Abstract
Histamine acts centrally to increase energy expenditure and reduce body weight by mechanisms not fully understood. It has been suggested that in the obese state hypothalamic histamine signaling is altered. Previous studies have also shown that histamine acting in the preoptic area controls thermoregulation. We aimed to study the influence of preoptic histamine on body temperature and energy homeostasis in control and obese mice. Activating histamine receptors in the preoptic area by increasing the concentration of endogenous histamine or by local injection of specific agonists induced an elevation of core body temperature and decreased respiratory exchange ratio (RER). In addition, the food intake was significantly decreased. The hyperthermic effect was associated with a rapid increase in mRNA expression of uncoupling proteins in thermogenic tissues, the most pronounced being that of uncoupling protein (UCP) 1 in brown adipose tissue and of UCP2 in white adipose tissue. In diet induced obese mice histamine had much diminished hyperthermic effects as well as reduced effect on RER. Similarly, the ability of preoptic histamine signaling to increase the expression of uncoupling proteins was abolished. We also found that the expression of mRNA encoding the H1 receptor subtype in the preoptic area was significantly lower in obese animals. These results indicate that histamine signaling in the preoptic area modulates energy homeostasis by regulating body temperature, metabolic parameters and food intake and that the obese state is associated with a decrease in neurotransmitter’s influence.
Keywords: histamine, thermoregulation, obesity, preoptic area, hyperthermia
1
A role of CNS histamine in thermoregulation has been established in various organisms from invertebrates (Hong et al., 2006) to lower vertebrates (Leger and Mathieson, 1997) as well as mammals. In the latter, the preoptic area/anterior hypothalamus, region which contains thermoregulatory neurons, is the main locus in which histamine affects body temperature (Green et al., 1976, Colboc et al., 1982). Our recent studies have identified the cellular mechanisms by which histamine controls core body temperature. In the median preoptic nucleus (MnPO) histamine acts directly onto two distinct populations of neurons: it excites glutamatergic neurons by activating H1 receptors and inhibits the activity of GABAergic neurons expressing H3 receptors (Lundius et al., 2010). At the level of the medial preoptic nucleus (MPON) histamine increases the firing rate of glutamatergic neurons by acting at both H1 and H2 subtype receptors, the latter being the predominant mechanism (Tabarean et al., 2012).
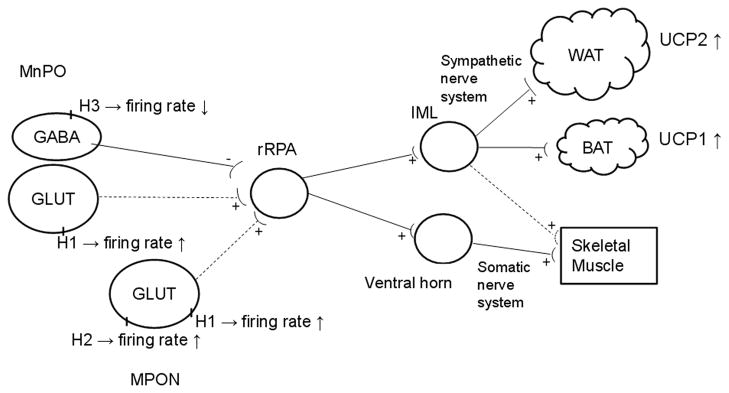
MnPO and MPON thermoregulatory neurons control thermoeffector processes via the sympathetic and the somatic nervous system (reviewed in Morrison and Nakamura, 2011). A simplified scheme of the thermoregulatory network and the processes activated is presented in Fig 1. The sympathetic nervous system innervates the brown adipose tissues (BAT) (reviewed in Morrison, 2011), the white adipose tissue (WAT) (reviewed in Bartness and Song, 2007) and possibly the skeletal muscles (Yoshitomi et al., 1998). Uncoupling protein (UCP) 1 plays a crucial role in brown adipose tissue (BAT) thermogenesis. Infusion of histamine in the third ventricle or in the preoptic area (POA) increases in BAT sympathetic nerve activity and in the UCP1 mRNA expression (Yasuda et al., 2004). The sympathetic nervous system also controls the expression of UCP2 and UCP3 in WAT and skeletal muscle (Yoshitomi et al., 1998). These uncoupling proteins appear to contribute little to adaptive thermogenesis, however they have important functions in energy metabolism. UCP2 is involved in shifting a given cell towards fatty acid fuel utilization (reviewed in Andrews, 2010). Interestingly, a decreased respiratory exchange ratio (RER) was observed in response to central histamine administration (Malmlof et al., 2005), however the mechanism involved is not known. UCP3 activation is necessary to sustain high metabolic rates in thermogenic tissues (Schrauwen et al., 2004, Nau et al., 2008). In pathophysiological conditions activation of UCP3 can result in significant heat production (Banks et al., 2009).
Fig. 1.
Simplified diagram of the neural pathways controlling thermoeffector mechanisms (Morrison and Nakamura, 2011). The diagram also presents the proposed mechanisms activated by histamine. GABAergic neurons in the MnPOA tonically inhibit sympathetic premotor neurons in the rostral raphe pallidus (rRPA). Histamine reduces the firing rates of GABAergic MnPO neurons (Lundius et al, 2010). This results in stimulation of the sympathetic output system. Activation of H1 and H2 receptors expressed by MnPO and MPON neurons, respectively, increased firing rates and stimulates the sympathetic neuron system. The dashed lines indicate that the respective projections have been suggested by physiological studies but have not been demonstrated directly yet. IML, intermediolateral cell column.
Numerous studies suggest a role of histamine signaling in obesity. Histamine-deficient animals (HDC−/−) develop obesity and have an impaired ability to express UCP1 in BAT (Fulop et al., 2003). Similarly H1R−/− and H3R−/− mice develop obesity (Masaki et al., 2001b, Takahashi et al., 2002). Other studies have reported a decreased level of hypothalamic histamine in obese animals (Itateyama et al., 2003). Here we have characterized in detail the influence of histamine agonism in the median and medial preoptic nuclei on parameters of energy metabolism and on the expression of uncoupling proteins in thermogenic tissues (BAT, white adipose tissue and skeletal muscle). We have also tested the hypothesis that histamine modulation of energy homeostasis is attenuated in obese mice.
2. Experimental procedures
All animal work was conducted in accordance with the National Institute of Health Guide for the care and use of laboratory animals (NIH publication No. 80-23). The procedures were approved by the Institutional Animal Care and Use Committee of the Scripps Research Institute. The standards are set forth by American Association for the Accreditation of Laboratory Animal Care (AAALAC) standards and the regulations set forth in the Animal Welfare Act. Efforts were made to minimize the number of animals used and their suffering.
2.1 Diet induced obesity
3–4 months old male C57/Bl6 mice littermates were split in two age-matched groups: one was fed a normal diet (control group) i.e. mouse chow containing 11 % fat (Harlan Teklad S-2335) and the other was fed a high fat diet (obese group) containing 60% fat (Research Diets, D12492). Mice were kept for 5 weeks on the high fat diet until they reached a body weight of at least 40 g. After five weeks the average weight for the obese group was 43±3 (n=78) while that of the control group was 34±3 (n=78).
2.2 Telemetry and POA injections
4–5 months old male wild-type C57BL/6 mice were anesthetized with isoflorane (induction 3–5%, maintenance 1–1.5%) and surgically implanted with radio telemetry devices (TA-F20, Data Sciences, Inc., St Paul, MN) into the peritoneal cavity for core body temperature (CBT) and motor activity measurement as previously described (Lundius et al., 2010). For brain injections, mice were first subject to stereotaxic placement of a guide cannula (26 Ga, 10 mm length) as previously described (Lundius et al., 2010, Sethi et al., 2011).
Coordinates for cannula (27 ga. 16 mm length) implants in the median preoptic nucleus (MnPO) were: 0.38 mm from Bregma and ventral 4.6 mm. Coordinates for cannula (27 ga. 16 mm length) implants in the medial preoptic nucleus (MPON) were: from Bregma −0.02 mm, 0.35 and −0.35 mm lateral, and ventral 4.75 mm (Paxinos and Franklin, 2001). The ambient temperature was maintained at ~26 ± 0.5 °C in a 12:12-h lightdark cycle controlled room (lights on 8:00 am). H1-3 receptor agonists or the histamine N-methyl transferase inhibitor SKF91488 were injected into the MnPO or bilaterally in the MPON. All substances injected were dissolved in sterile aCSF. Mice were handled for at least three days before the injection during 5 minutes every day for habituation. On the day of injections, mice were held and the injector (cannulae 33 Ga, 17 mm length) was placed inside the cannula. The injector was connected to a microsyringe (0.5 μL). The injected volume was 0.2 μL (rate 0.1 μL/min). After this procedure the animal was returned to the home cage. Injections were always performed at 12 am local time, during the “subjective light period”.
Mice were individually housed in a Plexiglas cage in a room maintained at 26 ± 0.5 °C on a 12:12 h light–dark cycle (lights on at 6:00 a.m.) with ad libitum access to food and water and allowed to recover from surgery for 2 weeks. The cages were positioned onto the receiver plates (RPC-1; Data Sciences) and radio signals from the implanted transmitter were recorded. Core body temperature (CBT) and motor activity were continuously monitored with a fully automated data acquisition system (Dataquest A.R.T., Data Sciences, Inc., St Paul, MN) for at least 24 h for baseline levels of temperature prior to experiments. Calculations were carried out with values collected 1 h after injection in order to exclude the stress-associated increase of CBT.
2.3 Histology
The cannula placement was always checked at the end of the experiments by blue pontamine dye injection. The animals were then killed and the brains were removed. The brains were incubated for 24 h in 4% paraformaldehyde and then for an additional 24 h in 30% sucrose at 4°C, and frozen. Tissue sections of 40 μm were cut after fixation on a freezing-stage microtome. Location of canulla placements were determined as described previously (Sanchez-Alavez et al., 2006). Only data from animals in which the correct placement of the canulla(s) was confirmed were included in this report.
2.4 Indirect calorimetry
Indirect calorimetry was performed as previously described (Sanchez-Alavez et al., 2010) using the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) on acclimated (for 2–3 days), singly-housed mice using a computer controlled, open-circuit system (Oxymax System, Columbus Instruments, Columbus, OH). Respiratory exchange ratio (RER) was calculated as VCO2/VO2. Data were recorded under ambient room temperature clamped at 26 °C, beginning from the onset of the light cycle. Heat production (kcal/h/g) was calculated using the formula = CV * VO2 * 1 L/1000 ml * 1 kg/1000 g where CV represents the caloric value (CV = 3.815 + 1.232 × RER) as described in (Gaidhu et al., 2011).
2.5 Statistics
The values reported are presented as mean ± standard deviation (S.D.). Each data point, in each condition, represents the mean of data collected from 6 mice for which the correct placement of the cannula was confirmed. Statistical significance of the results pooled from two groups was assessed with t-tests. One way analysis of variance (ANOVA) test (P<0.05) was used for comparison of multiple groups. The CBT and motor activity responses were compared using repeated measure ANOVA followed by Newman-Keuls post-test (P<0.05). Comparisons of cumulative distributions of food intake were done with Kolmogorov-Smirnov test (P< 0.05). Heat production and RER were quantified as area under the curve for the time interval 1–7 h after injection.
2.6 Tissue harvesting
The mice were killed by decapitation under sedation while under general anesthesia with isoflorane (5%). The interscapular brown adipose tissue, retroperitoneal white adipose tissue, quadriceps skeletal muscle and the brain were collected. The tissues were kept at −80 °C until the time of RNA extraction. Preoptic tissue was punched out from acute hypothalamic slices prepared as described previously (Lundius et al., 2010). The slices were prepared from control diet and obese mice that were not cannulated (n=6 each).
2.7 RT/QPCR
Total RNA was extracted using Qiagen RNAeasy kit manufacturer’s instructions. RNA extraction was conducted in dry ice. The concentration of RNA was determined using a nanodrop measuring the absorbance at 260 nm with reference to 280 nm. RNA was then stored at −80 °C. 4 μg RNA was then reverse-transcribed into first-complimentary DNA (cDNA) using Superscript III reverse transcriptase kit (Invitrogen, CA) according to manufacturer’s instructions. The reaction was inactivated by incubating it at 75°C for 10 minutes. In order to remove RNA complimentary 1 μl RNAse H was added to the reaction and was incubated at 37°C for 20 minutes. The concentration of prepared cDNA was measured using nanodrop measuring absorbance at 230 nm with reference to 280 nm. Quantitative PCR reactions were performed in duplicate using Roche light-cycler and the following parameters were used for 45 cycles (95°C for 10 minutes, 95°C for 5 seconds, 60°C for 5 seconds, 72°C for 8 seconds for 45 cycles; for melting: 95°C for 5 seconds, 65°C for 5 seconds; for cooling 40°C for 30 seconds). Each 20 μl of PCR reaction contained: 4 μl Sybr green (Roche, enzyme mixed according to manufacturer’s instructions), 9 μl PCR graded water, 2 μl 1 μM gene of interest primer and 5 μl diluted cDNA (1/30 dilution). Each reaction included a standard curve. To determine a standard curve, 1 μl of the non- diluted cDNA for all samples were mixed and a dilution of 1:100, 1:50 and 1: 25 was made. The standard curve is used for determining the efficiency of the primer and calculating the concentration of the mRNA amplified in the QPCR reaction. The concentration of each gene was normalized using the housekeeping gene GAPDH. The primers used are listed in Table 1.
Table 1.
Primers utilized for the QPCR reactions
| Primer | Forward sequence | Reverse sequence | Amplicon Size |
|---|---|---|---|
| UCP 1 | 5′-AGCCGGCTTAATGACTGGAG-3′ | 5′-TCTGTAGGCTGCCCAATGAAC-3′ | 211 bp |
| UCP 2 | 5′-CTACAAGACCATTGCACGAGAGG-3′ | 5′-AGCTGCTCAATAGGTGACAAACAT-3′ | 196 bp |
| UCP 3 | 5′-CAACCTTGGCTAGACGCACAG-3′ | 5′-TGGAGGTCCGAGGAGAGAGC-3′ | 141 bp |
| H1 | 5′-GGGAAAGGGAAACAGTCACA-3′ | 5′-ACTGTCGATCCACCAAGGTC-3′ | 195 bp |
| H2 | 5′-CAGCTTCCATCCTCAACCTC-3′ | 5′-GACCTGCACTTTGCACTTGA-3′ | 224 bp |
| H3 | 5′-CTTCAGCGTCCTTGGAGAAG-3′ | 5′-AGTGGCACAGTGGGTAGAGG-3′ | 264 bp |
| GAPDH | 5′-CTTCACCACCATGGAGAAGGC-3′ | 5′-GGCATGGACTGTGGTCATGAG-3′ | 131 |
3. Results
3.1 Effects of activation of histamine receptors in the MnPO or MPON on core body temperature, motor activity, RER and heat production
To increase the concentration of endogenous histamine we have injected locally the histamine N-methyl transferase inhibitor SKF91488. In different sets of mice we have also studied the effects of specific H1-3 agonists, with the doses chosen according to those found to be effective in our previous studies (Lundius et al., 2010, Tabarean et al., 2012).
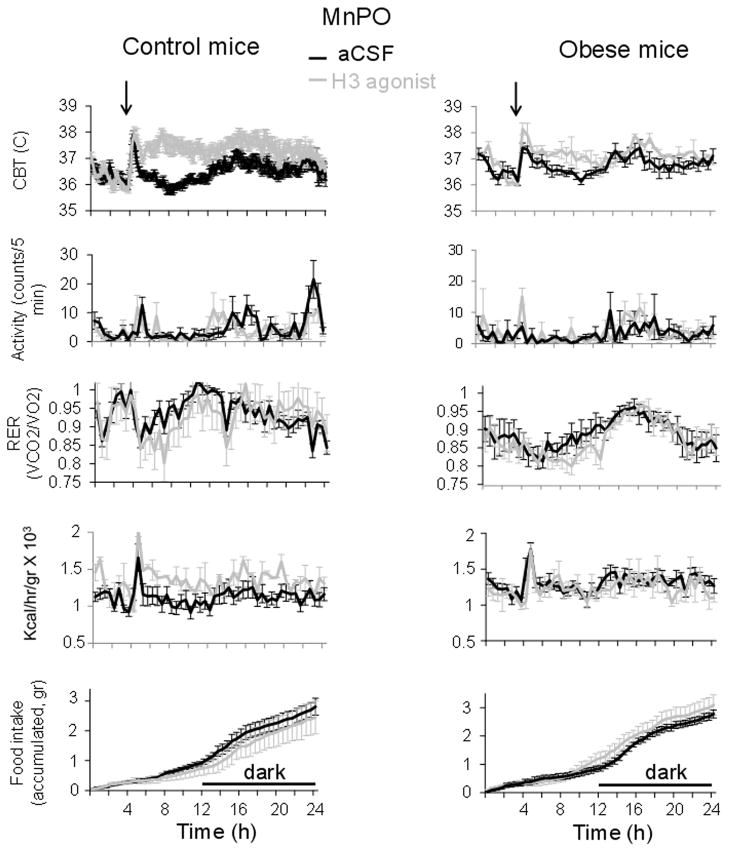
Injections were performed at 4 h into the light part of the day when animals are normally not active and were followed by a stress induced hyperthermia that lasted ~1h that was similar in animals treated with aCSF. However, as CBT returned to basal level in animals receiving vehicle, it remained on average ~ 1.6 °C higher in mice injected with SKF91488 (100 μM, 0.2 μl) intra-MnPO for about 6 h (Fig. 2) (n = 6 mice; p < 0.01). The difference in CBT between the two groups disappeared at onset of darkness when animals became active and aCSF injected mice displayed an increase in their CBT, and remained similar for the rest of the recording until the end of the dark cycle. Surprisingly, the drug also decreased the level of motor activity at the beginning of the dark phase (Fig. 2) (n = 6; p < 0.05).
Fig. 2. Effects of intra-MnPO injection of SKF91488 on CBT and energy homeostasis in control and diet-induced obese mice.
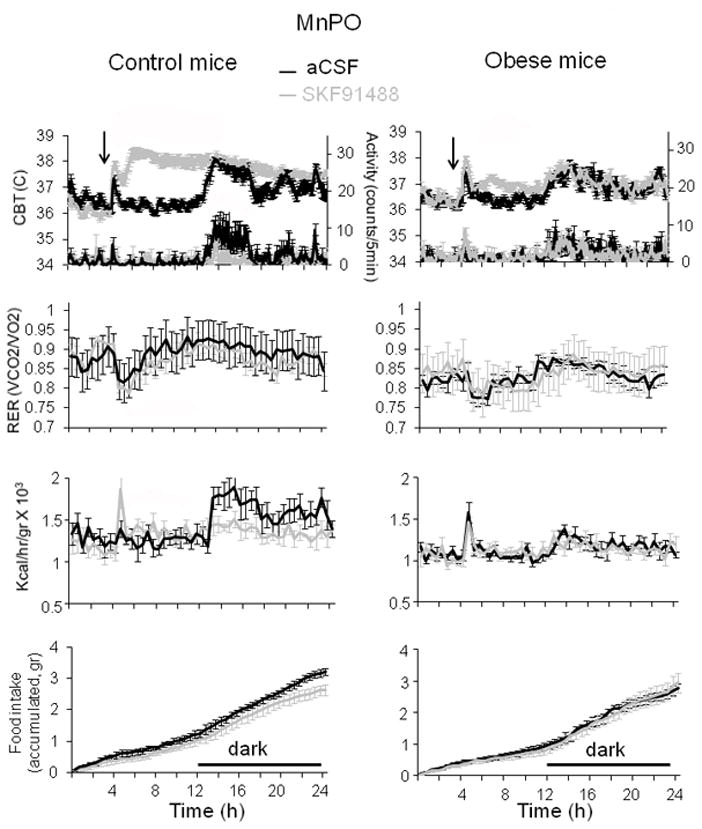
24 h profiles of CBT, motor activity (first row panels), RER (second row), heat production (third panel) and food intake (lower panel) recorded from control (left column) and obese mice (right column) injected with SKF91488 (100 μM, 0.2 μL) or aCSF in the MnPO. Injection was performed at time 4 h into the light part of the day (n = 6 mice for each treatment).
Hyperthermic responses were observed also with intra-MnPO injections of the H1 agonist betahistine (800 μM, 0.2 μl) (Fig 3) and the H3 agonist R-α-methylhistamine (10 μM, 0.2 μl) (Fig 4). Thus the agonists induced an average hyperthermia of 1.4 °C and 1.3 °C, respectively (n = 6; p < 0.01) during the light phase. The difference in CBT, relative to control mice, decreased significantly for both agonists at onset of darkness, however the hyperthermia was statistically significant only for mice injected with R-α-methylhistamine (Fig 4, n = 6 mice; p < 0.05). The drugs had no effect on MA during the light phase. Betahistine decreased the level of motor activity at the beginning of the dark phase (Fig 3) (n = 6; p < 0.05), as observed with SKF91488, while the H3 agonist had no such effect (Fig 4).
Fig. 3. Effects of intra-MnPO injection of betahistine on CBT and energy homeostasis in control and diet-induced obese mice.
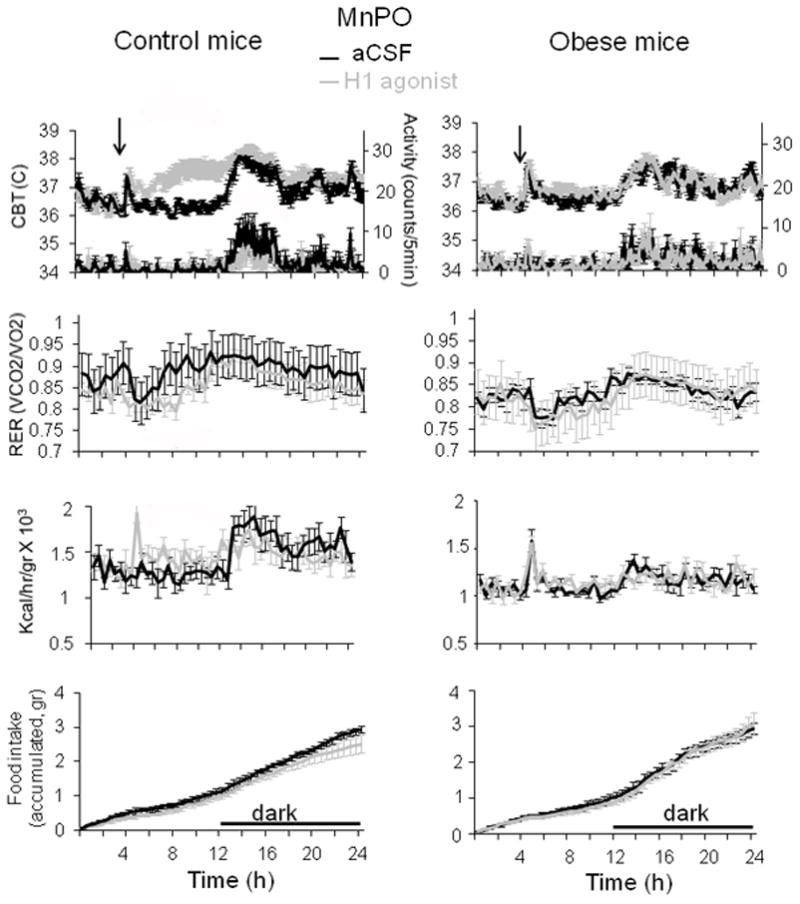
24 h profiles of CBT (upper panels), motor activity (second row), RER (third row), heat production (fourth row) and food intake (lower panels) recorded from control (left column) and obese mice (right column). The H1 agonist betahistine (800 μM, 0.2 μL) or aCSF were injected in the MnPO. Injection was performed at time 4 h into the light part of the day (n = 6 mice for each treatment).
Fig. 4. Effects of intra-MnPO injection of R-α-methylhistamine on CBT and energy homeostasis in control and diet-induced obese mice.
24 h profiles of CBT, motor activity (first row panels), RER (second row), heat production (third panel) and food intake (lower panel) recorded from control (left column) and obese mice (right column). The H3 agonist R-α-methylhistamine (20 μM, 0.2 μL) or aCSF were injected in the MnPO. Injection was performed at time 4 h into the light part of the day (n = 6 mice for each treatment).
All 3 drugs resulted in a decrease in the respiratory exchange ratio (RER) that lasted at least 6 hours post-injection. The average RER, quantified as area under the curve, was significantly smaller following injection of the drugs when compared to the control (n = 6; p < 0.05), for all three treatments. Similarly, the hyperthermic responses activated by the three drugs were associated with increased heat production (Figs 2–4) that lasted at least 6 hours. The average heat production for 6h post-injection, quantified as area under the curve, was significantly larger following injection of the drugs when compared to aCSF treatments (n = 6; p < 0.05).
We have then carried out identical experiments in diet-induced obese age-matched mice. 24 hours prior to experiments the mice were switched from high fat diet to normal diet to exclude a direct effect of the diet on the metabolic parameters. Obese mice displayed lower baseline RER, heat production and motor activity when compared with control littermates (n = 6; p < 0.05) (Figs 2–4). The hyperthermic effect was much reduced in obese mice averaging ~ 0.4, 0.2 and 0.5 °C for SKF91488 (100 μM, 0.2 μl), betahistine (800 μM, 0.2 μl) and R-α-methylhistamine (10 μM, 0.2 μl), respectively (Figs 2–4). Accordingly, the increase in heat production induced by the three treatments was abolished (Figs 2–4). In response to the three drugs the RER displayed a decreasing trend, however the difference was statistically significant when compared to the control only for the H3 agonist treatment for the interval 1–7 h following injection (Fig 4, n = 6; p<0.05).
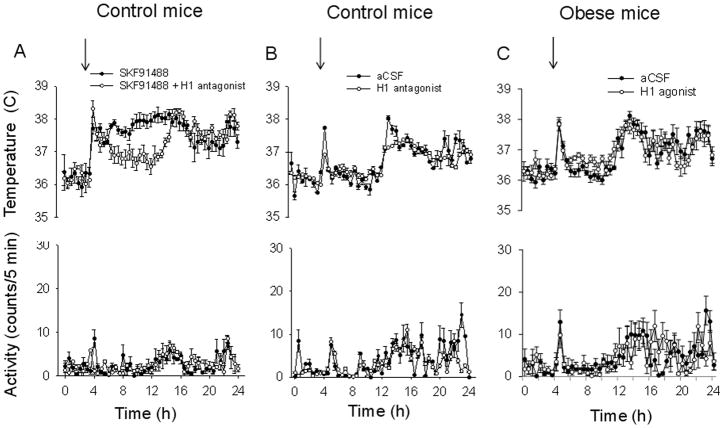
To further address the pharmacology of the responses described above we have studied the effect of the H1 antagonist trans triprolidine in a different set of control mice. The drug had little effect on CBT when administered alone intra-MnPO (30 nM, 0.2 μl) but significantly reduced the hyperthermic response to SKF91488 (100 μM, 0.2 μl) when the drugs were administered together (Fig 5A, B). The hyperthermia averaged 1.6 °C in control and 0.5 °C in the presence of trans triprolidine. We have also studied the effect of a second H1 agonist, 2-pyridylethylamine, in a set of obese mice. The agonist (300 μM, 0.2 μl) activated a hyperthermia that averaged 0.3 °C in obese mice (Fig 5C), a much smaller value than the 1.4 °C response observed in control mice (Lundius et al, 2010).
Fig. 5. Involvement of H1 receptors in the MnPO in the histamine responses of control and obese mice.
A. 24 h profiles of CBT (upper row) and motor activity (lower row) recorded from control mice. SKF91488 (100 μM, 0.2 μL) or SKF91488 (100 μM, 0.2 μL) + the H1 antagonist trans triprolidine (30 nM, 0.2 μL) were injected in the MnPO. Injection was performed at time 4 h into the light part of the day (n = 6 for each treatment).
B. 24 h profiles of CBT (upper row) and motor activity (lower row) recorded from control mice. The H1 antagonist trans triprolidine (30 nM, 0.2 μL) or aCSF were injected in the MnPO. Injection was performed at time 4 h into the light part of the day (n = 6 mice for each treatment).
C. 24 h profiles of CBT (upper row) and motor activity (lower row) recorded from obese mice. The H1 agonist 2-pyridylethylamine (300 μM, 0.2 μL) or aCSF were injected in the MnPO. Injection was performed at time 4 h into the light part of the day (n = 6 for each treatment).
Several studies suggest that the MPON plays an important and distinct role in thermoregulation (Nakamura et al., 2009, Yoshida et al., 2009, Tanaka et al., 2011). Recently we have shown that in this nucleus histamine modulates CBT by acting at H1 and H2 receptors, the latter being the dominant mechanism (Tabarean et al., 2012). We have therefore performed a similar set of experiments in the MPON. Bilateral injections of SKF91488 (100 μM, 0.2 μl) or H2 agonist dimaprit (10 μM, 0.2 μl) resulted in hyperthermia, when compared with aCSF injection that averaged ~ 1.3 °C (Fig 5) (n = 6; p < 0.05). As for the MnPO injections the difference in CBT relative to the control was much reduced once the dark phase started. The drugs did not change the level of motor activity (Fig 5) (n = 6; p > 0.1). The average heat production was significantly larger following injection of either drug when compared to aCSF treatment, but the difference was not statistically significant (n = 6; p > 0.05). The average RER was significantly smaller following injection of SKF91488 (100 μM, 0.2 μl) when compared to the control (n = 6; p < 0.05). In contrast, dimaprit had no effect on RER (n = 6; p > 0.1). To further test the role of H2 receptors in the responses described above we have studied the effect of the H2 antagonist tiotidine in a different set of control mice. The drug had little effect on CBT when administered alone intra-MPON (30 nM, 0.2 μl) but significantly reduced the hyperthermia (0.5 °C versus 1.3 °C) when administered together with SKF91488 (100 μM, 0.2 μl) (data not shown).
In obese littermates bilateral intra-MPON injection of SKF91488 (100 μM, 0.2 μl) or H2 agonist dimaprit (10 μM, 0.2 μl) resulted in much smaller hyperthermia. The increase in body temperature lasted ~ 6h and averaged ~0.4 °C for both drugs. In obese mice both drugs had no effect on motor activity, heat production or RER (Fig 5, n=6 p>0.1 for each).
3.2 Effects of activation of histamine receptors in the MnPO and MPON on feeding behavior
Previous studies have reported that i.c.v. injection of histamine decreases feeding (Ookuma et al., 1989, Ookuma et al., 1993). Since the preoptic area is not commonly considered as a region involved in the control of food intake we were surprised to observe that increasing the level of endogenous histamine with SKF91488 as well as injection of histaminergic agonists resulted in a significant decrease in food intake. Thus, both SKF91488 and betahistine injection in the MnPO resulted in a significant decrease in the cumulative food intake (Figs 3 and 4, K-S test, p<0.05). The H3 agonist R-α-methylhistamine also displayed a trend to decrease food intake but the distributions were not statistically different (K-S test, P>0.05) (Fig. 4). Similarly intra-MPON injections of SKF91488 resulted in a significant decrease in the cumulative food intake (Fig. 6, K-S test, p<0.05). In contrast, dimaprit was without effect on food intake (Fig. 6, K-S test, p>0.05). In obese mice the effects of histamine receptor activation on food intake were abolished (Figs. 2–4, 6). Surprisingly, H3 agonism in the MnPO and SKF91488 injection in the MPON displayed a tendency to increase food intake in obese mice. The effect of SKF91488 on food intake was specific for the MnPO and MPON, since injections in the lateral MPA (approximately 0.5 mm lateral to the MPON, bilaterally) didn’t increase food intake (data not shown).
Fig. 6. Effects of intra-MPON injection of SKF91488 or dimaprit on CBT and energy homeostasis in control and diet-induced obese mice.
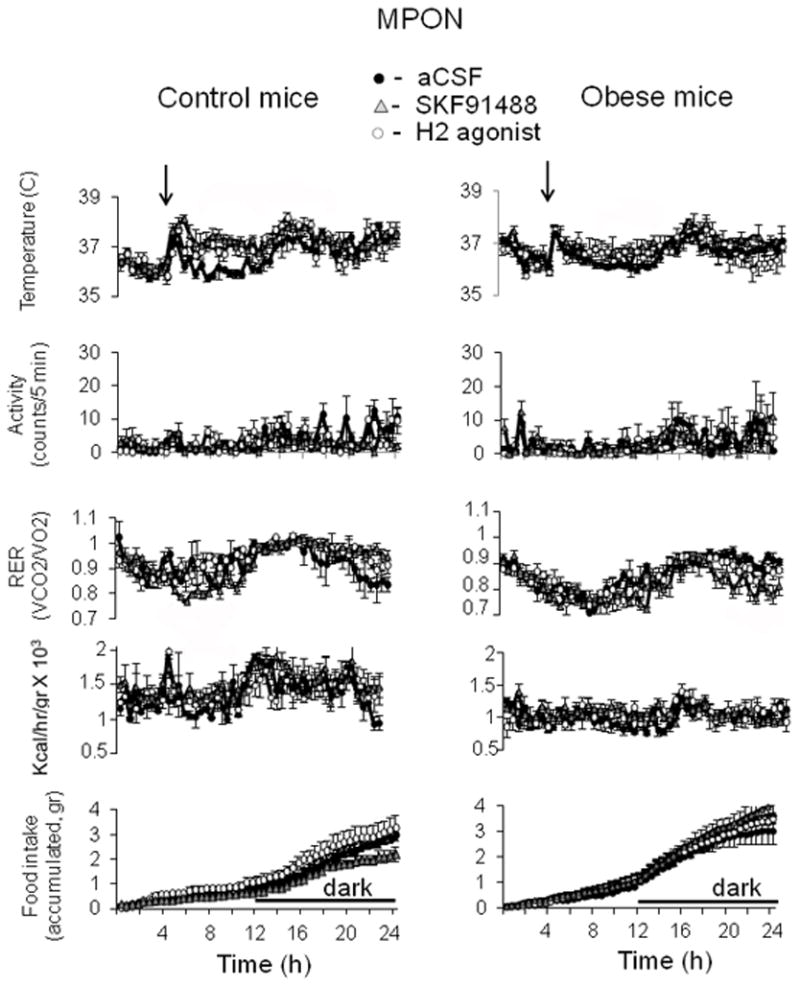
24 h profiles of CBT (upper panels), motor activity (second row), RER (third row), heat production (fourth row) and food intake (lower panels) recorded from control (left column) and obese mice (right column). SKF91488 (100 μM, 0.2 μL), the H2 agonist dimaprit (10 μM, 0.2 μL) or aCSF were injected bilaterally in the MPON. Injection was performed at time 4 h into the light part of the day (n = 6 for each treatment).
3.3 Effects of SKF91488 and histamine receptor agonists on the expression of uncoupling proteins UCP1-3 in thermogenic tissues
To quantify the expression of UCP1, 2 and 3 in thermogenic tissues in response to histamine agonism in the MnPO or MPON of control and obese mice we have repeated the treatments (Figs.2–4, 6) in the same groups of mice after allowing for 6 days of recovery. Obese mice were returned to high fat diet at the end of the first experiment. The CBT and motor activity were monitored with telemetry to verify that the responses were similar to those observed during the first experiment. At 4 h after injection the animals were killed and tissues were harvested as described in Methods. RNA was then extracted from BAT, WAT and skeletal muscle and reverse-transcribed.
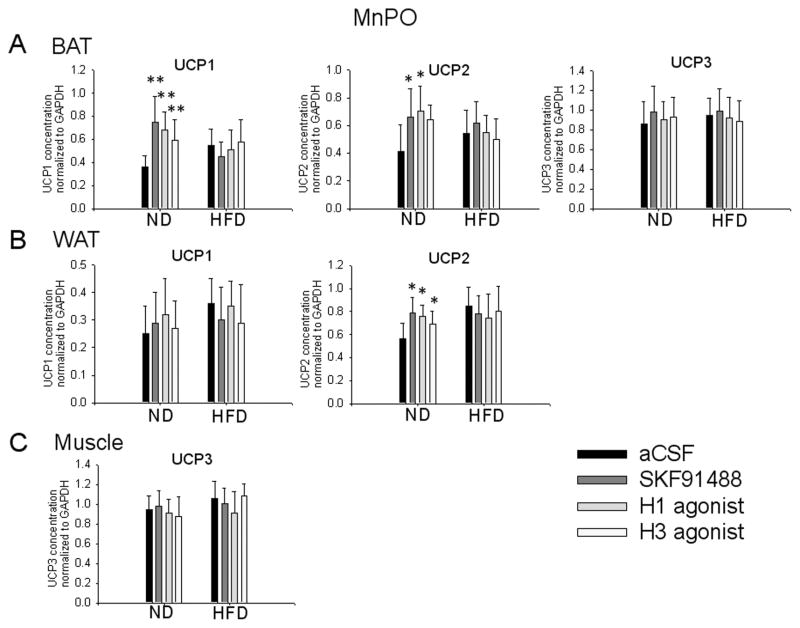
UCP1, 2, 3 and GADPH expression was measured using qPCR (Figs. 7, 8). When normalized with respect to GAPDH gene expression, the BAT levels of UCP1 were found to be significantly increased 4 h after intra-MnPO injection of SKF91488 (100 μM, 0.2 μl), or H1 agonist betahistine (800 μM, 0.2 μl) or the H3 agonist R-α-methylhistamine (10 μM, 0.2 μl) (Fig. 7A, n=6, ANOVA p<0.01 relative to aCSF). The same treatments resulted also in a significant increase in the expression of BAT UCP2 levels (Fig. 7A, n=6, ANOVA, p<0.05 relative to aCSF) and had no influence on the levels of BAT UCP3 expression (Fig. 7A, n=6, ANOVA, p>0.3 relative to aCSF). The three treatments also increased the WAT UCP2 levels (Fig. 7B, n=6, ANOVA, p<0.05 relative to aCSF). All three drugs displayed a trend to increase WAT UCP1 levels albeit it was not statistically significant (Fig. 7B, n=6, ANOVA, p>0.1 relative to aCSF). Skeletal muscle UCP3 levels were not changed (Fig. 7C, n=6, ANOVA, p>0.3 relative to aCSF).
Fig. 7. Effects of intra-MnPO injection of histaminergic agonists on the concentrations of mRNA encoding UCP1-3 in thermogenic tissues of control and diet-induced obese mice.
A–C. Bar graphs illustrating the concentrations of UCP1-3 in the BAT (A) UCP1 and 2 in the WAT (B) and UCP3 in the muscle (C) of mice injected intra-MnPO with aCSF or one of the following histaminergic agonists: SKF91488 (100 μM, 0.2 μL), R-α-methylhistamine (20 μM, 0.2 μL) or betahistine (500 μM, 0.2 μL). Values were normalized to those of GAPDH in the samples. Values represent the means ± S.D. (n = 6 for each treatment, *p < 0.05, **p < 0.01).
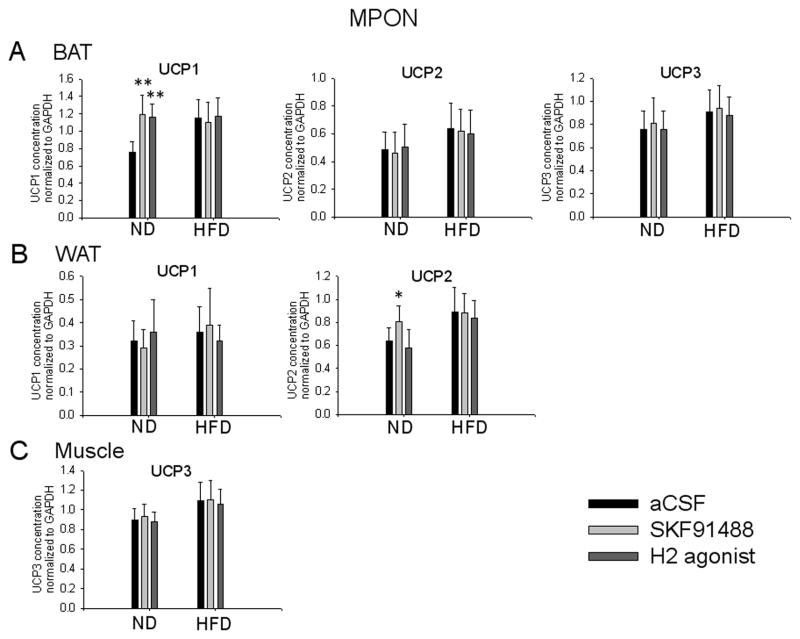
Fig. 8. Effects of intra-MPON injection of histaminergic agonists on the concentrations of mRNA encoding UCP1-3 in thermogenic tissues of control and diet-induced obese mice.
A–C. Bar graphs illustrating the concentrations of mRNA encoding UCP1-3 in the BAT (A) UCP1 and 2 in the WAT (B) and UCP3 in the muscle (C) of mice injected bilaterally intra-MPON with aCSF or one of the following histaminergic agonists: SKF91488 (100 μM, 0.2 μL) or dimaprit (10 μM, 0.2 μL). Values were normalized to those of GAPDH in the samples. Values represent the means ± S.D. (n = 6 for each treatment, *p < 0.05, **p < 0.01).
The concentrations of UCP1 and 2 in the BAT and WAT of obese mice were higher than those of control littermates (Fig. 7A, B, unpaired t-test, p<0.05). In contrast to the respective responses of control mice, intra-MnPO injections of SKF91488 (100 μM, 0.2 μl), or betahistine (800 μM, 0.2 μl) or R-α-methylhistamine (10 μM, 0.2 μl) were without effect on UCP1-3 expression in obese mice (Fig. 7A–C, n=6, ANOVA p>0.1 relative to aCSF).
We have also characterized the changes of UCP1-3 expression in response to bilateral intra-MPON injections of SKF91488 (100 μM, 0.2 μl) or H2 agonist dimaprit (10 μM, 0.2 μl). Both drugs increased the expression of BAT UCP1 (Fig. 8A, n=6, ANOVA p<0.01 relative to aCSF). SKF91488 also increased the concentration of UCP2 transcripts in WAT while dimaprit was without effect (Fig. 8B, n=6, ANOVA, p<0.05 and p>0.1 respectively, relative to aCSF). The two drugs had no other effect on UCP1-3 expression in any of the tissues studied (Fig. 8A–C, n=6, ANOVA p>0.1 relative to aCSF). The treatments had no effect on UCP1-3 expression in the BAT, WAT and skeletal muscle of obese mice (Fig 8A–C, n=6, ANOVA p>0.1 relative to aCSF)
3.4 Decreased concentrations of mRNA encoding the H1 receptor in the preoptic area of diet-induced obese mice
The responses to histamine receptor agonists were dramatically diminished in diet-induced obese mice therefore we have studied the expression of H1-3 receptors in the preoptic area. Acute brain slices were prepared from control and obese littermates (n=6 for each) that have not been cannulated. A region containing the MnPO and MnPO was punched out. Following RNA extraction and reverse H1-3 receptor and GAPDH expression were measured using qPCR. When normalized with respect to GAPDH transcript concentration, the concentration of H1 receptor transcripts was reduced by 42% in the obese mouse samples (t-test, P<0.01). In contrast, the levels for H2 and H3 receptor transcripts were not different (t-test, P>0.2).
4. Discussion
In this study we have established that increasing endogenous histamine concentrations in the MnPO and MPON using the histamine N-methyl transferase inhibitor SKF91488 results in a robust hyperthermia during the light phase of the circadian cycle as we have previously demonstrated using injections of exogenous histamine or H1-3 receptor subtype specific agonists (Lundius et al., 2010, Sethi et al., 2011, Tabarean et al., 2012). We have also confirmed that the hyperthermic effect was not associated with increased motor activity. Once the dark phase started and the mice became active the difference in CBT relative to the control (mice injected with aCSF) was much reduced or disappeared completely. Thus, SKF91488 or H1 agonist injections in the MnPO resulted in CBT slightly higher than the control during the dark phase, albeit the difference was not statistically significant (P>0.05). These treatments resulted, surprisingly, in reduced motor activity during the same time period, effect that may account for a lack of hyperthermia during the dark phase (relative to control mice) (Figs 1 and 2). In contrast, H3 agonist injection in the MnPO as well as SKF91488 or H2 agonist injections in MPON did not affect motor activity but induced a significant increase in CBT relative to the control, albeit of smaller amplitude than that observed during the light phase (Figs 4 and 6). The hyperthermic responses were associated with increased heat production during the light phase for all treatments. During the dark phase SKF91488 or H1 agonist injections in the MnPO resulted, surprisingly, in decreased heat production, probably due to lower motor activity. Since these mice displayed CBT at least as high as the controls these results suggest that the two drugs also activated heat conservation mechanisms.
Our observation of decreased motor activity during the active phase in response to H1 activation in the MnPO is similar to that reported for injections of exogenous histamine in the preoptic area of rats: an inhibitory influence on motor behaviors (Alvarez and Banzan, 1992). In contrast, histamine injected in the ventrolateral preoptic area was found to increase motor activity (Liu et al., 2010). These results indicate that our data were not contaminated by “spread”, since the ventrolateral preoptic area is in the vicinity of the sites targeted in this study (MnPO and MPON).
Measurement of respiratory exchange ratio (RER) demonstrated that both MnPO and MPON injections of SKF91488 resulted in a small but significant decrease in the average RER compared with vehicle treated mice, indicating a switch from glucose metabolism to increased fatty acid oxidation. While injections of H1 and H3 agonists in the MnPO had similar effect on RER with that of SKF91488, the H2 agonist applied intra-MPON did not modify RER suggesting that other histamine receptors may also play a role in this nucleus. Since the only other histamine receptor found in the MPON was the H1 receptor (Tabarean et al., 2012) it is likely that this receptor is responsible for the decrease in RER. These findings also indicate that increased thermogenesis is not always associated with a decrease in RER.
Quantification of the concentration of uncoupling proteins UCP1-3 in BAT, WAT and skeletal muscle revealed that histamine agonism in the MnPO resulted, within 4 hours, in a robust upregulation of UCP1 expression in BAT as well as of UCP2 in WAT. There were also trends for increased UCP2 expression in BAT and UCP1 expression in WAT. Histamine agonism in the MPON resulted in similar upregulation of UCP1 transcripts in BAT. SKF91488 but not dimaprit resulted in increased UCP2 expression in WAT. These results further suggest that decreased RER is associated with increased expression of UCP2, rather than with increased thermogenesis. In contrast to a previous report of upregulated UCP3 expression in response to central histamine injection (Masaki et al., 2001a) we have not found such changes in this study. It is possible that such changes occur over longer treatments (days instead of hours) or that different hypothalamic sites control the respective changes in UCP3 expression.
Preoptic thermoregulatory neurons control the expression of UCP1 in BAT by regulating the sympathetic nerve activity, a mechanism that is well documented (reviewed in Morrison, 2011). It has been shown that histamine applied intracerebroventricular or intra-PO activates both the sympathetic nerve system and UCP1 expression (Yasuda et al., 2004). Here we have demonstrated that either the MnPO or the MPON can drive thermogenesis, but differential mechanisms are involved: in the former nucleus H1 or H3 receptors are activated while H2 receptors play the main role in the latter.
The mechanism by which histamine influences the expression of UCP1 and 2 in WAT is less clear. The sympathetic nerve system also innervates WAT and plays a role in lipid mobilization (Bowers et al., 2004). Central histamine administration has been shown to activate sympathetic nerves innervating WAT and to induce lipolysis (Yoshimatsu et al., 2002, Shen et al., 2007, Shen et al., 2008), thus, it is likely that activation of the sympathetic nerve system by histamine also accounts for the changes observed in WAT. In this study we have identified the MnPO and MPON as sites where histamine signaling increases lipid utilization and the upregulation of UCP2 in WAT and BAT as a likely mechanism. Since UCP1 is a marker of brown adipocytes and precursor cells, its increased expression in WAT observed here suggests that such cells, interspersed in this tissue, are stimulated by preoptic histamine agonism. It is interesting to note that differential effects of the histamine receptor agonists on thermogenesis/UCP1 expression on one hand and RER/UCP2 expression on the other, suggest an anatomical dissociation of the neural pathways mediating the effects on BAT and WAT, respectively, at the level of the preoptic area.
4.1 Role of hypothalamic histamine in feeding behavior
Hypothalamic histamine has also been involved in suppressing feeding behavior, (Ookuma et al., 1989, Ookuma et al., 1993), with the H1 receptor being the main effector (Masaki et al., 2004). Histamine applied in the the ventromedial hypothalamus (VMH) or the paraventricular nucleus (PVN) decreased food intake (Ookuma et al., 1989, Ookuma et al., 1993). In this study we have found that histamine agonism in the MnPO and MPON also induced a small but significant decrease in food intake, effect likely due to activation of H1 receptors. It is interesting to note that also other studies have indicated a role of the preoptic area in controlling feeding behavior (Patterson et al., 2006, Santollo et al., 2011). The neural networks that mediate the modulation of feeding by the preoptic area remain to be determined.
4.2 Altered preoptic histamine signaling in obesity
The most striking finding in our study was that diet-induced obese littermates displayed much diminished responses to histamine agonism in both MnPO and MPON. Thus, the changes in CBT, motor activity, RER, heat production were much reduced in obese mice compared with control littermates. These changes were not due to decreased concentration of endogenous histamine, since responses to injections of histamine receptor agonists were similarly attenuated. Also we would like to note that diet-induced obese animals have the ability to generate equal or higher fever to endogenous pathogen (Pohl et al., 2009), therefore the decreased histaminergic responses observed here cannot be explained by impaired thermogenesis.
While the obese mice displayed increased levels of UCP1-3 transcripts in BAT, WAT and skeletal muscle as previously reported (Fromme and Klingenspor, 2011), the upregulation of UCP1 and UCP2 transcripts in BAT and WAT in response to activation of histamine receptors in MnPO or MPON was abolished. We have further revealed that the expression of H1 receptor transcripts in the preoptic area is much decreased in obese mice, mechanism that may account at least in part for the changes in the efficacy of histamine in the preoptic nuclei. However, the expression of H2 and H3 receptors was not changed in obese mice, suggesting that other mechanisms are involved in their diminished responsiveness to H2 and H3 specific agonists.
In conclusion our results reveal a robust decrease in histamine’s efficacy as an acute modulator of thermoregulation in diet-induced obese mice and raise the possibility of a causal relationship between this alteration and the development of an energy imbalance. On the other hand our data also suggest that increasing histaminergic agonism in obesity may have a limited effect on energy expenditure, however, studies utilizing chronic agonist treatments will be required to conclusively address this potential caveat.
Highlights.
Increased concentration of endogenous histamine in the median or medial preoptic nuclei results in hyperthermia
Diet-induced obese mice displayed much diminished responses to histamine agonism in the preoptic nuclei
The concentration of H1 receptor transcripts in the preoptic area of obese mice was lower than in control littermates
Acknowledgments
We would like to thank Viktor Zhukov for excellent technical support. This work was supported by National Institutes of Health Grant NS060799 (I.V.T.).
Abbreviations
- MnPO
median preoptic nucleus
- MPON
medial preoptic nucleus
- BAT
brown adipose tissue
- WAT
white adipose tissue
- CBT
core body temperature
- RER
respiratory exchange ratio
- UCP
uncoupling protein
- aCSF
artificial cerebrospinal fluid
Footnotes
Authors’ contributions: I.V.T. designed and supervised the study. J.S., M.S-A. and I.V.T. carried out experiments and analyzed data. I.V.T. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez EO, Banzan AM. The role of histamine in the anterior hypothalamus and its functional interaction with the hippocampus on exploratory behavior in adult male rats. Behav Brain Res. 1992;48:127–133. doi: 10.1016/s0166-4328(05)80148-2. [DOI] [PubMed] [Google Scholar]
- Andrews ZB. Uncoupling protein-2 and the potential link between metabolism and longevity. Curr Aging Sci. 2010;3:102–112. doi: 10.2174/1874609811003020102. [DOI] [PubMed] [Google Scholar]
- Banks ML, Buzard SK, Gehret CM, Monroy AN, Kenaston MA, Mills EM, Sprague JE. Pharmacodynamic characterization of insulin on MDMA-induced thermogenesis. Eur J Pharmacol. 2009;615:257–261. doi: 10.1016/j.ejphar.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Festuccia WT, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1167–1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- Colboc O, Protais P, Costentin J. Histamine-induced rise in core temperature of chloral-anaesthetized rats: mediation by H2-receptors located in the preopticus area of hypothalamus. Neuropharmacology. 1982;21:45–50. doi: 10.1016/0028-3908(82)90209-x. [DOI] [PubMed] [Google Scholar]
- Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1–8. doi: 10.1152/ajpregu.00411.2010. [DOI] [PubMed] [Google Scholar]
- Fulop AK, Foldes A, Buzas E, Hegyi K, Miklos IH, Romics L, Kleiber M, Nagy A, Falus A, Kovacs KJ. Hyperleptinemia, visceral adiposity, and decreased glucose tolerance in mice with a targeted disruption of the histidine decarboxylase gene. Endocrinology. 2003;144:4306–4314. doi: 10.1210/en.2003-0222. [DOI] [PubMed] [Google Scholar]
- Gaidhu MP, Frontini A, Hung S, Pistor K, Cinti S, Ceddia RB. Chronic AMP-kinase activation with AICAR reduces adiposity by remodeling adipocyte metabolism and increasing leptin sensitivity. J Lipid Res. 2011;52:1702–1711. doi: 10.1194/jlr.M015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MD, Cox B, Lomax P. Sites and mechanisms of action of histamine in the central thermoregulatory pathways of the rat. Neuropharmacology. 1976;15:321–324. doi: 10.1016/0028-3908(76)90136-2. [DOI] [PubMed] [Google Scholar]
- Hong ST, Bang S, Paik D, Kang J, Hwang S, Jeon K, Chun B, Hyun S, Lee Y, Kim J. Histamine and its receptors modulate temperature-preference behaviors in Drosophila. J Neurosci. 2006;26:7245–7256. doi: 10.1523/JNEUROSCI.5426-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itateyama E, Chiba S, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine in genetically obese animals: its implication of leptin action in the brain. Exp Biol Med (Maywood) 2003;228:1132–1137. doi: 10.1177/153537020322801006. [DOI] [PubMed] [Google Scholar]
- Leger JP, Mathieson WB. Development of bombesin-like and histamine-like innervation in the bullfrog (Rana catesbeiana) central nervous system. Brain Behav Evol. 1997;49:63–77. doi: 10.1159/000112982. [DOI] [PubMed] [Google Scholar]
- Liu YW, Li J, Ye JH. Histamine regulates activities of neurons in the ventrolateral preoptic nucleus. J Physiol. 2010;588:4103–4116. doi: 10.1113/jphysiol.2010.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci. 2010;30:4369–4381. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmlof K, Zaragoza F, Golozoubova V, Refsgaard HH, Cremers T, Raun K, Wulff BS, Johansen PB, Westerink B, Rimvall K. Influence of a selective histamine H3 receptor antagonist on hypothalamic neural activity, food intake and body weight. Int J Obes (Lond) 2005;29:1402–1412. doi: 10.1038/sj.ijo.0803036. [DOI] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Yasuda T, Noguchi H, Kakuma T, Watanabe T, Sakata T, Yoshimatsu H. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004;53:2250–2260. doi: 10.2337/diabetes.53.9.2250. [DOI] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H, Chiba S, Watanabe T, Sakata T. Central infusion of histamine reduces fat accumulation and upregulates UCP family in leptin-resistant obese mice. Diabetes. 2001a;50:376–384. doi: 10.2337/diabetes.50.2.376. [DOI] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H, Chiba S, Watanabe T, Sakata T. Targeted disruption of histamine H1-receptor attenuates regulatory effects of leptin on feeding, adiposity, and UCP family in mice. Diabetes. 2001b;50:385–391. doi: 10.2337/diabetes.50.2.385. [DOI] [PubMed] [Google Scholar]
- Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol. 2011;110:1137–1149. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161:614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau K, Fromme T, Meyer CW, von Praun C, Heldmaier G, Klingenspor M. Brown adipose tissue specific lack of uncoupling protein 3 is associated with impaired cold tolerance and reduced transcript levels of metabolic genes. J Comp Physiol B. 2008;178:269–277. doi: 10.1007/s00360-007-0219-7. [DOI] [PubMed] [Google Scholar]
- Ookuma K, Sakata T, Fukagawa K, Yoshimatsu H, Kurokawa M, Machidori H, Fujimoto K. Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res. 1993;628:235–242. doi: 10.1016/0006-8993(93)90960-u. [DOI] [PubMed] [Google Scholar]
- Ookuma K, Yoshimatsu H, Sakata T, Fujimoto K, Fukagawa F. Hypothalamic sites of neuronal histamine action on food intake by rats. Brain Res. 1989;490:268–275. doi: 10.1016/0006-8993(89)90244-8. [DOI] [PubMed] [Google Scholar]
- Patterson M, Murphy KG, Thompson EL, Smith KL, Meeran K, Ghatei MA, Bloom SR. Microinjection of galanin-like peptide into the medial preoptic area stimulates food intake in adult male rats. J Neuroendocrinol. 2006;18:742–747. doi: 10.1111/j.1365-2826.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin BJ. The mouse brain in stereotaxic coordinates. San Diego: Academic; 2001. [Google Scholar]
- Pohl J, Woodside B, Luheshi GN. Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology. 2009;150:4901–4910. doi: 10.1210/en.2009-0526. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1β. PNAS. 2006;103:2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Osborn O, Mitsukawa K, Schaefer J, Dubins J, Holmberg KH, Klein I, Klaus J, Gomez LF, Kolb H, Secrest J, Jochems J, Myashiro K, Buckley P, Hadcock JR, Eberwine J, Conti B, Bartfai T. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010;59:43–50. doi: 10.2337/db09-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Torregrossa AM, Eckel LA. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Horm Behav. 2011;60:86–93. doi: 10.1016/j.yhbeh.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P, Hardie DG, Roorda B, Clapham JC, Abuin A, Thomason-Hughes M, Green K, Frederik PM, Hesselink MK. Improved glucose homeostasis in mice overexpressing human UCP3: a role for AMP-kinase? Int J Obes Relat Metab Disord. 2004;28:824–828. doi: 10.1038/sj.ijo.0802629. [DOI] [PubMed] [Google Scholar]
- Sethi J, Sanchez-Alavez M, Tabarean IV. Kv4.2 mediates histamine modulation of preoptic neuron activity and body temperature. PLoS One. 2011;6:e29134. doi: 10.1371/journal.pone.0029134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett. 2007;416:193–197. doi: 10.1016/j.neulet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Shen J, Tanida M, Yao JF, Niijima A, Nagai K. Biphasic effects of orexin-A on autonomic nerve activity and lipolysis. Neurosci Lett. 2008;444:166–171. doi: 10.1016/j.neulet.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Sanchez-Alavez M, Sethi J. Mechanism of H2 histamine receptor dependent modulation of body temperature and neuronal activity in the medial preoptic nucleus Neuropharmacology. 2012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Suwa H, Ishikawa T, Kotani H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J Clin Invest. 2002;110:1791–1799. doi: 10.1172/JCI200215784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, McKinley MJ, McAllen RM. Preoptic-raphe connections for thermoregulatory vasomotor control. J Neurosci. 2011;31:5078–5088. doi: 10.1523/JNEUROSCI.6433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Masaki T, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine regulates sympathetic nerve activity and expression of uncoupling protein 1 mRNA in brown adipose tissue in rats. Neuroscience. 2004;125:535–540. doi: 10.1016/j.neuroscience.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu H, Tsuda K, Niijima A, Tatsukawa M, Chiba S, Sakata T. Histidine induces lipolysis through sympathetic nerve in white adipose tissue. Eur J Clin Invest. 2002;32:236–241. doi: 10.1046/j.1365-2362.2002.00972.x. [DOI] [PubMed] [Google Scholar]
- Yoshitomi H, Yamazaki K, Abe S, Tanaka I. Differential regulation of mouse uncoupling proteins among brown adipose tissue, white adipose tissue, and skeletal muscle in chronic beta 3 adrenergic receptor agonist treatment. Biochem Biophys Res Commun. 1998;253:85–91. doi: 10.1006/bbrc.1998.9746. [DOI] [PubMed] [Google Scholar]