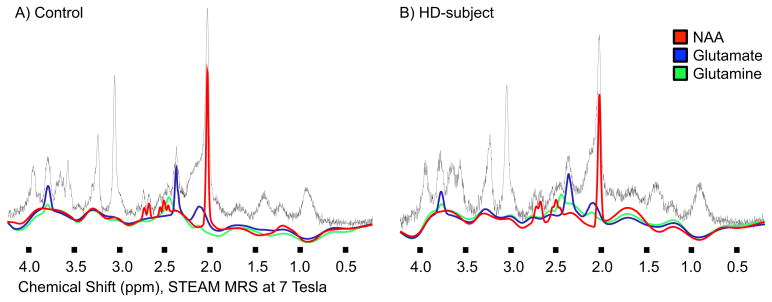
Figure 2.
Spectral fit obtained with LCModel for NAA, glutamate and glutamine, examples for one unaffected control and one HD subject.
Table 3.
Metabolite concentrations measured by MRS, data are presented as mean (standard deviation). Abbreviations: N-acetylaspartate (NAA), γ-aminobutyric acid (GABA), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (Ins), N-acetylaspartylglutamate (NAAG), phosphoethanolamine (PE), taurine (Tau), total choline (glycerophosphocholine and phosphocholine), total creatine (phosphocreatine and creatine).
| Metabolite Concentrations (i.U.) | CRLB | |||
|---|---|---|---|---|
|
|
||||
| Controls | Early HD | T-test (p) | Entire Sample | |
| NAA | 14.2 (1.5) | 12.9 (1.1) | * 0.02 | 3.46 (1.1) |
| GABA | 2.79 (0.7) | 2.64 (0.5) | 0.54 | 15.0 (3.1) |
| Gln | 3.77 (0.8) | 3.48 (0.8) | 0.38 | 15.0 (4.1) |
| Glu | 15.3 (1.8) | 13.8 (1.23 | * 0.02 | 5.17 (0.5) |
| GSH | 1.65 (0.4) | 1.49 (0.2) | 0.29 | 16.0 (3.1) |
| mI | 6.65 (1.1) | 6.59 (1.1) | 0.90 | 9.21 (1.2) |
| NAAG | 3.00 (0.9) | 2.91 (0.7) | 0.78 | 12.5 (3.0) |
| PE | 4.19 (0.7) | 3.67 (0.6) | 0.07 | 14.7 (2.6) |
| Tau | 2.14 (0.5) | 2.33 (0.4) | 0.32 | 15.9 (3.4) |
| Total Choline | 2.30 (0.4) | 2.18 (0.2) | 0.34 | 6.46 (0.9) |
| Total Creatine | 13.2 (0.9) | 12.7 (0.9) | 0.15 | 3.00 (0.3) |
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 17.0, SPSS Inc, Chicago, IL, USA). Group differences of clinical measures and brain volumes derived from segmentation were assessed using independent sample t-tests. Analysis of covariance (ANCOVA) was used to test for metabolite group differences with age as a covariate and also to test for an interaction effect of age and disease-status. To confirm specificity of differences of metabolite levels, metabolites differing in the initial ANCOVA were in addition converted to Z-scores (z=(x-μ)/σ) indicating distance from controls (mean) in standard deviations and compared to total choline Z-scores using independent sample t-tests. Metabolite concentrations were tested for normal distribution and applicability of parametric testing by applying a one-sample Kolmogorov-Smirnov test, resulting in confirmation of the null hypothesis. Statistics were performed using the SPSS statistical software package for Windows, Version 17.0. We predicted that NAA would be lower; other tests were exploratory. Metabolites with significantly different concentrations between controls and HD subjects were tested in a secondary analysis for correlation with clinical measures using linear regression to estimate coefficients of determination (r2) and correlation coefficients (Pearson’s r). Significant correlations were additionally tested for an impact of age, sex and years of education by using these parameters as covariates.
Results
Subject characteristics
Group demographics for the 24 individuals included in this study (twelve unaffected controls, twelve HD subjects) are displayed in Table 1. Two of the twelve subjects expansion positive for HD had motor signs (e.g. QNE chorea subscores: 7 and 8) consistent with manifest HD, the other ten were in the premanifest or prodromal phase resulting in an overall sample representing a very early stage of HD (average estimated years to onset: 7.7 years (SD 3.1), disease burden score: 369.8 (SD 97.3)). The twelve subjects expansion positive for HD differed significantly from controls in their cognitive performance as assessed by MOCA scores (controls: 28.5 (SD 2.2); HD mutation positives: 25.0 (SD 4.7), p=0.03), there were no further group differences in either of the demographic and disease parameters assessed.
Group comparisons of brain morphology between controls and HD subjects
The assessed measures of brain morphology indicate a slight tendency toward reduced putaminal and caudate volumes and increased ventricular-volumes in the group of early HD subjects versus controls, but differences, including cingulate subvolumes, were not significant (Table 2). Also whole brain ratios of cerebral gray- (GM), white-matter (WM) and cerebro spinal fluid (CSF) did not differ between the groups assessed indicating similar tissue distributions (group averages controls and early HD, respectively, (SD): GM/WM: 1.05(0.07); 0.99(0.09); p=0.12; GM/CSF: 2.61(0.36); 2.42(0.38); p=0.20; WM/CSF:2.5(0.32); 2.43(0.34); p=0.60).
Table 2.
Volumes (ml) derived from tissue segmentation of the T1-weighted MPRAGE scans for controls and HD-subjects. Data are presented as mean (standard deviation).
| Controls | Early HD | T-test (p) | |
|---|---|---|---|
|
|
|||
| Sulcal CSF | 180 (27) | 183 (20) | 0.81 |
| Ventricles | 17.2 (8.9) | 22.0 (14.7) | 0.34 |
| Cerebellar GM | 56.8 (10) | 59.5 (17) | 0.64 |
| Cerebral GM | 466 (63) | 436 (43) | 0.18 |
| Caudate | 8.30 (1.1) | 7.90 (3.0) | 0.64 |
| Thalamus | 20.6 (1.8) | 20.0 (1.9) | 0.42 |
| Putamen | 9.10 (0.8) | 8.80 (1.0) | 0.46 |
| Brainstem | 21.7 (2.3) | 21.3 (3.0) | 0.65 |
| Cerebellar WM | 21.5 (2.6) | 22.1 (3.1) | 0.60 |
| Cerebral WM | 448 (69.6) | 440 (46.1) | 0.75 |
| Anterior Cingulate Gyrus | 9.95 (2.12) | 9.75 (1.13) | 0.80 |
| Anterior-Middle Cingulate | 5.52 (1.01) | 5.08 (0.43) | 0.22 |
| Posterior-Middle Cingulate | 5.2 (0.92) | 4.88 (0.59) | 0.36 |
| Dorsal Posterior Cingulate | 3.18 (0.91) | 2.67 (0.24) | 0.10 |
| Ventral Posterior Cingulate | 1.4 (0.33) | 1.27 (0.23) | 0.30 |
Quality of MRS measures
The 24 individuals studied showed for 11 measures analyzed with MRS spectra with sufficient fit quality as estimated using LCModel (CRLB <= 20%, Table 3). We were not able to achieve sufficient signal quality for the following eight metabolites included in the basis set: Alanine, ascorbate, aspartate, glucose, glycine, guanidinoacetate, lactate, scyllo-inositol. MRS-voxel specific segmentation for each participant indicated similar shares of CSF, GM and WM in controls and HD-subjects within the volume assessed (CSF/GM/WM, mean%, SD): controls 0.40%(0.19)/20.3%(1.9)/79.3%; HD-subjects 0.35% (0.24)/20.1% (1.74)/79.6% (1.59).
Group comparison of metabolite levels between controls and HD subjects
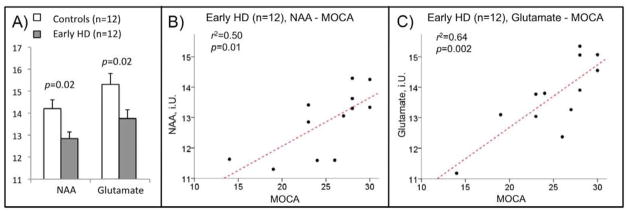
We found significantly lower NAA in HD subjects versus controls (−9.6%, p=0.02). Of the other 10 metabolites fulfilling criteria of sufficient signal quality, only glutamate showed significantly lower levels in the HD sample (−10.1%, p=0.02) (Table 3). No significant interaction effect between age and group differences could be observed for NAA (F(2,23)=0.34, p=0.73) and glutamate (F2,23)=0.82, p=0.49). Z-scores indicating distance to controls were significantly higher in both NAA (−1.24 SD, p=0.13) and glutamate (−1.27 SD, p=0.11) than for total choline (−0.33 SD) and indicate specificity of the observed alterations versus global changes. Group differences increased when a subset of the HD subjects with mild cognitive dysfunction (MOCA<26, n=5; mean NAA (SD): 12.2 (0.9), mean glutamate (SD): 12.9 (1.1)) was compared to controls (NAA: −18.8%, p=0.005; glutamate: −18.7%, p=0.006).
Secondary regression analysis with cognitive dysfunction in HD subjects
By applying linear regression analysis, a relation between MOCA-scores with NAA (r2=0.50, p=0.01, Pearson’s r=0.71) and glutamate levels (r2=0.64, p=0.002, Pearson’s r=0.80) could be observed (Figure 3). There was no significant relationship measurable between NAA or glutamate with MMSE, NART-VIQ, NART-FISQ, CAG repeat length, disease burden score, QNE-total, QNE-chorea, HAM-D or BDI.
Figure 3.
Group means of NAA and glutamate levels measured by MRS in controls and HD-subjects (A); linear regression for the HD-sample of MOCA scores with NAA (B) and glutamate (C).
Discussion
The aim of this study was to identify metabolic brain alterations in an early phase of HD that relate to cognitive decline, and might be used as biomarkers for disease progression and neuroprotective treatment approaches in the prodromal or early disease phase. We focused on the PCC as a brain region centrally involved in cognitive control and early neurodegeneration 16, 31, 32, 50–53, and tested NAA and a broad range of other brain metabolites for group differences between controls and early HD subjects. Metabolites significantly differing between both groups were tested in a secondary analysis for correlations with clinical measures of cognitive function and also HD status.
To our knowledge, this is the first study to perform MRS on the PCC in an HD sample. This region was mainly selected because of its central role in cognitive functions particularly affected in early HD 3, 29, 54, 55, but also because of the fact that the PCC is only affected by subtle volume loss compared to other brain areas such as frontal lobes or striatum 13, 16, 56, 57, which simplifies MRS analysis. Methodological strengths of this study include normalization of metabolites to unsuppressed water, which has been shown earlier to reflect biochemical changes more reliably than total creatine ratios and therefore resulting in more valid measures particularly in HD 24. In addition, we used high field strength of 7T, which yields significantly higher signal to noise ratio and spectral resolution than lower field-strengths of 1.5T or 4T 30, thereby increasing sensitivity of the analysis. This is consistent with our results, as we could identify 11 metabolites at high signal quality for the 7.2ml PCC voxel analyzed, reflected by CRLB significantly below 20% (Table 3). There were no significant differences in cingulate volumes or GM/WM/CSF tissue distribution for the voxel measured by MRS between both samples, therefore minimizing the risk of bias through partial volume effects in our study. Weaknesses of the study include in particular the small sample size and also the solely cross-sectional design, which necessitate follow-up studies. Furthermore the fact that inferences on pathology before manifestation of motor symptoms have to be made with caution due to the heterogeneity of the studied sample, as both prodromal HD subjects and individuals at an early stage of HD were included.
We tested a group of twelve early HD subjects including a broad spectrum of disease burden, reflected by accordingly distributed clinical measures of neurological and cognitive symptoms (Table 1). These were compared for group differences with twelve healthy control subjects, which were matched for factors that might affect the statistical analysis including age, sex, ethnicity and education. Due to its homogeneous matching of cases and controls, with sufficient spread of quantitative clinical markers in the early HD sample, our sample appears well suited to detect relevant alterations of brain metabolite levels. The MOCA test assesses different types of cognitive abilities reported to be affected at early stages in HD, including orientation, short-term memory, language abilities, visuospatial ability and unlike the Mini-Mental State Exam (MMSE), a widely used method of screening for dementia, the MOCA includes a test of executive function 29, 42, 43. Lower MOCA-scores in the sample of subjects with the HD mutation are consistent with earlier reports on cognitive deficits occurring very early in the course of HD 3, 29, 54, 55. The fact, that we do not find significant brain atrophy of striatal structures such as putamen and caudate nucleus (Table 2), as it has been shown to be characteristical for HD 11–13, 16, underlines the generally early stage of disease progression in the studied population, but also lack of power to detect subtle effects due to small sample size. If the sample size were larger, we would expect to detect structural changes; it is striking that MRS at 7T has the power to detect changes even in such a small group, suggesting consistent alterations due to HD observable at high signal to noise with the applied protocol.
By testing a total of 11 brain metabolites found to be measurable at sufficient quality, we find significant differences between controls and early HD subjects for NAA and glutamate levels in a voxel located in the PCC-region. Specificity of these findings for NAA and glutamate versus global alterations is supported by the fact that differences to control were significantly larger than for total-choline measures, where no alterations in the context of HD have been reported at this point. These differences were more pronounced in a subgroup of early HD subjects with cognitive dysfunction (MOCA<26) and appear independent from age effects or global non-specific effects on metabolite levels. Secondary analysis indicates a relation of both NAA and glutamate with cognitive performance (MOCA-scores) in early HD (Figure 3). CRLB and also spectra suggest sufficient signal quality at 7T to resolve glutamine from glutamate (Table 3 and Figure 2). However, a low degree of contamination of the glutamate signal by glutamine is nevertheless possible. While we expected to see changes in NAA, the glutamate finding is more exploratory and needs to be interpreted with caution as it does not remain significant after correction for multiple testing.
Although earlier studies did not include the PCC, our findings nevertheless appear to be consistent with reports on reduced NAA and glutamate in HD 24, 25. While we find reduced levels of glutamate to be related to cognitive dysfunction in HD-subjects, earlier literature supports glutamatergic excitotoxicity as a significant mechanism in the pathogenesis of HD 8, 22, 58. For neurons glutamatergic excitotoxicity has been suggested to result from increased levels of glutamate as a neurotransmitter but also possibly through increased sensitivity of glutamate receptors in a context of generally lower abundance of glutamate 59, 60. The glutamate levels measured by our MRS approach however, are not specific for synaptic transmission, but rather reflect cellular integrity of viable neurons which may decrease with progressing HD. We did not find relationships between metabolite levels and measures of HD-status such as disease burden score or CAG-repeat length or neurological symptoms, which has been reported for populations including subjects at later stages of HD 19, 24, 25.
While impaired cognitive performance, which progresses to dementia in later phases, is a frequent finding in early HD 5, 6, 8, 29, 61, to our knowledge there are no published studies reporting a relation between altered brain metabolites and cognitive decline in HD. Earlier MRS-studies testing mainly striatal and other subcortical areas, did not find significant correlations between brain metabolites and cognitive performance 24, 25. However, a recent study applying structural MRI suggests a relationship between reduced PCC volume and impaired visual working memory in early HD 16. This fits well with considerations about an important role of the PCC in cognitive processing 51, 53, 62, 63 and a concatenation of data on PCC dysfunction being related to cognitive decline in other neurodegenerative disorders including Alzheimer’s and Parkinson’s Disease 31–35, 64. These data are consistent with our findings of a relationship between NAA and glutamate levels with cognitive dysfunction in early HD, and might indicate common patterns of brain system impairment in neurodegenerative diseases generally. Furthermore, low glutamate in the PCC has recently been suggested as a marker of cognitive decline in schizophrenia 65, which might be additional evidence for the relation of PCC neuronal integrity with cognitive performance in chronic CNS diseases. While our data indicate changes of PCC NAA and glutamate-levels in parallel with cognitive decline in HD, further studies are necessary to clarify the neural mechanisms implied and particularly how these may relate to the metabolite alterations observable by MRS.
In summary, the present study provides first evidence of reduced NAA and glutamate levels in the PCC of HD gene mutation carriers, which relate to decreased cognitive performance and possibly precede structural brain changes. However, further studies are necessary to replicate our findings and also to evaluate their applicability as longitudinal biomarkers for disease progression in HD. Considering the congruence of our findings with earlier reports on other neurodegenerative disorders, the alterations here reported might be generally applicable to syndromes of progressive cognitive decline and serve as outcome biomarkers for potential neuroprotective therapy approaches.
Acknowledgments
This study was made possible by grant support from NINDS NS16375, NIH-NCRR P41-RR015241 and P50AG005146. Dr. Paul G. Unschuld is supported by NIH-T32MH015330. We thank all HD-subjects and controls for their study participation. We thank Nadine Yoritomo and Morgan Writhenour of the Baltimore Huntington’s Disease Center (BHDC) at Johns Hopkins Hospital for support in study organization. We thank Terri Brawner, Ivana Kusevic and Kathleen Kahl of the F.M.Kirby Research Center and Guillermo Verduzco of the Division of Psychiatric Neuroimaging for their technical assistance. The National Center for Research Resources (NCRR) is a component of the National Institutes of Health (NIH). The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Equipment used in the study is manufactured by Philips. Dr. van Zijl receives grant support from Philips, is a paid lecturer for Philips Medical Systems, and is the inventor of technology that is licensed to Philips. This arrangement has been approved by Johns Hopkins University in accordance with its conflict of interest policies.
Author Roles
-
Research Project
Conception: PGU, CAR
Organization: PGU, MS, GR, CAR
Execution: PGU, RAEE, AC, XL, RLM
-
Statistical Analysis
Design: PGU, CAR
Execution: PGU, RAEE, AC, XL, XW, KO
Review and Critique: PGU, RAEE, AC, XL, MS, XW, KO, JB, SSB, GR, RLM, PCMvZ, PBB, CAR
-
Manuscript
Writing of the first draft: PGU
Review and Critique: RAEE, AC, XL, MS, XW, KO, JB, SSB, GR, RLM, PCMvZ, PBB, CAR
Full Financial Disclosure of All Authors for the preceding 12 months
Paul G. Unschuld
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: NIH-T32MH015330 | Other: none |
Richard A.E. Edden
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: One lecture to Eli Lilly & Co in 2011 | Royalties: none |
| Grants: NIH-NCRR P41-RR015241 and P50AG005146 | Other: none |
Aaron Carass
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
Xinyang Liu
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Brigham and Women’s Hospital, Harvard Medical School |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
Megan Shanahan
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
Xin Wang
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
Kenichi Oishi
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: NIH R21AG033774, R01HD065955-A1 | Other: none |
Jason Brandt
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: related to neuropsychological injuries |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: Alere San Diego, Inc.; Copper Ridge Institute |
| Honoraria: none | Royalties: Psychological Assessment Resources, Inc. |
| Grants: R01-AG007370-16A2, P50-NS016375, U01-AG014260, Seattle Institute for Biomedical and Clinical Research BJ18-BAL-A | Other: none |
Susan Bassett
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: NIH RO1 AG16324, RO1AG021804, P50 NS38377 | Other: none |
Graham Redgrave
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: none | Other: none |
Russell L. Margolis
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: NS16375, NIH NS16375, NS061099, NS060118, NS052592, NS066111, MH086703, MH087874, Medivation, Pfizer (grant funding and licensing fee) | Other: none |
Peter C.M. Van Zijl
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Kennedy Krieger Institute, Johns Hopkins |
| Partnerships: none | Contracts: none |
| Honoraria: Philips Medical Systems | Royalties: none |
| Grants: Philips Medical Systems, NIH-NCRR P41-RR015241 and P50AG005146 | Other: none |
Peter B. Barker
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: none | Expert Testimony: none |
| Advisory Boards: none | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: none | Royalties: none |
| Grants: NIH-NCRR P41-RR015241 and P50AG005146 | Other: none |
Christopher A. Ross
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: Alnylam/Medtronics, Isis, Zenobia | Expert Testimony: none |
| Advisory Boards: Vertex | Employment: Johns Hopkins University |
| Partnerships: none | Contracts: none |
| Honoraria: MRC | Royalties: none |
| Grants: NINDS NS16375 | Other: none |
Footnotes
Financial Disclosure/Conflict of Interest concerning the research related to the manuscript: Equipment used in the study is manufactured by Philips. Dr. van Zijl receives grant support from Philips, is a paid lecturer for Philips and the inventor of technology that is licensed to Philips. This arrangement has been approved by Johns Hopkins in accordance with its conflict of interest policies.
References
- 1.Brandt J, Bylsma FW, Gross R, Stine OC, Ranen N, Ross CA. Trinucleotide repeat length and clinical progression in Huntington’s disease. Neurology. 1996;46(2):527–531. doi: 10.1212/wnl.46.2.527. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence AD, Hodges JR, Rosser AE, et al. Evidence for specific cognitive deficits in preclinical Huntington’s disease. Brain. 1998;121 ( Pt 7):1329–1341. doi: 10.1093/brain/121.7.1329. [DOI] [PubMed] [Google Scholar]
- 3.Marder K, Zhao H, Myers RH, et al. Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology. 2000;54(2):452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- 4.Tabrizi SJ, Scahill RI, Durr A, et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- 5.Stout JC, Paulsen JS, Queller S, et al. Neurocognitive signs in prodromal Huntington disease. Neuropsychology. 2011;25(1):1–14. doi: 10.1037/a0020937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsen JS. Cognitive Impairment in Huntington Disease: Diagnosis and Treatment. Curr Neurol Neurosci Rep. 2011 doi: 10.1007/s11910-011-0215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gusella JF, Wexler NS, Conneally PM, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306(5940):234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- 8.Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis toclinical treatment. Lancet Neurol. 2011;10(1):83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 9.Gusella JF, McNeil S, Persichetti F, et al. Huntington’s disease. Cold Spring Harb Symp Quant Biol. 1996;61:615–626. [PubMed] [Google Scholar]
- 10.Gasser T, Bressman S, Durr A, Higgins J, Klockgether T, Myers RH. State of the art review: molecular diagnosis of inherited movement disorders. Movement Disorders Society task force on molecular diagnosis. Mov Disord. 2003;18(1):3–18. doi: 10.1002/mds.10338. [DOI] [PubMed] [Google Scholar]
- 11.Aylward EH. Change in MRI striatal volumes as a biomarker in preclinical Huntington’s disease. Brain Res Bull. 2007;72(2–3):152–158. doi: 10.1016/j.brainresbull.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Aylward EH, Nopoulos PC, Ross CA, et al. Longitudinal change in regional brain volumes in prodromal Huntington disease. J Neurol Neurosurg Psychiatry. 2010 doi: 10.1136/jnnp.2010.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulsen JS, Magnotta VA, Mikos AE, et al. Brain structure in preclinical Huntington’s disease. Biol Psychiatry. 2006;59(1):57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65(5):745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- 15.Rosas HD, Salat DH, Lee SY, et al. Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain. 2008;131(Pt 4):1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobbs NZ, Pedrick AV, Say MJ, et al. The structural involvement of the cingulate cortex in premanifest and early Huntington’s disease. Mov Disord. 2011;26(9):1684–1690. doi: 10.1002/mds.23747. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins BG, Klivenyi P, Kustermann E, et al. Nonlinear decrease over time in N-acetyl aspartate levels in the absence of neuronal loss and increases in glutamine and glucose in transgenic Huntington’s disease mice. J Neurochem. 2000;74(5):2108–2119. doi: 10.1046/j.1471-4159.2000.0742108.x. [DOI] [PubMed] [Google Scholar]
- 18.Bender A, Auer DP, Merl T, et al. Creatine supplementation lowers brain glutamate levels in Huntington’s disease. J Neurol. 2005;252(1):36–41. doi: 10.1007/s00415-005-0595-4. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins BG, Rosas HD, Chen YC, et al. 1H NMR spectroscopy studies of Huntington’s disease: correlations with CAG repeat numbers. Neurology. 1998;50(5):1357–1365. doi: 10.1212/wnl.50.5.1357. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds NC, Jr, Prost RW, Mark LP. Heterogeneity in 1H-MRS profiles of presymptomatic and early manifest Huntington’s disease. Brain Res. 2005;1031(1):82–89. doi: 10.1016/j.brainres.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Pernaute R, Garcia-Segura JM, del Barrio Alba A, Viano J, de Yebenes JG. Clinical correlation of striatal 1H MRS changes in Huntington’s disease. Neurology. 1999;53(4):806–812. doi: 10.1212/wnl.53.4.806. [DOI] [PubMed] [Google Scholar]
- 22.Taylor-Robinson SD, Weeks RA, Bryant DJ, et al. Proton magnetic resonance spectroscopy in Huntington’s disease: evidence in favour of the glutamate excitotoxic theory. Mov Disord. 1996;11(2):167–173. doi: 10.1002/mds.870110209. [DOI] [PubMed] [Google Scholar]
- 23.van Oostrom JC, Sijens PE, Roos RA, Leenders KL. 1H magnetic resonance spectroscopy in preclinical Huntington disease. Brain Res. 2007;1168:67–71. doi: 10.1016/j.brainres.2007.05.082. [DOI] [PubMed] [Google Scholar]
- 24.Sturrock A, Laule C, Decolongon J, et al. Magnetic resonance spectroscopy biomarkers in premanifest and early Huntington disease. Neurology. 2010;75(19):1702–1710. doi: 10.1212/WNL.0b013e3181fc27e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Bogaard SJ, Dumas EM, Teeuwisse WM, et al. Exploratory 7-Tesla magnetic resonance spectroscopy in Huntington’s disease provides in vivo evidence for impaired energy metabolism. J Neurol. 2011 doi: 10.1007/s00415-011-6099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabrizi SJ, Langbehn DR, Leavitt BR, et al. Biologicaland clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hersch SM, Rosas HD. Biomarkers to Enable the Development of Neuroprotective Therapies for Huntington’s Disease. 2011 [PubMed] [Google Scholar]
- 28.Weir DW, Sturrock A, Leavitt BR. Development of biomarkers for Huntington’s disease. Lancet Neurol. 2011;10(6):573–590. doi: 10.1016/S1474-4422(11)70070-9. [DOI] [PubMed] [Google Scholar]
- 29.Brandt J, Inscore AB, Ward J, et al. Neuropsychological deficits in Huntington’s disease gene carriers and correlates of early “conversion”. J Neuropsychiatry Clin Neurosci. 2008;20(4):466–472. doi: 10.1176/appi.neuropsych.20.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62(4):868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choo IH, Lee DY, Oh JS, et al. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2010;31(5):772–779. doi: 10.1016/j.neurobiolaging.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagust WJ, Landau SM, Shaw LM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73(15):1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 35.Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110 ( Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- 36.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 37.Penney JB, Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol. 1997;41(5):689–692. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- 38.Folstein SE, Jensen B, Leigh RJ, Folstein MF. The measurement of abnormal movement: methods developed for Huntington’s disease. Neurobehav Toxicol Teratol. 1983;5(6):605–609. [PubMed] [Google Scholar]
- 39.World_Medical_Association. Declaration of Helsinki. Law Med Health Care. 1991;19(3–4):264–265. [PubMed] [Google Scholar]
- 40.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 43.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 44.Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8(6):847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- 45.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med. 2001;45(5):765–769. doi: 10.1002/mrm.1104. [DOI] [PubMed] [Google Scholar]
- 46.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med. 1989;9(1):79–93. doi: 10.1002/mrm.1910090110. [DOI] [PubMed] [Google Scholar]
- 47.Bazin PL, Pham DL. Topology-preserving tissue classification of magnetic resonance brain images. IEEE Trans Med Imaging. 2007;26(4):487–496. doi: 10.1109/TMI.2007.893283. [DOI] [PubMed] [Google Scholar]
- 48.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 49.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 50.Hirono N, Mori E, Ishii K, et al. Hypofunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64(4):552–554. doi: 10.1136/jnnp.64.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nielsen FA, Balslev D, Hansen LK. Mining the posterior cingulate: segregation between memory and pain components. Neuroimage. 2005;27(3):520–532. doi: 10.1016/j.neuroimage.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Y, Dougherty JH, Jr, Hubner KF, Bai B, Cannon RL, Hutson RK. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer’s disease and mild cognitive impairment. Alzheimers Dement. 2008;4(4):265–270. doi: 10.1016/j.jalz.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31(9):3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brandt J, Shpritz B, Codori AM, Margolis R, Rosenblatt A. Neuropsychological manifestations of the genetic mutation for Huntington’s disease in presymptomatic individuals. J Int Neuropsychol Soc. 2002;8(7):918–924. doi: 10.1017/s1355617702870060. [DOI] [PubMed] [Google Scholar]
- 55.Lawrence AD, Sahakian BJ, Hodges JR, Rosser AE, Lange KW, Robbins TW. Executive and mnemonic functions in early Huntington’s disease. Brain. 1996;119 ( Pt 5):1633–1645. doi: 10.1093/brain/119.5.1633. [DOI] [PubMed] [Google Scholar]
- 56.Aylward EH, Brandt J, Codori AM, Mangus RS, Barta PE, Harris GJ. Reduced basal ganglia volume associated with the gene for Huntington’s disease in asymptomatic at-risk persons. Neurology. 1994;44(5):823–828. doi: 10.1212/wnl.44.5.823. [DOI] [PubMed] [Google Scholar]
- 57.Aylward EH, Anderson NB, Bylsma FW, et al. Frontal lobe volume in patients with Huntington’s disease. Neurology. 1998;50(1):252–258. doi: 10.1212/wnl.50.1.252. [DOI] [PubMed] [Google Scholar]
- 58.Taylor-Robinson SD, Weeks RA, Sargentoni J, et al. Evidence for glutamate excitotoxicity in Huntington’s disease with proton magnetic resonance spectroscopy. Lancet. 1994;343(8906):1170. doi: 10.1016/s0140-6736(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 59.Estrada Sanchez AM, Mejia-Toiber J, Massieu L. Excitotoxic neuronal death and the pathogenesis of Huntington’s disease. Arch Med Res. 2008;39(3):265–276. doi: 10.1016/j.arcmed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Roze E, Saudou F, Caboche J. Pathophysiology of Huntington’s disease: from huntingtin functions to potential treatments. Curr Opin Neurol. 2008;21(4):497–503. doi: 10.1097/WCO.0b013e328304b692. [DOI] [PubMed] [Google Scholar]
- 61.Walker FO. Huntington’s disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 62.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 63.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camicioli RM, Korzan JR, Foster SL, et al. Posterior cingulate metabolic changes occur in Parkinson’s disease patients without dementia. Neurosci Lett. 2004;354(3):177–180. doi: 10.1016/j.neulet.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 65.Bustillo JR, Chen H, Gasparovic C, et al. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 Tesla. Biol Psychiatry. 2011;69(1):19–27. doi: 10.1016/j.biopsych.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]