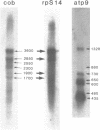
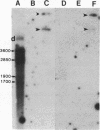
Abstract
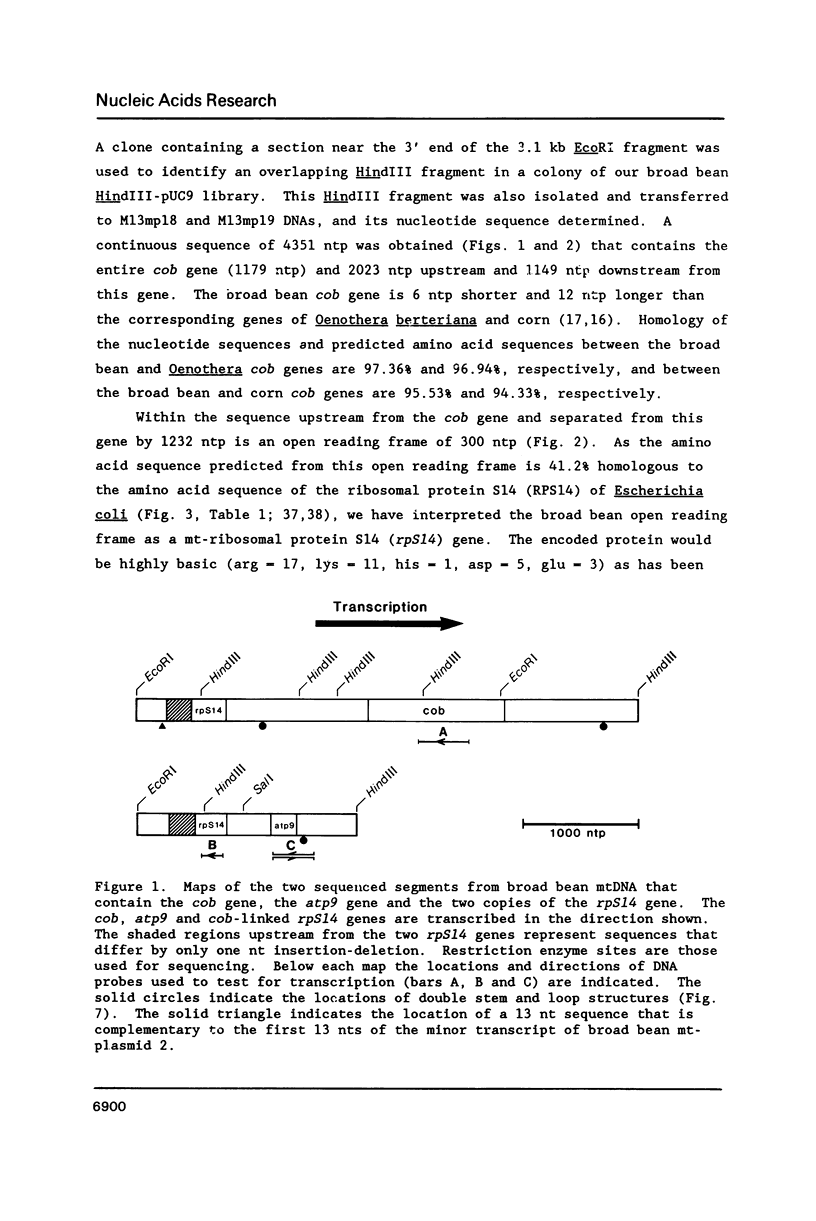
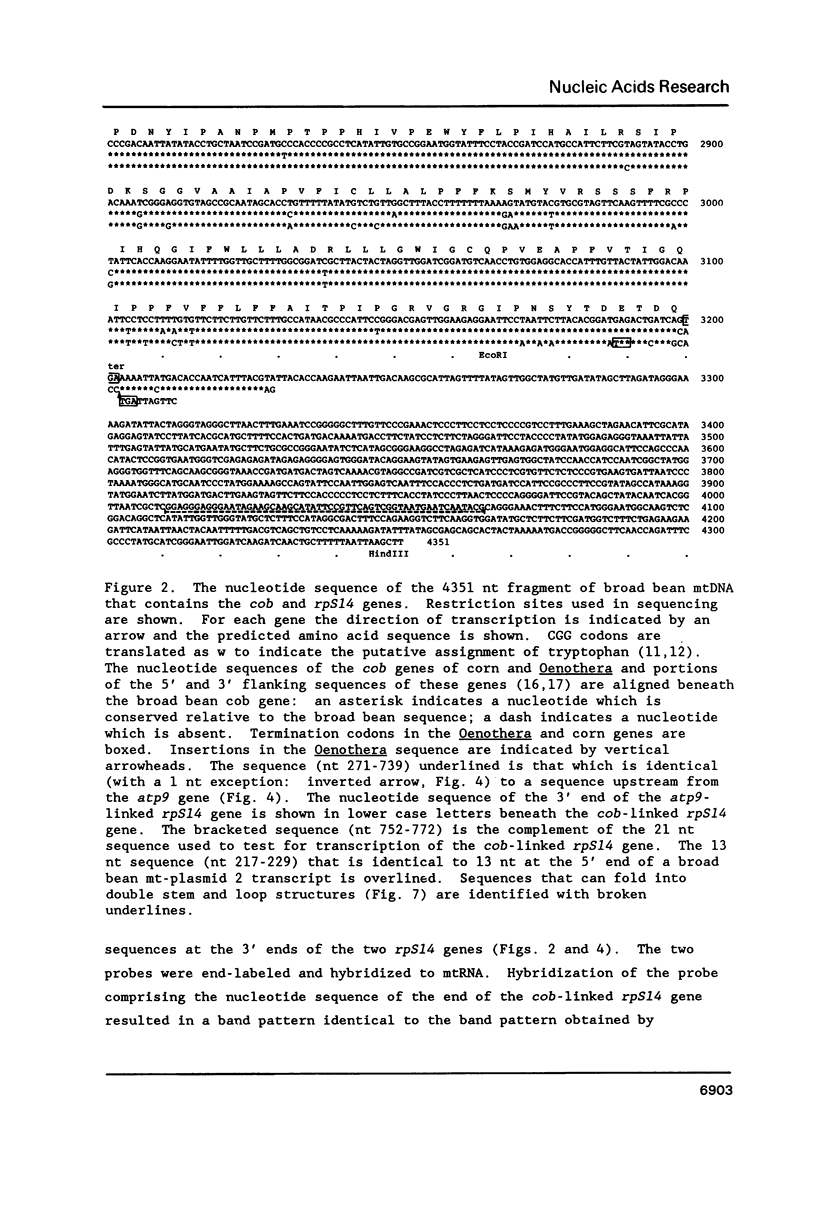
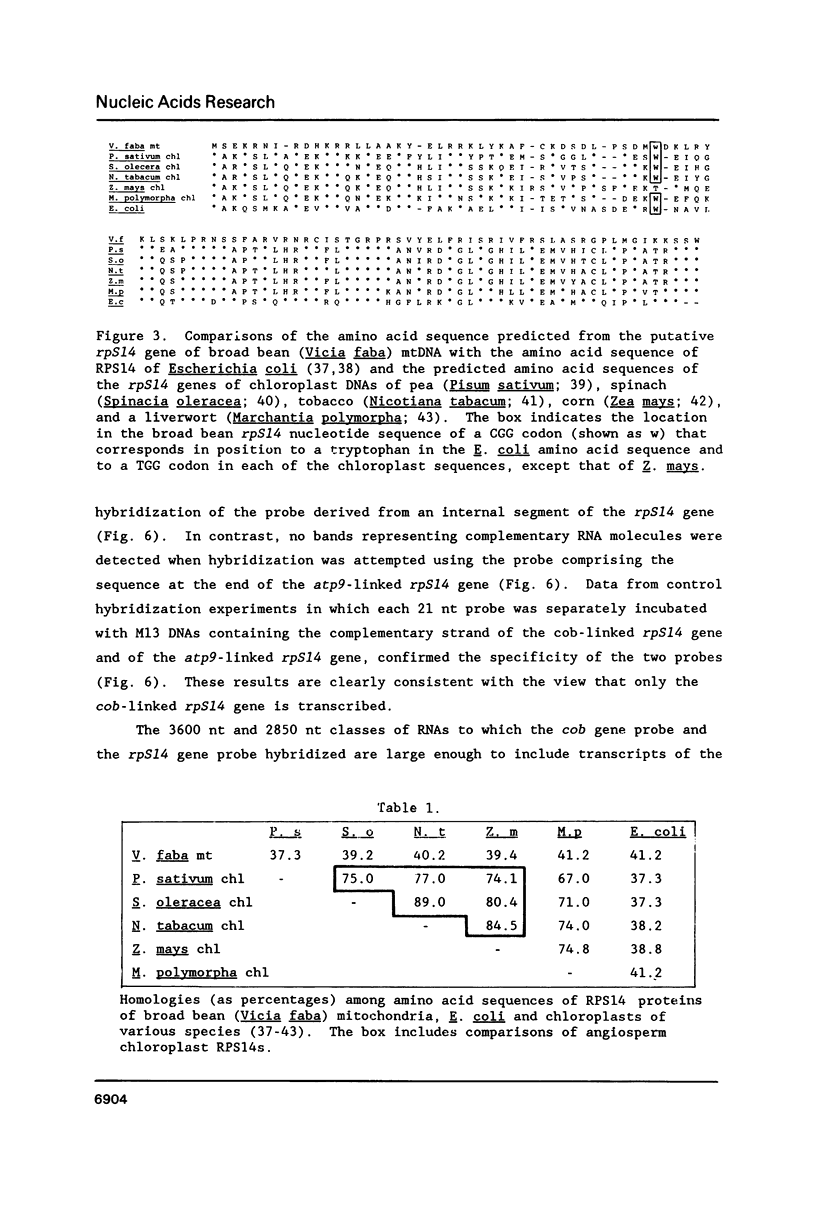
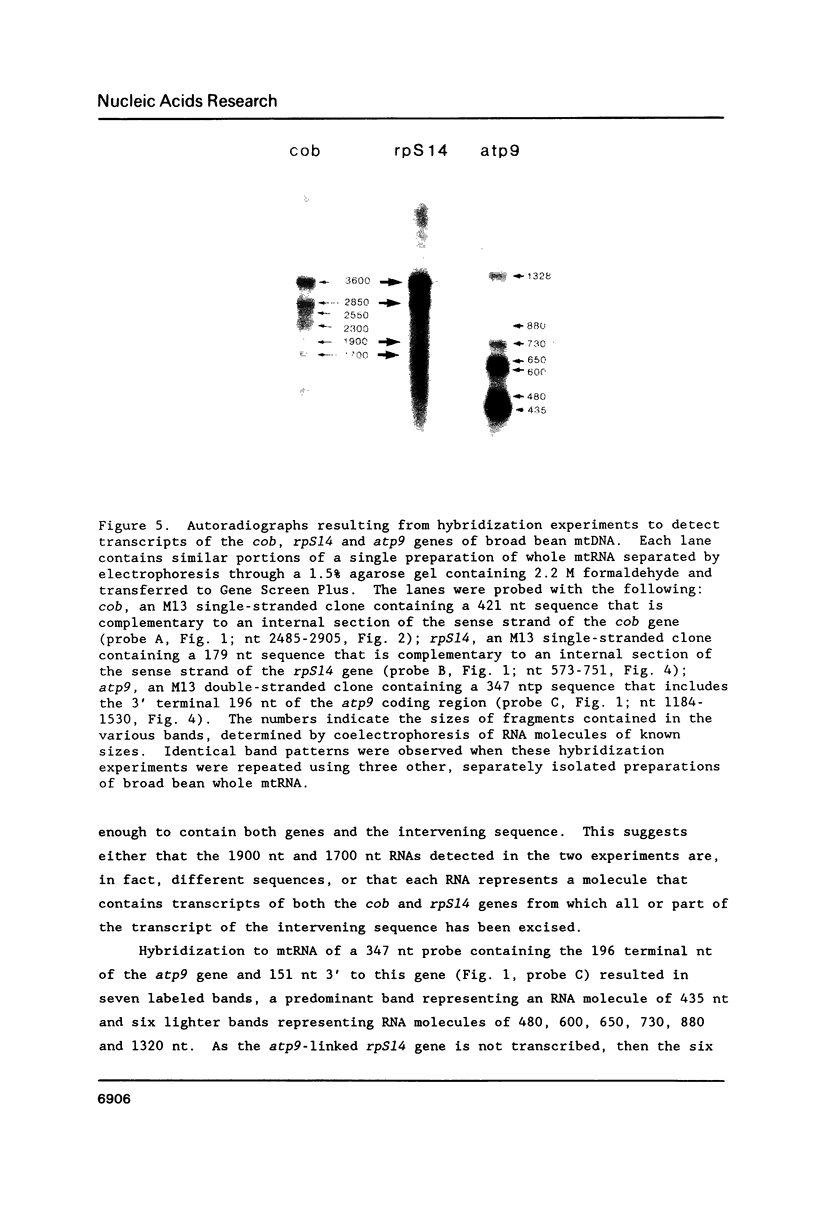
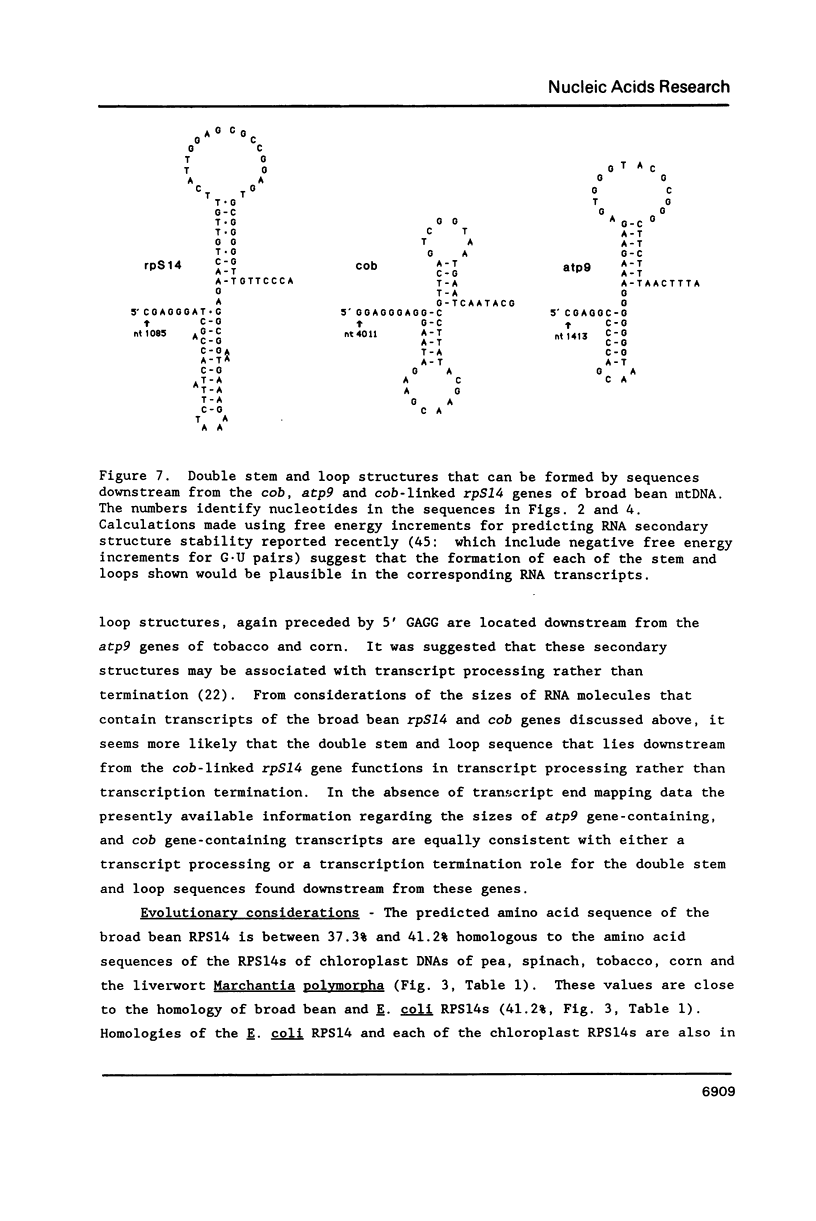
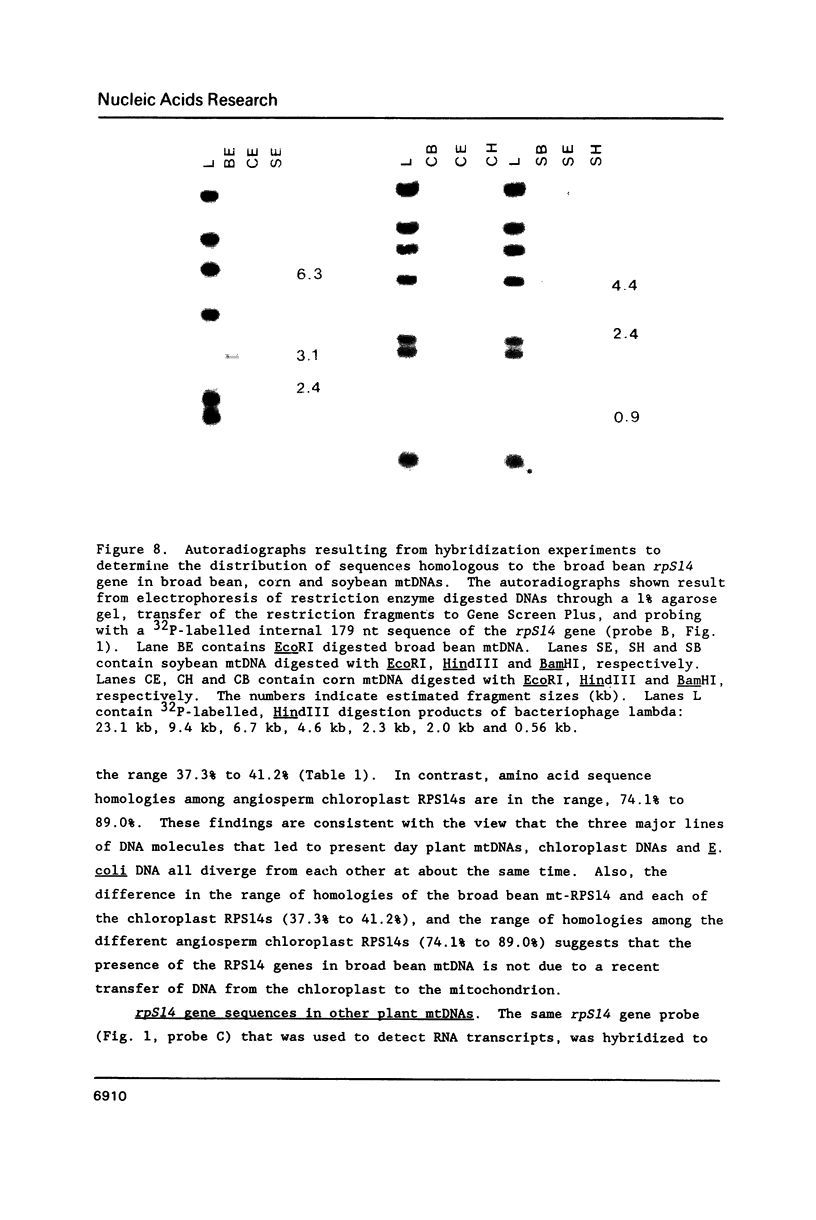
Broad bean (Vicia faba) mtDNA contains an open reading frame with a predicted amino acid sequence that is 41% homologous to the ribosomal protein S14 (RPS14) of Escherichia coli, and which is located 1232 ntp upstream from a gene for cytochrome b (cob). A second putative rpS14 gene occurs in broad bean mtDNA, 344 ntp upstream from a gene for ATPase subunit 9 (atp9). However, the atp9-linked rpS14 gene is 12 codons shorter than the cob-linked rpS14 gene. Sequence homology is found upstream (for 218 ntp) but not downstream from the two rpS14 genes. Transcripts were detected in broad bean mtRNA only for the cob-linked rpS14 gene. All RNA molecules that include a transcript of the rpS14 gene also include a transcript of the cob gene. Sequences homologous to the broad bean mitochondrial rpS14 gene were detected in soybean mtDNA, but not in corn mtDNA. Relationships between the amino acid sequences of RPS14s encoded in broad bean mtDNA, in chloroplast DNAs of various angiosperms, and in E. coli are consistent with the view that the ancestral lines of these three kinds of DNA diverged from each other within a relatively short time period.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bland M. M., Levings C. S., 3rd, Matzinger D. F. The tobacco mitochondrial ATPase subunit 9 gene is closely linked to an open reading frame for a ribosomal protein. Mol Gen Genet. 1986 Jul;204(1):8–16. doi: 10.1007/BF00330180. [DOI] [PubMed] [Google Scholar]
- Boer P. H., McIntosh J. E., Gray M. W., Bonen L. The wheat mitochondrial gene for apocytochrome b: absence of a prokaryotic ribosome binding site. Nucleic Acids Res. 1985 Apr 11;13(7):2281–2292. doi: 10.1093/nar/13.7.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Boer P. H., Gray M. W. The wheat cytochrome oxidase subunit II gene has an intron insert and three radical amino acid changes relative to maize. EMBO J. 1984 Nov;3(11):2531–2536. doi: 10.1002/j.1460-2075.1984.tb02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L. The mitochondrial S13 ribosomal protein gene is silent in wheat embryos and seedlings. Nucleic Acids Res. 1987 Dec 23;15(24):10393–10404. doi: 10.1093/nar/15.24.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D. O., Goddard J. M., Martin S. C., Fauron C. M., Wolstenholme D. R. Drosophila mitochondrial DNA: a novel gene order. Nucleic Acids Res. 1982 Nov 11;10(21):6619–6637. doi: 10.1093/nar/10.21.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dawson A. J., Jones V. P., Leaver C. J. The apocytochrome b gene in maize mitochondria does not contain introns and is preceded by a potential ribosome binding site. EMBO J. 1984 Sep;3(9):2107–2113. doi: 10.1002/j.1460-2075.1984.tb02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Levings C. S., Timothy D. H. Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol. 1985 Nov;79(3):914–919. doi: 10.1104/pp.79.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey R. E., Schuster A. M., Levings C. S., Timothy D. H. Nucleotide sequence of F(0)-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1015–1019. doi: 10.1073/pnas.82.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri L., Hill W. E., Wittmann H. G., Wittmann-Liebold B. Ribosomal proteins: their structure and spatial arrangement in prokaryotic ribosomes. Adv Protein Chem. 1984;36:1–78. doi: 10.1016/s0065-3233(08)60295-8. [DOI] [PubMed] [Google Scholar]
- Hack E., Leaver C. J. The alpha-subunit of the maize F(1)-ATPase is synthesised in the mitochondrion. EMBO J. 1983;2(10):1783–1789. doi: 10.1002/j.1460-2075.1983.tb01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesel R., Brennicke A. Cytochrome oxidase subunit II gene in mitochondria of Oenothera has no intron. EMBO J. 1983;2(12):2173–2178. doi: 10.1002/j.1460-2075.1983.tb01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesel R., Schobel W., Schuster W., Brennicke A. The cytochrome oxidase subunit I and subunit III genes in Oenothera mitochondria are transcribed from identical promoter sequences. EMBO J. 1987 Jan;6(1):29–34. doi: 10.1002/j.1460-2075.1987.tb04714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G. F. A systemic DNA sequencing strategy. J Mol Biol. 1982 Jul 5;158(3):539–549. doi: 10.1016/0022-2836(82)90213-3. [DOI] [PubMed] [Google Scholar]
- Isaac P. G., Brennicke A., Dunbar S. M., Leaver C. J. The mitochondrial genome of fertile maize (Zea mays L.) contains two copies of the gene encoding the alpha-subunit of the F1-ATPase. Curr Genet. 1985;10(4):321–328. doi: 10.1007/BF00365628. [DOI] [PubMed] [Google Scholar]
- Isaac P. G., Jones V. P., Leaver C. J. The maize cytochrome c oxidase subunit I gene: sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J. 1985 Jul;4(7):1617–1623. doi: 10.1002/j.1460-2075.1985.tb03828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T., Moon E., Wu R. Cytochrome oxidase subunit II gene of rice has an insertion sequence within the intron. Nucleic Acids Res. 1984 Oct 11;12(19):7305–7315. doi: 10.1093/nar/12.19.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Fauron C. M. The physical map and organisation of the mitochondrial genome from the fertile cytoplasm of maize. Nucleic Acids Res. 1984 Dec 21;12(24):9249–9261. doi: 10.1093/nar/12.24.9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon E., Kao T. H., Wu R. Pea cytochrome oxidase subunit II gene has no intron and generates two mRNA transcripts with different 5'-termini. Nucleic Acids Res. 1985 May 10;13(9):3195–3212. doi: 10.1093/nar/13.9.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikami A., Nakamura K. Structure and expression of pea mitochondrial F1ATPase alpha-subunit gene and its pseudogene involved in homologous recombination. J Biochem. 1987 Apr;101(4):967–976. doi: 10.1093/oxfordjournals.jbchem.a121966. [DOI] [PubMed] [Google Scholar]
- Ohgushi M., Wada A. 'Molten-globule state': a compact form of globular proteins with mobile side-chains. FEBS Lett. 1983 Nov 28;164(1):21–24. doi: 10.1016/0014-5793(83)80010-6. [DOI] [PubMed] [Google Scholar]
- Schuster W., Hiesel R., Isaac P. G., Leaver C. J., Brennicke A. Transcript termini of messenger RNAs in higher plant mitochondria. Nucleic Acids Res. 1986 Aug 11;14(15):5943–5954. doi: 10.1093/nar/14.15.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I. D., Isaac P. G., Leaver C. J. Stoichiometric differences in DNA molecules containing the atpA gene suggest mechanisms for the generation of mitochondrial genome diversity in maize. EMBO J. 1987 Apr;6(4):865–869. doi: 10.1002/j.1460-2075.1987.tb04832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Fraser A. R., Laroche J., Gartner-Kepkay K. E., Zouros E. Atypical mitochondrial DNA from the deep-sea scallop Placopecten magellanicus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7595–7599. doi: 10.1073/pnas.84.21.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Inokuchi H., Ohyama K., Ozeki H. Nucleotide sequence of Marchantia polymorpha chloroplast DNA: a region possibly encoding three tRNAs and three proteins including a homologue of E. coli ribosomal protein S14. Nucleic Acids Res. 1984 Dec 21;12(24):9551–9565. doi: 10.1093/nar/12.24.9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahleithner J. A., Wolstenholme D. R. Mitochondrial plasmid DNAs of broad bean: nucleotide sequences, complex secondary structures, and transcription. Curr Genet. 1987;12(1):55–67. doi: 10.1007/BF00420728. [DOI] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Gross N. J. The form and size of mitochondrial DNA of the red bean, Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1968 Sep;61(1):245–252. doi: 10.1073/pnas.61.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R., Dierks P. M. Sequence and transcription analysis of the Petunia mitochondrial gene for the ATP synthase proteolipid subunit. Nucleic Acids Res. 1986 Oct 24;14(20):7995–8006. doi: 10.1093/nar/14.20.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]