Abstract
Incompatibility group IncA/C plasmids are large, low copy, theta-replicating plasmids that have been described in the literature for over 40 years. However, they have only recently been intensively studied on the genomic level because of their associations with the emergence of multidrug resistance in enteric pathogens of humans and animals. These plasmids are unique among other enterobacterial plasmids in many aspects, including their modular structure and gene content. While the IncA/C plasmid genome structure has now been well defined, many questions remain pertaining to their basic biological mechanisms of dissemination and regulation. Here, we discuss the history of IncA/C plasmids in light of our recent understanding of their population distribution, genomics, and effects on host bacteria.
Keywords: ICE, IncA/C, emergence, plasmid, regulation, resistance
Since the discovery of the first multidrug-resistant Shigella strains in 1955, plasmids encoding multidrug resistance have been extensively studied and appreciated for their diversity and plasticity.1 Among the large number of defined plasmid incompatibility (Inc.) groups is IncA/C, which have been described in the literature for over 40 years but until recently were not extensively studied. IncA/C plasmids were first identified among multidrug resistant Aeromonas hydrophila and Vibrio spp causing disease in cultured fish in the 1970s, where it was hypothesized that such plasmids emerged in response to the common practice of therapeutic antibiotic treatment in response to disease.2,3 Their appearance coincided with the use of antibiotics by cultured fish and soft-shelled turtle farms. This provided the first evidence that IncA/C plasmids might have emerged in response to antibiotic use in animal agriculture.
In the 1990s, the plasmid-encoded β-lactamase gene blaCMY-2 was identified among Klebsiella pneumoniae,4 and several reports in the early 2000s identified blaCMY-2 on a conjugative element in Escherichia coli and Salmonella enterica from food-producing animals.5-10 This was followed by the identification of blaCMY-2 in clinical S. enterica and K. pneumoniae of humans.11,12 The application of plasmid genome sequencing and replicon typing revealed that blaCMY-2 was often localized to IncA/C plasmids, and that these plasmids also encoded for resistance to numerous other antimicrobials.13-16 Studies by the US. Food and Drug Administration examining clinical isolates from 1940–2000 showed that IncA/C plasmids and their multidrug resistance phenotypes emerged in S. enterica serovar Newport isolates after 1980.17 Thus, the literature would suggest an emergence of this plasmid type in food animal and human enteric bacteria originating from an environmental bacterial reservoir, followed by the rapid acquisition of resistance gene modules associated with antibiotics used in animal agriculture and human therapy. Circulation of IncA/C plasmids in Gram-negative pathogens is now common, and these plasmids bring with them the ability to encode resistance to broad arrays of antimicrobial agents.
Precisely how IncA/C plasmids have emerged among enteric bacteria is a difficult question to address, but it is certainly not surprising. These plasmids apparently have an extremely broad host range,18 coupled with an ability to spread via conjugative transfer within bacterial communities. While the conjugal transfer of some IncA/C plasmid variants between and among E. coli and Salmonella in vitro is a subject of debate,19 there are documented cases of the short-term ability of these plasmids to disseminate in hospital settings. For example, a 2-year outbreak study in a Tunisian hospital involving multidrug-resistant Providencia stuartii identified IncA/C plasmid variants that had circulated among three bacterial clones over this period.20
Comparative IncA/C plasmid sequencing has revealed that they possess at least three hotspots for the integration of mobile genetic elements.13-16 IncA/C plasmids now have been sequenced from isolates of E. coli, S. enterica, Vibrio cholerae, Yersinia pestis, Photobacterium damselae subsp piscicida, Yersinia ruckeri, and Klebsiella pneumonia. Multiple lineages of IncA/C plasmids have subsequently been identified, with each containing unique arrays of mobile genetic elements within their hotspot regions. Since the entrance of IncA/C plasmids into human and animal enterobacterial populations, the hotspot regions of this plasmid have apparently rapidly evolved. A case in point is the recent finding that IncA/C plasmids acquiring the blaNDM-1 gene encoding the New Delhi metallo-β-lactamase have successfully disseminated among clinical K. pneumoniae and E. coli originating in India.21,22 Therefore, it seems likely that both horizontal gene transfer and clonal dissemination must play an important role in the success of IncA/C plasmids, although the abundance of these plasmids in the environment and their full range of bacterial hosts is not yet appreciated.
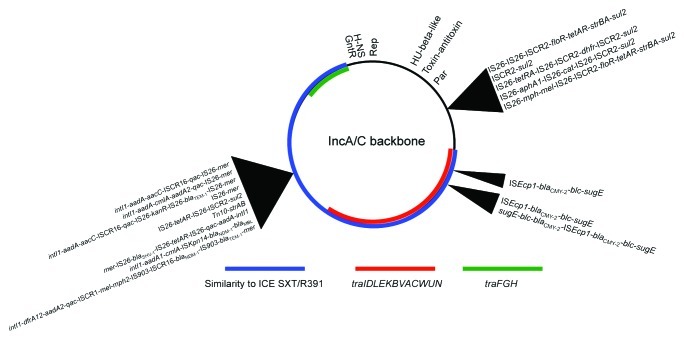
There are a number of interesting traits of IncA/C plasmids that make them unique among known enterobacterial plasmid types. First, their genome structure is remarkable. These plasmids have an iteron-containing theta replicon, at least three integrative hotspots capable of acquiring a variety of mobile genetic elements, a putative transfer region resembling that of an integrative conjugative element, a number of putative transcriptional regulators belonging to various classes, and an even larger number of hypothetical genes that appear to be derived from a variety of different sources (Fig. 1).13-16,23,24 Remarkably, the core backbone of the numerous IncA/C plasmid genomes described in the literature thus far shares 99% nucleotide sequence similarity, with substantial differences only occurring within the resistance-associated genes of their hotspot regions. With the growing number of unpublished genome sequences, however, this level of conservation is not always observed. For example, recent IncA/C plasmid sequences have been deposited from Aeromonas hydrophila (FJ705807), Xenorhabdus nematophila (FN667743), and Aeromonas salmonicida (CP000645), and these plasmids share only 72–94% nucleotide sequence similarity in their core backbones with the previously mentioned plasmids. It can be hypothesized, then, that the diversity of bacterial species harboring IncA/C plasmids is vastly underappreciated, as is the genetic diversity within this plasmid group. This previously unrecognized diversity also suggests that the aforementioned plasmids sharing high conservation in their backbone regions have a relatively recent ancestry, supporting the notion that IncA/C plasmids of enteric bacteria originate from a common ancestral plasmid type. These findings further support the rapid dissemination of these plasmids between enteric bacteria and a rapid evolution via recombinational events.
Figure 1. Backbone structure of IncA/C plasmids. Integration hotspots are depicted with black arrows, and inserted modules of sequenced IncA/C plasmids are summarized next to the arrows. The replicon (Rep), transcriptional regulators (HU-β-like, GntR, and H-NS), and plasmid stability regions (Toxin-antitoxin and Par) of the IncA/C backbone are depicted in the circular map. Regions with similarity to integrative conjugative element (ICE) SXT/R391 and putative transfer regions are colored according to the legend.
IncA/C plasmids have been shown to modulate changes to their bacterial host chromosomes. For example, IncA/C plasmids confer the mobilization in trans of chromosomal mobile elements, as demonstrated in Salmonella enterica using chromosomal island SGI1.25 They are also capable of integration into the bacterial chromosome.26 On the transcriptional level, we have demonstrated effects on the E. coli chromosome after the acquisition of an IncA/C plasmid, the most notable effect being the upregulation of the multiple antimicrobial resistance (mar) locus involved in low-level resistance to multiple antimicrobial agents.27 Thus, the ability of these plasmids to interact with the bacterial host using multiple regulatory mechanisms and to mobilize other genetic elements suggests that the broad host range of IncA/C plasmids may be enabled not only by its basic replicon but also through transcriptional regulation and extensive genetic recombination.
IncA/C plasmids possess a number of putative transcriptional regulators with sequence similarity (albeit low) to proteins of the H-NS, HU-β, Xre, LysR, GntR and LuxR families.16 The presence of such proteins on large, low copy plasmids is not unexpected. Other large plasmids, such as Inc.P, Inc.H and Inc.X, also encode H-NS-like proteins and other transcriptional regulators.26,28,29 Some of these proteins have been shown to exhibit silencing effects on the bacterial chromosome, suggesting that this might be a key activity in the successful dissemination of these plasmids to a broad range of bacterial hosts. However, the high number of putative regulatory genes that IncA/C plasmids possess suggests that strict regulation of both the plasmid and naïve host chromosome is critical for their broad-host dissemination. Interestingly, the closest database matches to these protein sequences are from non-enteric bacteria such as Vibrio spp, Aeromonas spp, etc., indicating that the most recent long-term hosts of these plasmids may indeed have been soil and water bacteria.16 It is therefore tempting to speculate that these plasmids are well suited to persist in certain subsets of reservoir bacteria, in which they provide a lower imposed fitness cost to their bacterial host and are better able to persist in a microbial community. It has been demonstrated that IncA/C plasmids in hosts such as E. coli and Salmonella might require selective pressure for their long-term persistence.30 However, if other reservoir bacteria are available as better hosts for IncA/C plasmids when resistance phenotypes are not required, a daunting scenario emerges where many suitable reservoirs for this plasmid might exist in any given ecological niche. This would afford IncA/C plasmids the opportunity to reside in a microbial community in the absence of benefit to an enteric host, persist, and subsequently emerge in pathogens whenever conditions require.
Many questions remain related to IncA/C plasmid biology. What is the nature of the IncA/C plasmid-bacteria relationship, and how does this relationship differ in enteric bacteria vs. the assumed long-term hosts of these plasmids? Notably, all studies examining IncA/C plasmid biology thus far have utilized a limited number of bacterial species and strains. It is evident, though, that additional hosts should be considered when studying its mechanisms of transfer and stability. Another lingering question is how frequently, and to what extent, does recombination occur on these plasmids? Is their dissemination due primarily to the emergence of highly successful clones or frequent horizontal gene transfer events? Given their apparently recent introduction into enteric bacteria of humans and animals, are we in the beginning stages of a long battle with these plasmids in efforts to circumvent the dissemination of antimicrobial resistance? Furthermore, what led to this scenario and does it represent an epidemic threat to the treatment of Gram-negative bacterial infection? In the world of mobile genetic elements, IncA/C plasmids are both fascinating and concerning and there is much to be learned from them.
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/19626
References
- 1.Watanabe T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki T, Egusa S, Kimura T, Watanabe T. Detection of R factors in naturally occurring Aeromonas salmonicida strains. Appl Microbiol. 1971;22:716–7. doi: 10.1128/am.22.4.716-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wantanabe T, Aoki T, Ogata Y, Egusa S. Anbtibiotics and drug resistance in animals. R factors related to fish culturing. Ann N Y Acad Sci. 1971;182:383–410. doi: 10.1111/j.1749-6632.1971.tb30674.x. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Stemplinger I, Jungwirth R, Giamarellou H. Characterization of the plasmidic beta-lactamase CMY-2, which is responsible for cephamycin resistance. Antimicrob Agents Chemother. 1996;40:221–4. doi: 10.1128/aac.40.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen KJ, Poppe C. Occurrence and characterization of resistance to extended-spectrum cephalosporins mediated by beta-lactamase CMY-2 in Salmonella isolated from food-producing animals in Canada. Can J Vet Res. 2002;66:137–44. [PMC free article] [PubMed] [Google Scholar]
- 6.Giles WP, Benson AK, Olson ME, Hutkins RW, Whichard JM, Winokur PL, et al. DNA sequence analysis of regions surrounding blaCMY-2 from multiple Salmonella plasmid backbones. Antimicrob Agents Chemother. 2004;48:2845–52. doi: 10.1128/AAC.48.8.2845-2852.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang MS, Besser TE, Call DR. Variability in the region downstream of the blaCMY-2 beta-lactamase gene in Escherichia coli and Salmonella enterica plasmids. Antimicrob Agents Chemother. 2006;50:1590–3. doi: 10.1128/AAC.50.4.1590-1593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diarrassouba F, Diarra MS, Bach S, Delaquis P, Pritchard J, Topp E, et al. Antibiotic resistance and virulence genes in commensal Escherichia coli and Salmonella isolates from commercial broiler chicken farms. J Food Prot. 2007;70:1316–27. doi: 10.4315/0362-028x-70.6.1316. [DOI] [PubMed] [Google Scholar]
- 9.Daniels JB, Call DR, Besser TE. Molecular epidemiology of blaCMY-2 plasmids carried by Salmonella enterica and Escherichia coli isolates from cattle in the Pacific Northwest. Appl Environ Microbiol. 2007;73:8005–11. doi: 10.1128/AEM.01325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YN, Peng J, Wang Q, Pei ZF, Zhang WJ, Niu ZX. Appearance of blaCMY-2)gene-positive Salmonella isolates of pig origin in China. Int J Antimicrob Agents. 2008;31:292–3. doi: 10.1016/j.ijantimicag.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi MB, Leon V, Canche C, Perez C, Zhao S, Hubert SK, et al. Rapid and widespread dissemination of multidrug-resistant blaCMY-2 Salmonella Typhimurium in Mexico. J Antimicrob Chemother. 2007;60:398–401. doi: 10.1093/jac/dkm168. [DOI] [PubMed] [Google Scholar]
- 12.Ding H, Yang Y, Lu Q, Wang Y, Chen Y, Deng L, et al. The prevalence of plasmid-mediated AmpC beta-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae from five children’s hospitals in China. Eur J Clin Microbiol Infect Dis. 2008;27:915–21. doi: 10.1007/s10096-008-0532-4. [DOI] [PubMed] [Google Scholar]
- 13.Welch TJ, Fricke WF, McDermott PF, White DG, Rosso ML, Rasko DA, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One. 2007;2:e309. doi: 10.1371/journal.pone.0000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fricke WF, Welch TJ, McDermott PF, Mammel MK, LeClerc JE, White DG, et al. Comparative genomics of the IncA/C multidrug resistance plasmid family. J Bacteriol. 2009;191:4750–7. doi: 10.1128/JB.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Call DR, Singer RS, Meng D, Broschat SL, Orfe LH, Anderson JM, et al. blaCMY-2-positive IncA/C plasmids from Escherichia coli and Salmonella enterica are a distinct component of a larger lineage of plasmids. Antimicrob Agents Chemother. 2010;54:590–6. doi: 10.1128/AAC.00055-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fern´ndez-Alarco´n C, Singer RS, Johnson TJ. Comparative genomics of multidrug resistance-encoding IncA/C plasmids from commensal and pathogenic Escherichia coli from multiple animal sources. PLoS One. 2011;6:e23415. doi: 10.1371/journal.pone.0023415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh AZ. S., Sabo, J.L., Abbott, J.W., Fields, P.I., Mcdermott, P.F. Plasmid replicon typing of historical Salmonella Newport: 1940-2000. Annual Meeting for the American Society for Microbiology. New Orleans, LA, 2011. [Google Scholar]
- 18.Suzuki H, Yano H, Brown CJ, Top EM. Predicting plasmid promiscuity based on genomic signature. J Bacteriol. 2010;192:6045–55. doi: 10.1128/JB.00277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole TL, Edrington TS, Brichta-Harhay DM, Carattoli A, Anderson RC, Nisbet DJ. Conjugative transferability of the A/C plasmids from Salmonella enterica isolates that possess or lack bla(CMY) in the A/C plasmid backbone. Foodborne Pathog Dis. 2009;6:1185–94. doi: 10.1089/fpd.2009.0316. [DOI] [PubMed] [Google Scholar]
- 20.Arpin C, Thabet L, Yassine H, Messadi A, Boukadida J, Dubois V, et al. Evolution of an Incompatibility Group IncA/C Plasmid Harboring blaCMY-16 and qnrA6 Genes and its transfer through three clones of Providencia stuartii during a 2-year Outbreak in a Tunisian Burn Unit. Antimicrob Agents Chemother. 2011 doi: 10.1128/AAC.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carattoli A, Villa L, Poirel L, Bonnin RA, Nordmann P. Evolution of IncA/C blaCMY-2 carrying plasmids by acquisition of the blaNDM-1 carbapenemase gene. Antimicrob Agents Chemother. 2011 doi: 10.1128/AAC.05116-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chihara S, Okuzumi K, Yamamoto Y, Oikawa S, Hishinuma A. First case of New Delhi metallo-beta-lactamase 1-producing Escherichia coli infection in Japan. Clin Infect Dis. 2011;52:153–4. doi: 10.1093/cid/ciq054. [DOI] [PubMed] [Google Scholar]
- 23.Llanes C, Gabant P, Couturier M, Michel-Briand Y. Cloning and characterization of the Inc A/C plasmid RA1 replicon. J Bacteriol. 1994;176:3403–7. doi: 10.1128/jb.176.11.3403-3407.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llanes C, Gabant P, Couturier M, Bayer L, Plesiat P. Molecular analysis of the replication elements of the broad-host-range RepA/C replicon. Plasmid. 1996;36:26–35. doi: 10.1006/plas.1996.0028. [DOI] [PubMed] [Google Scholar]
- 25.Douard G, Praud K, Cloeckaert A, Doublet B. The Salmonella genomic island 1 is specifically mobilized in trans by the IncA/C multidrug resistance plasmid family. PLoS One. 2010;5:e15302. doi: 10.1371/journal.pone.0015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun CS, Suzuki C, Naito K, Takeda T, Takahashi Y, Sai F, et al. Pmr, a histone-like protein H1 (H-NS) family protein encoded by the IncP-7 plasmid pCAR1, is a key global regulator that alters host function. J Bacteriol. 2010;192:4720–31. doi: 10.1128/JB.00591-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson TJ, Danzeisen J. L., Fernandez-Alarcon, C., and Lang, K.S. The mar locus of Escherichia coli is modulated through acquisition of a multidrug resistance-encoding IncA/C plasmid. Annual Meeting of the American Society for Microbiology. New Orleans, LA, 2011. [Google Scholar]
- 28.Doyle M, Fookes M, Ivens A, Mangan MW, Wain J, Dorman CJ. An H-NS-like stealth protein aids horizontal DNA transmission in bacteria. Science. 2007;315:251–2. doi: 10.1126/science.1137550. [DOI] [PubMed] [Google Scholar]
- 29.Dillon SC, Cameron AD, Hokamp K, Lucchini S, Hinton JC, Dorman CJ. Genome-wide analysis of the H-NS and Sfh regulatory networks in Salmonella Typhimurium identifies a plasmid-encoded transcription silencing mechanism. Mol Microbiol. 2010;76:1250–65. doi: 10.1111/j.1365-2958.2010.07173.x. [DOI] [PubMed] [Google Scholar]
- 30.Subbiah M, Top EM, Shah DH, Call DR. Selection pressure required for long-term persistence of blaCMY-2-positive IncA/C plasmids. Appl Environ Microbiol. 2011;77:4486–93. doi: 10.1128/AEM.02788-10. [DOI] [PMC free article] [PubMed] [Google Scholar]