Abstract
The phylogenetic relationship between the whitefly Bemisia afer (Priesner & Hosny) (Hemiptera: Aleyrodidae) from China and other populations among the world were analyzed based on the mitochondrial cytochrome oxidase I (mtCOI) gene. Phylogenetic analysis of mtCOI sequences and those of reference B. afer sequences showed that the populations of the species could be separated into 5 clades (I–V). There were at least two clades of the species from China (IV and V). These data suggested that B. afer might be a species complex. The Chinese B. afer populations were most divergent with B. afer from the United Kingdom and African countries. The distance between the Chinese B. afer (IV and V) and clades I, II, and III is more than 32%, while the distance among clades I, II, III is lower than 7.7%. A new set of primers specific to B. afer was designed to amplify a region of approximately 400 bp to discriminate B. afer from other Bemisia species in China based on mtCOI sequences.
Keywords: mitochondrial cytochrome oxidase I, molecular markers
Introduction
During recent years, the outbreak of Bemisia tabaci (Priesner & Hosny) (Hemiptera: Aleyrodidae) and the serious damage caused by this whitefly pest in many countries have required researchers to study the biological and ecological characteristics of and effective control strategies against it (Brown et al. 2000; Brown 2007; De Barro et al. 2000, 2003). The identification of species in the genus Bemisia is the basis of this research, but the taxonomy of whiteflies has long been problematic because of similarities in the morphology of pupae and adults. Pupae of Bemisia species exhibit phenotypic variation in response to differences in leaf surface topology and to environmental and physical factors (Maruthi et al. 2007).
In China, B. tabaci became the major pest of the fiber crops, ornamental plants and vegetables (Chu et al. 2006, 2007, 2008). During field research, a Bemisia species, which was difficult to distinguish from B. tabaci, was discovered on Broussonetia papyrifera (Linn.) Vent. (Urticales: Moraceae). Based on the morphological characteristics of pupae and adults, the species was identified as B. afer. As B. tabaci has several close relatives and numerous biotypes, B. afer also is likely to have many forms and cryptic species. Earlier studies indicated that B. afer exhibits much greater morphological variation than does B. tabaci and its variants (Anderson et al. 2001; Maruthi et al. 2007). The Chinese B. afer has slightly different morphology compared to B. afer from other geographical locations, and it is expected that these differences would be reflected at the molecular level.
The mitochondrial cytochrome oxidase I (mtCOI) gene has been used extensively as a molecular marker to identify B. tabaci variants that exhibit rich biological differences (Frohlich et al. 1999; Hsieh et al. 2007) but lack distinguishing morphological features. Previous studies have shown that mtCOI sequences also are informative for identifying B. afer variants, which lack distinguishing morphological features (Maruthi et al. 2007). In this study, the mtCOI gene of B. afer was sequenced using the primer set (C1-J-2195 and L2-N-3014) that has been used extensively on B. tabaci, and the fragments were also sequenced. The phylogenetic relationships among the world populations were analyzed. The infection status of an endosymbiont Wolbachia of Chinese B. afer was studied because it often causes reproductive incompatibilities between infected and uninfected hosts, which can affect the divergence of mtDNA and can facilitate or even cause host speciation (Werren 1997; Ballard et al. 2004; Shoemaker et al. 2004). Finally, the specific primers to Chinese B. afer were designed based on the sequences of the mtCOI gene of Chinese B. afer and B. tabaci biotypes B and Q.
The objectives of the paper are: 1) to further analyze the phylogenetic relationships, based on the mitochondria COI gene, between Chinese populations of B. afer on B. papyrifera with other populations of B. afer from the United Kingdom and African countries and to discuss the relationship between the divergence of B. afer and the endosymbiont, Wolbachia; 2) to develop a rapid molecular marker based on the mtCOI gene to distinguish B. afer from B. tabaci biotypes B and Q, which are the predominate biotypes in China, especially the biotypes in the Shandong province. The aim is to contribute to the understanding of the systematic status of B. afer populations in China and the genetic differentiation of B. afer worldwide.
Materials and Methods
Collection of the samples and species identification
During 2006 and 2007, pupae and adults of Bemisia species on B. papyrifera were collected alive and placed singly into tubes containing 95% ethanol. The species were identified based on the pupae and adults.
DNA extraction and PCR
Genomic DNA was extracted from individual adults according to the method described previously by Frohlich et al. (1999). Polymerase chain reaction (PCR) was employed to amplify fragments of the B. afer mitochondrial COI gene (800–820 bp), using parameters and PCR primers (C1-J-2195 and L2-N-3014) as described by Frohlich et al. (1999).
PCR assays were conducted using 2 µl of each template DNA in a total reaction volume of 25 µl. PCR conditions follow Frohlich et al. (1999), with 1 unit of Taq DNA polymerase. PCR products were separated on 1.0% agarose gel. The bands were visualized by ethidium bromide staining and viewed with a UV light source.
Cytochrome oxidase I sequencing and phylogenetic analysis
PCR products were purified using an EZ Spin Column DNA Gel Extraction Kit (Sangon Technology Company, www.sangon.com/index.htm) according to the manufacturer's instructions. The DNA sequence for each PCR product was determined from the 5'end at the Sangon Technology Company. The mtCOI sequences determined were deposited in GenBank.
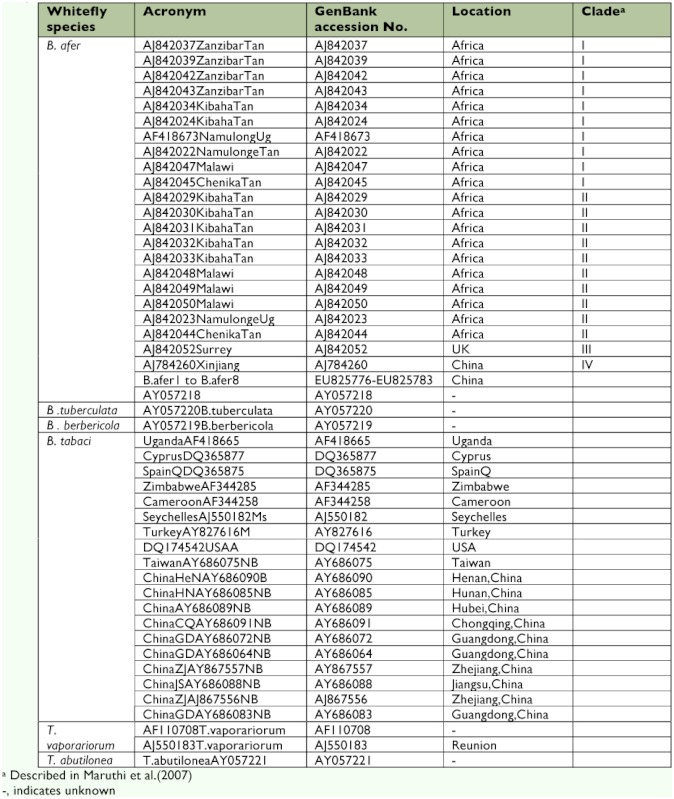
Phylogenetic analysis included all available mtCOI sequences from GenBank and sequences from this study, with the sequences of Bemisia tabaci (mainly including the indigenous biotypes from China), B. tuberculata, B. berbericola, Trialeurodes vaporariorum, and Trialeurodes abutilonea as the outgroup (Table 1). The mtCOI sequences were aligned using the CLUSTAL W algorithm (Thompson et al. 1994). The aligned mtCOI sequences of ∼ 600 bp are presented. Distances based on the mtCOI sequences of ∼600 bp were calculated based on the Kimura 2-parameter model using MEGA 4.1 (Tamura et al. 2007). The ME (Molecular Evolution) and MP (Maximum Parsimony) algorithms available in MEGA 4.1 were used to infer phylogenetic relationships from the sequences. One thousand Bootstrap replicates were performed for each analysis.
Table 1.
Detail of Bemisia afer sample and other whiteflies species collections and their mtCOI gene sequences used in the study.
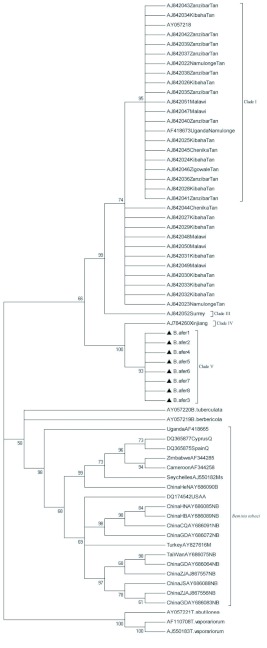
On the basis of the results of phylogenetic analysis, the B. afer specimens were separated into five subclades. The sequences in the subclades were selected to further calculate distances within and between group average calculations using MEGA 4.1.
Wolbachia detection of Chinese B. afer
All B. afer were also screened for Wolbachia infection by PCR, employing the primers wsp81F and wsp691R (Zhou et al. 1998), which amplify part of the Wolbachia surface protein gene (wsp). The study included 15 Chinese B. afer individuals, and the PCR was repeated three times.
Development of the specific diagnostic test
Experiments showed that the primers C1-J-2195 and L2-N-3014 did not always amplify products of the expected size, although the DNA was useful. A set of primers specific to the B. afer mtCOI gene was designed, which included the newly designed forward primer Bafer-J2 (5′-GTTAGTTTTGGGGATTAGTC-3′) by aligning in CLUSTAL W (Thompson et al. 1994) and the reverse primer L2-N-3014 (5′-TCCAATGCACTAATCTGCCATATTA-3′). The other whitefly species (Table 2) were used in PCR reactions to test the specificity of primers to B. afer. The PCR reaction mix followed the method previously described by Frohlich et al. (1999). Reactions used the following PCR program: 94° C for 2 min; followed by 30 cycles of 94° C for 1 min, 54° C for 1 min, and 72° C for 1 min; and ending with 72° C for 5 min. PCR products were separated on 1.0% agarose gel. The bands were visualized by ethidium bromide staining and viewed with a UV light source.
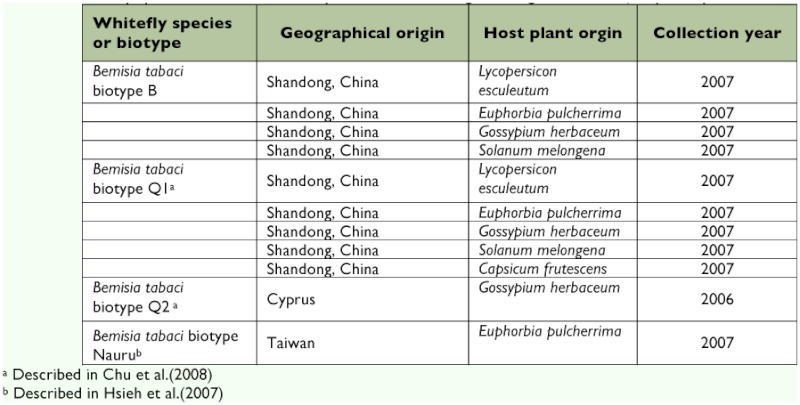
Table 2.
Whitefly species used in the PCR amplification of mtCOI gene using the Bemisia afer specific primers
Results
Morphology of B. afer
Adults of New World specimens of B. afer have the upper and lower eyes completely separate in both sexes, as contrasted with B. tabaci adults,which have one ommatidium connection. Some B. afer from the Macaronesian Islands have the male eyes connected by one ommatidium but separated in the female, while others from different hosts have both eyes separate as in the North American forms (RJ Gill, unpublished data). In the specimens for this study, the female had a one ommatidium connection, while the male had both eyes connected positively with one ommatidium, but also another ommatidium almost but not touching. In the adults, there were clear morphological differences.
Phylogenetic analysis of B.afer
A total of 8 B. afer mtCOI gene sequences of about 600 bases were obtained from populations in Shandong, China during 2006 and 2007. The GenBank accession numbers are EU825776 to EU825783. The phylogenetic tree generated with the Minimum Evolution (ME) method is shown in Figure 1. The tree generated with MP (Maximum Parsimony) method (not shown) is similar to Figure 1. The tree that was generated by heuristic research had 65% confidence level. Based on the trees, B. afer could be separated into 5 clades. The populations from Xinjiang, China (AJ784260Xinjiang) and Shandong, China (Bafer 1–Bafer8) were grouped in clade IV and V, respectively. The Chinese B. afer populations (clade IV and V) were most divergent with clades I, II, and III.
Figure 1.
ME (Minimum Evolution) tree based on ∼600-bp fragment of the mtCOI sequences. Numbers at nodes indicate bootstrap scores using 1000 replicates. Abbreviations are as described in Table 1.  indicates sequences obtained in this study. High quality figures are available online.
indicates sequences obtained in this study. High quality figures are available online.
Genetic differentiation of the B.afer in China and Wolbachia infection
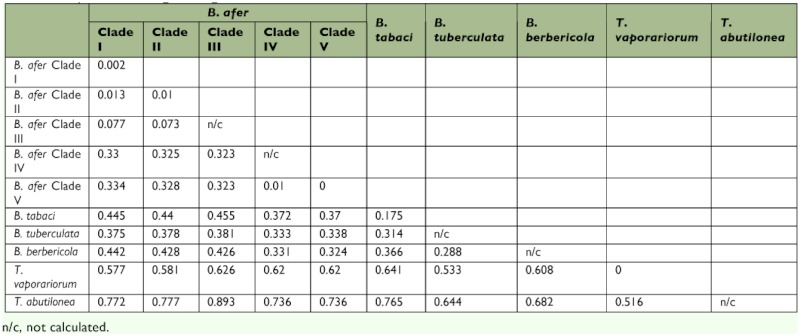
Average distances within and between clades based on mtCOI of whiteflies are summarized in Table 3. The Chinese B. afer populations were most divergent with B.afer from the United Kingdom and African countries. The distance between the Chinese B. afer (clade IV and V) and clades I, II, and III was more than 32%), while the distance among clades I, II, and III were lower than 7.7%. No Wolbachia could be detected in Chinese populations of B.afer (clade V).
Table 3.
Average distance within and between clades of whiteflies based on mtCOI. The genetic distance among the haplotypes within each clade is presented along the diagonal.
Specific primers for the B. afer in China
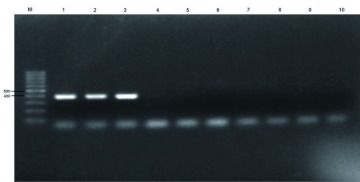
Figure 2 shows the PCR products generated from the DNA of several whitefly species using Bafer-J2 and L2-N-3014 primers. A band of approx 400 bp was obtained from DNA of B. afer from Shandong, China. No specific PCR products were obtained from DNA of B. tabaci biotype B, Q1, Q2, or Nauru.
Figure 2.
PCR products generated using the Bemisia afer specific primers (Bafer-J2 and L2-N-3014) (lanes 1–3); Bemisia tabaci biotype B (lanes 4–5), biotype Q1 (lanes 6–7), biotype Q2 (lane 8–9), biotype Naura (lane 10). M: 100 bp molecular weight marker, the sizes of which are shown on the left. High quality figures are available online.
Discussion
The phylogenetic analysis based on the mtCOI sequences suggested that the B. afer is a species complex that includes many genetically divergent clades. The result based on the molecular marker is consistent with the analysis based on the morphological characteristics.
This study revealed the presence of at least 5 clades in B. afer worldwide. The endosymbiont Wolbachia was not detected in B. afer and may not have affected the evolution of the Chinese B. afer (clade V). Studies with higher sample sizes are required and detailed examinations of morphological and molecular characters are necessary to understand the B. afer species complex.
In China, there are at least two clades of B. afer based on the mtCOI sequences. Although this study confirmed the presence of B. afer in China, the biology of the Chinese species, including host ranges, is still unknown and should be further studied. This study shows that a simple, PCR-based technique is sufficient for the reliable identification of B. afer using a new primer pair designed to amplify a portion of the mtCOI gene, which has been shown to be specific to the Chinese B. afer (clade V).
Acknowledgements
We would like to acknowledge Dr. Ian Denholm (Rothamsted Experimental Station) and Chia-Hung Hsieh (National Taiwan University Taiwan University) for providing whitefly samples for the experiments. This work was funded by the Outstanding Youth Science Foundation of Shandong Province (JQ200811), the Key Project of Chinese National Programs for Fundamental Research and Development (2009CB119200), the National Natural Science Foundation of China (30771410).
Abbreviations
- mtCOI
mitochondrial cytochrome oxidase I gene
References
- Anderson PK, Martin JH, Hernandez P, Lagnaout A. Bemisia afer sens. lat. (Homoptera: Aleyrodidae) outbreak in the Americas. Florida Entomologist. 2001;84:316–317. [Google Scholar]
- Ballard JWO, Whitlock MC. The incomplete natural history of mitochondria. Molecular Ecology. 2004;13:729–744. doi: 10.1046/j.1365-294x.2003.02063.x. [DOI] [PubMed] [Google Scholar]
- Brown JK. The Bemisia tabaci complex: Genetic and phenotypic variability drives begomovirus spread and virus diversification. Plant Disease APSNet. 2007. Available from: http://www.apsnet.org/online/feature/btabaci/
- Brown JK, Frohlich DR, Rosell RC. The sweetpotato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex. Annual Review of Entomology. 1995;40:511–534. [Google Scholar]
- Chu D, Jiang T, Liu GX, Jiang DF, Tao YL, Fan ZX, Zhou HX, Bi YP. Biotype status and distribution of Bemisia tabaci (Hemiptera: Aleyrodidae) in Shandong province of China based on mitochondrial DNA markers. Environmental Entomology. 2007;36:1290–1295. doi: 10.1603/0046-225x(2007)36[1290:bsadob]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chu D, Wan FH, Tao YL, Liu GX, Fan ZX, Bi YP. Genetic differentiation of Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q based on mitochondrial DNA markers. Insect Science. 2008;15:115–123. [Google Scholar]
- Chu D, Zhang YJ, Brown JK, Cong B, Xu BY, Wu QJ, Zhu GR. The introduction of the exotic Q biotype of Bemisia tabaci (Gennadius) from the Mediterranean region into China on ornamental crops. Florida Entomologist. 2006;89:168–174. [Google Scholar]
- De Barro PJ, Driver F, Trueman JWH, Curran J. Phylogenetic relationships of world populations of Bemisia tabaci (Gennadius) using ribosomal ITS1. Molecular Phylogenetics and Evolution. 2000;16:29–36. doi: 10.1006/mpev.1999.0768. [DOI] [PubMed] [Google Scholar]
- De Barro PJ, Scott KD, Graham GC, Lange CL, Schutze MK. Isolation and characterization of microsatellite loci in Bemisia tabaci. Molecular Ecology Notes. 2003;3:42–43. [Google Scholar]
- Frohlich DR, Torres-Jerez I, Bedford ID, Markham PG, Brown JK. A phylogeographical analysis of Bemisia tabaci species complex based on mitochondrial DNA markers. Molecular Ecology. 1999;8:1683–1691. doi: 10.1046/j.1365-294x.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Wang CH, Ko CC. Evidence from molecular markers and population genetic analyses suggest recent invasions of the Western North Pacific region by biotypes B and Q of Bemisia tabaci (Gennadius). Environmental Entomology. 2007;36:952–961. doi: 10.1603/0046-225x(2007)36[952:efmmap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Maruthi MN, Rekha AR, Sseruwagi P, Hillocks RJ. Mitochondrial DNA variability and development of a PCR diagnostic test for populations of the whitefly Bemisia afer (Priesner and Hosny). Molecular Biotechnology. 2007;35:31–40. doi: 10.1385/mb:35:1:31. [DOI] [PubMed] [Google Scholar]
- Shoemaker DD, Dyer KA, Ahrens M, McAbee K, Jaenike J. Decreased diversity but increased substitution rate in host mtDNA as a consequence of a Wolbachia endosymbiont infection. Genetics. 2004;168:2049–2058. doi: 10.1534/genetics.104.030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positionsspecific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Annual Review of Entomology. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Zhou W, Rousset F, O'Neill S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proceedings of the Royal Society B: Biological Sciences. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]