Abstract
Objective
Bilateral severe-to-profound sensorineural hearing loss is a standard criterion for cochlear implantation. Increasingly, patients are implanted in one ear and continue to use a hearing aid in the non-implanted ear to improve abilities such as sound localization and speech understanding in noise. Patients with severe-to-profound hearing loss in one ear and a more moderate hearing loss in the other ear (i.e., asymmetric hearing) are not typically considered candidates for cochlear implantation. Amplification in the poorer ear is often unsuccessful due to limited benefit, restricting the patient to unilateral listening from the better ear alone. The purpose of this study was to determine if patients with asymmetric hearing loss could benefit from cochlear implantation in the poorer ear with continued use of a hearing aid in the better ear.
Design
Ten adults with asymmetric hearing between ears participated. In the poorer ear, all participants met cochlear implant candidacy guidelines; seven had postlingual onset and three had pre/perilingual onset of severe-to-profound hearing loss. All had open-set speech recognition in the better hearing ear. Assessment measures included word and sentence recognition in quiet, sentence recognition in fixed noise (four-talker babble) and in diffuse restaurant noise using an adaptive procedure, localization of word stimuli and a hearing handicap scale. Participants were evaluated pre-implant with hearing aids and post-implant with the implant alone, the hearing aid alone in the better ear and bimodally (the implant and hearing aid in combination). Postlingual participants were evaluated at six months post-implant and pre/perilingual participants were evaluated at six and 12 months post-implant. Data analysis compared results 1) of the poorer hearing ear pre-implant (with hearing aid) and post-implant (with cochlear implant), 2) with the device(s) used for everyday listening pre- and post-implant and, 3) between the hearing aid-alone and bimodal listening conditions post-implant.
Results
The postlingual participants showed significant improvements in speech recognition after six months cochlear implant use in the poorer ear. Five postlingual participants had a bimodal advantage over the hearing aid-alone condition on at least one test measure. On average, the postlingual participants had significantly improved localization with bimodal input compared to the hearing aid-alone. Only one pre/perilingual participant had open-set speech recognition with the cochlear implant. This participant had better hearing than the other two pre/perilingual participants in both the poorer and better ear. Localization abilities were not significantly different between the bimodal and hearing aid-alone conditions for the pre/perilingual participants. Mean hearing handicap ratings improved post-implant for all participants indicating perceived benefit in everyday life with the addition of the cochlear implant.
Conclusions
Patients with asymmetric hearing loss who are not typical cochlear implant candidates can benefit from using a cochlear implant in the poorer ear with continued use of a hearing aid in the better ear. For this group of ten, the seven postlingually deafened participants showed greater benefits with the cochlear implant than the pre/perilingual participants; however, further study is needed to determine maximum benefit for those with early onset of hearing loss.
Keywords: Asymmetric hearing loss, Bilateral, Bimodal, Cochlear implant, Speech recognition
Introduction
Current cochlear implant (CI) candidacy includes severe-to-profound sensorineural hearing loss in both ears and poor speech recognition in the best aided condition. When these audiologic criteria are met, cochlear implantation in at least one ear is often recommended and considered standard clinical care. A growing number of patients have received bilateral CIs to alleviate the negative effects of severe-to-profound hearing loss. Bilateral input to the auditory system enhances the potential for binaural processing which relies on head shadow, binaural squelch, binaural summation, and localization abilities (Bronkhorst & Plomp 1988, 1989; Colburn et al. 2006). Bilateral implantation, therefore, has the potential to improve speech recognition in quiet, in noise and from a distance. In addition, bilateral implantation can improve localization of sound in the environment and reduce the overall effort expended during communication (Eapen et al. 2009; Laske et al. 2009; Litovsky et al. 2009; Noble 2010; Preece 2010; for reviews see Ching et al. 2007; Firszt et al. 2008; Sammeth et al. 2011). Bilateral CI studies that include subjective assessments suggest that patient satisfaction is high (Laske et al. 2009; Noble 2010; Peters et al. 2010) which is confirmed by clinical experience; most adults who have received bilateral implants would not return to wearing just one device. Although individual results vary, the findings generally support the use of bilateral CIs to improve hearing abilities in every day life. Bilateral implantation in adults is now accepted as a consideration in clinical practice and has consensus support nationally (Balkany et al. 2008) and internationally (Offeciers et al. 2005; Craddock et al. 2008).
Often patients who have slightly more hearing in one ear choose to receive a CI in the poorer ear and keep a hearing aid (HA) in the better ear, referred to as bimodal stimulation. In these cases, bilateral input is provided with acoustic amplification from one ear and electric stimulation from the opposite ear. As noted with bilateral implantation in adults, bimodal listening has resulted in improvements in speech recognition, localization and everyday functional abilities (El Fata et al. 2009; Fitzpatrick et al. 2009; Potts et al. 2009; for reviews see Ching et al. 2007; Firszt et al. 2008; Sammeth et al. 2011). Although participants in these studies have one ear that is “aidable”, the amount of residual hearing is generally poor and may be centered at one or two low frequencies. Compared with unilateral cochlear implantation, many recipients prefer the combination of devices and describe a more natural sound quality, a balanced input, and even improvements in recognition and appraisal of music (Kong et al. 2005; Gfeller et al. 2008).
Many patients function with noticeable asymmetry in hearing between ears, for example severe-to-profound hearing loss in one ear but only mild-to-moderate, moderate, or moderate-to-severe hearing loss in the other. The better hearing ear is fit with a HA, and the poorer ear often remains unaided. Typically, the poorer ear has not been considered for cochlear implantation due to the presence of a better hearing ear. In this case, hearing input remains asymmetric, essentially unilateral to the better ear, and the ability to restore binaural processing is lost. Listening with just one ear results in poor speech understanding in noise (McLeod et al. 2008; Wie et al. 2010) and diminished sound localization (Humes et al. 1980; Abel et al. 1982) even when the good ear has normal thresholds. Those with asymmetric hearing loss, as described here, are at an even greater disadvantage, since their better ear does not have normal hearing.
The overall goal of this research is to evaluate behavioral outcomes in individuals who have asymmetric hearing loss between ears and receive a CI in the poorer ear. The current study examines speech recognition, localization and measures of hearing handicap, both pre- and post-implant, in ten participants. The findings begin to explore a current clinical question; that is, whether CI candidacy criteria should be expanded in the case of asymmetric hearing loss to include treatment of the ear with severe-to-profound hearing loss. In addition, the results begin to identify patient variables that may influence outcomes and should be considered in future studies.
Materials and Methods
This study was approved by the Human Research Protection Office (HRPO # 201012936) at Washington University School of Medicine (WUSM).
Participants
Participants were ten adults with asymmetric hearing loss between ears (i.e. severe-to-profound hearing loss [SPHL] in one ear and better hearing in the other) who ranged in age from 26 to 82 years with a mean age of 53 years and standard deviation (SD) of 24.4. A summary of each participant's hearing history is provided in Table 1. In the poorer ear, participants P1-P3 and P7-P10 had postlingual onset of SPHL, whereas P4-P6 had pre/perilinguistic onset of SPHL. Participant 5's hearing loss in the poorer ear was formally identified when entering kindergarten, but she reports a possibility of congenital onset. Two of the participants with postlingual onset of SPHL (P7 and P9) and all of the pre/perilinguistic onset participants (P4-P6) had no HA experience in the poorer ear. The other participants had used a HA in the poorer ear; however, only P1 was still using a HA in the poorer ear at the time of the CI evaluation. All participants had relatively long-term HA use in the better ear, except P5 who was fit with a HA as part of the CI candidacy evaluation and P10 who was fit with a HA 11 months prior to cochlear implantation. All patients reported difficulty hearing in daily life and were referred for a CI evaluation by their audiologist or otolaryngologist. Participants were evaluated for CI candidacy by teams at three medical centers: WUSM (P1, P2, P3, P4, P7), Midwest Ear Institute, Kansas City (MEI; P5, P8, P9, P10), and University of Texas, Dallas (UTD; P6).
Table 1.
Demographic information for each participant.
| Participant | Age at Implant | Etiology | Age at Onset of HL (P/B) | Age HA Use Began (P/B) | Age HA Use Ended (P) | Duration SPHL CI Ear (P) | Hearing Aid (B) |
|---|---|---|---|---|---|---|---|
| P1 | 45 | Unknown | 25 / 25 | 25 / 25 | 45 | 1 | Widex Diva 19 |
| P2 | 28 | Meniere's | 19 / 19 | 22 / 22 | 24 | 1.5 | Beltone LINQ |
| P3 | 59 | Familial | 20 / 20 | 23 / 28 | 56 | 10 | Phonak Naida V SP |
| P4 | 28 | Unknown | 3 / 3 | Never / 3 | n/a | 25 | Unitron Power |
| P5 | 28 | Unknown/EVA | 5* / 23 | Never / 28 | n/a | 23 | Starkey S Series |
| P6 | 26 | Meningitis | 7 months | Never / 1 | n/a | 25.5 | Widex Inteo 19 |
| P7 | 77 | Unknown | 37 / 57 | Never / 67 | n/a | 40 | Widex Mind 440 |
| P8 | 82 | Familial/Noise | 62 / 62 | 63 / 77 | 77 | 6 | Starkey S Series |
| P9 | 80 | Meniere's/Noise | 47 / 25 | Never / 63 | n/a | 34 | Phonak Eleva |
| P10 | 76 | Unknown | 61 / 61 | 69 / 75 | 72 | 6 | Starkey S Series |
Possibly congenital
Note: EVA = enlarged vestibular aqueduct; HL = hearing loss; P = poorer hearing ear; B = better hearing ear; HA = hearing aid; SPHL = severe-to-profound hearing loss; CI = cochlear implant
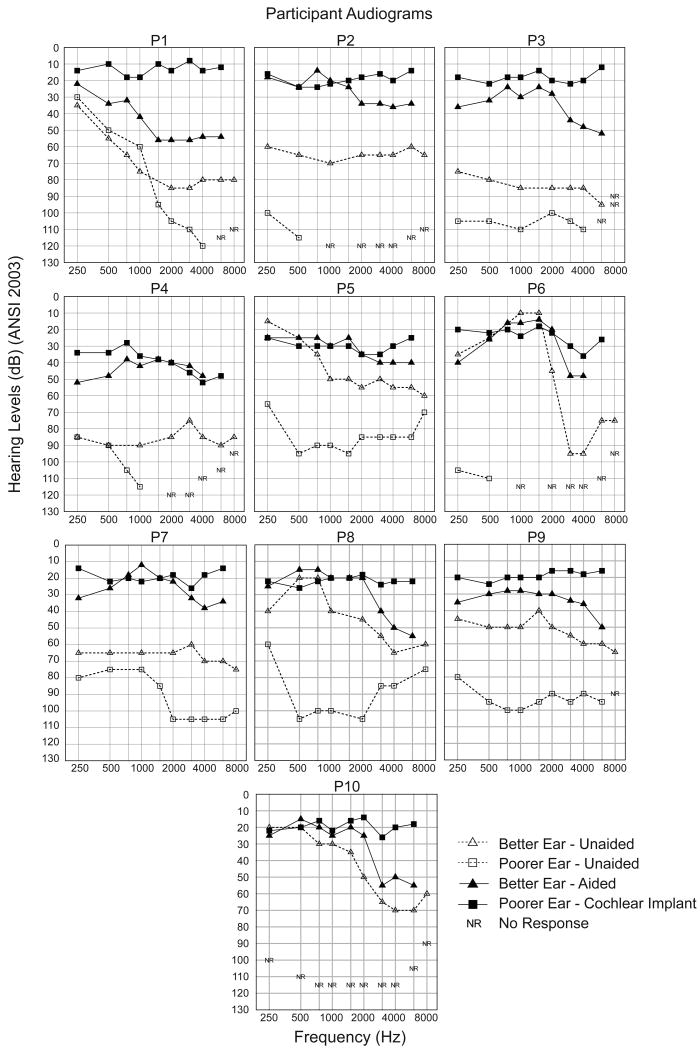
Each participant's audiogram prior to cochlear implantation is shown in Figure 1 (better ear = open triangles with dashed lines, poorer ear = open squares with dashed lines). The unaided pure tone average (PTA of .5, 1, 2 kHz) in the better ear for each participant ranged from 27 to 88 dB HL with a mean of 56 dB HL (SD = 21.7). The PTA in the poorer ear ranged from 72 to 120 dB HL with a mean of 101 dB HL (SD = 16.1). For each participant, aided sound-field threshold levels obtained in the better ear using 2 dB increments of frequency modulated (FM) tones are shown (filled triangles with solid lines) in Figure 1. The aided, three-frequency average in the better ear ranged from 18 to 44 dB HL with a mean of 29 dB HL (SD = 9.2). The participants' amplification worn in the better ear is described in Table 1. Recall that P1 was the only participant who wore a HA in the poorer ear at the time of the CI evaluation. Aided sound-field thresholds for P1's poorer ear ranged from 22 to 56 dB HL at .25-8 kHz with a three-frequency average of 45 dB HL. Four other participants (P2, P3, P8, P10) had used a HA in the poorer ear previously but discontinued use several years earlier due to lack of benefit. An attempt to fit a HA to the poorer ear was included as part of the CI evaluation process for the participants who were not currently using a HA. Although they were willing to wear the HA during the evaluation, they all described the input as unpleasant and in some cases non-auditory.
Figure 1.
Individual audiometric thresholds as a function of frequency are shown for each participant. Unaided thresholds are open symbols with dashed lines, the better ear as triangles and the poorer ear as squares. Aided better ear thresholds are filled triangles with solid lines. Poorer ear post-implant thresholds with a CI are filled squares with solid lines.
Aided speech recognition scores were obtained during the initial CI evaluation to determine candidacy. All participants had open-set speech recognition in the better ear scoring at least 50% on either a sentence or monosyllabic word test. Only P1, P7 and P9 had any aided speech recognition in the poorer ear, scoring between 7% and 23% for monosyllabic words (P1-23%, P7-13%, P9-7%). With little or no open-set speech recognition in the poorer ear, cochlear implantation was recommended by each of the participant's CI centers.
Speech Recognition and Localization Measures
The test battery was designed to include materials and conditions that reflect real-life listening situations, avoid ceiling and floor effects, whenever possible, and have the potential to be replicated in a clinical setting. The protocol included word and sentence measures in quiet and background noise, using either fixed or adaptive noise, with noise being either four-talker babble or restaurant noise. Consonant-Vowel Nucleus-Consonant (CNC) Monosyllabic Words (Peterson & Lehiste, 1962) spoken by a male talker were presented at 60 dB SPL. Hearing In Noise Test (HINT) sentences (Nilsson et al. 1994) and TIMIT sentences (Lamel et al. 1986; Dorman et al. 2005; King et al. 2011) were presented at 60 dB SPL at a +8 dB signal-to-noise ratio (SNR) using four-talker babble. For both measures, the sentences and noise were presented from the same loudspeaker located approximately three feet in front of the listener. The TIMIT sentences were also presented in quiet at 50 dB SPL. The HINT sentences are four to six words in length, are spoken clearly by a male talker and have been used widely with CI recipients. TIMIT sentences range from four to eight words, include both male and female talkers, a variety of regional dialects, and several speaking rates. Compared to the HINT sentences, the TIMIT sentences are more difficult as well as more representative of real-life listening situations because of the variety of speakers and lack of predictability.
The R-SPACE™ (Revit et al. 2002; Compton-Conley et al. 2004) is a laboratory sound system designed to reproduce real-world noise conditions. The listener was surrounded by eight loudspeakers from which recorded restaurant noise was presented (i.e. dishes clanking, people talking) at a fixed level of 60 dB SPL. The long-term spectrum of the restaurant noise was similar to the speech shaped noise from the original HINT test (Compton-Conley et al. 2004). The HINT sentences were presented from 0° azimuth at a level that was varied in an adaptive manner resulting in a Reception Threshold for Sentences (RTS) which represented the SNR for 50% accuracy. The adaptive procedure began with the first of 20 sentences administered at +12 dB SNR (72 dB SPL) and followed the adaptive procedure outlined in the HINT Test Manual (Nilsson et al. 1994). If the first sentence was not understood correctly, the sentence was repeated at a higher (easier) SNR until the participant correctly repeated the sentence. If a participant was incorrect at the highest SNR, the list was scored at floor level (+22 dB). Once the first sentence was correct in its entirety, the SNR for the next sentence was decreased by 4 dB. Throughout the test, the SNR was decreased (made more difficult) after each sentence that was repeated correctly and increased (made easier) after each incorrect response. The step size was 4 dB for the first 4 sentences and 2 dB for the last 16 sentences. The presentation SNR was determined for what would be the 21st sentence and included in the final score calculation. The SNR values obtained using 2 dB step sizes (sentences 5-21) were averaged to obtain an SNR-50.
To assess localization, monosyllabic words were presented randomly from a 15 loudspeaker array at a roved 60 dB SPL (± 3 dB) level. Loudspeakers were arranged in an arc, 10° apart, with ten active and five inactive speakers. The participant was positioned approximately three feet in front of the center loudspeaker. Ten words were presented from each of the active loudspeakers. Although words were used as the stimuli, the participant was not asked to repeat the word, but only to identify the speaker location for each presentation. A root mean score (RMS) error was calculated for each test condition. The RMS error was the mean difference in degrees between the location of the sound source and the source identified by the participant. The formula calculated the square of the difference between the location and the sound source for each trial. These values were summed and divided by the number of trials (100). The square root of that number resulted in the RMS error for the respective test condition.
All testing was performed at WUSM for P1, P2, P3, P4, P6 and P7. The other participants (P5, P8, P9 and P10) were tested at MEI except for a single session (post-implant) in which localization and R-Space testing was performed at WUSM. Test conditions and lists (two for each test) were pseudo randomized for each participant and each test interval. All testing was conducted in a soundbooth and one individual calibrated the test stimuli to ensure consistency between both centers and uniform calibration of the sound pressure level (SPL) of the stimuli.
Test Intervals and Device Fitting Procedures
Participants were evaluated pre-implant and will be followed at several intervals post-implant; here we report data for the pre-implant and six-month post-implant test sessions for all participants as well as 12-month results for the three pre/perilingual participants. (Twelve month results are not yet available for all postlingual participants.) The results are presented for the better ear alone, poorer ear alone and in the participants' everyday listening condition. Pre-implant, all participants used their own HA for testing the better ear. Participants 2-10 used a clinic-owned HA for testing the poorer ear, and P1 used her own HA. The pre-implant everyday listening condition for P1 was bilateral HAs. For the remaining participants, the pre-implant everyday listening condition was a HA in the better ear alone. Post-implant, all participants continued using their own HA for testing the better ear, the CI for testing the poorer ear, and the two together (bimodal) for the everyday listening condition. Table 2 provides a summary of the devices used at the pre-implant and post-implant test intervals. Testing the poorer ear alone was always conducted with the better ear blocked (EAR foam plug and Howard Leight Hearing Protection ear muff). Prior to testing, standard ANSI S3.22-1996 electroacoustic assessment was used to ensure proper HA function. The HA fittings were verified to meet NAL targets using the Audioscan Verifit system for 60 or 65 dB input. Fitting parameters were adjusted to provide audibility for soft speech (55 dB SPL) and comfort for loud sounds (75 dB SPL). Tolerance and comfort issues limited the amount of gain that could be provided in the high frequencies for some patients.
Table 2.
Device use conditions for participants.
| Condition | P1 | P2-10 |
|---|---|---|
|
| ||
| Pre-Implant | ||
| Better Ear | Own HA | Own HA |
| Poorer Ear | Own HA | Clinic HA |
| Everyday | Bilateral HAs | Better Ear HA |
|
| ||
| Post-Implant | ||
| Better Ear | Own HA | Own HA |
| Poorer Ear | CI | CI |
| Everyday | Bimodal | Bimodal |
Note: HA = hearing aid; CI = cochlear implant
In the implanted ear, each participant's speech processor was programmed by an experienced CI audiologist. A variety of programming parameters were adjusted after the initial activation to ensure optimal speech understanding in everyday life. Participants wore their preferred speech processor program and settings for all testing. Table 3 shows the implant type and programming parameters used by each participant. All except two of the participants used a Nucleus device (Contour Advance or 512). Participant 4 used the Advanced Bionics 90K and P5 the Med-El Sonata 100. Each participant had 3D reconstructions of spiral CT scans (Skinner et al. 2007) that confirmed full electrode array insertion within the cochlea.
Table 3.
Cochlear implant type and parameters for each participant.
| Participant | Cochlear Implant | Processor | # Active Channels | Strategy | Rate per Channel | Total Stim Rate |
|---|---|---|---|---|---|---|
| P1 | Nucleus Contour Advance | Freedom | 21 | ACE | 1800 | 14400 |
| P2 | Nucleus Contour Advance | Freedom | 22 | ACE | 1800 | 14400 |
| P3 | Nucleus 512 | 810 | 22 | ACE | 1800 | 14400 |
| P4 | Advanced Bionics 90K/Hifocus 1j | Harmony | 8 | HiRes-S | 2900 | 23300 |
| P5 | Med-El Sonata100 | OPUS 2 | 10 | FSP | 2256 | 22560 |
| P6 | Nucleus 512 | 810 | 22 | ACE | 500 | 4000 |
| P7 | Nucleus 512 | 810 | 20 | ACE | 900 | 7200 |
| P8 | Nucleus 512 | 810 | 21 | ACE | 900 | 7200 |
| P9 | Nucleus 512 | 810 | 20 | ACE | 900 | 7200 |
| P10 | Nucleus 512 | 810 | 19 | ACE | 900 | 7200 |
Note: # = number; ACE = advanced combined encoder; HiRes-S = high resolution with sequential stimulation; FSP = fine structure processing; Stim = stimulation
Participant Rating of Hearing Disability
In addition to the speech recognition measures, the Speech, Spatial and Qualities of Hearing Scale (SSQ; Gatehouse & Noble 2004) was administered at the pre- and post-implant intervals. This questionnaire assesses hearing disability as rated by the participant, includes a broad range of domains, and reflects the individual's perception of functioning in real-world situations. Section I (hearing of Speech) probes speech recognition in a variety of sound environments with varying degrees of talker visibility. Section II (Spatial hearing) examines three components of spatial hearing, sound direction, distance and movement. Section III (Qualities of sound) considers segregation of sounds, naturalness and listening effort. When answering the questions, either pre-implant or post-implant, all participants were instructed to consider their everyday listening condition (see Table 2).
Data Analysis
Several comparisons were of interest. First, to evaluate changes in the poorer ear after receiving an implant, scores pre-implant with a HA were compared to scores post-implant with a CI. Second, to evaluate the impact of adding a CI, the everyday listening condition pre-implant (either one or two hearing aids) was compared to the everyday listening condition post-implant (bimodal). Finally, the HA-alone condition (better ear) was compared to the bimodal condition at post-implant intervals. Paired t-tests were used for comparisons with group postlingual participant data. A binomial model (Thornton & Raffin 1978; Carney & Schlauch 2007) was used for individual CNC, HINT and TIMIT data with significant differences defined as those at the 0.05 level. For individual HINT sentence scores presented in the R-SPACE™, a critical difference of 1.4 dB based on the 95% confidence interval was used (Compton-Conley et al. 2004). Localization results were analyzed with paired t-tests to compare group postlingual participant RMS error scores. In addition, individual localization responses were calculated as the mean and standard deviation of responses at each source speaker location. Ordinary least squares (OLS) regression with correction of standard errors for unequal variance between conditions was used to compare the slopes of the fitted lines. This method accounted for differences in variance as well as differences in slope. For the SSQ, a 2 (interval) × 3 (section) repeated measures ANOVA was used to analyze group results for the postlingual participants. Individual results were analyzed using the categorization system developed by Noble et al. (2009).
Results
Sound-Field Thresholds
Figure 1 displays FM tone, sound-field threshold levels from .25-6 kHz with the CI (filled squares and solid lines) as well as with the HA in the opposite ear for each participant (filled triangles and solid lines). Recall P1-P3 and P7-P10 had postlingual onset of SPHL in the poorer ear, whereas P4-P6 had pre/perilingual onset of SPHL. The CI sound-field threshold levels obtained at the six-month interval for the postlingual participants were near 20 dB HL across the frequency range. The CI sound-field threshold levels for P5 and P6 were between 20 and 40 dB HL across the frequency range; P4 had thresholds between 42-52 dB HL at 3000-6000 Hz. Tolerance issues prevented the pre/perilingual participants from obtaining the desired sound-field thresholds of 20-30 dB HL at all frequencies. The CI provided better audibility for at least part of the frequency range for all participants compared to the HA in the opposite ear.
Speech Recognition - Participants with Postlingual Onset of SPHL
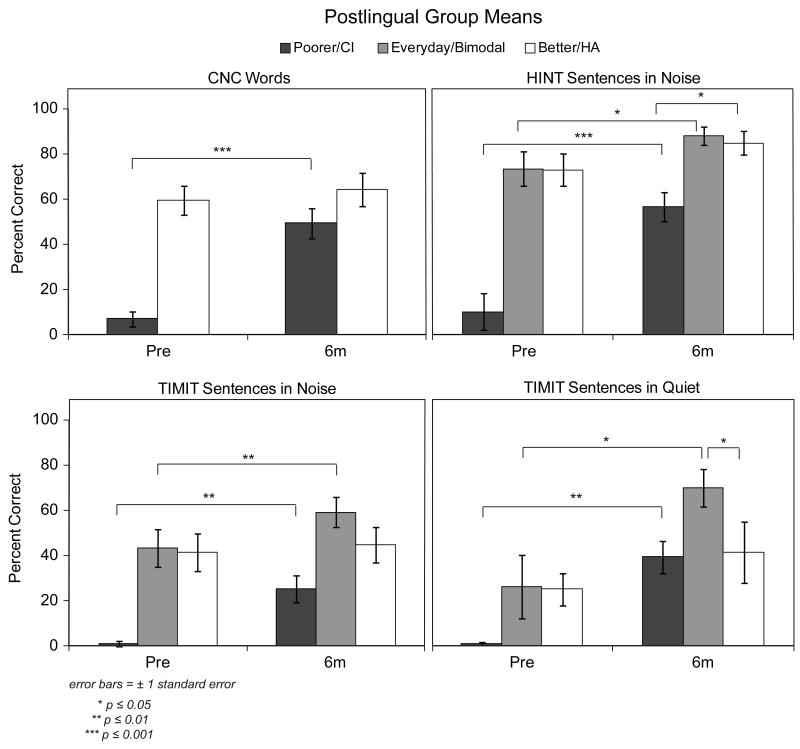
Group mean speech recognition scores for the seven participants with postlingual onset of SPHL are shown in Figure 2. Pre-implant scores are shown on the left of each panel and scores at the six-month interval are shown on the right. For this figure as well as Figures 3-6, the black bars represent group mean scores for the poorer ear. The white bar displays mean scores for the better ear alone with a HA. The gray bars (e.g., HINT and TIMIT) represent mean scores for the participants' everyday listening condition. Recall, pre-implant, the everyday listening condition was bilateral HAs for P1 and a single HA in the better ear for P2-P10. Post-implant, the everyday listening condition was bimodal (HA + CI) for all participants. The mean scores for participants with postlingual onset of SPHL show a substantial and significant improvement between the poorer hearing ear pre-implant and that same ear in the CI-alone condition at the six-month interval on all four test measures CNC t(6) = -10.56, p ≤ 0.001; HINT in noise t(6) = -10.56; p ≤ 0.001; TIMIT in noise t(6) = -5.11, p ≤ 0.01; TIMIT in quiet t(6) = -5.99, p ≤ 0.001. For each test, the group mean and range of scores at the pre-implant and post-implant intervals for the poorer ear are provided in Table 4. The group mean pattern was true at the individual level as well; all postlingual participants had a significant increase in speech recognition post-implant on all four measures (p ≤ 0.05).
Figure 2.
Group mean speech recognition scores (CNC words, HINT sentences in noise, TIMIT sentences in noise and TIMIT sentences in quiet) pre-implant and at six months post-implant are shown for the seven postlingual participants. Scores are shown in black for the poorer hearing ear that was implanted, in white for the better hearing ear with a HA, and in gray for the participants' everyday listening condition (bimodal at the post-implant interval).
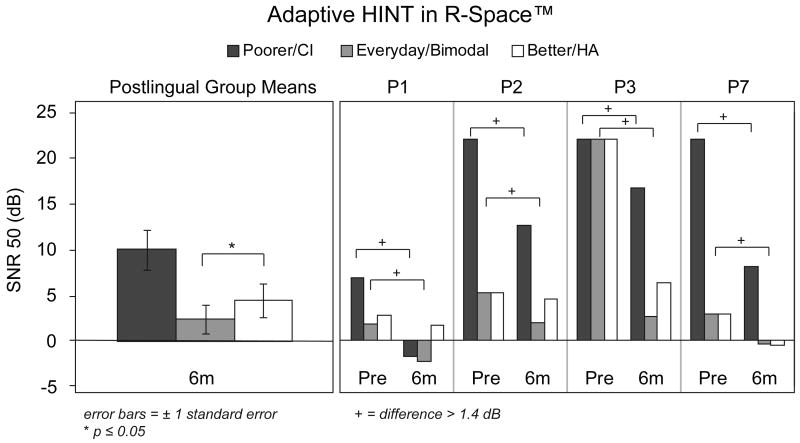
Figure 3.
Group mean adaptive HINT scores in restaurant noise at the six-month interval for all postlingual participants are shown in the left panel. Individual participants' scores are shown in the right panel for the four participants who had both pre- and post-implant testing. Scores are expressed as SNR and are shown in black for the poorer hearing ear that was implanted, in white for the better hearing ear with a HA, and in gray for the participants' everyday listening condition (bimodal at the post-implant interval).
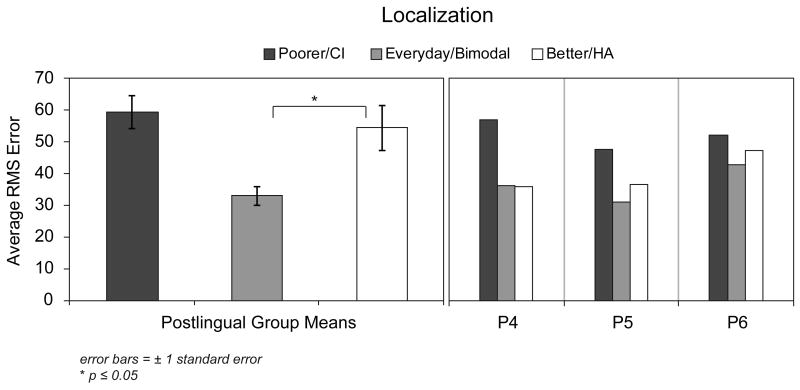
Figure 6.
Group mean RMS error scores are shown in the left panel for the postlingual participants. Individual RMS error scores for the pre/perilingual participants are shown in the right panel. Scores are shown in black for the poorer hearing ear that was implanted, in white for the better hearing ear with a HA, and in gray for the participants' everyday listening condition (bimodal).
Table 4.
Group mean and range of scores in percent correct for the poorer ear alone at the pre-implant (HA) and post-implant (CI) intervals for postlingual participants.
| Test Measures | Pre-implant Mean | Pre-implant Range | Post-implant Mean | Post-implant Range |
|---|---|---|---|---|
| CNC words | 6 | 0-23 | 50 | 29-79 |
| HINT sentences in noise | 10 | 0-58 | 57 | 32-87 |
| TIMIT sentences in noise | 1 | 0-8 | 25 | 12-57 |
| TIMIT sentences in quiet | 1 | 0-4 | 39 | 22-75 |
Comparisons between the pre-implant and post-implant everyday listening conditions indicated significantly higher group mean scores post-implant for all three sentences tests (HINT in noise t(6) = -3.29; p ≤ 0.05; TIMIT in noise t(6) = -2.72, p ≤ 0.05; TIMIT in quiet t(6) = -5.27, p ≤ 0.01). Analysis of this comparison for individual participants indicated significantly higher scores in the post-implant bimodal condition than in the pre-implant everyday listening condition for all participants with TIMIT sentences in quiet, for one participant (P2) with HINT sentences in noise, and for two participants (P2 and P3) with TIMIT sentences in noise (p ≤ 0.05).
At the six month interval, the bimodal condition (gray bars) produced higher group mean scores than the HA-alone condition (white bars) for HINT and TIMIT sentences. However, the improvement was only significant for TIMIT sentences in quiet (50 dB SPL), t(6) = -2.98, p ≤ 0.05. Analysis of individual scores (bimodal compared to HA-alone) indicated significantly better bimodal performance for two participants (P2 and P3) on the TIMIT sentences in quiet and for one participant (P1) on TIMIT sentences in noise (p ≤ 0.05). Individual participants' scores indicated no significant difference between bimodal and HA-alone scores for HINT sentences in noise (p > 0.05). Ceiling effects with the HA alone were present for all except P9, so it is not surprising that no differences were measured between the two conditions for this test.
As noted in the methods, the adaptive HINT Sentence Test was administered in the R-SPACE™ (available at the WUSM site but not the MEI site). Testing simulated listening in a restaurant environment and the score obtained was the SNR at which the participant could repeat 50% of the sentences correctly (SNR-50; lower scores are better). Both pre- and post-implant results were obtained for the four participants with postlingual onset of SPHL who were followed at WUSM. The three postlingual MEI participants traveled to WUSM for a single post-implant test session at six months. Figure 3 provides group mean results for the six-month interval for all seven participants in the panel on the left and individual results for the four participants with pre- and post-implant results in the panel on the right. At the six-month interval, the lowest average SNR was in the bimodal condition. The means indicate significantly better (lower) SNR scores in the bimodal compared to HA-alone condition, t(6) = 3.02, p ≤ 0.05. The individual results for the four participants with pre- and post-implant scores indicate improvement for all four participants when comparing the poorer ear alone pre-implant and the CI-alone condition post-implant. Likewise, the post-implant bimodal results were better than the pre-implant everyday results for all four participants.
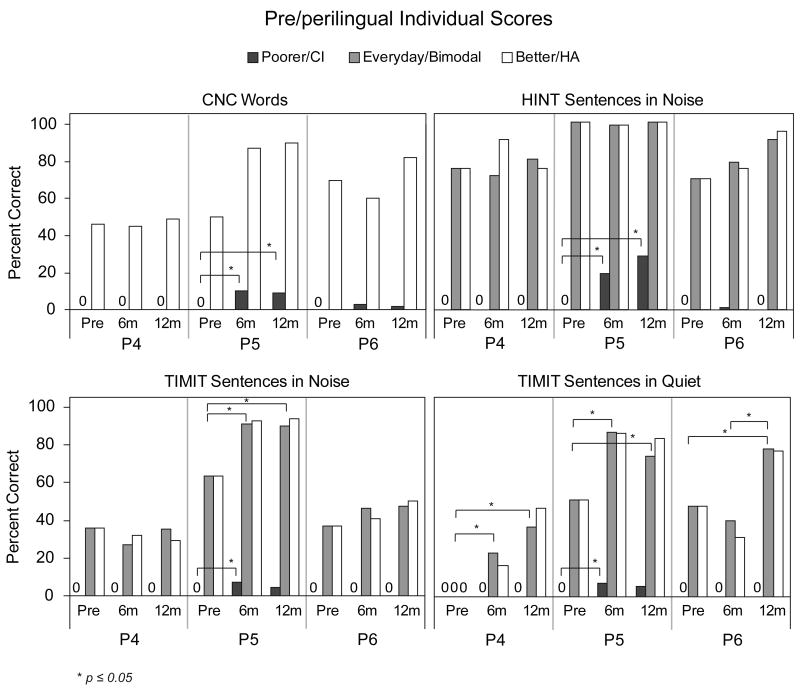
Speech Recognition - Participants with Pre/Perilingual Onset of SPHL
Speech recognition scores (CNC Words, HINT sentences in noise, TIMIT sentences in noise and TIMIT sentences in quiet) are shown in Figure 4 for the three individual participants with pre/perilingual onset of SPHL at the pre-implant, six-month and 12-month intervals. For the poorer ear, P5 had a small but statistically significant improvement from pre-implant to six months post-implant for all four measures (p ≤ 0.05), whereas P4 and P6 showed little if any speech recognition either pre- or post-implant in the poorer ear. At the 12-month interval, P5 had a small but nonsignificant increase for HINT sentences in noise compared to her score at six-months post-implant (p > 0.05). Scores on the other measures were stable. Participant 5 also demonstrated significant improvement in the everyday listening condition post-implant (bimodal) compared to pre-implant for TIMIT sentences in noise and in quiet, as did P4 and P6 for TIMIT sentences in quiet (p ≤ 0.05).
Figure 4.
Individual participants' speech recognition scores (CNC words, HINT sentences in noise, TIMIT sentences in noise and TIMIT sentences in quiet) are shown for the three pre/perilingual participants pre-implant, and at six and 12 months post-implant. Scores are shown in black for the poorer hearing ear that was implanted, in white for the better hearing ear with a HA, and in gray for the participants' everyday listening condition (bimodal at the post-implant interval).
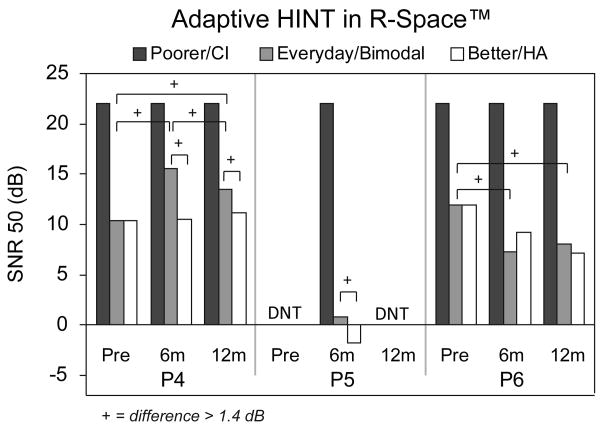
Figure 5 shows the SNR from the adaptive HINT Sentence test in the R-Space™ for the three pre/perilingual participants. Results for P4 and P6 were obtained at the pre-implant, six-month and 12-month intervals and for P5 at the six-month interval. None of these participants were able to understand sentences in restaurant noise at the highest SNR (+22 dB) in the poorer ear at any tested interval. Participant 6 scored better in the everyday condition at six and 12-months post-implant compared to pre-implant. For P4, scores in the bimodal condition post-implant were poorer than those obtained in the everyday condition pre-implant. Both P4 and P5 scored worse in the bimodal condition compared to the HA-alone condition at the post-implant intervals.
Figure 5.
Individual pre/perilingual participants' adaptive HINT scores in restaurant noise are shown. Scores are expressed as SNR and are shown in black for the poorer hearing ear that was implanted, in white for the better hearing ear with a HA, and in gray for the participants' everyday listening condition (bimodal at the post-implant interval).
Better Ear HA alone over time - All Participants
Performance in the better ear with a HA was not expected to change over time since all participants had stable hearing thresholds and most had considerable HA experience in the better ear. However, several participants demonstrated improvement in the post-implant HA-alone condition over time. For example, four postlingual participants had improvement on at least one measure from pre-implant to six months post-implant and all three pre/perilingual participants improved on at least one measure from pre-implant to the 12-month interval.
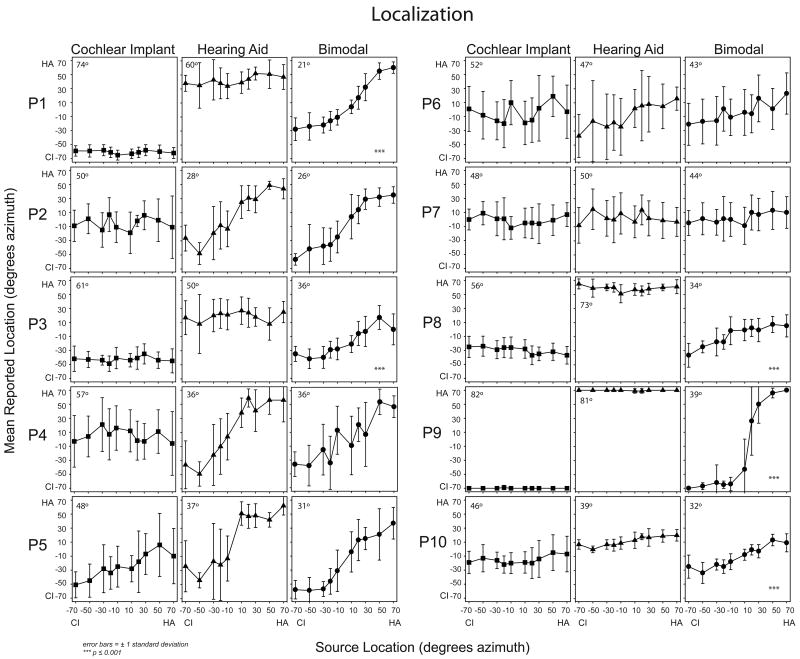
Localization
Figure 6 shows the mean six-month RMS error scores for postlingual participants on the left and individual RMS error scores for pre/perilingual participants on the right. For the postlingual participants, there was a significant improvement in the bimodal compared to the HA-alone condition; t(6) = 3.13, p ≤ 0.05. For the pre/perilingual participants, the RMS error for the bimodal condition was similar to or slightly lower than the HA-alone RMS error. In Figure 7, the localization plots show the mean response and standard deviation in degrees azimuth for each of the 10 active loudspeakers used to present the stimuli. The location of the stimulus is shown along the x-axis and the response along the y-axis. Perfect localization responses would be graphed as a diagonal line from the lower left hand corner to the upper right hand corner. To aid in interpretation, all data are plotted as if the participant had the CI in the left ear and the HA in the right ear (i.e. the negative numbers reflect loudspeaker locations to the CI side and positive numbers reflect those to the HA side). The RMS error score is also indicated in the upper left hand corner of each plot. Results of the OLS regression with correction of standard errors for unequal variance indicated that the slope of the bimodal condition line was significantly different than the HA-alone condition line for five participants: P1, F(1,294) = 97.6; P3, F(1,294) = 24.4; P8, F(1,294) = 59.7; P9 F(1,294) = 454.2; and P10 F(1,294) = 24.7; for all five, p ≤ 0.001. In other words, these five postlingually deafened participants demonstrated significantly improved localization in the bimodal compared to the HA-alone condition. Visual inspection of the results indicated that four of these (P1, P3, P8, P9) and to some extent P10 perceived sound as originating on the side of the device for either the HA-alone or the CI-alone conditions. The participants who did not have a significant difference between HA-alone and bimodal localization performance fell into two basic patterns. Three participants (P2, P4, P5) were able to localize to some extent with the HA alone and this ability was similar to the bimodal condition. The other two participants (P6, P7) were unable to localize irrespective of listening condition.
Figure 7.
Individual participants' localization results are shown for the conditions CI-alone (filled squares, left panels), HA-alone (filled triangles, middle panels), and bimodal (filled circles, right panels) at six months post-implant. Symbols represent mean responses in degrees azimuth. X-axis represents the location of the stimuli and the Y-axis, the location of the response. The RMS error score for each participant and condition is noted in the upper left hand corner of each panel. Significant differences between the HA-alone and bimodal conditions are indicated with asterisks in the lower right hand corner of the bimodal panels.
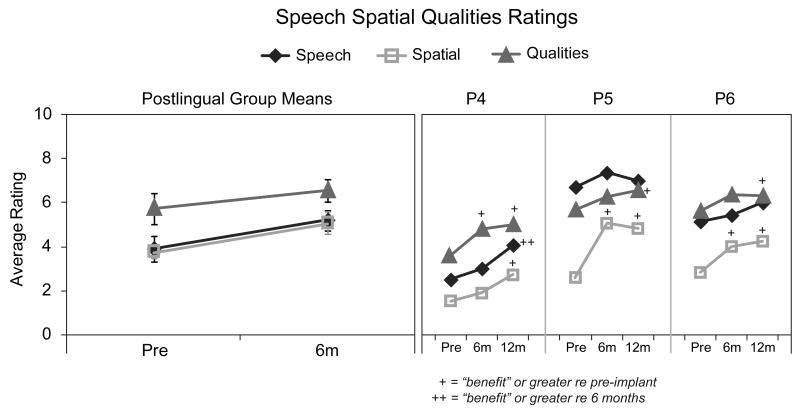
Speech, Spatial and Qualities of Hearing Scale
Figure 8 shows ratings pre- and post-implant for each of the three sections of the SSQ; Speech Hearing, Spatial Hearing, and Sound Qualities. On the left are mean ratings for the participants with postlingual onset of SPHL and on the right are the individual ratings for the three participants with pre/perilingual onset of SPHL. Values are shown with the diamond symbol for the Speech, the square symbol for the Spatial, and the triangle symbol for the Qualities sections. Results of a 2 (interval) × 3 (section) repeated measures ANOVA for postlingual participant group mean scores indicated a significant main effect for both interval, F(2,6) = 14.47, p ≤ 0.01, and section, F(2,12) = 5.05, p ≤ 0.05. Post hoc comparisons identified the Speech and Spatial sections as significantly different from the Qualities section (p ≤ 0.05). For individuals, rating changes between test intervals were classified according to Noble and colleagues as follows: ≤ 1 indicates “no change”; +1-2 indicates “benefit”; +2-4 “high benefit”, +> 4 “very high benefit” and > 1 in the negative direction indicates “negative benefit” (Noble et al. 2009; Noble 2010). All postlingual participants reported “benefit” from pre- to post-implant. All except P10 noted improvement on the Speech section (ranging from “benefit” to “very high benefit”). All except P1 noted improvement on the Spatial section (ranging from “benefit” to “high benefit”). Three participants reported improvement on the Qualities section (P2 and P8 “benefit”; P7 “very high benefit”).
Figure 8.
Average ratings for the three sections of the SSQ (Speech as diamonds, Spatial as squares, Quality as triangles) for postlingual participants are shown in the left panel. Individual results for pre/perilingual participants are shown in the right panel at pre-implant and at six and 12 months post-implant.
All three pre/perilingual participants reported “benefit” in Spatial hearing over time. Participants 4 and 6 noted an improvement (“benefit”) between the pre-implant and 12-month intervals. Participant 5 reported improvement (“high benefit”) by the six-month interval. Participant 4 was the only participant who reported improvements on the Speech and Qualities sections (“benefit”) from pre-implant to 12 months.
Discussion
Sound Detection
An important benefit from cochlear implantation is the ability to detect sound at soft and even very soft levels across the frequency range (.25-6 kHz). Although detection does not ensure speech recognition, if a speech cue is not audible, it will not be understood. The ability to detect sound in the poorer ear was substantially improved for all participants with the CI. Additionally, the CI provided better detection for part or all of the frequency range compared to the aided sound-field thresholds of the better ear (Figure 1). The three pre/perilingual participants had somewhat worse sound-field thresholds compared to the postlingual participants. In particular, P5 and P6's thresholds were similar to the postlingual participant's thresholds through 1500 and 2000 Hz, respectively, but were elevated in the high frequencies. Participant 4's thresholds were elevated across the frequency range but were also worse in the high frequencies compared to the low and mid frequency range. Clinically, individuals with pre/perilingual onset of SPHL can have tolerance issues that may prevent obtaining sound-field levels less than 30 dB HL across the frequency range.
Word and Sentence Recognition in Postlingual Participants
The participants with postlingual onset of SPHL showed substantial improvement in word and sentence recognition scores at the six-month post-implant test interval compared to pre-implant scores for the ear that was implanted. Not surprisingly, these seven participants demonstrated CI-alone speech recognition scores that are similar to the range of traditional postlingual CI recipients who have SPHL in both ears. These same participants showed better scores in the bimodal condition on sentence measures, both in quiet and in noise, compared to scores in their pre-implant everyday listening condition (Figure 2). The poorest post-implant HA-alone score for this group was for TIMIT sentences at a soft level (50 dB SPL) in quiet. The addition of a CI resulted in significant improvement over the HA-alone condition. This may be due in part to a summation effect but improved detection levels provided by the CI may also account for this enhancement. It is also notable that HA-alone performance for this group on the HINT sentences in noise, on average, was above 80%, limiting demonstration of a bimodal benefit. A ceiling effect was not present in the HA-alone condition for the more challenging TIMIT sentences in noise (mean score of 45%); therefore, a bimodal effect could be measured. Although the bimodal mean was 14 percentage points higher than the HA-alone mean, the difference did not reach statistical significance (p = 0.064). The HINT sentences presented adaptively in the R-Space™ did identify significant bimodal benefit over the HA-alone condition at the six-month interval.
Word and Sentence Recognition in Pre/Perilingual Participants
In contrast to the postlingual participants, the participants with pre/perilinguistic onset of SPHL demonstrated little to no CI-alone speech recognition after 12 months of CI use. In spite of this, all three continue to use their CIs regularly. Only P5 showed some ability to recognize speech with audition alone in the implanted ear (e.g., 9% on CNC words, 29% on HINT sentences in noise, 5% on TIMIT sentences in quiet and noise at 12-months post-implant). Several reasons may account for the differences between this individual and the other two pre/perilingual participants. First, she had more hearing in the poorer ear than either P4 or P6; however, like P4 and P6, she never wore a HA in the poorer ear. Second, P5 had more hearing in the better ear as well as a later onset of HL in that ear compared to P4 and P6. It is possible that her experience with a more intact auditory system was a factor in her improved CI performance. Third, she consistently wore the implant from the initial activation and was highly motivated to learn to hear through her implant. Participant 4 struggled initially as she adjusted to the new sound provided by the CI. This was partially due to her noisy daily environment which included taking care of her three young children. Evidenced by her scores, the addition of the CI seemed to result in poorer speech recognition abilities compared to the HA-alone condition, particularly in noise. Patients with asymmetric hearing must alter their strategies for coping with difficult listening situations once a CI is received. For example, turning the better ear away from a competing noise prior to implantation would have reduced the noise to the only-hearing ear; however, after implantation, unwanted/competing noise may be introduced regardless of head position. Participant 6 had relatively consistent device use as she learned to listen in the bimodal condition, but could not always use the CI and HA together for select work situations. For example, when talking on the phone with her better (HA) ear, the sounds she heard in the implanted ear were initially distracting; she had no prior experience ignoring other sounds while on the phone.
The reports from these three participants remind us that adapting to an implant is a process that takes time, experience, and practice, especially when there have been long periods of auditory deprivation in the ear being implanted. Participants 4-6 had between 23 and 25.5 years of deprivation in the implanted ear; P7 and P9 also had extended periods of deprivation (40 and 34 years, respectively) in the poorer ear; however, their onset of SPHL was postlingual. It is common that longer periods of time are required to maximize potential benefits of implantation for traditional CI recipients with pre/perilinguistic onset of SPHL compared to those with postlinguistic onset (Litovsky et al. 2004; Nava et al. 2009). These three participants had meaningful residual hearing in the non-implanted ear, which distinguishes them from traditional CI recipients with bilateral pre/perilinguistic onset of SPHL. Additional longitudinal data are needed to determine whether outcomes will be different for patients with pre/perilinguistic onset of SPHL in only one ear versus SPHL in both ears. Another characteristic of participants 4-6 is that each had no HA experience in the ear to be implanted. (Again, P7 and P9 also did not use amplification in the poorer ear; however the hearing loss was postlingual.) The lack of amplification in the poorer ear is likely due to the large asymmetry between ears from childhood. Clinically, it is more difficult to achieve a successful HA fitting in the poorer ear for individuals with large differences in hearing abilities between ears. Exploration is warranted as to whether benefits can be achieved for this population if there is no HA experience in the poorer ear, even when the other ear has more hearing and has been consistently amplified. Given the results of P4-P6, a more earnest attempt should be made to evaluate amplification for the poorer ear during childhood, and if unsuccessful, to consider cochlear implantation.
Changes in Speech Recognition for the Better Hearing Ear
There were several instances of improved scores with the better ear in the HA-alone condition post-implant compared to the pre-implant test interval. For example, P5's scores improved on most measures. Other participants improved on one or two measures. In the case of P5, the improvements may be due to the additional HA experience in the better ear. This patient was first fit with a HA several weeks prior to her enrollment in the study. However, the other participants had worn a HA in the better ear for longer periods of time and improvement in HA-alone scores was not expected post-implantation. These improvements are likely due to increased familiarity with the tasks and test environment. Another possibility, particularly for P2, P3 and P6 who demonstrated bimodal benefit, may be that experience, or in the case of P2 and P3 renewed experience with binaural processing (as a result of using the implant and the HA together), enhanced the abilities of the better ear via the HA. Whether binaural listening can improve single ear perception remains speculative but could be considered in a future longitudinal study with additional participants.
Effectiveness of Test Battery
The test measures selected for this study included materials and conditions that were designed to reflect real-life listening situations and were chosen to avoid floor and ceiling effects. Sentences that change in structure, are spoken by different male and female talkers, and use a variety of rates better reflect conversations that one might encounter throughout the day. Background noise that includes people talking or restaurant noise also simulates the listening environments patients report as most difficult in everyday experiences. Evaluation of speech recognition in simulated diffuse noise with multiple surrounding loudspeakers, as with the restaurant noise and R-SPACE™, re-creates a familiar environment that CI recipients encounter during family and work gatherings. At the same time, the wide range of individual abilities makes it difficult to find measures that do not result in floor or ceiling effects for at least some patients. This is exacerbated for this study population, where there is wide discrepancy in ability between the two ears. For example, the HINT sentences in noise at a +8 SNR resulted in ceiling effects for the HA ear, which negated the ability to identify bimodal improvement. However, it was one measure for which perilingually deafened P5 was able to recognize speech using the CI alone, scoring higher on this measure than any others. Although adaptive procedures as used with the R-SPACE™ can avoid ceiling effects, floor effects were seen in the participants' poorer ears at both pre- and post-implant intervals. Protocols that include multiple measures of speech recognition, localization and hearing handicap better capture the abilities of this patient population. To clinically monitor progress over time in the CI-alone condition, sentence materials that range in difficulty may be necessary (e.g. HINT sentences in quiet for patients with limited speech understanding and adaptive measures in noise for patients who perform near ceiling on easier tests in quiet).
Localization
Difficulty with localization and spatial hearing is a known result for individuals with unilateral hearing loss (Humes et al. 1980, Abel et al. 1982). It is also a reported frustration of patients with asymmetric hearing loss between ears (Noble & Gatehouse 2004, Newman et al. 1997). For the participants in the current study, the group mean results (Figure 6) and individual results for five of the seven postlingually deafened participants (Figure 7) demonstrated significantly improved localization in the bimodal compared to HA-alone condition. In the bimodal condition P1, P3, P8 and P9 were able to, in varying degrees of accuracy, estimate the sound source all along the loudspeaker array, whereas with either device alone, all stimuli were heard as originating from only the side of the device. Other studies have shown localization improvements in the bimodal condition versus either ear alone for some postlingual adults (Ching et al. 2004, 2007; Seeber et al. 2004; Dunn et al. 2005; Potts et al. 2009). For the most part, those study participants had less hearing in the HA ear than the participants in the current study. Both Seeber et al. (2004) and Dunn et al. (2005) reported individual results for their adult study participants (samples of 11 and 12, respectively), all of whom were postlingually deafened. The results of both studies were similar in that three participants localized fairly well and a similar number determined the side of presentation for stimuli presented from the far right or left. The participant with the best localization ability studied by Seeber and colleagues (2004) had the most residual hearing in the HA ear, with thresholds in the same range as the asymmetric participants in the current study.
Speech, Spatial and Qualities of Hearing Scale
Of interest were the participants' subjective reports following cochlear implantation of the poorer ear. Significant improvement pre- to post-implant was observed for Spatial and Speech hearing with the postlingual participants and for Spatial hearing for all three pre/perilingual participants. On the whole, Qualities of hearing had the smallest change between pre-implant and six months post-implant intervals although four participants rated Qualities as improved. This may be partially due to the fact that Qualities was rated fairly high at the pre-implant interval. None of the group mean ratings for the three sections worsened following implantation. Some individual participants did report “negative benefit” on individual questions. For example, P1 and P7 reported a less natural quality for some sounds. Participant 5 reported a need to concentrate more at times and difficulty ignoring other sounds when attending to one sound in particular. However, no participant had overall “negative benefit” for any of the three sections and all participants had overall “benefit” (> +1) on at least one of the three sections. Even though there was considerable improvement post-implant, the average ratings remained far below the maximum rating of 10, a rating that indicates little to no disability perceived by the individual. Therefore, even with a HA and a CI combined, perceived communication difficulties persisted. This is consistent with a study by Donaldson et al. (2009) where CI participants, including those who used a HA on the contralateral ear, reported difficulties in adverse listening conditions.
CI Candidacy for Patients with Asymmetric Hearing Loss
Our study results indicate that even though the better ear performance did not meet traditional CI candidacy criteria, the majority of participants benefitted from a CI in the poorer ear. This supports consideration for cochlear implantation in the poorer ear of adults with one ear outside the traditional CI candidacy criteria, especially those with postlingual onset of hearing loss. Perhaps individual ear scores should be considered rather than only “best-aided” bilateral speech recognition scores for the speech recognition component of the CI evaluation process. In most cases of asymmetric hearing loss where one ear is in the severe-to-profound hearing loss range, a “best-aided” bilateral speech recognition score probably reflects the better hearing ear. With the possible exception of P5, the better hearing ear of the current study sample still demonstrated reduced speech understanding compared to adults with normal hearing, as noted by the reduced scores for CNC words, TIMIT sentences in quiet and noise, and the adaptive HINT in the R-SPACE™. Therefore, examination of each ear and treatment with the best sensory device available for an individual ear and patient may be prudent if the goal is optimal hearing in daily living. This is especially true given that we do not fully understand the critical time period for acquiring binaural processing and to what extent binaural abilities can be achieved in the presence of different audiometric profiles such as asymmetric hearing loss.
Patients with pre/perilingual onset of SPHL and no HA experience in the poorer ear present a more challenging clinical question. The limited benefit of speech recognition provided by the CI for the three participants with pre/perilingual deafness brings into question the advisability of cochlear implantation for patients with this profile. However, looking beyond speech recognition scores to measures that reflect daily communication demonstrates that cochlear implantation can be beneficial to this population. All three of these participants consistently use their CIs and reported improvements in everyday listening on the SSQ. Participants 5 and 6 noted “benefit” and “high benefit”, respectively, for Spatial hearing and P4 reported “benefit” or “high benefit” on all three sections of the SSQ. The reports of benefit in everyday life are consistent with the participants' willingness to wear the CI daily.
Pre-implant counseling and appropriate expectations are key components for successful implantation of all prospective CI recipients. However, if implantation of a congenitally deaf ear that has not received stimulation is considered, counseling and expectations are critical in the decision making and potential outcome. All three of the pre/perilingual participants in this study had been counseled extensively and were motivated. The preliminary results from this study are encouraging but also indicate that further investigation is warranted. Additional participants followed over a longer period of time will improve clarity of expected outcomes and factors that affect variability in this patient population.
Acknowledgments
We would like to acknowledge Lisa Potts, Karen Mispagel, Brenda Gotter, Sallie Vanderhoof and Kristen Lewis for assistance with data collection and Chris Brenner for assistance with graphics. We thank Dorina Kallogjeri for statistical support and Tim Holden for calibrating the test equipment and stimuli. We appreciate our patients' time and participation in this study.
This work was supported by R01DC009010 from the National Institute on Deafness and Other Communication Disorders. Dorina Kallogjeri, MD, MPH was supported by the P30 Research Center for Auditory and Vestibular Studies and the National Institutes of Health (NIDCD P30DC04665).
References
- Abel SM, Birt D, Mclean JA. Sound localization in hearing-impaired listeners. In: Gatehouse RW, editor. Localization of sound: theory and applications. Groten, CT: Amphora Press; 1982. pp. 207–219. [Google Scholar]
- Balkany T, Hodges A, Telischi F, Hoffman R, Madell J, Parisier S, Gantz B, Tyler R, Peters R, Litovsky R. William House Cochlear Implant Study Group: position statement on bilateral cochlear implantation. Otol Neurotol. 2008;29:107–108. doi: 10.1097/mao.0b013e318163d2ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronkhorst AW, Plomp R. The effect of head-induced interaural time and level differences on speech intelligibility in noise. J Acoust Soc Am. 1988;83:1508–1516. doi: 10.1121/1.395906. [DOI] [PubMed] [Google Scholar]
- Bronkhorst AW, Plomp R. Binaural speech intelligibility in noise for hearing-impaired listeners. J Acoust Soc Am. 1989;86:1374–1383. doi: 10.1121/1.398697. [DOI] [PubMed] [Google Scholar]
- Carney E, Schlauch RS. Critical difference table for word recognition testing derived using computer simulation. J Speech Lang Hear Res. 2007;50:1203–1209. doi: 10.1044/1092-4388(2007/084). [DOI] [PubMed] [Google Scholar]
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25:9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- Ching TYC, van Wanrooy E, Dillion H. Binaural-bimodal fitting or bilateral implantation for managing severe to profound deafness: A review. Trends Amplif. 2007;11:161–192. doi: 10.1177/1084713807304357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn HS, Shinn-Cunningham B, Kidd G, Durlach N. The perceptual consequences of binaural hearing. Int J Audiol. 2006;45:S34–S44. doi: 10.1080/14992020600782642. [DOI] [PubMed] [Google Scholar]
- Compton-Conley CL, Neuman AC, Killion MC, Levitt H. Performance of directional microphones for hearing aids: real-world versus simulation. J Am Acad Audiol. 2004;15:440–55. doi: 10.3766/jaaa.15.6.5. [DOI] [PubMed] [Google Scholar]
- Craddock L, Brinton J, Shakeel RS, Balkany TJ. Bilateral cochlear implantation: the British Cochlear Implant Group position. Cochlear Implants Int. 2008;9:65–69. doi: 10.1179/cim.2008.9.2.65. [DOI] [PubMed] [Google Scholar]
- Donaldson GS, Chisolm TH, Blasco GP, Shinnick LJ, Ketter KJ, Krause JC. BKB-SIN and ANL predict perceived communication ability in cochlear implant users. Ear Hear. 2009;30:401–410. doi: 10.1097/AUD.0b013e3181a16379. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Spahr AJ, Loizou PC, Dana CJ, Schmidt JS. Acoustic simulations of combined electric and acoustic hearing (EAS) Ear Hear. 2005;26:371–80. doi: 10.1097/00003446-200508000-00001. [DOI] [PubMed] [Google Scholar]
- Dunn CC, Tyler RS, Witt SA. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. J Speech Lang Hear Res. 2005;48:668–680. doi: 10.1044/1092-4388(2005/046). [DOI] [PubMed] [Google Scholar]
- Eapen RJ, Buss E, Adunka MC, Pillsbury HC, 3rd, Buchman CA. Hearing-in-noise benefits after bilateral simultaneous cochlear implantation continue to improve 4 years after implantation. Otol Neurotol. 2009;30:153–9. doi: 10.1097/mao.0b013e3181925025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fata F, James CJ, Laborde ML, Fraysse B. How much residual hearing is ‘useful’ for music perception with cochlear implants? Audiol Neurootol. 2009;14:14–21. doi: 10.1159/000206491. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Reeder RM, Skinner MW. Restoring hearing symmetry with two cochlear implants or one cochlear implant and a contralateral hearing aid. J Rehabil Res Dev. 2008;45:749–68. doi: 10.1682/jrrd.2007.08.0120. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick E, Olds J, Durieux-Smith A, McCrae R, Schramm D, Gaboury I. Pediatric cochlear implantation: how much hearing is too much? Int J Audiol. 2009;48:91–7. doi: 10.1080/14992020802516541. [DOI] [PubMed] [Google Scholar]
- Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ) Int J Audiol. 2004;43:85–99. doi: 10.1080/14992020400050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfeller K, Oleson J, Knutson JF, Breheny P, Driscoll V, Olszewski C. Multivariate predictors of music perception and appraisal by adult cochlear implant users. J Am Acad Audiol. 2008;19(2):120–134. doi: 10.3766/jaaa.19.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Allen SK, Bess FH. Horizontal sound localization skills of unilaterally hearing-impaired children. Audiology. 1980;19:508–18. doi: 10.3109/00206098009070082. [DOI] [PubMed] [Google Scholar]
- King S, Firszt JB, Reeder RM, Holden LK, Strube M. Evaluation of TIMIT sentence list equivalency with adult cochlear implant recipients. J Am Acad Audiol. 2011 doi: 10.3766/jaaa.23.5.3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Stickney G, Zeng F. Speech and melody recognition in binaurally combined acoustic and electric hearing. J Acoust Soc Am. 2005;117:1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- Lamel FL, Kassel RH, Seneff S. Speech database development: design and analysis of the acoustic-phonetic corpus. Proceedings of DARPA Speech Recognition Workshop, Report No. SAIC-86\1546 1986 [Google Scholar]
- Laske RD, Veraguth D, Dillier N, Binkert A, Holzmann D, Huber AM. Subjective and objective results after bilateral cochlear implantation in adults. Otol Neurotol. 2009;30:313–8. doi: 10.1097/MAO.0b013e31819bd7e6. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 2009;30:419–431. doi: 10.1097/AUD.0b013e3181a165be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J, Peters R, Lake J, Johnstone P, Yu G. Bilateral cochlear implants in adults and children. Arch Otolaryngol Head Neck Surg. 2004;130:648–655. doi: 10.1001/archotol.130.5.648. [DOI] [PubMed] [Google Scholar]
- McLeod B, Upfold L, Taylor A. Self reported hearing difficulties following excision of vestibular schwannoma. Int J Audiol. 2008;47:420–430. doi: 10.1080/14992020802033083. [DOI] [PubMed] [Google Scholar]
- Nava E, Bottari D, Bonfioli F, Portioli G, Beltrame MA, Pavani F. Spatial hearing with a single cochlear implant in late-implanted adults. Hear Res. 2009;47:928–32. doi: 10.1016/j.heares.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Newman CW, Jacobson GP, Hug GA, Sandridge SA. Perceived hearing handicap of patients with unilateral or mild hearing loss. Ann Otol Rhinol Laryngol. 1997;106:210–4. doi: 10.1177/000348949710600305. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Soli SD, Sullivan JA. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95:1085–99. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- Noble W, Gatehouse S. Interaural asymmetry of hearing loss, Speech, Spatial and Qualities of Hearing Scale (SSQ) disabilities, and handicap. Int J Audiol. 2004;43:100–114. doi: 10.1080/14992020400050015. [DOI] [PubMed] [Google Scholar]
- Noble W, Tyler RS, Dunn CC, Bhullar N. Younger- and older-age adults with unilateral and bilateral cochlear implants: Speech and spatial hearing self-ratings and performance. Otology and Neurotology. 2009;30:921–929. doi: 10.1097/MAO.0b013e3181b76b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W. Assessing binaural hearing: results using the speech, spatial and qualities of hearing scale. J Am Acad Audiol. 2010;21:568–74. doi: 10.3766/jaaa.21.9.2. [DOI] [PubMed] [Google Scholar]
- Offeciers E, Morera C, Müller J, Huarte A, Shallop J, Cavallé L. International consensus on bilateral cochlear implants and bimodal stimulation. Acta Otolaryngol. 2005;125:918–919. doi: 10.1080/00016480510044412. [DOI] [PubMed] [Google Scholar]
- Peters BR, Wyss J, Manrique M. Worldwide trends in bilateral cochlear implantation. The Laryngoscope. 2010;120:S17–S44. doi: 10.1002/lary.20859. [DOI] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Potts LG, Skinner MW, Litovsky RA, Strube MJ, Kuk F. Recognition and localization of speech by adult cochlear implant recipients wearing a digital hearing aid in the nonimplanted ear (bimodal hearing) J Am Acad Audiol. 2009;20:353–73. doi: 10.3766/jaaa.20.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece J. Sound localization by cochlear implant users. Seminars in Hearing. 2010;31:37–46. [Google Scholar]
- Revit LJ, Schulein RB, Julsrom SD. Toward accurate assessment of real-world hearing aid benefit. Hear Rev. 2002;9:34–38. 51. [Google Scholar]
- Sammeth CA, Bundy SM, Miller DA. Bimodal hearing or bilateral cochlear implants: a review of the research literature. Seminars in Hearing. 2011;32:3–31. [Google Scholar]
- Seeber BU, Baumann U, Fastl H. Localization ability with bimodal hearing aids and bilateral cochlear implants. J Acoust Soc Am. 2004;116:1698–1709. doi: 10.1121/1.1776192. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Holden TA, Whiting BR, Voie AH, Brunsden B, Neely JG, Saxton EA, Hullar TE, Finley CC. In vivo estimates of the position of Advanced Bionics' electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol. 2007;116:1–24. [PubMed] [Google Scholar]
- Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978;21:507–18. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- Wie OB, Pripp AH, Tvete O. Unilateral deafness in adults: Effects on communication and social interaction. Ann Otol Rhinol Laryngol. 2010;119:772–781. [PubMed] [Google Scholar]