Abstract
Extraocular muscles (EOMs) have unique calcium handling properties, yet little is known about the dynamics of calcium events underlying ultrafast and tonic contractions in myofibers of intact EOMs. Superior oblique EOMs of juvenile chickens were dissected with their nerve attached, maintained in oxygenated Krebs buffer, and loaded with fluo-4. Spontaneous and nerve stimulation-evoked calcium transients were recorded and, following calcium imaging, some EOMs were double-labeled with rhodamine-conjugated alpha-bungarotoxin (rhBTX) to identify EOM myofiber types. EOMs showed two main types of spontaneous calcium transients, one slow type (calcium waves with 1/2max duration of 2–12 s, velocity of 25–50 μm/s) and two fast “flash-like” types (Type 1, 30–90 ms; Type 2, 90–150 ms 1/2max duration). Single pulse nerve stimulation evoked fast calcium transients identical to the fast (Type 1) calcium transients. Calcium waves were accompanied by a local myofiber contraction that followed the calcium transient wavefront. The magnitude of calcium-wave induced myofiber contraction far exceeded those of movement induced by nerve stimulation and associated fast calcium transients. Tetrodotoxin eliminated nerve-evoked transients, but not spontaneous transients. Alpha-bungarotoxin eliminated both spontaneous and nerve-evoked fast calcium transients, but not calcium waves, and caffeine increased wave activity. Calcium waves were observed in myofibers lacking spontaneous or evoked fast transients, suggestive of multiply-innervated myofibers, and this was confirmed by double-labeling with rhBTX. We propose that the abundant spontaneous calcium transients and calcium waves with localized contractions that do not depend on innervation may contribute to intrinsic generation of tonic functions of EOMs.
Keywords: extraocular muscle, skeletal muscle, calcium, calcium imaging, oculomotor, myofiber, fluo-4
1. Introduction
The extraocular muscles (EOMs) perform a diverse range of functions that include superfast saccades as well as slow tonic movements (Eggers, 1992; Spencer and Porter, 2006). EOMs can contract and relax extremely fast, with speeds that are similar to those of ultrafast sound-producing muscles. EOMs sustain individual twitch contractions at frequencies of 300–400 Hz without complete tetanic fusion (for review, see Li et al., 2011). The very fast contraction-relaxation cycles of EOMs differ from those in limb skeletal muscle.
The duration of muscle contraction depends primarily on changes in cytosolic free Ca2+ concentration that increases rapidly and initiates contraction (Berchtold et al., 2000). Total muscle Ca2+ content is up to 40-fold higher in EOMs than in limb skeletal muscle (Porter and Karathanasis, 1999). Fast contracting muscles must be capable of rapid relaxation, and indeed, the key calcium pump (calcium ATPase, SERCA) is abundantly expressed in the sarcoplasmic reticulum (SR) of EOMs (Jacoby and Ko, 1993; Porter and Karathanasis, 1999). The extraordinarily fast contraction and relaxation of EOMs are correlated with the superior ability of EOMs to maintain calcium homeostasis (Khurana et al., 1995; Andrade et al., 2005; Zeiger et al., 2010). Ca2+ in EOMs is amplified by the SR but differs from limb skeletal muscles in ryanodine receptor composition (O'Brien et al., 1993) and in gene expression for proteins involved in calcium homeostasis (Fischer et al., 2002; Zeiger et al., 2010).
One major question is how calcium handling contributes to the diverse functions of EOMs. EOMs not only contract and relax rapidly, but they are also composed of two different types of myofibers, singly-innervated myofibers, SIFs (~80–90%), and multiply-innervated myofibers, MIFs (~10–20%). The MIFs are thought to be tonic fibers that primarily produce slow, graded tension and are involved in fine foveating movements (Eggers, 1992; Spencer and Porter, 2006). Traditional concepts of eye movements teach that mechanically simple EOMs rotate the globe under explicit neural control of every kinematic nuance. Recent work on orbital biomechanics and pulley theory challenges this concept and emphasizes the role of peripheral biomechanics in tasks previously believed to be micromanaged by brainstem neurophysiology (Demer, 2006; Miller, 2007). Despite the rapid contraction and relaxation speed of EOMs and their unique tonic functions, little is known about calcium transients in intact EOMs. To our knowledge, calcium transients have only been imaged in EOM myotubes in culture (Zeiger et al., 2010) and in whole EOM (Andrade et al., 2005), but not in individual myofibers in intact EOMs.
Here we examined calcium transients in EOMs from chicken and additional mammalian species (mouse, rabbit) using fluo-4 (Gee et al., 2000). We focused much of our work on chicken, because the avian superior oblique muscle is easier to prepare into nerve-muscle preparations suitable for electrical stimulation and calcium imaging. Detailed information is available about muscle force, number of endplates, motor neurons, and myofibers in chicken EOMs of this age (Sohal et al., 1985; Hatton and von Bartheld, 1999; Croes and von Bartheld, 2005; Baryshnikova et al., 2007; Croes et al., 2007; Li et al., 2010, 2011). We describe two types of spontaneous and nerve-stimulation evoked calcium transients in avian EOMs, and we compare their physiological and pharmacological characteristics with the range of calcium transients previously reported in developing mammalian myotubes in culture (Flucher and Andrews, 1993; Jaimovich et al., 2000; Powell et al., 2001; Eltit et al., 2004; Campbell et al., 2006; Casas et al., 2010). We provide evidence for abundant spontaneous activity in EOMs, including calcium waves in MIFs with surprisingly large contractions – larger than nerve-stimulated contractions of SIFs. These findings open new and exciting avenues to better understand how EOMs may implement complex mechanical articulations without exquisite brainstem control, as postulated by modern theories of orbital biomechanics (Demer, 2006; Miller, 2007).
2. Material and methods
2.1. Animals
We used sixty male chickens at the age of 6–24 days after hatching (Hatchery: Ideal Poultry Breeding Farms, Cameron, TX), three C57BL/6 mice (two adults and one infant nine-day-old pup), and two New Zealand white male rabbits (2–3 kg; Western Oregon Rabbit Co, Philomath, Oregon). Animals were kept in cages with constant temperature and humidity in a ventilated room with a 12 hour light/dark cycle. Food and water were provided ad libitum. The Institutional Animal Care and Use Committee of the University of Nevada, Reno approved these experimental procedures.
2.2. Muscle dissection
Chickens were killed by decapitation, mice by isoflurane inhalation and cervical dislocation, and rabbits by an intravenous overdose of euthasol. From each chicken, the right superior oblique muscle was collected between posthatch days 6 and 24. This extraocular muscle (EOM) was gently dissected from the orbit, together with the distal inserting sclera and the proximal inserting bone fragment, and with the distal 1–1.5 cm of the innervating trochlear nerve intact. After dissection, the entire nerve-muscle preparation was placed into a Sylgard-lined 10 cm Petri dish containing oxygenated Krebs buffer. The adipose and connective tissues surrounding the EOM were carefully removed. In order to flatten the muscle in the dish, 3–4 medium-sized pins (50 μm diameter) were inserted through the sclera piece and the bone fragment, and 2–4 micropins (20 μm diameter) were inserted into both the medial and lateral edges of the muscle. The EOMs were dissected and pinned to the dish in their entirety (from proximal tendon to distal tendon). The trochlear nerve was straightened and pinned onto the dish (Fig. 1A). The superior oblique muscles from infant and adult mice and adult rabbits were collected using a similar method as described above. Care was taken to free the muscle from the cartilaginous trochlea. In muscles obtained from mice, fewer pins were used due to the smaller size of the EOM.
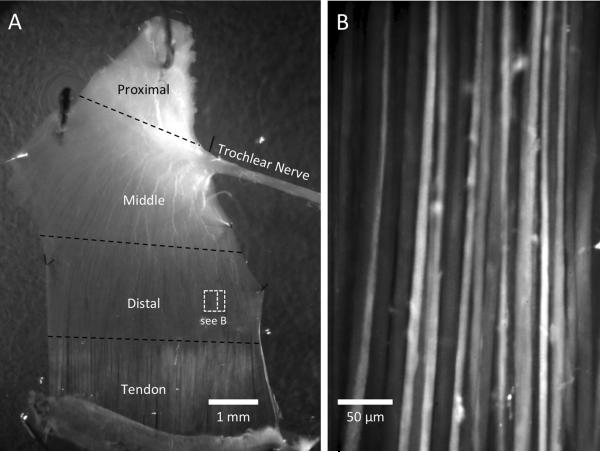
Fig. 1.
A–B. Extraocular muscle (EOM) preparation and fluo-4 loaded myofibers. A. A chicken superior oblique EOM is pinned to the bottom of a 35-mm dish (dorsal surface). The trochlear nerve is indicated and its branching fibers in the proximal and middle segments are clearly visible. The transition from myofiber to tendon is apparent by the lighter color of the tendon. The thickness of the EOM decreases from proximal to distal. The relative size of the field of view (FOV) used for calcium imaging is shown by the dashed square in the distal region. The larger rectangle within the square represents the size of the image in (B). B. Representative image of fluo-4 dye-loaded extraocular myofibers in the distal segment of a superior oblique muscle from a 9-day old chicken.
2.3. Fluorescent dye loading, electrical stimulation and imaging
All procedures were conducted either at room temperature or at 37°C. After dissection, EOMs were incubated in 50 nM fluo-4 AM (Molecular Probes, Eugene, OR), dissolved in 0.02% dimethyl sulfoxide (DMSO) and 0.01% of the non-toxic detergent Cremophor EL (Sigma-Aldrich, St. Louis, MO) for 20–60 minutes at room temperature (see Bayguinov et al., 2010). Then the tissues were rinsed with fresh oxygenated Krebs buffer for 10 minutes to remove excessive dye. Fluowas excited at 488 nm using Lambda LS (Sutter Instrument Company, Novato, CA) on a Nikon Eclipse E600FN upright fluorescence microscope that was equipped with a Nikon Fluor 20x water-immersion lens. The scope was also equipped with a TRITC filter (530–560 nm excitation, 590–650 nm emission) to visualize rhodamine-conjugated α-bungarotoxin (rhBTX, see below). Real-time videos were recorded using an Andor iXON +897 camera (Andor Technology, Belfast, UK). Images were acquired using Andor Solis 4.14 (Andor Technology, Belfast, UK) at 32 Hz for 1000 or 3000 frames with a resolution of 512 × 512 pixels (410 × 410 μm). EOMs displaying spontaneous calcium transients were recorded, then stimulated with a Grass SD9 stimulator (Grass Medical Instruments, West Warwick, RI) by applying either a single electrical pulse (15–30 Volt, 0.2 ms duration) or a train of pulses (5–25 Hz) to the innervating nerve. The Krebs–Ringer bicarbonate solution contained (in mM): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.5; and glucose, 11.5 (continuously gassed with 3% CO2–97% O2, pH 7.3–7.4) and was maintained at either 37°C or 22°C (room temperature). In selected cases, the whole EOM or the distal EOM segment (outside the endplate area) was treated with rhBTX (2 μg/ml) at the end of the experiment (after recording Ca2+ activity), and the myofibers of interest within the same view field were recorded using the TRITC fluorescence filter on the microscope.
To reduce the amplitude of the mechanical contraction after nerve stimulation, we pre-treated some EOMs with N-benzyl-p-toluene sulphonamide (BTS, 10 μM, Tokyo Chemical Industry Co, LTD, Japan). Although this treatment reduced muscle contractions as expected (Pinniger et al., 2005; Caputo and Bolanos, 2008), it interfered in our EOMs with the calcium events (reduced spontaneous activity and reduced nerve-evoked calcium transients, data not shown), despite the relatively low dose used (Hollingworth et al., 2008), and therefore we did not routinely use BTS in our protocol.
2.4. Image processing and analysis
Raw Solis.tiff stacks were analyzed on a MacPro desktop computer (Apple Inc., Cupertino, CA) using an in-house analysis software (Volumetry6a & VolumetryG7mv, G. W. Hennig). Movement of tissues in both the x and y direction were tracked, and the stacks were stabilized to allow for Ca2+ signals in individual regions of interest (ROIs) to be measured. Fluo-4 signals are reported as average intensity inside a ROI in 16-bit intensity units (i.u.). We constructed spatio-temporal maps (ST maps; e.g. Figs. 2B, E; 3B, C; 4B, D) by drawing rectangular ROIs over areas and averaging pixel intensities perpendicular (default) or parallel (indicated in text) to the long axis of the myofibers (see Bayguinov et al., 2010). The length and velocity of slow Ca2+ transients were calculated by applying a threshold to demarcate calcium waves followed by particle analysis (diameter and length; see Fig. 4 for example). We define propagating slow Ca2+ transients to be synonymous with “calcium waves.” Demarcated Ca2+ waves were used to create spatio-temporal (ST) objects using an iso-surfacing (marching cubes) algorithm. Duration of the calcium transients was measured at half maximum intensity level (1/2max).
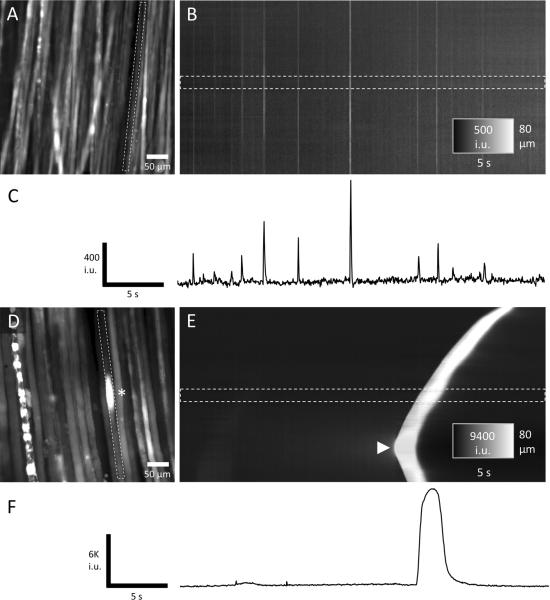
Fig. 2.
A–F. Two main types of Ca2+ transients observed in chicken extraocular muscle (EOM). A. An example of a field of view (FOV) from the distal region of a chicken EOM loaded with fluo-4. Fast Ca2+ transients were observed in this region and were measured in one muscle fiber (see dashed rectangle). The intervals between these fast Ca2+ transients and their amplitudes were variable as shown in the spatiotemporal (ST) map (B) and time course (C). In contrast, slow Ca2+ transients (calcium waves) only occurred in distinct segments of a myofiber (see asterisk in FOV in D) and propagated slowly in the longitudinal axis. In this example, a calcium wave was initiated in a myofiber towards the bottom of the FOV (see arrowhead in the ST map in E) and propagated in both directions. Ca2+ waves had much greater amplitudes and durations (10–20×) compared to the fast Ca2+ transients (F).
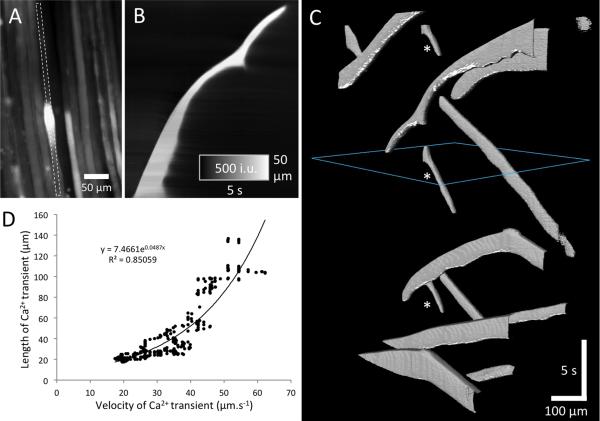
Fig. 3.
A–D. Characteristics of Ca2+ waves. A. Field of view (FOV) showing a Ca2+ wave in an individual EOM myofiber. The propagation of the Ca2+ wave was unusually variable and slowed down dramatically towards the top of the FOV as shown in the ST map in B. The pattern of propagation of all (12) Ca2+ waves in the FOV is represented by ST objects in C. Note that very few myofibers displayed more than one Ca2+ wave during the recording period of 32 s (see asterisks in C), and that Ca2+ waves initiated within the FOV often traveled in both directions along the myofiber away from the site of initiation (“V-shaped” ST objects). The relationship between the longitudinal length of a Ca2+ wave and its velocity was exponential (D).
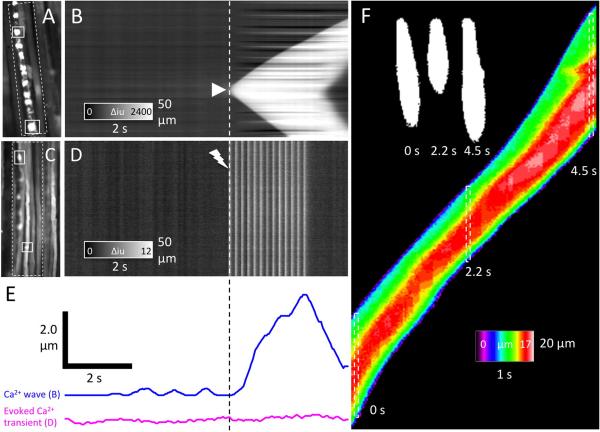
Fig. 4.
A–D. Ca2+ waves and contractions. A and C show fields of view (FOV) of chicken EOM preparations. An ST map was constructed by averaging the fluorescence intensities perpendicular to the long axis of adjacent myofibers in the area encompassed by the white dashed rectangle (B and D). A Ca2+ initiated in the rightmost myofiber (arrowhead in ST map in B) that caused a contraction after a delay of ~200 ms (blue trace in E). The contraction was more easily measured in the leftmost myofiber as the distance between the two white tracking rectangles in A. Fast Ca2+ transients evoked by a train of electrical impulses (C and ST Map in D) did not result in any appreciable distortion of muscle fibers (see E, magenta trace). The distortion due to longitudinal contraction could also be visualized by examining the shape of the Ca2+ wave as shown in F. The “bulging” of the myofiber, most likely due to longitudinal compression, was maximal 0.6–0.8 s after elevated Ca2+ levels were detected. The diameter of the myofiber made visible by the Ca2+ wave was color-coded, such that the normal resting diameter of the myofiber (~10 μm) was colored green and dilated regions were colored red (15–17 μm; see F). In this example, the Ca2+ wave propagated from the bottom to the top of the FOV at different speeds (see silhouettes at 0, 2.2 and 4.5 s in F), resulting in an elongation of the “teardrop” shape at higher velocities.
2.5. Treatment with pharmacological agents
EOMs were screened under the fluorescent microscope for regions with well-loaded straight muscle fibers and frequent fast spontaneous activities (mostly within the distal segment as shown in Fig. 1). The Petri dish was then secured to the stage to prevent movement. Thereafter, videos of baseline (control) activity were recorded. After several recordings, one of the following inhibitors or other pharmacological agents were added to the dish: 2-aminoethyl diphenyl borate (2-APB), caffeine, lidocaine, ryanodine or tetrodotoxin (Sigma, MI, USA); carbonyl cyanide p-(trifluromethoxy)phenyl-hydrazone (FCCP, Tocris MI, USA); alpha-bungarotoxin (Invitrogen, Carlsbad, CA, USA) or tetramethylrhodamine-conjugated alpha-bungarotoxin (rhBTX, Molecular Probes, Eugene, OR, USA), with final concentrations described in the text. Electrical stimuli were applied to the nerve trunk to verify the effects of various channel inhibitors.
2.6. Statistics
The n values represent the number of animals on which observations were made. Regression analysis and frequency histograms were performed using Excel (Microsoft, Redmond, WA). One-way ANOVA and Scheffe post-hoc tests were used for statistical comparisons. A p<0.05 was considered significant.
3. Results
We focused our analysis on the “flat” distal segment of the extraocular muscle (EOM) that inserts on the globe, where the myofibers fan out and form a relatively thin layer (Fig. 1A). The trochlear nerve was clearly visible, including its nerve fibers branching throughout the proximal and middle segments (Fig. 1A). Most of the myofibers in the chicken EOMs contained fluo-4 after dye loading, as shown in Fig. 1B. Myofibers were similarly dye-loaded in mouse and rabbit EOMs (data not shown). A total of 38 chicken EOMs, three mouse EOMs and one rabbit EOM were successfully analyzed, with 10–15 videos for each muscle, each video of 32 seconds duration, containing about 30–50 myofibers per field of view (FOV). Most of the data we present are derived from superior oblique EOMs of 7–20 day old chickens, unless indicated otherwise. Spontaneous calcium transients were seen in most of the chicken EOMs, and also in developing, early postnatal mouse EOM. In chicken EOMs, we observed two main types of calcium transients. The first were relatively abundant, rapid (“flash”-like) transients, presumably involving the entire myofiber (Fig. 2A–C and supplementary material: video 1). The second were less frequent, propagated at slow velocities, and were localized to distinct segments of the myofibers (see Fig. 2D–F and supplementary material: video 2). We use the terminology “calcium waves” for these slowly propagating calcium transients.
3.1 Ca2+ waves
Slowly propagating Ca2+ transients were seen in about 20% (total >200 myofibers) of the examined muscles. These waves lasted 2–12 s and had a relatively fast upstroke, long “plateau” period, followed by a slow exponential decline in Ca2+-induced fluorescence back to resting levels (see Fig. 2F). Calcium waves occurred in myofibers of all sizes and were not restricted to large- or small-sized myofibers. The entire wave propagated along the imaged region of the myofiber at a velocity of 17–62 μm.s−1 (mean of 34.4 ± 5.2 μm.s−1, SEM), but the speed could be highly variable even within individual myofibers (Fig. 3A–C). Interestingly, the longitudinal length of the Ca2+ wave that propagation increased. Analysis of the propagation velocity (leading edge of the transient) and longitudinal length of calcium waves revealed an exponential relationship (R2 = 0.85; Fig. 3D). Repetitive Ca2+ waves were only occasionally observed (see asterisks in Fig. 3C). Ca2+ waves often propagated in both directions away from the site of initiation (see “V-shaped” ST-objects towards the bottom of Fig. 3C and supplementary material: video 2). In several other cases, calcium waves traveling in opposite directions towards each other collided and annihilated each other (data not shown).
Most calcium waves were associated with a local myofiber contraction that followed the leading edge of the calcium wave after a short delay (200–400 ms; see Fig. 4A–B, E). Curiously, as long as the propagation velocity of these Ca2+ waves was slow, the contraction initiated by the leading edge of the Ca2+ wave distorted the myofiber before the remainder of the Ca2+ transient had time to propagate past that position, creating a “teardrop” effect (see silhouettes in Fig. 4F). Shape analysis of Ca2+ waves (diameter encoded as a color in ST map; Fig. 4F) showed that the position of the maximum circumferential dilation (~50% greater than resting diameter; most likely due to longitudinal compression) was located further towards the rear of the wave when the velocity of propagation increased (see separation of normal diameter “green” areas and dilated “red” areas at the top of the ST map in Fig. 4F). However, the time between an area of a myofiber being first exposed to high Ca2+ levels and the time at which it reached maximal circumferential dilation was relatively constant (0.6–0.8 s). This type of analysis allows us to quantify distortion of individual myofibers within the syncytium when traditional particle tracking methods cannot be used. This is important for future work that will attempt to better understand how contractions in individual myofibers contribute to overall force generation in EOMs (see discussion).
Calcium waves sometimes originated and propagated away from a region that displayed “spark-like” activity (similar to “fast-localized transients” FLT, Flucher and Andrews, 1993) at the myofiber membrane (supplementary material: video 4). On multiple occasions, the wave originated from a site that appeared to be localized close to innervating nerve fibers (either visualized as small active irregularly shaped structures adjacent to myofibers, or verified by subsequent demonstration of en-grappe innervation through rhBTX double-labeling; see below and Fig. 5). However, there was no consistent relationship apparent between en-grappe innervation and the origination of the calcium wave, and nerve stimulation did not evoke these slow waves (see below).
Fig. 5.
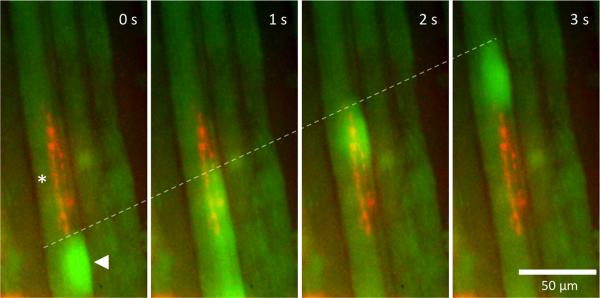
Ca2+ waves and myofiber innervation. Panels (0–3 s) show the initiation (see arrowhead) and propagation (see dashed white line) of a calcium wave upwards from a region in an individual myofiber. The preparation was subsequently double-labeled with rh-aBTX and was shown to contain typical “engrappe” innervation (red fluorescence) close to the site of initiation of the calcium wave (see asterisk). This provides direct evidence that slow calcium transients occur predominantly, if not exclusively, in MIF-type myofibers.
3.2. Fast Ca2+ transients
Fast spontaneous Ca2+ transients were observed in nearly all of the 38 chicken EOMs examined (Fig. 6A, B). They were absent in the three adult mammalian EOMs, but were seen in one limb skeletal muscle. They were similar to previously described “action potential (AP)-induced calcium transients” in chick and mouse myotubes (Flucher and Andrews, 1993), except that they were shorter in 1/2max duration (typically about 30–150 ms) than the transients of 200–500 ms duration reported by Flucher and Andrews (1993). The occurrence of the fast Ca2+ transients in individual myofibers was variable (approximately 1–20/min), however their occurrence over the entire FOV (about 40 myofiber segments) could be as high as three transients per second (see supplementary video 1). Fast Ca2+ transients were seen both at 37°C as well as at room temperature. They propagated at a velocity that exceeded the acquisition rate of the camera (32 fps) and therefore must be equal to, or exceed 13 mm.s−1 along a myofiber. Spontaneous fast calcium transients did not evoke any measurable myofiber contraction in our preparation (data not shown).
Fig. 6.
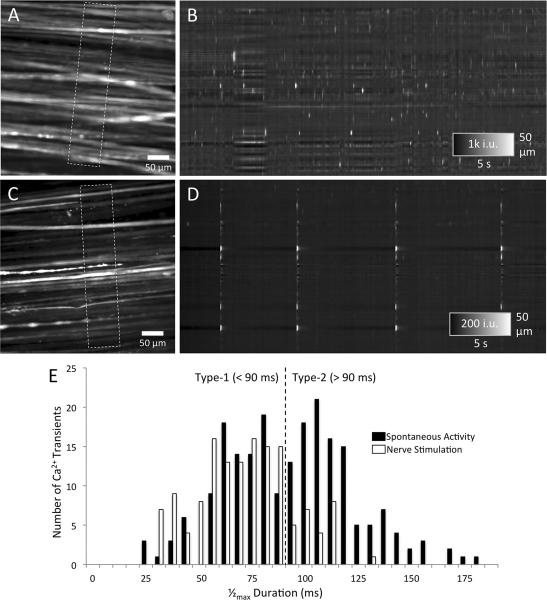
A–E. Spontaneous and nerve-evoked fast Ca2+ transients. A shows a FOV from which an ST map was constructed by averaging Ca2+ intensities in myofibers parallel to their long axis (see dashed rectangle). B shows the pattern of fast Ca2+ transients over time in ~15 active myofibers. Note the variable intensity and interval of fast Ca2+ transients both within individual myofibers (a single horizontal row) and between multiple myofibers (multiple rows). C shows a FOV in an experiment in which four pulses of electrical nerve stimulation were applied (~ every 5–8 s). Note the synchronous activation of many myofibers in the ST map shown in D. The frequency histogram of half-maximal duration shows a bi-phasic distribution (E; black bars) with two peaks located at ~75 ms and ~110 ms and a trough at ~90 ms. Faster events (<90 ms) were described as Type-1, and slower events (>90 ms) as Type-2 fast Ca2+ transients. Nerve-evoked fast Ca2+ transients were predominantly Type-1 (<90 ms). Histogram data were compiled from 350 transients examined in seven EOMs.
Analysis of the waveform of 350 fast Ca2+ transients revealed two types of fast Ca2+ transients based on their duration. Type-1 fast Ca2+ transients had a 1/2max duration of <90 ms, while Type-2 fast Ca2+ transients had a 1/2max duration of >90 ms as revealed by the bi-phasic distribution in Fig. 6E. Using Rayleigh's criterion for resolution to distinguish between two Gaussian curves, the separation between the two peaks is sufficient (i.e. dip is > 26.4%) to recognize them as two distinguishable curves. Similarly, the 1/2max durations of Ca2+ transients evoked by nerve stimulation had a unimodal distribution that closely resembled the Type-1 distribution and was distinct from the Type-2 distribution. Overall, the Type-1 fast Ca2+ transients comprised approximately half of the fast spontaneous transients.
The two types of fast transients were occasionally observed in the same myofiber and therefore do not correlate with distinct myofiber types. Fast Ca2+ transients, of either type, rarely occurred simultaneously in adjacent myofibers, and did not seem to be correlated with each other. Fast transients would be expected only in MIFs, not in SIFs, since MIFs typically do not generate action potentials (APs, Jacoby et al., 1989; Eggers, 1992). Consistent with this expectation, we did not observe simultaneous or subsequent occurrence of fast and slow Ca2+ transients, despite careful analysis of about 200 cases of slow Ca2+ transients (calcium waves) and about 10 times more cases of fast transients. Thus, these data support the notion that MIFs rather than SIFs exhibit calcium waves, while only SIFs can propagate APs and fast (evoked and spontaneous) calcium transients.
Rapid, “flash”-like calcium transients could be evoked by electrical stimulation of the trochlear nerve. Stimulation with a single pulse typically and reliably resulted in a rapid calcium transient that appeared to be identical to the characteristics of the spontaneous Type-1 fast calcium transients (Fig. 6C, D and white bars in E). There was little indication of fatigue in EOMs when trochlear nerves were stimulated with frequencies of 1 Hz for 90 seconds (Fig. 7A). At stimulation frequencies at 10–15 Hz, the fast transients followed the stimulation (all peaks visible). When a more rapid train of pulses was delivered (20 Hz), a pattern was observed that shows an intermediate stage between partial and complete “fusion” (Fig. 7B), as has also been reported in skeletal muscle (Baylor and Hollingworth, 2012). Figure 7C shows responses from a mouse EOM to electrical stimulation of the nerve; similar data were obtained for chicken EOMs. Not all myofibers across the EOM were activated by electrical stimulation. Unlike the fast calcium transients, there was no indication that nerve stimulation evoked calcium waves. In one case, nerve stimulation showed calcium release within MIF-type en-grappe nerve endings, but this did not result in either fast calcium transients or calcium waves within the myofiber (data not shown). Single twitch and train-pulse evoked calcium transients resulted in only minimal myofiber contraction (movement) in presumptive SIFs in our isometric preparation (Fig. 4C–E, supplementary material: video 3), in contrast to the substantial movement that was induced by calcium waves in presumptive MIFs (Fig. 4A–B, E).
Fig. 7.
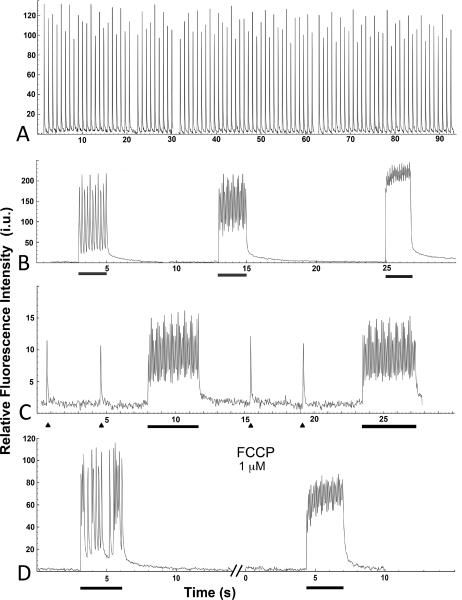
A–D. Nerve-evoked stimulation of EOMs by a single pulse and train of pulses. A. Graph shows consistent amplitude of transients, without any significant fatigue, in a chicken EOM that was stimulated for 90 seconds with a 1 Hz pulse train, 30V. B. Traces of higher frequency stimulation (2 seconds duration, bar) with a train of pulses at 5, 10, and 20 Hz (from left to right) in a chicken EOM. Note the undulating amplitudes and increases in baseline with increasing frequencies. C. Traces of calcium transients evoked by two separate single pulses (triangle), followed by a train of pulses (at 10 Hz, for 4 seconds, bar) in an adult mouse EOM. Note again the undulating amplitudes during the train of pulses. D. The mitochondrial uncoupler, FCCP, increases the baseline and reduces the amplitude in EOMs after a 4 min delay, when stimulated with a train of pulses at 10 Hz for 3 seconds (bar). In all traces, the y-axis shows relative fluorescence in intensity units (i.u.).
3.3. Physiological and morphological analysis of calcium waves
To confirm the notion that slow calcium transients occurred predominantly in MIF-type rather than SIF type myofibers, the whole EOM or the distal EOM segment (outside the endplate area) was treated with rhBTX after recording Ca2+ activity, and the myofibers of interest within the same view field were recorded using the TRITC fluorescence filter on the microscope. Treatment with rhBTX produced labeling of myofibers that was typical for en-grappe innervation (Hess, 1961; Hess and Pilar, 1963), and individual MIFs were identified based on this label. Images were then superimposed with frames from the previous movies of the calcium activity in the same field of view to identify the type of myofiber (MIF or SIF). Figure 5 (and supplementary material: video 4) show an example of such double-labeling. We demonstrate that MIF-type myofibers display calcium waves, but we cannot exclude the possibility that some SIF-type myofibers also display slow calcium transients, as the absence of rhBTX-label along a restricted myofiber segment does not allow one to conclude that it is a SIF-type myofiber. This possibility, however, seems unlikely, because we did not observe a single convincing case of a myofiber with a calcium wave that displayed a spontaneous or evoked fast transient in a previous or subsequent recording session, as mentioned above.
3.4. Neurally-mediated activation of calcium transients
To determine the mechanisms involved in the generation and propagation of the observed calcium transients, we tested the effects of several key pharmacological agents. Tetrodotoxin (TTX; 2–5 μM) was applied directly to the nerve stump and along the nerve trunk during the recording of spontaneous activity as well as prior to nerve-stimulation of the muscle. TTX abolished nerve-evoked fast calcium transients in less than 3 minutes (data not shown), but did not have an effect on spontaneous (either fast or slow) activity, even after prolonged (10–16 min) diffusion and equilibration of the TTX throughout the muscle. We also tested lidocaine (2 mM for 3 min) which blocks some TTX-resistant sodium channels. Similar effects as those observed after the addition of TTX were observed. Since MIFs in EOMs do not generate propagating action potentials (Jacoby et al., 1989; Eggers, 1992), it is unlikely that fast spontaneous calcium transients occur in MIFs. Alpha-bungarotoxin (αBTX; nicotinic acetylcholine receptor blocker, 2 mM low dose, or 5 mM high dose) was used to determine whether the spontaneous or evoked fast calcium transients required neuromuscular transmission utilizing the nicotinic acetylcholine receptor. Spontaneous and nerve-evoked fast calcium transients were abolished in the αBTX treated EOMs (n=3), indicating that activation of the acetylcholine receptor on the postsynaptic endplate is required for spontaneous and nerve-stimulation evoked fast calcium transients. The αBTX did not abolish the calcium waves, demonstrating that activation of the nicotinic acetylcholine receptor is not required for the spontaneous slow transients (calcium waves), while this receptor is essential for the nerve-evoked and spontaneous fast transients. Leakage of acetylcholine from the endplate (Eggers, 1992) appears to be sufficient to induce spontaneous calcium transients, but such leakage in a non-quantal fashion obviously requires functional acetylcholine receptors to induce APs in SIF-type myofibers of EOMs. Since calcium waves induced robust localized myofiber contractions (Fig. 4A–B, E), we measured and directly compared the movement of individual myofibers elicited either by a calcium wave or an evoked twitch or tetanic stimulation. As shown in Fig. 4E, the calcium wave-evoked contraction of myofibers (presumptive MIFs) was substantially larger than the nerve stimulation-evoked contraction of (presumptive) SIFs. These findings suggest, but do not prove, that calcium wave-induced MIF contraction, and not only nerve-evoked contractions, may be functionally relevant.
3.5. Role of intracellular calcium stores
Calcium release from the SR is thought to be required for the spontaneous and nerve-evoked calcium transients. When we applied a high concentration of ryanodine (10 μM), dye intensity levels rapidly saturated. This seemed to eliminate both spontaneous (slow and fast) and evoked fast calcium transients and often also caused muscle contraction. The rapid saturation limited the use of the drug in making conclusions about the mechanism. Caffeine potentiates calcium-induced calcium release in limb skeletal muscle myotubes (Flucher and Andrews, 1993). To determine whether caffeine has a similar effect in EOM myofibers as it does in skeletal muscle, we used either a low dose (2 mM) or a higher dose (5 mM). At the low dose, caffeine induced Ca2+ waves in less than 1 minute. With the high dose of caffeine, myofibers became saturated with calcium (myofibers turned completely “white”), similar to the effect of ryanodine. Therefore, the response to caffeine in whole EOMs differs significantly from that of cultured myotubes (Klein et al., 1992; Flucher and Andrews, 1993; Endo, 2009).
Since many EOM myofiber types contain an unusually large amount of mitochondria, thought to be a major calcium store (Fischer et al., 2002; Andrade et al., 2005), we examined whether mitochondria contribute significantly to the calcium transients we observed in EOMs. When we applied the mitochondrial inhibitor FCCP (1 μM; uncoupler of oxidative phosphorylation in mitochondria; Caputo and Bolanos, 2008), there was no effect on transients in individual myofibers (data not shown), but after a 2–4 minute delay, the baseline calcium-induced fluorescence increased and the amplitude of transients decreased with tetanic stimulation of pulses at 10 Hz (Fig. 7D). Mitochondrial calcium handling may have a role in EOM myofiber activation, although this effect appears to occur only at higher frequencies of activation.
To determine whether IP3-gated intracellular calcium stores may be involved in the generation of the calcium waves (Powell et al., 2001), we applied 2-APB, an inositol 1,4,5-trisphosphate (IP3) receptor blocker (100 μM; Johnston et al., 2005). This drug was previously shown to be equally effective as xestospongin in inhibiting chicken muscle IP3 receptors (Jordan et al., 2005). 2-APB had no effect on the generation of calcium waves in EOMs (data not shown).
4. Discussion
The current study was undertaken to characterize the types of calcium transients present in myofibers of intact juvenile extraocular muscles (EOMs). We found that EOMs contain distinct types of calcium transients, two fast types and one slow type, and that the slow calcium transients (calcium waves) elicit remarkably robust localized myofiber contractions. We propose that the localized contractions elicited by the calcium waves may contribute to the tonic, gaze-holding functions of EOMs, some of which are now believed to be intrinsic EOM responses, not directly controlled by neural input (Demer, 2006; Miller, 2007).
EOMs differ from limb skeletal muscles in that they have a unique composition of myofibers: singly and multiply innervated fibers (SIFs and MIFs respectively, Spencer and Porter, 2006), and they display extremely short contraction/relaxation cycles (Li et al., 2011). Their enhanced calcium handling capacities are believed to enable or assist these functions (Porter and Karathanasis, 1999; Andrade et al., 2005; Zeiger et al., 2010). Previous studies on EOMs have been limited to the examination of calcium transients in whole muscle (Andrade et al., 2005) or immature models (cultured myotubes, Zeiger et al., 2010). In our work, we have examined spontaneous and nerve-stimulation evoked calcium transients in single, identified mature myofibers in a syncytium which greatly increases the spatial and temporal characterization of these events. We made use of inherent advantages of avian EOMs for calcium imaging; chicken EOMs, in particular the superior oblique muscle, can be readily dissected with the trochlear nerve intact, allowing us to assess calcium transients and contractions elicited by nerve stimulation rather than direct muscle stimulation. Due to the spread and thinning of the distal segment of the EOMs over the globe, it is not necessary to skin or peel off individual myofiber bundles for imaging, as is commonly done for limb skeletal muscle (Andrade et al., 2005, but see Casas et al., 2010; Baylor and Hollingworth, 2012). The use of 7–20 day old chickens corresponds to the age when the oculomotor system is largely mature (Croes et al., 2007).
Multiple types of calcium transients
Previous work has identified multiple types of calcium transients in skeletal muscle, with considerable variability between animal species, muscle cell types, ages, and in-vitro versus in-vivo conditions (Flucher and Andrews, 1993; Jaimovich et al., 2000; Powell et al., 2001; Eltit et al., 2004; Campbell et al., 2006; Cheng and Lederer, 2008; Hollingworth et al., 2008; Casas et al., 2010). Spontaneous calcium transients differed in duration, amplitude, propagation velocity and pharmacological properties. Evoked calcium transients are primarily of the faster types (Table 1). Our study focused on the “global transients” rather than the discretely localized sparks or embers (Schneider and Ward, 2002; Csernoch, 2007; Cheng and Lederer, 2008). We demonstrate abundant spontaneous calcium transients in 1–3 week old chicken EOMs, in contrast to adult mammalian skeletal muscle that lacks spontaneous activity, unless myofibers are substantially modified (e.g., enzyme-dissociated, dialyzed, cut, permeabilized, cultured, or subjected to osmotic stress, Cheng and Lederer, 2008; Hollingworth et al., 2008; Endo, 2009). We discuss two types of calcium transients in EOMs: slowly propagating calcium transients (calcium waves) and fast “flash”-like transients (either spontaneous or nerve stimulation-evoked).
Table 1.
Properties of calcium transients in skeletal myotubes or myofibers
| Name of Transient (T) | Cell Type | Duration1 | Spontaneous/Evoked? | Area [μm] or Speed [μm/s] | References |
|---|---|---|---|---|---|
| Spark | Myofiber: Mammal Amphibian: | 15 ms | only when permeabilized Spontaneous | 2–4 μm | Cheng & Lederer, 2008 |
| Myoplasmic free Ca2+ transient | Mouse | 4–5 ms | AP-induced (local electrodes) | ? | Hollingworth et al., 2008 |
| Fast localized =FLT | young myotubes | 50–100 ms | Spontaneous | 20–50 μm, local | Flucher & Andrews, 1993 |
| 50–200 ms | Campbell et al., 2006 | ||||
| AP-induced | C2 | 200–500 ms | Spontaneous, Electrical field | Flucher & Andrews, 1993 | |
| Fast signal | NLT | 1 s | Electrical field | ? | Eltit et al., 2004 |
| Wave | C2 mouse Chick embryo leg/breast | 1–2 s | Caffeine-induced | 35–70 μm/s | Flucher & Andrews, 1993 |
| Short duration =SDT | embr. myocyte Xenopus | 2 s | Spontaneous | ? | Campbell et al., 2006 |
| Long duration =LDT | embr. myocyte Xenopus | 25–84 s | Caffeine-induced | ? | Campbell et al., 2006 |
| ~80 s | |||||
| Slow signal | NLT | >200 s | Electrical field | ? | Eltit et al., 2004 |
|
| |||||
| Flash (type-1) | EOM | 30–90 ms | Spontaneous, Nerve stimulation | >13,000 μm/s | current study |
| Flash (type-2) | EOM | 90–150 ms | Spontaneous | >13,000 μm/s | current study |
| Calcium wave | EOM | 2–12 s | Spontaneous | ~ 25–50 μm/s | current study |
AP, action potential; embr, embryonic; EOM, extraocular muscle; NLT, Normal Line transfected with the Large T antigen (muscle cell line); ?, not determined.
Note that the current study measured the duration of calcium transients at half maximum intensity level; other studies measured the duration of transients from onset of peak to baseline. Since our transients had both a rapid rise and decay phase, this does not significantly alter the durations or the conclusions made.
Calcium waves
A variety of slow Ca2+ transients have been described in skeletal myofibers (Table 1). The duration of the slow transients (2–12 s) we observed falls between the shorter caffeine-induced “wave” (1–2 s, Flucher and Andrews, 1993) or “short duration transient,” SDT (1–2 s, Campbell et al., 2006) and the much longer “long duration transient,” LDT (25–84 s, Campbell et al., 2006, Table 1) or the delayed “slow signal” (NLT, Eltit et al., 2004, Casas et al., 2010, >200 s, Table 1). Thus, calcium waves in chicken EOMs are distinct from those in limb skeletal muscle. Some of these differences may reflect the different degrees of differentiation of cultured myotubes (slower calcium uptake) vs. mature myofibers (more rapid calcium uptake).
In previous studies, delayed and slow calcium waves (25–200 s) in cultured myocytes were found to be ryanodine-insensitive, but IP3 receptor dependent (Jaimovich et al., 2000; Powell et al., 2001; Eltit et al., 2004; Campbell et al., 2006; Casas et al., 2010). This differs from calcium waves in EOMs that were not reduced by the IP3 receptor blocker 2-APB. The calcium waves observed in our study were similar to (although of longer duration than) those described in limb skeletal muscle myotubes after the addition of caffeine (Flucher and Andrews, 1993).
While calcium waves have been previously described, they are often considered preparation artifacts of cultured and isolated, denervated myofibers, and most of such slow calcium transients were not accompanied by local myofiber contractions and movement. Therefore, since we imaged minimally manipulated myofibers within intact tissue and at physiological temperature, it is important to emphasize that our work provides some of the strongest evidence to date that calcium waves do occur in vivo and are not necessarily artifacts. In previous work, although caffeine-induced waves showed contractions (Flucher and Andrews, 1993), calcium waves in limb skeletal myotubes did not elicit contractile activity (Jaimovich et al., 2000). Our work on EOMs is the first to demonstrate that spontaneous slow calcium transients in intact myofibers are accompanied by robust localized contractions. The distortion produced by calcium wave-induced contractions in an active myofiber was considerably greater than those elicited by fast transients (Fig. 4A–E) and could be detected in adjacent myofibers at a distance of up to 30 μm, consistent with reports that myofibers in the EOM are coupled within an elastic network (Demer et al., 1995; Osanai et al., 2009). The lack of appreciable movement in SIFs after twitch or tetanic nerve stimulation illustrates the difference between a calcium wave-induced localized MIF contraction and an isometric contraction in a pinned EOM preparation. At the point of maximal wave-induced contraction (located at some distance behind the leading edge of the Ca2+ wave) there was a pronounced increase in the diameter (“dilation”) of the myofiber that impacted upon the adjacent myofibers. This is likely due to longitudinal compression that causes the cell to “bulge” out circumferentially at the site of maximal stress. While it has been shown that Ca2+ is involved in the regulation of cell volume (McCarty and O'Neil, 1992), these changes take minutes to reach maximal effect (~20% Δ volume; Liu and Persson, 2005) and are unlikely to explain the rapid and localized expansion and “contraction” back to normal diameters (<2 s) that we observed in response to calcium waves. The characteristics of the “teardrop”-shaped Ca2+ waves offer a way to measure distortions in single active myofibers in intact EOMs without using surface markers or other fixed landscape points.
As mentioned above, only SIFs but not MIFs propagate APs; therefore, myofibers with spontaneous or evoked fast calcium transients most likely are SIFs. To confirm that Ca2+ waves were indeed localized to MIFs, we double-labeled some EOM preparations with fluorescent α-bungarotoxin (rhBTX) after calcium imaging. We found that MIF-type innervation is present in some myofibers that showed Ca2+ wave activity (Fig. 5). Calcium waves in MIF-type myofibers are consistent with the notion that MIFs are involved in tonic contractions, even though they lack propagated action potentials (Chiarandini and Stefani, 1979; Jacoby et al., 1989). Accordingly, although we do not have direct evidence, we propose that calcium waves may contribute to the tonic adjustments of eye position that are believed to be carried out by MIF-type myofibers (Eggers, 1992; Spencer and Porter, 2006). Ca2+ waves observed in our study occurred asynchronously across multiple myofibers and even within individual myofibers, similar to the graded, tonic pattern of activity in postural skeletal muscles (e.g. soleus; Mosher et al., 1973), blood vessels (Jaggar et al., 2000) and intestinal smooth muscle (Hennig et al., 2002). The summation of a large number of slow asynchronous contractions in individual myofibers may enable the EOM to maintain a specific degree of tonic contraction even in the presence of fast saccadic movements. While traditional models of ocular motility assumed that the brainstem precisely controls every aspect of eye movement, modern theories of orbital biomechanics postulate that some features of the EOMs are capable of operating without direct brainstem control (Demer, 2006; Miller, 2007). One may envision that calcium waves and their tonic contractions contribute to function within pulley mechanics, maintaining vector forces in response to local conditions and requirements, or they may help to optimize myofiber length (Scott, 1994). It should be explored whether calcium waves and local contractions may be involved in differences between horizontal rectus motoneuron behavior and horizontal rectus EOM force (Miller et al., 2011). Although speculative at this point, calcium wave-induced contractions independent of direct neural control may contribute to mechanisms by which the peripheral effector organ operates within such a model of orbital biomechanics. Future studies will address this possibility.
Fast calcium transients
The fast Ca2+ transients we observed (30–150 ms 1/2max duration) were similar in duration to the “FLT” transient (50–200 ms; Flucher and Andrews, 1993; Campbell et al., 2006), but those we observed occurred throughout entire cells, rather than being discretely localized as previously reported (Flucher and Andrews, 1993; Campbell et al., 2006). They were also considerably shorter in duration compared to the action potential (AP)-induced transient (200–500 ms in duration; Flucher and Andrews, 1993), and the so-called “fast signal” (1000 ms; Eltit et al., 2004; see Table 1). In our study, two types of fast Ca2+ transient could be distinguished based on their half maximal duration, which we named Type-1 and Type-2. Their significance is currently unclear, but they do not appear to be correlated with distinct myofiber types, since both subtypes were found in the same myofiber. The mechanism underlying the two types of fast Ca2+ transients is unlikely to be due to multiple APs prolonging the event, as more than two peaks would be expected in the frequency distribution corresponding to different numbers of APs if this were the case. Faster acquisition rates may allow us to better distinguish waveform differences between Type-1 and Type-2 fast Ca2+ transients. Note that most other studies did not use 1/2max duration calculations to estimate the duration of calcium transients (Table 1).
The properties of the fast Ca2+ transients we saw in EOMs are consistent with AP-induced, acetylcholine-receptor dependent calcium release from SR as they were abolished by α-bungarotoxin (αBTX). Since MIFs do not generate propagating muscle APs (Chiarandini and Stefani, 1979; Jacoby et al., 1989; Eggers, 1992), both spontaneous and nerve-evoked fast transients would be expected to occur in SIFs rather than MIFs. Indeed, we did not observe a single convincing myofiber with both slow and fast calcium transients, consistent with the notion that only SIFs generate APs and propagate fast calcium transients, while only MIFs have calcium wave activity (Jacoby et al., 1989; Spencer and Porter, 2006). TTX and lidocaine rapidly eliminated nerve-evoked calcium transients. The failure to block spontaneous fast transients with these drugs is most likely due to differences in sodium channels, including TTX sensitivity, between nerve and muscle (Bondi et al., 1986). The function of spontaneous activity in EOMs is unclear, but such intracellular Ca2+ transients may regulate biochemical pathways, gene expression and plasticity (McCormick, 1999).
In previous work, the mitochondrial uncoupling agent FCCP reduced calcium signals in whole EOM (Andrade et al., 2005). In our study, FCCP had a relatively small, but consistent effect on elevating the baseline in individual myofibers after tetanus-like stimulation, indicating that mitochondrial buffering of calcium may be involved in its sequestration (Vanden Berghe et al., 2002). However, given the lack of effects on individual transients, mitochondrial calcium buffering does not seem to be a major factor in the generation of fast Ca2+ transients. Calcium waves were not assessed for the effect of FCCP, and it is possible that mitochondria are involved as previously shown in mammalian skeletal myotubes (Eisner et al., 2010).
Calcium handling and growth factors
Little is known about the mechanisms that would explain why calcium handling in EOMs is so efficient compared to other skeletal muscles, but increasing evidence indicates that growth factors such as insulin-like growth factor-1 (IGF-1) are involved. IGF-1 is expressed at significantly higher levels in EOMs than in other skeletal muscles (Fischer et al., 2002; Feng and von Bartheld, 2011). IGF-1 prevents the age-dependent decrease in charge movement and intracellular calcium in skeletal muscle (Wang et al., 2002), induces intracellular calcium signals (Espinosa et al., 2004), and regulates transcription of dihydropyridine receptors as well as parvalbumin – both of which control calcium (Renganathan et al., 1997; Latres et al., 2005). Further, IGF-1 shortens the half-relaxation time of EOM contraction (Li et al., 2011). We are currently examining how growth factors affect calcium transients in EOMs. Our preliminary data indicate that IGF-1 acutely and dramatically increases calcium waves in EOMs. Abnormal trophic feedback may be involved in strabismus (von Bartheld et al., 2010). Strabismic EOMs were shown to contract slower than normal EOMs (Lennerstrand, 2007), and strabismic human EOMs significantly down-regulate several calcium-related genes (Altick, Feng, Schlauch, Johnson, von Bartheld, manuscript submitted). A better understanding of calcium handling in EOMs may inform about the causes or parameters of EOM dysfunction in strabismic conditions.
Supplementary Material
Spontaneous fast calcium transients in chicken EOMs. The left side shows the field of view from which a ST map (right side upper: tan ROI) and traces (right side lower: red, green and blue ROIs) were extracted.
Spontaneous slow calcium transients (calcium waves) in chicken EOMs. The left side shows the FOV in which Ca2+ waves propagate along myofibers. The ST objects in the cube on the right side represent the pattern of propagation of these Ca2+ waves. Note that the passage of time is in the vertical axis.
Example of a twitch and pulse train of nerve stimulation-evoked fast calcium transients in a chicken EOM. Note the minimal contraction movement in this isometric, pinned EOM preparation.
The first calcium wave (green) initiates in a myofiber from a chicken EOM near an innervation site identified to be MIF-type with rh-BTX (red).
Highlights
Extraocular myofibers display two types of spontaneous calcium transients
Slow calcium transients can generate local myofiber contractions
Slow calcium transients occur in multiply-innervated myofibers
Extraocular muscles may use slow calcium transients to adjust tonic functions
BULLET POINTS
Extraocular myofibers display two types of spontaneous calcium transients
Slow calcium transients can generate local myofiber contractions
Slow calcium transients occur in multiply-innervated myofibers
Extraocular muscles may use slow calcium transients to implement tonic functions
Acknowledgments
The authors thank Drs. James Kenyon and Joseph Demer for helpful comments and advice. Our work was supported by NIH grants EY012841 and DK045713; imaging was done in core facilities supported by NIH COBRE grants RR018751, GM103513, RR024210 and GM103554.
Supported by: NIH grant EY012841 (CSvB), NIH grant DK45713 (TKS), and NIH COBRE grants RR018751, GM103513, RR024210, and GM103554
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade FH, McMullen CA, Rumbaut RE. Mitochondria are fast Ca2+ sinks in rat extraocular muscles: a novel regulatory influence on contractile function and metabolism. Invest. Ophthalmol. Vis. Sci. 2005;46:4541–4547. doi: 10.1167/iovs.05-0809. [DOI] [PubMed] [Google Scholar]
- Baryshnikova LM, Croes SA, von Bartheld CS. Classification and development of myofiber types in the superior oblique extraocular muscle of chicken. Anat. Rec. (Hoboken) 2007;290:1526–1541. doi: 10.1002/ar.20614. [DOI] [PubMed] [Google Scholar]
- Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J. Physiol. 2010;588:4453–4474. doi: 10.1113/jphysiol.2010.196824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Intracellular calcium movements during excitation-contraction coupling in mammalian slow-twitch and fast-twitch muscle fibers. J. Gen. Physiol. 2012;139:261–272. doi: 10.1085/jgp.201210773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold MW, Brinkmeier H, Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- Bondi AY, Chiarandini DJ, Jacoby J. Induction of action potentials by denervation of tonic fibres in rat extraocular muscles. J. Physiol. 1986;374:165–178. doi: 10.1113/jphysiol.1986.sp016073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NR, Podugu SP, Ferrari MB. Spatiotemporal characterization of short versus long duration calcium transients in embryonic muscle and their role in myofibrillogenesis. Dev. Biol. 2006;292:253–264. doi: 10.1016/j.ydbio.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Caputo C, Bolaños P. Effect of mitochondria poisoning by FCCP on Ca2+ signaling in mouse skeletal muscle fibers. Pflugers Arch. 2008;455:733–743. doi: 10.1007/s00424-007-0317-0. [DOI] [PubMed] [Google Scholar]
- Caputo C, Bolaños P, Escobar AL. Fast calcium removal during single twitches in amphibian skeletal muscle fibres. J. Muscle Res. Cell. Motil. 1999;20:555–567. doi: 10.1023/a:1005526202747. [DOI] [PubMed] [Google Scholar]
- Casas M, Figueroa R, Jorquera G, Escobar M, Molgó J, Jaimovich E. IP(3)-dependent, post-tetanic calcium transients induced by electrostimulation of adult skeletal muscle fibers. J. Gen. Physiol. 2010;136:455–467. doi: 10.1085/jgp.200910397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ. Calcium sparks. Physiol. Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- Chiarandini DJ, Stefani E. Electrophysiological identification of two types of fibres in rat extraocular muscles. J. Physiol. 1979;290:453–465. doi: 10.1113/jphysiol.1979.sp012783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croes SA, von Bartheld CS. Development of the neuromuscular junction in extraocular muscles of white Leghorn chicks. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005;282:110–119. doi: 10.1002/ar.a.20155. [DOI] [PubMed] [Google Scholar]
- Croes SA, Baryshnikova LM, Kaluskar SS, von Bartheld CS. Acute and long-term effects of botulinum neurotoxin on the function and structure of developing extraocular muscles. Neurobiol. Dis. 2007;25:649–664. doi: 10.1016/j.nbd.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernoch L. Sparks and embers of skeletal muscle: the exciting events of contractile activation. Pflugers Arch. 2007;454:869–878. doi: 10.1007/s00424-007-0244-0. [DOI] [PubMed] [Google Scholar]
- Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr. Opin. Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest. Ophthalmol. Vis. Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- Eggers HM. Functional anatomy of the extraocular muscles (chapter 31) In: Tasman W, Jaeger EA, editors. Duane's Foundations of Clinical Ophthalmology. revised ed vol. 1. JB Lippincott; Philadelphia: 1992. [Google Scholar]
- Eisner V, Parra V, Lavandero S, Hidalgo C, Jaimovich E. Mitochondria fine-tune the slow Ca(2+) transients induced by electrical stimulation of skeletal myotubes. Cell Calcium. 2010;48:358–370. doi: 10.1016/j.ceca.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Eltit JM, Hidalgo J, Liberona JL, Jaimovich E. Slow calcium signals after tetanic electrical stimulation in skeletal myotubes. Biophys. J. 2004;86:3042–3051. doi: 10.1016/S0006-3495(04)74353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 2009;89:1153–1176. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- Espinosa A, Estrada M, Jaimovich E. IGF-I and insulin induce different intracellular calcium signals in skeletal muscle cells. J. Endocrinol. 2004;182:339–352. doi: 10.1677/joe.0.1820339. [DOI] [PubMed] [Google Scholar]
- Feng CY, von Bartheld CS. Expression of insulin-like growth factor 1 isoforms in the rabbit oculomotor system. Growth Horm. IGF Res. 2011;21:228–232. doi: 10.1016/j.ghir.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MD, Gorospe JR, Felder E, Bogdanovich S, Pedrosa-Domellöf F, Ahima RS, Rubinstein NA, Hoffman EP, Khurana TS. Expression profiling reveals metabolic and structural components of extraocular muscles. Physiol. Genomics. 2002;9:71–84. doi: 10.1152/physiolgenomics.00115.2001. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Andrews SB. Characterization of spontaneous and action potential-induced calcium transients in developing myotubes in vitro. Cell Motil. Cytoskeleton. 1993;25:143–157. doi: 10.1002/cm.970250204. [DOI] [PubMed] [Google Scholar]
- Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/ceca.1999.0095. [DOI] [PubMed] [Google Scholar]
- Hatton WJ, von Bartheld CS. Analysis of cell death in the trochlear nucleus of the chick embryo: calibration of the optical disector counting method reveals systematic bias. J. Comp. Neurol. 1999;409:169–186. [PubMed] [Google Scholar]
- Hennig GW, Smith CB, O'Shea DM, Smith TK. Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J. Physiol. 2002;543:233–253. doi: 10.1113/jphysiol.2002.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. Structural differences of fast and slow extrafusal muscle fibres and their nerve endings in chickens. J. Physiol. 1961;157:221–231. doi: 10.1113/jphysiol.1961.sp006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A, Pilar G. Slow fibres in the extraocular muscles of the cat. J. Physiol. 1963;169:780–798. doi: 10.1113/jphysiol.1963.sp007296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S, Zeiger U, Baylor SM. Comparison of the myoplasmic calcium transient elicited by an action potential in intact fibres of mdx and normal mice. J. Physiol. 2008;586:5063–5075. doi: 10.1113/jphysiol.2008.160507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby J, Chiarandini DJ, Stefani E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J. Neurophysiol. 1989;61:116–125. doi: 10.1152/jn.1989.61.1.116. [DOI] [PubMed] [Google Scholar]
- Jacoby J, Ko K. Sarcoplasmic reticulum fast Ca(2+)-pump and myosin heavy chain expression in extraocular muscles. Invest. Ophthalmol. Vis. Sci. 1993;34:2848–2858. [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am. J. Physiol. Cell. Physiol. 2000;278:C235–256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Jaimovich E, Reyes R, Liberona JL, Powell JA. IP(3) receptors, IP(3) transients, and nucleus-associated Ca(2+) signals in cultured skeletal muscle. Am. J. Physiol. Cell. Physiol. 2000;278:C998–C1010. doi: 10.1152/ajpcell.2000.278.5.C998. [DOI] [PubMed] [Google Scholar]
- Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J. Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan T, Jiang H, Li H, DiMario JX. Regulation of skeletal muscle fiber type and slow myosin heavy chain 2 gene expression by inositol trisphosphate receptor 1. J. Cell Sci. 2005;118:2295–302. doi: 10.1242/jcs.02341. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Prendergast RA, Alameddine HS, Tomé FM, Fardeau M, Arahata K, Sugita H, Kunkel LM. Absence of extraocular muscle pathology in Duchenne's muscular dystrophy: role for calcium homeostasis in extraocular muscle sparing. J. Exp. Med. 1995;182:467–475. doi: 10.1084/jem.182.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MG, Simon BJ, Schneider MF. Effects of procaine and caffeine on calcium release from the sarcoplasmic reticulum in frog skeletal muscle. J. Physiol. 1992;453:341–366. doi: 10.1113/jphysiol.1992.sp019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J. Biol. Chem. 2005;280:2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G. Strabismus and eye muscle function. Acta Ophthalmol. Scand. 2007;85:711–723. doi: 10.1111/j.1600-0420.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- Li T, Wiggins LM, von Bartheld CS. Insulin-like growth factor-1 and cardiotrophin 1 increase strength and mass of extraocular muscle in juvenile chicken. Invest. Ophthalmol. Vis. Sci. 2010;51:2479–2486. doi: 10.1167/iovs.09-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Feng CY, von Bartheld CS. How to make rapid eye movements “rapid”: the role of growth factors for muscle contractile properties. Pflugers Arch. 2011;461:373–386. doi: 10.1007/s00424-011-0925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Persson EG. Simultaneous changes of cell volume and cytosolic calcium concentration in macula densa cells caused by alterations of luminal NaCl concentration. J. Physiol. 2005;563:895–901. doi: 10.1113/jphysiol.2004.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Frueh BR, Williams DA. Contractile properties of single skinned fibres from the extraocular muscles, the levator and superior rectus, of the rabbit. J. Physiol. 1994;475:337–346. doi: 10.1113/jphysiol.1994.sp020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiol. Rev. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Spontaneous activity: signal or noise? Science. 1999;285:541–543. doi: 10.1126/science.285.5427.541. [DOI] [PubMed] [Google Scholar]
- Miller JM. Understanding and misunderstanding extraocular muscle pulleys. J. Vis. 2007;7(10):1–15. doi: 10.1167/7.11.10. [DOI] [PubMed] [Google Scholar]
- Miller JM, Davison RC, Gamlin PD. Motor nucleus activity fails to predict extraocular muscle forces in ocular convergence. J. Neurophysiol. 2011;105:2863–2873. doi: 10.1152/jn.00935.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CG, Gerlach RL, Stuart DG. Soleus and anterior tibial motor units of the cat. Brain Res. 1973;44:1–11. doi: 10.1016/0006-8993(72)90361-7. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Meissner G, Block BA. The fastest contracting muscles of nonmammalian vertebrates express only one isoform of the ryanodine receptor. Biophys. J. 1993;65:2418–2427. doi: 10.1016/S0006-3495(93)81303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai H, Murakami G, Ohtsuka A, Suzuki D, Nakagawa T, Tatsumi H. Histotopographical study of human periocular elastic fibers using aldehyde-fuchsin staining with special reference to the sleeve and pulley system for extraocular rectus muscles. Anat. Sci. Int. 2009;84:129–140. doi: 10.1007/s12565-009-0017-2. [DOI] [PubMed] [Google Scholar]
- Pinniger GJ, Bruton JD, Westerblad H, Ranatunga KW. Effects of a myosin-II inhibitor (N-benzyl-p-toluene sulphonamide, BTS) on contractile characteristics of intact fast-twitch mammalian muscle fibres. J. Muscle Res. Cell Motil. 2005;26:135–141. doi: 10.1007/s10974-005-2679-2. [DOI] [PubMed] [Google Scholar]
- Porter JD, Karathanasis P. The development of extraocular muscle calcium homeostasis parallels visuomotor system maturation. Biochem. Biophys. Res. Commun. 1999;257:678–683. doi: 10.1006/bbrc.1999.0536. [DOI] [PubMed] [Google Scholar]
- Powell JA, Carrasco MA, Adams DS, Drouet B, Rios J, Müller M, Estrada M, Jaimovich E. IP(3) receptor function and localization in myotubes: an unexplored Ca(2+) signaling pathway in skeletal muscle. J. Cell Sci. 2001;114:3673–3683. doi: 10.1242/jcs.114.20.3673. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Messi ML, Schwartz R, Delbono O. Overexpression of hIGF-1 exclusively in skeletal muscle increases the number of dihydropyridine receptors in adult transgenic mice. FEBS Lett. 1997;417:13–16. doi: 10.1016/s0014-5793(97)01225-8. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Ward CW. Initiation and termination of calcium sparks in skeletal muscle. Front. Biosci. 2002;7:d1212–22. doi: 10.2741/A834. [DOI] [PubMed] [Google Scholar]
- Scott AB. Change of eye muscle sarcomeres according to eye position. J. Pediatr. Ophthalmol. Strabismus. 1994;31:85–88. doi: 10.3928/0191-3913-19940301-05. [DOI] [PubMed] [Google Scholar]
- Sohal GS, Knox TS, Allen JC, Jr., Arumugam T, Campbell LR, Yamashita T. Development of the trochlear nucleus in quail and comparative study of the trochlear nucleus, nerve, and innervation of the superior oblique muscle in quail, chick, and duck. J. Comp. Neurol. 1985;239:227–236. doi: 10.1002/cne.902390209. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog. Brain Res. 2006;151:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe P, Kenyon JL, Smith TK. Mitochondrial Ca2+ uptake regulates the excitability of myenteric neurons. J. Neurosci. 2002;22:6962–6971. doi: 10.1523/JNEUROSCI.22-16-06962.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Croes SA, Johnson LA. Strabismus. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. Elsevier; San Diego: 2010. pp. 525–532. [Google Scholar]
- Wang ZM, Messi ML, Delbono O. Sustained overexpression of IGF-1 prevents age-dependent decrease in charge movement and intracellular Ca(2+) in mouse skeletal muscle. Biophys. J. 2002;82:1338–1344. doi: 10.1016/S0006-3495(02)75489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger U, Mitchell CH, Khurana TS. Superior calcium homeostasis of extraocular muscles. Exp. Eye Res. 2010;91:613–622. doi: 10.1016/j.exer.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spontaneous fast calcium transients in chicken EOMs. The left side shows the field of view from which a ST map (right side upper: tan ROI) and traces (right side lower: red, green and blue ROIs) were extracted.
Spontaneous slow calcium transients (calcium waves) in chicken EOMs. The left side shows the FOV in which Ca2+ waves propagate along myofibers. The ST objects in the cube on the right side represent the pattern of propagation of these Ca2+ waves. Note that the passage of time is in the vertical axis.
Example of a twitch and pulse train of nerve stimulation-evoked fast calcium transients in a chicken EOM. Note the minimal contraction movement in this isometric, pinned EOM preparation.
The first calcium wave (green) initiates in a myofiber from a chicken EOM near an innervation site identified to be MIF-type with rh-BTX (red).