Abstract
Adenosine is an important neuromodulator, known to interact with both dopaminergic and glutamatergic systems to influence psychostimulant action. In the present study, we examined the effects of ATL444, a novel adenosine receptor antagonist, on motivation for cocaine in male and female rats. Adult male and female Sprague-Dawley rats were trained to self-administer cocaine (1.5 mg/kg/infusion) on a fixed-ratio 1 schedule with a daily maximum of 20 infusions. Following 5 consecutive sessions during which all 20 available infusions were obtained, motivation for cocaine (0.5 mg/kg/infusion) was assessed under a progressive ratio (PR) schedule, and once responding stabilized, the effect of treatment with ATL444 (0, 15, and 30 mg/kg, i.p.) was examined. As a control, we also assessed its effects on PR responding for sucrose. Binding studies revealed that ATL 444 was 3-fold, 25-fold, and 400-fold more selective for the A2A receptor as compared to A1, A2B, and A3 receptors, respectively. ATL444 produced a significant increase in motivation for cocaine on the day of treatment in females with a trend for an increase in males. In addition, over the two PR sessions following ATL444 treatment a significant decrease in responding was observed in males but not females. Responding for sucrose was unaffected by ATL444 treatment. Our results reveal that adenosine receptor blockade may mediate both acute increases in the reinforcing effects of cocaine, and longer term inhibitory effects on cocaine reinforcement that differ according to sex.
Keywords: Cocaine, Self-administration, adenosine receptors, sex difference
1. Introduction
Adenosine is a purine nucleoside that is widely distributed throughout the central nervous system and is recognized as a modulator of neurotransmitter release and neuronal excitability. Its physiological effects are mediated through the activation of four receptor types (A1, A2A, A2B, and A3). Antagonistic interactions exist between different subtypes of adenosine and dopamine receptors (Ferre et al., 1997). Adenosine, acting on both A1 and A2A receptors modulates dopaminergic neurotransmission through its effects on dopamine release and functional interactions between adenosine and dopamine receptors. Adenosine A1 receptors, which are expressed widely throughout the brain, co-localize with dopamine D1 receptors (Ferret et al., 1994; Gines et al., 2000). The A2A receptor is highly expressed in the striatum, primarily in GABAergic striato-pallidal projection neurons that also express dopamine D2 receptors (Augood and Emerson, 1994; Fink et al., 1992;Pollack et al., 1993; Schiffmann et al., 1991) and to a lesser extent in excitatory synapses of cortico-striatal terminals (Svenningson et al., 1999). A2A receptors have been shown to interact with several neurotransmitter receptors, including dopamine D2 and metabotropic glutamate subtype 5 (mGluR5) receptors (Ferre et al., 1991; 2002; Fink et al., 1992), with evidence for both antagonistic and synergistic effects. Although little is known regarding the role of adenosine A1 receptors in psychostimulant action and addiction-related behaviors, a large number of studies have demonstrated a role for A2A receptors. Baldo et al. (1999) have demonstrated that A2A agonists elevate brain stimulation reward thresholds, while antagonists reverse this effect, suggesting that A2A receptors are involved in the mesolimbic system regulation of reward, and signaling at this receptor is increasingly recognized as a possible therapeutic target for addiction (for reviews, see Brown and Short, 2008; Ferre et al., 2007; Shen and Chen, 2009).
A2A-D2 receptor heterodimers, through which A2A receptors might act to antagonize D2 receptor signaling, have been hypothesized to mediate A2A receptor effects on psychostimulant reward. A2A-D2 receptor interactions have been demonstrated in vitro (Canals et al., 2003; Fuxe et al., 1998; Hillion et al., 2002; Marcellino et al., 2010), and functionally antagonistic interactions between A2A receptors and cocaine-mediated behaviors involving D2 receptors (Adams et al., 2001; Kita et al., 1999; Ushijima et al., 1995) have been reported. Given findings showing that D2 receptor antagonism attenuates cocaine's reinforcing and addiction-related properties (Anderson et al. 2006; Mantsch et al. 2010; Milivojevic et al. 2004; Xi and Gardner 2007; but see Xue et al. 2011), adenosine A2A receptors may be a potential therapeutic target for cocaine addiction treatment. Several pharmacological studies indicate that adenosine A2A receptors influence the behavioral response to cocaine, although the direction of these effects has been inconsistent. For example, A2A receptor antagonists have been shown to increase cocaine sensitization and enhance discriminative-stimulus effects of cocaine (Filip et al., 2006; Justinova et al., 2003), whereas agonists reduce cocaine sensitization (Filip et al., 2006). Stimulation of A2A receptors also reduces reinstatement of cocaine seeking elicited by cocaine and cocaine-conditioned cues (Batchell and Self, 2009) while an A1/A2A receptor antagonist has been shown to reinstate cocaine-seeking behavior (Weerts and Griffiths, 2003). However, there have been very few studies characterizing the effects of adenosine receptor antagonism on ongoing cocaine self-administration. Three studies have addressed the role of A2A receptors in self-administering animals, and they have yielded mixed results. Justinova et al. (2010) found that in squirrel monkeys, cocaine self-administration on a fixed-ratio 10 schedule was not affected by treatment with the adenosine A2A receptor antagonist MSX-3. Soria et al. (2006) reported that motivation for cocaine was decreased in A2A receptor knockouts, while Knapp et al. (2001) found that initiation of cocaine self-administration on a fixed ratio 5 (FR5) schedule was reduced by treatment with adenosine A2A receptor agonists. This latter experiment is furthermore difficult to interpret due to the possibility that the agonist may have mimicked the effects of drug to produce satiation. A primary goal of our study was therefore to more fully characterize the effects of adenosine receptor antagonism in self-administering animals by examining its effects on motivation for cocaine using a progressive ratio (PR) schedule which is believed to be a more sensitive measure of changes in reinforcement efficacy than the fixed-ratio schedule.
A second goal of this work was to compare the effects of adenosine receptor antagonism on motivation for cocaine between males and females. The vast majority of treatment studies for cocaine addiction in animals have focused exclusively on males. However, sex differences have been demonstrated in many behavioral measures of cocaine addiction, including the motivation to self-administer low doses of cocaine as assessed by responding on a PR schedule (Carroll et al., 2002; Lynch and Taylor, 2004; Roberts et al., 1989). Moreover, dopaminergic transmission has been shown to differ according to sex. Release of dopamine in the striatum following cocaine is greater in females (Walker et al., 2006), and differential effects of both dopamine D1 and D2 receptor manipulation with respect to acute cocaine induced behaviors have been reported in male and female rats (Festa et al., 2006; Schindler and Carmona, 2002; Walker et al., 2006). Given the role that adenosine receptors play in modulating dopamine signaling, it is likely that the effects of adenosine receptor antagonism will also vary according to sex. Although sex differences in the effects of A1 antagonism have been reported following withdrawal from ethanol (Butler et al., 2008, 2009), to date no studies have examined sex differences in the effects of A2A receptor antagonism in addiction models.
In the present study, we examined the effect of a preferential A2A receptor antagonist on motivation to obtain cocaine infusions in actively self-administering animals. Specifically, the effects of pretreatment with systemic injection of the adenosine receptor antagonist ATL444 were tested in Sprague-Dawley rats responding for cocaine under a PR schedule. In addition, we evaluated the effects of ATL444 in both males and females in order to determine the existence of sex differences in the response to adenosine receptor inhibition. Separate groups of male and female rats responding for sucrose pellets were used to test for behavioral specificity.
2. Methods
2.1. Subjects
Male and female Sprague-Dawley rats (approximately 90 days old and weighing 380-410 g (males) or 280-310 g (females) were obtained from Charles River Laboratories. Animals were housed in operant conditioning chambers (Med-Associates, Inc., St. Albans, VT) in a temperature (20-22° C) and humidity (40-70 %) controlled vivarium, and were maintained in a 12- hour light: 12-hour dark cycle (lights on 0700, off 1900h). Food (Purina rat chow) and water were available ad libitum for the duration of the study. In order to facilitate acquisition of cocaine self-administration, after an acclimation period of at least 3 days following arrival, rats were briefly trained to lever press for sucrose pellets on a fixed-ratio 1 schedule. Training was considered to be complete after two consecutive 24-hour sessions during which 100 or more sucrose pellets were obtained. Following training, rats were anesthetized with a combination of ketamine (60 mg/kg) and pentobarbital (Nembutal, 5 mg/kg) and implanted with a silicone catheter into the right jugular vein as previously described (Lynch, 2008). During 3 days of recovery from surgery, animals received intravenous gentamicin (2 mg) followed by 0.1 ml heparinized saline (8.3 IU heparin/ml 0.9% physiological saline) to prevent infection and ensure catheter patency. Throughout the self-administration period animal health was monitored daily, and rats were weighed and catheters flushed with heparinized saline 3 times per week. All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and protocols were approved by the University of Virginia's Animal Care and Use Committee.
2.2. Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse (Research Triangle Park, NC) and was dissolved to a concentration of 0.7 mg/ml in sterile 0.9% saline and delivered at a constant rate of 0.025 ml/s through a 10 ml syringe housed in a motorized syringe pump (Med-Associates, Inc., St. Albans, VT). The dose of cocaine/infusion, either 1.5 mg/kg or 0.5 mg/kg, was held constant across subjects while infusion duration varied according to body weight (1 s/100 g). Cocaine solutions were made fresh weekly and refrigerated, but were delivered from the syringes at room temperature. ATL444, a preferential antagonist of A2A receptor adenosine receptors developed by Dogwood Pharmaceuticals, Inc., was dissolved in a 1.0 ml solution containing 10% DMSO/10% cremophor/80% saline.
2.3. Chemistry
ATL444 was synthesized using Comparative Molecular Field Analysis (CoMFA; Tripos, Inc.), a widely used 3D qualitative structure-activity relationship (QSAR) methodology, along with a host of similar compounds designed as agonists and antagonists of the A2A receptor over a wide range of KDs (Sun et al., 2007). The structure of ATL444 and the general synthetic scheme for this class of substituted adenine compounds are shown in Figure 1. Briefly, guanosine, 4.1, is acetylated to protect the ribose during reductive chlorination by POCl3/diethylaniline to form 6-chloroguanosine, 4.3. Non-aqueous diazotization in the presence of elemental iodine in diiodomethane is a standard route to the protected 6-chloro-2-iodonebularine, 4.4. Heating in methanolic ammonia deprotects the sugar and displaces the 6-chloro substituent to form 2-iodoadensoine, 4.5. Palladium-catalyzed coupling of 4.5 with a terminal alkyne generates 2-alkynyladenosine, 4.6. The sugar moiety is cleaved with acid to form the 9H-adenine, 4.7. Alkylation with a halide completes the synthesis of target 2,9-disubstituted adenine, 4.8. The requisite alkyne synthesis (Fig. 1., bottom panel) occurs as follows: starting from the Boc-protected methanol compound, the acetylene group is installed by displacing the tosylated alcohol using lithium acetylide. The Boc group is then removed using TFA and ATL444 is realized by treating the cycloalkylketone with ethynylmagnesium bromide. The structural features of ATL444 include the lack of a 7-ribose moiety normally required for agonist activity. ATL444 has also been shown to cross the blood-brain barrier and was originally identified as a drug candidate for Parkinson's disease (Adenosine Therapeutics, LLC internal data), producing motor stimulant activity consistent with blockade of A2A receptors as previously demonstrated in rodent models of Parkinson's disease (Ferre et al., 1997; Hauber et al., 1998; Pinna et al., 1996).
Fig. 1.
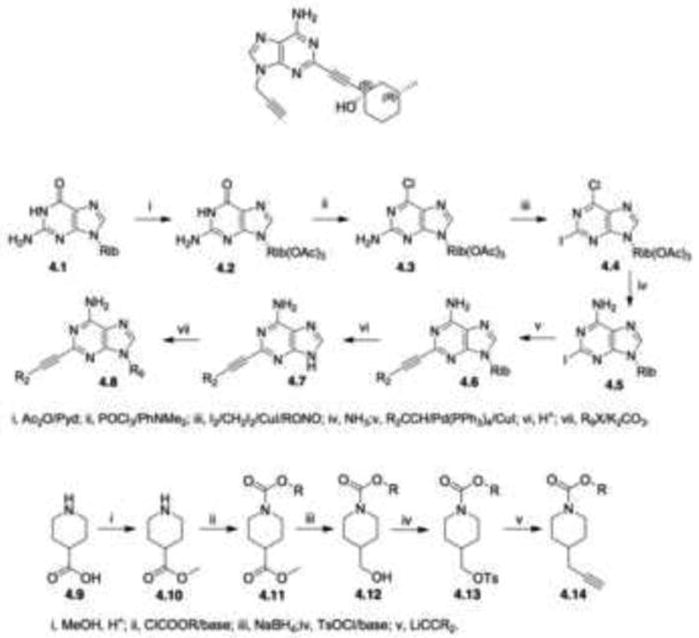
Structure and synthetic scheme for ATL444. Top panel: Structure of ATL444. Middle and bottom panels: Outline of the general synthesis scheme for ATL444. Middle panel: Synthesis of the target 2,9-disubstituted adenine. Bottom panel: Alkyne synthesis.
2.4. ATL444 Competition Binding Experiments
The binding methodology has been described previously (Sullivan et al., 1999). In brief, all subtypes of recombinant rat adenosine receptors were stably expressed in HEK-293 cells. Crude membrane preparations from these transfected cells were diluted in HE buffer (50 mM HEPES; 1 mM EDTA pH 7.4) at concentrations ranging from 2-50 μg/tube in a volume of 150 μL and adenosine deaminase added at 2 U/mL. Dilutions of the test article were prepared at 10× concentration in HE containing 10% DMSO. An appropriate radioligand (125I-ABA for A1A receptor and A3A receptor, 125I-ZM241385 for A2A receptor, or 125I-ABOPX for A2B receptor) was diluted in HE containing 14.7 mM MgCl2. Diluted test article (25 μL) was added to each membrane sample (150 μL). The radioligand was added in a 75 μL volume, the tubes incubated for 1.5 – 3.0 hours at room temperature and then filtered through glass fiber filters and counted in a Wallac Wizard 1470 gamma counter (Perkin Elmer, Boston MA). The non-specific binding of radiolabeled ligand was measured in the presence of the non-selective adenosine receptor agonist, NECA (100 μM). Within each assay, a minimum of three repeats were analyzed. Competition binding curves were constructed and IC50 values calculated using a 4-parameter logistic fit (PRISM 5.0, GraphPad Software, San Diego, CA). The value of Ki for the displacement of radioligand binding by agonist was calculated using the Cheng-Prusoff equation (Cheng and Prusoff, 1973).
2.5. Cocaine Self-Administration Procedure
Rats (n=10 males, n=10 females) were trained to self-administer cocaine (1.5 mg/kg per infusion) under a fixed-ratio 1 schedule with a maximum number of 20 infusions available per day. This relatively high dose of cocaine was used to ensure rapid and maximal rates of acquisition. Each response on the left lever under the daily session resulted in an infusion of cocaine and was accompanied by the sound of the infusion pump and illumination of a stimulus light above the lever for the duration of the infusion. Responses on the right lever were recorded, but not reinforced. After two consecutive days during which all 20 available infusions were obtained, the dose of cocaine available was lowered to 0.5 mg/kg per infusion since pharmacological manipulations are more readily revealed under low to moderate dose conditions. After 3 additional days during which all 20 available infusions were obtained, rats were given access to cocaine under a PR schedule, with daily 23-hour sessions initiated at 1200h. With this schedule, the response requirement to receive a cocaine infusion increases throughout the session until responding ceases. The steps were as follows: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, etc. (for reference see Arnold and Roberts, 1997). Under these conditions, responding typically ceases within 2-3 hours. Breakpoints were defined as the final ratio completed (i.e., number of infusions delivered) each session. Once responding was stable under the PR schedule (defined as 3 consecutive sessions with no increasing or decreasing trends in the number of infusions obtained, and in which self-administration behavior did not vary by more than 3 breakpoints), the adenosine A2A receptor antagonist ATL444 was administered via intraperitoneal (IP) injection 20 minutes before the start of the PR session. Each animal received injections of vehicle, 15 mg/kg and 30 mg/kg ATL444. These doses were selected based on previous pharmacokinetic and pharmacodynamic work done with ATL444 showing that at these doses stimulate locomotor activity beginning within 5 minutes of treatment, reaching a peak within 20 minutes, and lasting for approximately 60 minutes (Adenosine Therapeutics, LLC internal data). The order of dose presentation was counterbalanced and at least 5 days of stable PR sessions separated each injection. Daily sessions were conducted 7 days per week.
2.6. Sucrose Controls
Rats (n=6 males, n=5 females) were trained to self-administer sucrose pellets on an FR1 schedule until (100 pellets were obtained over 2 consecutive days, and then placed on a PR schedule for 2 hours per day with daily sessions beginning at 1000h. PR steps were identical to those used for cocaine and all animals reached their breakpoint for sucrose responding within the two-hour session limit. IP injections of ATL444 (15 and 30 mg/kg) and vehicle were administered as described above. As with the cocaine self-administering animals, sucrose controls were ad lib fed.
2.7. Data Analysis
The effect of ATL444 injections on PR responding for cocaine in males and females was determined by comparing percent change from baseline responding using repeated measures ANOVA. Mean baseline was obtained by averaging the number of cocaine infusions across each of the 3 days preceding the test day. Statistical analyses were performed with PASW 18 (SPSS, Inc.). Within-subjects factors were baseline change in the number of cocaine infusions self-administered during the PR sessions beginning the day of treatment and ending 3 days after treatment. Posthoc comparisons were made using the Bonferroni corrected t-test. The alpha level for statistical significance was set at 0.05.
3. Results
The results of competition binding experiments to all 4 subtypes of rat adenosine receptor using the A2A receptor antagonist, ATL444, are shown in Table 1. The specific radioligand and number of replicates for each receptor subtype are indicated. For each receptor subtype the Ki is given in nanomolar. The data indicate that ATL444 binds with high affinity to the rat A2A receptor. The Ki of ATL444 for the A2A receptor was determined to be 2.5 ± 0.8 nM (mean ± SD), and for the A1 receptor, A2B receptor and A3 receptor was determined to be 7.0 ± 0.7 nM, 61.8 ± 9.3 nM and >1000 nM, respectively. ATL444 exhibited 3-fold selectivity for the A2A receptor as compared to the A1 receptor, 25-fold selectivity for the A2A receptor as compared to the A2B receptor, and more than 400-fold selectivity for the A2A receptor compared to the A3 receptor.
Table 1.
Characterization of the adenosine A2A receptor antagonist, ATL444, by radioligand binding in recombinant rat receptors (mean ± SD).
| Ki (nM) | ||||
|---|---|---|---|---|
|
| ||||
| Receptor | A1 | A2A | A2B | A3 |
| Radioliganda | 125I-ABA | 125I-ZM241385 | 125I-ABOPX | 125I-ABA |
| ATL444 | 7.0 ± 0.7 (n=5) |
2.5 ± 0.8 (n=3) |
61.8 ± 9.3 (n=4) |
>1000 (n=5) |
Chemical names of radioligands used: 125I-ABA = N6– (4-amino-3-[125I]iodobenzyl)adenosine; 125I-ZM241385 = [125I]-4-(2-[7-amino-2-2-furyl1,2,4triacolo2,3-a-1,3,5triazin-5-yl-amino]ethyl)phenol; 125I-ABOPX=3-(3-[125 I]iodo-4-aminobenzyl)-8-(4-oxyacetate)phenyl-1-propylxanthine.
Consistent with previous research, breakpoints at baseline were higher in females than males (14.0 ± 1.0, 12.0 ± 0.8, respectively). Although this difference did not reach statistical significance under these moderate dose conditions (Fig. 2, top panels), the effects of ATL-444 in males and females were analyzed as percent change from baseline to correct for baseline sex differences. ATL444 treatment resulted in time- and sex-dependent changes in cocaine self-administration (sex, F1,46=8.9, P<0.01; day, F3,138=23.8, P<0.001; day by dose, F6,138=23.8, P<0.001; and sex by dose F2,46=4.0, P<0.05). On the day of treatment, there was in an increase in the number of cocaine infusions selfadministered from baseline (overall effect of dose, F2,46=11.3, P<0.001). Within females, PR responding on the day of treatment with 15 and 30 mg/kg ATL444 was significantly increased from baseline (22 ± 4% and 18 ± 1%, respectively; P's<0.01). Although responding also tended to increase in males on the day of treatment (15 ± 4% and 13 ± 10% for the 15 and the 30 mg/kg dose, respectively), these changes did not reach statistical significance (P=0.08). In contrast, in males we saw a persistent decrease in PR responding for cocaine following treatment with ATL444, but no significant changes in the days following treatment in females (P>0.05). Posthoc comparison within males revealed that this effect was due to a significant decrease in responding on the 2 days following treatment with the 15 mg/kg dose (Post 1, P<0.01; Post 2, P<0.05) and a significant decrease on the 2nd day after treatment following the 30 mg/kg dose (P<0.05).
Fig. 2.
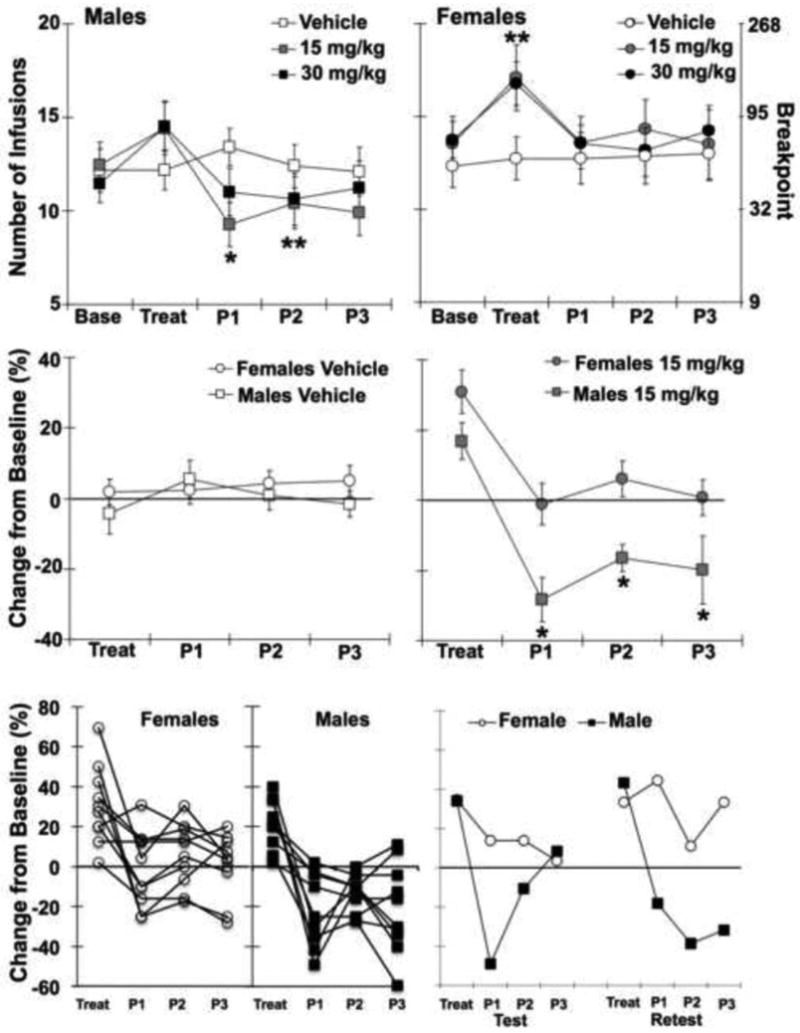
Effect of ATL-444 on PR responding for cocaine. Top panels: mean (SEM) infusions and corresponding breakpoints observed for the baseline session (Base), the day of treatment (Treat) and the 3 post-treatment sessions that followed (P1, 2, 3) in males (left) and females (right). * indicates significant difference between vehicle and 15 mg/kg treatment group. ** indicates significant difference between vehicle and both 15 and 30 mg/kg treatment groups. Middle panels: mean (SEM) percent change from baseline number of cocaine infusions on the day of treatment (Treat) and for the 3 sessions that followed treatment (P1, 2, 3) with vehicle (left) or 15 mg/kg ATL-444 (right). * represents a significant difference between males and females. Lower left panel: individual female (left) and male (right) values for percent change from baseline number of cocaine infusions on the day of treatment with 15 mg/kg ATL444 (Treat) and for the 3 sessions that followed treatment (P1, 2, 3). Lower right panel: replicate of the effects of the 15 mg/kg dose of ATL444 in a representative male and female that received a repeat treatment with this same dose.
These sex and time effects at the 0 and 15 mg/kg dose of ATL-444 are further illustrated in Fig. 2 (middle and lower left panels). Although no significant sex or time-dependent changes were observed following treatment with vehicle (Fig.2, middle left panel; P>0.05), within the 15 mg/kg dose, there was a significant effect of day (F3,36=11.7, P<0.001) and sex (F1,12=7.1, P<0.05) with males, but not females, showing a reduction in cocaine intake on the days following treatment (Fig.2, middle right panel). Post hoc comparison between males and females revealed a significant sex difference on the first 2 days following treatment (P<0.05). No significant sex difference was observed at the 30 mg/kg dose (data not shown). A scatter plot of the results at the 15 mg/kg dose for each individual animal is shown in Fig. 2 (lower left panel).
In order to further establish these time and sex-dependent changes following ATL444 treatment, two animals were given a second treatment with the 15 mg/kg dose. The second round of treatment was tested after the animals had returned to pre-treatment baseline. As shown in Fig. 2 (lower right panel), these findings replicate the sex and time-dependent changes observed for the group after a single treatment. For both the initial treatment and at retest, responding was initially increased in both the male and female on the day of treatment, but decreased only in the male on the days following treatment.
As a control for non-specific effects of A2A receptor blockade on PR responding, we also measured its effect on responding for sucrose under a PR schedule (Table 2). Average levels of responding did not differ between cocaine and sucrose self-administering animals, with mean number of deliveries of 12.5 ± 0.37 and 11.5 ± 1.42, respectively. As there was no significant overall effect of sex, data for males and females were pooled. No significant overall effects of treatment were found, indicating that the observed effects of ATL444 treatment on PR responding were specific to cocaine.
Table 2.
Mean (±SEM) number of sucrose deliveries at baseline (Base), the day of treatment (Treatment) with ATL-444 (15 mg/kg or 30 mg/kg) or vehicle, and on 3 post-treatment sessions that followed (Post 1, 2, 3). Data for males and females are pooled.
| Treatment | Base | Treatment | Post 1 | Post 2 | Post 3 |
|---|---|---|---|---|---|
| Vehicle | 10.8 ± 0.8 | 10.1 ± 0.9 | 11.3 ± 0.7 | 10.6 ± 0.8 | 11.1 ± 0.6 |
| 15 mg/kg | 11.0 ± 0.5 | 11.9 ± 0.7 | 10.7 ± 0.6 | 11.4 ± 0.7 | 11.6 ± 0.7 |
| 30 mg/kg | 12.4 ± 0.8 | 12.6 ± 0.9 | 11.4 ± 1.0 | 11.7± 1.2 | 11.1 ± 1.3 |
4. Discussion
The findings of this study demonstrate that preferential blockade of adenosine A2A/A1 receptors with ATL444 produces time- and sex-specific changes in motivation to obtain cocaine in self-administering animals. In females, ATL444 treatment produced an acute increase in motivation for cocaine on the day of treatment, but no persistent changes beyond the day of treatment. In males, although motivation for cocaine also tended to be higher on the day of treatment, this increase was followed by a decrease in motivation that persisted for two days following treatment. That these effects were observed for cocaine, but not sucrose, indicates that they are not due to non-specific effects of ATL444, but instead are specific to cocaine reinforcement.
The acute increase in PR responding for cocaine after ATL444 treatment is in agreement with previous studies that have examined the effects of different A2A receptor antagonists on acute cocaine-related behaviors. The A2A receptor antagonist MSX-3 has been shown to increase cocaine-induced locomotor activity and sensitization in rats (Filip et al., 2006). Another A2A antagonist, DMPX (3,7-dimethyl-1-propargylxanthine), and was found to enhance cocaine-induced hyperactivity in mice (Poleszak and Malec, 2002). Concomitant with the increase in behavioral responses to cocaine associated with adenosine A2A receptor antagonists, stimulation of A2A receptors has been shown to inhibit cocaine-induced conditioned place preference (Poleszak and Malec, 2002), attenuate sensitization to cocaine (Filip et al., 2006), and inhibit the initiation of cocaine self-administration on an FR5 schedule (Knapp et al., 2001). Taken together, these pharmacological data support a role of acute A2A receptor activation in opposing and A2A receptor blockade in stimulating the reinforcing effects of cocaine. Interestingly, dose-dependent effects of these A2A receptor antagonists were not found in previous studies, and we likewise saw no difference in the enhancement of PR responding by 15 or 30 mg/kg dose of ATL444. The reasons for this lack of dose-dependent effects remain unclear. Furthermore, while ATL444 did not significantly elevate PR breakpoints in male rats, it is possible that males may be less sensitive than females to ATL444, and a significant effect might be seen in males at a slightly higher dose.
One of the most intriguing aspects of our study is the persistent decrease in motivation for cocaine observed in males following ATL444 treatment. This type of longer term decrease in self-administration behavior has not been reported in previous pharmacological studies with adenosine antagonists, which have been limited to the examination of acute cocaine-induced locomotor effects, conditioned place preference, and fixed ratio responding on the day of treatment. However, global or forebrain-specific genetic inactivation of A2A receptors in mice produces a decrease in cocaine self-administration under both fixed-ratio and PR schedules (Chen et al., 2000; Soria et al., 2006), and in a recent study by Justinova et al. (2010), MSX-3 did not affect fixed-ratio responding for a low dose of cocaine in squirrel monkeys. While the reasons for the discrepancies among these genetic and pharmacological data are not entirely clear, it has been suggested that A2A receptor inactivation may play a differential role in psychostimulant effects depending on which populations of striatal A2A neurons are involved, and this may be affected by molecular adaptations that occur over time in the cortico-accumbens pathways involved in drug taking and seeking behavior (Brown and Short, 2008; Shen et al., 2008). In support of this idea, striatum-specific A2A receptor knockout mice (Shen et al., 2008) show an increase in cocaine-induced psychomotor activity. Therefore two populations of A2A receptors may differentially affect striatal neurotransmission and psychostimulant effects. Selective inactivation of striatal A2A receptors enhances psychostimulant effects, while forebrain or extra-striatal A2A receptor inactivation produces inhibitory effects (Brown and Short, 2008; Shen et al., 2008). Although the specific effects of ATL44 on the populations of pre- and post-synaptic A2A receptors in these different brain regions are unknown, it is possible that ATL444 exerts differential time-dependent effects on striatal and extra-striatal A2A receptors, to increase and decrease motivation for cocaine, respectively. Another possibility is that the time-dependent effects of ATL444 may be partially mediated through its effects at the A1 receptor. Although no studies have directly examined the role of A1 receptors in cocaine self-administration, there is evidence, albeit controversial, that the A1 receptor may influence cocaine reward. For example, one study showed that A1 receptor antagonism potentiates the discriminative stimulus effects of cocaine (Justinova et al., 2003), and another showed that such treatment decreased cocaine-induced place preference (Poleszak and Malec, 2002). Although these results are inconclusive for a role of A1 receptors in cocaine-mediated behavior, we cannot exclude them as a potential mechanism for our current findings. In addition, adenosine A1 and A2A receptors, which are colocalized in rat motor nerve terminals, have been shown to exert opposite regulatory actions on neurotransmitter release (Correia-De-Sa et al., 1996), and more recently, opposite modulatory roles for adenosine A1 and A2A receptors on dopamine and glutamate release in the nucleus accumbens have been described (Quarta et al., 2004).
The persistent decrease in motivation for cocaine that we found in males following antagonist treatment was not observed in females. Although the mechanism for this sex difference is not yet known, previous work suggests that it may be a result of increased sensitivity of the D2/A2A receptor system in females. Following either extended access cocaine self-administration or under the PR schedule using lower-doses than the one used in the present study, females show a higher motivation for cocaine than males (Carroll et al., 2002; Lynch and Taylor, 2004; Roberts et al., 1989) as well as higher levels of subsequent reinstatement responding (Lynch and Carroll, 2000). Dopaminergic signaling is believed to be the primary mediator of cocaine reinforcement, and among several molecular adaptations that occur in response to cocaine, females show signs of greater dopamine D2 signaling in the medial prefrontal cortex compared to males (Sun et al., 2010). Marcellino et al. (2007) have shown that A2A receptors increase in the NAc following withdrawal from cocaine self-administration, and they suggest that this A2A receptor up-regulation occurs as a compensatory response to increased D2 signaling. Thus, females may also show greater cocaine-induced A2A receptor increases compared to males, and hence altered D2/A2A receptor dynamics.
Ovarian hormones may also play a role in modulating the D2/A2A receptor interactions that -differentially affect cocaine self-administration in males versus females. D2 receptors in the striatum of females have been shown to be down-regulated by estradiol (Bazett and Becker, 1994). Therefore, depending on estrous cycle phase females may have different D2 receptor availability and responses to A2A receptor antagonism. Although we did assess for estrous cycle phase in the current study, the variability in cycle phase both within and between females tested here did not allow for an analysis of the effects of ATL-444 by estrous cycle phase. Future studies will be necessary to explore the relationship between levels of ovarian hormones and the effects of A2A signaling on cocaine self-administration.
5. Conclusions
In conclusion, the present findings underscore the important role played by adenosine receptor signaling in motivation to self-administer cocaine and suggest that A2A/A1 receptors may be an effective therapeutic target for cocaine addiction in males, but not females. Our results give evidence for two different effects of A2A/A1 receptor blockade: an acute increase in the motivation to self-administer cocaine, followed by a longer-term decrease in motivation that exhibits a sex difference. These differential time- and sex-dependent responses to A2A/A1 receptor blockade should be considered in the development of any pharmacological treatment strategy.
Highlights.
Adenosine A2A receptor blockade alters the motivation to self-administer cocaine in rats in a time- and sex-dependent manner.
In females, ATL44, a preferential A2A antagonist, acutely increased motivation for cocaine.
In males, ATL444 treatment produced long-term decreases in motivation for cocaine.
Differential responses to A2A receptor blockade should be considered in the development of treatment strategies for cocaine addiction.
Acknowledgments
We would like to thank Robert Thompson for technical assistance. This work was supported by NIDA grant R01 DA024716 (WJL) and SBIR grant 1R43DA022110-01 (JMR & AB). JMR and AB are employed by Dogwood Pharmaceuticals, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JU, Careri JM, Efferen TR, Rotrosen J. Differential effects of dopamine antagonists on locomotor activity, conditioned activity and conditioned place preference induced by cocaine in rats. Behav Pharmacol. 2001;12:603–11. doi: 10.1097/00008877-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Peirce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacol. 2006;31:1452–61. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–7. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Emson PC. Adenosine A2a receptor mRNA is expressed by enkephalin cells but not by somatostatin cells in rat striatum: a co-expression study. Brain Res Mol Brain Res. 1994;22:204–10. doi: 10.1016/0169-328x(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Effects of adenosine A2A receptor stimulation on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2009;206:469–78. doi: 10.1007/s00213-009-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Koob GA, Markou A. Role of adenosine A2 receptors in brain stimulation reward under baseline conditions and during cocaine withdrawal in rats. J Neurosci. 1999;19:11017–26. doi: 10.1523/JNEUROSCI.19-24-11017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–72. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Brown RM, Short JL. Adenosine A(2A) receptors and their role in drug addiction. J Pharm Pharmacol. 2008;60:1409–30. doi: 10.1211/jpp/60.11.0001. [DOI] [PubMed] [Google Scholar]
- Butler TR, Smith KJ, Self RL, Braden BB, Prendergast MA. Sex differences in the neurotoxic effects of adenosine A1 receptor antagonism during ethanol withdrawal: reversal with an A1 receptor agonist or an NMDA receptor antagonist. Alcohol Clin Exp Res. 2008;32:1260–70. doi: 10.1111/j.1530-0277.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Smith KJ, Berry JN, Sharrett-Field LJ, Prendergast MA. Sex differences in caffeine neurotoxicity following chronic ethanol exposure and withdrawal. Alcohol Alcohol. 2009;44:567–74. doi: 10.1093/alcalc/agp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, et al. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–9. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–13. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, et al. Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A(2A) adenosine receptors. Neuroscience. 2000;97:195–204. doi: 10.1016/s0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharm. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Correia-De-Sa P, Timoteo MA, Ribeiro JA. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J Neurophysiol. 1996;76:3910–9. doi: 10.1152/jn.1996.76.6.3910. [DOI] [PubMed] [Google Scholar]
- Fenu S, Pinna A, Ongini E, Morelli M. Adenosine A2A receptor antagonism potentiates L-DOPA-induced turning behavior and c-fos expression in 6-hydroxydopamine-lesioned rats. Eur J Pharmacol. 1997;321:143–7. doi: 10.1016/s0014-2999(96)00944-2. [DOI] [PubMed] [Google Scholar]
- Ferré S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci USA. 1991;88:7238–41. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Gimenze-Llort L, Finnman UB, Martinez E, Scotti de Carolis A, et al. Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport. 1994;6:73–6. doi: 10.1097/00001756-199412300-00020. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–7. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueño J, Gutiérrez MA, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA. 2002;99:11940–5. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Diamond I, Goldberg SR, Yao L, Hourani SM, Huang ZL, et al. Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog Neurobiol. 2007;83:332–47. doi: 10.1016/j.pneurobio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Jenab S, Weiner J, Nazarian A, Niyomchai T, Russo SJ, et al. Cocaine-induced sex differences in D1 receptor activation and binding levels after acute cocaine administration. Brain Res Bull. 2006;68:277–84. doi: 10.1016/j.brainresbull.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Przegaliński E, Muller CE, Agnati L, et al. Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res. 2006;1077:67–80. doi: 10.1016/j.brainres.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–95. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004;61:857–72. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Mol Brain Res Rev. 1998;26:258–73. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Ginés S, Hillion J, Torvinen M. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci USA. 2000;97:8606–11. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W, Nagel J, Sauer R, Muller CE. Motor effects induced by a blockade of adenosine A2A receptors in the caudate-putamen. Neuroreport. 1998;9:1803–6. doi: 10.1097/00001756-199806010-00024. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–7. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Segal PN, Antoniou K, Solinas M, Pappas LA, et al. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–86. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Redhi GH, Mascia P, Stroik J, Quarta D, et al. Reinforcing effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addiction Biology. 2011;16:405–15. doi: 10.1111/j.1369-1600.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K, Shiratani T, Takenouchi K, Fukuzako H, Takigawa M. Effects of D1 and D2 dopamine receptor antagonists on cocaine-induced self-stimulation and locomotor activity in rats. Eur Neuropsychopharmacol. 1999;9:1–7. doi: 10.1016/s0924-977x(97)00098-9. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Cottam N, Ciraulo DA, Kornetsky C. Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol Biochem Behav. 2001;68:797–803. doi: 10.1016/s0091-3057(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197:237–46. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–51. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Wisniewski S, Vranjkovic O, Peters C, Becker A, Valetine A, et al. Levo-tetrahydropalmatine attenuates cocaine self-administration under a progressive ratio schedule and cocaine discrimination in rats. Pharmacol Biochem Behav. 2010;97:310–6. doi: 10.1016/j.pbb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Roberts DC, Navarro G, Filip M, Agnati L, Lluís C, et al. Increase in A2A receptors in the nucleus accumbens after extended cocaine self-administration and its disappearance after cocaine withdrawal. Brain Res. 2007;1143:208–20. doi: 10.1016/j.brainres.2007.01.079. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Navarro G, Sahlholm K, Nilsson J, Agnati LF, Canela EI, et al. Cocaine produces D2R-mediatd conformational changes in the adenosine A(2A)R-dopamine D2R heteromer. Biochem Biophys Res Commun. 2010;394:988–92. doi: 10.1016/j.bbrc.2010.03.104. [DOI] [PubMed] [Google Scholar]
- Milivojevic N, Krisch I, Sket D, Zivin M. The dopamine D1 receptor agonist and D2 receptor antagonist LEK-8829 attenuates reinstatement of cocaine-seeking in rats. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:576–82. doi: 10.1007/s00210-004-0937-2. [DOI] [PubMed] [Google Scholar]
- Pinna A, diChiara G, Wardas J, Morelli M. Blockade of A2a adenosine receptors positively modulates turning behavior and c-Fos expression induced by D1 agonists in dopamine-denervated rats. Eur J Neurosci. 1996;8:1176–81. doi: 10.1111/j.1460-9568.1996.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Malec D. Adenosine receptor ligands and cocaine in conditioned place preference (CPP) test in rats. Pol J Pharmacol. 2002;54:119–26. [PubMed] [Google Scholar]
- Pollack AE, Harrison MB, Wooten GF, Fink JS. Differential localization of A2a adenosine receptor mRNA with D1 and D2 dopamine receptor mRNA in striatal output pathways following a selective lesion of striatonigral neurons. Brain Res. 1993;631:161–6. doi: 10.1016/0006-8993(93)91204-6. [DOI] [PubMed] [Google Scholar]
- Quarta D, Ferre S, Solinas M, You ZB, Hockemeyer J, Popoli P, et al. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure J Neurochem. 2004;88:1151–8. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Libert F, Vassart G, Vanderhaeghen JJ. Distribution of adenosine A2 receptor mRNA in the human brain. Neurosci Lett. 1991;130:177–81. doi: 10.1016/0304-3940(91)90391-6. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav. 2002;72:857–63. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Shen HY, Chen JF. Adenosine A(2A) receptors in psychopharmacology: modulators of behavior, mood and cognition. Curr Neuropharmacol. 2009;7:195–206. doi: 10.2174/157015909789152191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, et al. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008;28:2970–5. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Castañé A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2006;31:978–87. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Linden J, Buster BL, Scheld WM. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with the type IV phosphodiesterase inhibitor, rolipram. J Infect Dis. 1999;180:1550–60. doi: 10.1086/315084. [DOI] [PubMed] [Google Scholar]
- Sun WC, Moore JN, Hurley DJ, Vandenplass ML, Linden JM, Murray TF. Pharmacologic characterization of novel adenosine A2A receptor agonists in equine neutrophils. Am J Vet Res. 2007;68:981–7. doi: 10.2460/ajvr.68.9.981. [DOI] [PubMed] [Google Scholar]
- Sun WL, Festa ED, Jenab S, Quinones-Jenab V. Sex differences in dopamine D2-like receptor-mediated G-protein activation in the medial prefrontal cortex after cocaine. Ethn Dis. 2010;20:88–91. [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–96. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Ushijima I, Carino MA, Horita A. Involvement of D1 and D2 dopamine systems in the behavioral effects of cocaine in rats. Pharmacol Biochem Behav. 1995;52:737–41. doi: 10.1016/0091-3057(95)00167-u. [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Griffiths RR. The adenosine receptor antagonist CGS15943 reinstates cocaine-seeking behavior and maintains self-administration in baboons. Psychopharmacology (Berl) 2003;168:155–63. doi: 10.1007/s00213-003-1410-5. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–59. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Rebec GV, Sun W. Activation of D2-like receptors in rat ventral tegmental area inhibits cocaine-reinstated drug-seeking behavior. Eur J Neurosci. 2011;33:1291–8. doi: 10.1111/j.1460-9568.2010.07591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


