Abstract
Background
Protein from plant, as opposed to animal, sources may be preferred in chronic kidney disease (CKD), due to lower bioavailability of phosphate and lower nonvolatile acid load.
Study Design
Observational cross-sectional study.
Setting & Participants
2938 participants with chronic kidney disease and information on dietary intake at the baseline visit in the Chronic Renal Insufficiency Cohort Study.
Predictors
Percentage of total protein from plant sources (% plant protein) was determined by scoring individual food items from the National Cancer Institute Diet History Questionnaire (DHQ).
Outcomes
Metabolic parameters, including serum phosphate, bicarbonate (HCO3), potassium, and albumin, plasma fibroblast growth factor 23 (FGF23), and parathyroid hormone (PTH), and hemoglobin.
Measurements
We modeled the association between % plant protein and metabolic parameters using linear regression. Models were adjusted for age, sex, race, diabetes, body mass index, eGFR, income, smoking, total energy intake, total protein intake, 24 hour urinary sodium, use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers and use of diuretics.
Results
Higher % plant protein was associated with lower FGF23 (p=0.05) and higher HCO3 (p=0.01), but not with serum phosphate or PTH (p=0.9 and 0.5, respectively). Higher % plant protein was not associated with higher serum potassium (p=0.2), lower serum albumin (p=0.2) or lower hemoglobin (p=0.3). The associations of % plant protein with FGF23 and HCO3 did not differ by diabetes status, sex, race, CKD stage (2/3 vs. 4/5) or total protein intake (≤ 0.8 g/kg/d vs. >0.8 g/kg/d) (p-interaction > 0.10 for each).
Limitations
Cross-sectional study; Determination of % plant protein using the DHQ has not been validated.
Conclusions
Consumption of a higher percentage of protein from plant sources may lower FGF23 and raise HCO3 in patients with CKD.
Keywords: chronic kidney disease, nutrition, mineral metabolism, acidosis
Introduction
High dietary protein intake may adversely affect metabolic parameters in patients with chronic kidney disease (CKD) due to high loads of phosphate and nonvolatile acid 1–3. Although reduced overall protein intake may slow CKD progression and improve metabolic parameters in patients with CKD, this may be difficult to achieve and sustain in practice 4–7. In addition, there is concern among nephrologists that low protein diets may place patients at risk for protein energy malnutrition, a strong risk factor for death in patients as they approach end stage renal disease (ESRD) 8–10.
Protein derived from plant sources, as compared with animal sources, may have less adverse impact on metabolic risk factors in CKD 11, 12. Phosphate from plant-based proteins is complexed in the form of phytic acid, which is less digestible in humans and thus, is less bioavailable, than animal-based proteins 11, 13. A recent, small feeding study of 9 patients with CKD demonstrated that consuming a vegetarian, as opposed to a meat-based diet, resulted in reduced levels of serum phosphate and fibroblast growth factor 23 (FGF23) 12. These findings require confirmation in a larger, more diverse patient population consuming a range of diets. Additionally, sulfate containing amino acids, which contribute directly to nonvolatile acid load, are more abundant in animal-based, as opposed to plant-based proteins 14. For this reason higher relative consumption of plant-based proteins may lower the nonvolatile acid load, and may improve serum bicarbonate levels, but this has not been previously studied.
In this study, we evaluated the association between the percentage of protein intake from plant sources and metabolic risk factors for adverse outcomes in CKD, such as serum phosphate, FGF23, parathyroid hormone (PTH), and serum bicarbonate in a large, diverse cohort of patients with CKD consuming their usual diets.
Methods
Study Population
The Chronic Renal Insufficiency Cohort (CRIC) study is a prospective cohort study which enrolled 3612 adult participants (ages 21 to 74 years) with mild to moderate CKD (eGFR 20 to 70 mL/min/1.73 m2) across seven clinical centers in the United States between 2003 and 2006. Exclusion criteria included institutionalization, inability to give informed consent, pregnancy, polycystic kidney disease, previous treatment with dialysis for greater than 1 month, and other severe medical conditions (New York Heart Association class III or IV heart failure, cirrhosis, HIV/AIDS, previous organ or bone marrow transplant, immunotherapy for renal disease or vasculitis within past 6 months, previous chemotherapy for systemic cancer in last 2 years, previous multiple myeloma or renal carcinoma), as previously described 15. To increase representation of Hispanics, an additional 327 participants were enrolled from a single center between January 2006 and October 2008 as part of an ancillary study (HCRIC). Of all CRIC and HCRIC participants, 2938 with a dietary assessment available at study baseline were included in this analysis. This study was purely observational in nature. Participants did not undergo any dietary modification or receive dietary advice from the study staff as a component of CRIC. The study protocol was approved by all participating centers and participants provided written informed consent.
Data Collection
Dietary assessment was performed at the first study visit using a previously validated food frequency questionnaire, the National Cancer Institute Diet History Questionnaire (DHQ). Dietary intakes were estimated from participant responses using Diet*Calc software 16. To determine the relative contribution of plant and animal protein sources to total protein intake, 255 foods from the DHQ were evaluated by two independent investigators (JJS, CAMA). The percentage of animal protein in a given food item was estimated by referring to common recipes or dominant national brands, in the case of pre-packaged foods. Disagreement between reviewers was resolved through consensus. The percentage of animal protein attributed to the food item was then multiplied by the total protein content of the food in grams to determine the total animal protein content in grams. The remaining protein was assigned as the total plant protein content. Estimated daily animal and plant protein intakes were determined for each participant and expressed as a percentage of total daily protein intake.
Laboratory parameters were measured at the first study visit in a central laboratory. Plasma PTH was measured using a total intact assay (Scantibodies, Santee, CA). Plasma FGF23 was measured using a second generation C-terminal assay (Immutopics, San Clemente, CA; intra-assay coefficient of variation <5%). Routine laboratories including serum creatinine, bicarbonate, potassium, phosphate, and albumin, hemoglobin and urinary sodium, potassium and phosphate were measured using standard clinical assays. Glomerular filtration rate was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation 17.
Sociodemographics, medical history, and body mass index were assessed at study baseline. Diabetes was defined as fasting glucose ≥ 126 mg/dL and/or the use of insulin or oral hypoglycemic medications. Active vitamin D sterols included calcitriol, doxercalciferol and paricalcitol. Phosphate binding medications included calcium as well as non-calcium based binders. Alkali supplements included compounds containing calcium citrate, sodium bicarbonate, sodium citrate, and potassium citrate.
Statistical Analysis
The distributions of the exposure variable (i.e. percentage of protein from plant sources) and all outcome variables (serum phosphate, plasma FGF23, 24 hour urine phosphate, PTH, serum bicarbonate, serum albumin, serum potassium and hemoglobin) were assessed for normality and outliers. FGF23 and PTH were log transformed to approximate a normal distribution. The percentage of protein from plant sources was categorized in quintiles. Characteristics of the study population were compared across quintiles of the percentage of protein from plant sources using ANOVA (continuous variables) or Chi-square test (categorical variables).
The association between the percentage of protein from plant sources and all outcome variables was modeled using linear regression with the percentage of protein from plant sources treated as both a continuous and categorical variable. Models were adjusted for potential confounders identified a priori including age, sex, race, diabetes, body mass index, eGFR, income, smoking, total energy intake, total protein intake, 24 hour urinary sodium, use of angiotensin converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARB) and use of diuretics. Pre-specified interactions were explored between the percentage of protein from plant sources and diabetic status, sex, race, CKD stage (2 and 3 vs. 4 and 5) and total dietary protein intake (≤ 0.8 g/kg/d vs. >0.8 g/kg/d) using stratified models and formally tested by putting interaction terms in the model. As a sensitivity analysis these associations were also tested among a subpopulation excluding participants who were using active vitamin D, phosphate binding agents or alkali supplements.
All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Overall, the study population consisted of 45% diabetics, the mean age was 58 years (range 21 to 75 years), and the median eGFR was 44 mL/min/1.73 m2 (interquartile range 34 to 55 mL/min/1.73 m2). Fifteen percent of participants had stage 2 CKD, 67% stage 3 CKD, 17% stage 4 CKD and 0.1% stage 5 CKD. Median total protein intake in the study population was 63.7 g/day (interquartile range 45.4 to 88.0 g/day) with median of 0.7 g/kg/day after scaling to body weight (interquartile range 0.5 to 1.0 g/kg/day). Median intake of protein from animal sources was 41.8 g/day (interquartile range 28.0 to 61.1 g/day) and from plant sources was 20.7 g/day (interquartile range 14.9 to 28.8 g/day), resulting in a median percentage of total protein from plant sources (percent plant protein) of 33% (interquartile range 26 to 41%).
Characteristics of the study population stratified by quintiles of percent plant protein are presented in Table 1. Quintiles of percent plant protein were associated with many demographic variables, but were not strongly associated with either comorbid diseases, such as diabetes (p=0.12), cardiovascular disease (p=0.75), or hypertension (p=0.19), or with severity of CKD based on eGFR (p=0.64). Percent plant protein intake was associated with greater use of phosphate binders and active vitamin D sterols, although use of these medications was low in this cohort with only 7.4% using phosphate binders and 3.4% using active vitamin D overall.
Table 1.
Characteristics of study population stratified by quintiles of percentage of total protein intake from plant sources (n=2938)
| Quintiles of Percent Plant Protein | P-value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Characteristic | <24% | 24–29% | 30–35% | 36–44% | >44% | |
| Mean ± SD or n (%) | (n=587) | (n=588) | (n=588) | (n=587) | (n=588) | |
| Demographics | ||||||
|
| ||||||
| Age (years) | 56.5 ± 11.3 | 57.8 ± 11.1 | 58.5 ± 11.3 | 59.5 ± 9.9 | 59.6 ± 10.9 | <0.001 |
| Female sex | 232 (39.5%) | 272 (46.3%) | 263 (44.7%) | 302 (51.4%) | 321 (54.6%) | <0.001 |
| Race/ethnicity | <0.001 | |||||
| Non-hispanic white | 279 (47.5%) | 313 (53.2%) | 309 (52.6%) | 293 (49.9%) | 254 (43.2%) | |
| Non-hispanic black | 257 (43.8%) | 248 (42.2%) | 228 (38.8%) | 248 (42.2%) | 249 (42.3%) | |
| Hispanic | 39 (6.6%) | 17 (2.9%) | 27 (4.6%) | 22 (3.7%) | 30 (5.1%) | |
| Other | 12 (2.0%) | 10 (1.7%) | 24 (4.1%) | 24 (4.1%) | 55 (9.4%) | |
| Income† | 0.02 | |||||
| $20,000 or under | 152 (25.9%) | 148 (25.2%) | 137 (23.3%) | 149 (25.4%) | 173 (29.4%) | |
| $20,001– $50,000 | 146 (24.9%) | 152 (25.9%) | 145 (24.7%) | 144 (24.5%) | 152 (25.9%) | |
| $50,000 – $100,000 | 124 (21.1%) | 117 (19.9%) | 144 (24.5%) | 137 (23.3%) | 105 (17.9%) | |
| More than $100,000 | 84 (14.3%) | 86 (14.6%) | 60 (10.2%) | 67 (11.4%) | 53 (9.0%) | |
|
| ||||||
| Clinical characteristics | ||||||
|
| ||||||
| Diabetes | 278 (47.4%) | 271 (46.1%) | 261 (44.4%) | 267 (45.5%) | 236 (40.1%) | 0.12 |
| Cardiovascular disease | 199 (33.9%) | 188 (32.0%) | 184 (31.3%) | 202 (34.4%) | 189 (32.1%) | 0.75 |
| Hypertension | 516 (87.9%) | 505 (85.9%) | 494 (84.0%) | 494 (84.2%) | 491 (83.5%) | 0.19 |
| Body mass index (kg/m2) | 33.2 ± 8.0 | 32.8 ± 8.2 | 31.8 ± 7.3 | 31.9 ± 7.7 | 30.0 ± 7.3 | <0.001 |
| Estimated GFR (mL/min/1.73m2) | 45.6 ± 14.7 | 45.1 ± 15.5 | 44.7 ± 15.5 | 45.3 ± 14.7 | 44.4 ± 14.7 | 0.64 |
|
| ||||||
| Medications | ||||||
|
| ||||||
| ACE inhibitors/ARB | 411 (70.5%) | 422 (72.1%) | 401 (68.7%) | 390 (66.7%) | 385 (65.8%) | 0.11 |
| Diuretics | 350 (60.0%) | 376 (64.3%) | 333 (57.0%) | 335 (57.3%) | 333 (56.9%) | 0.05 |
| Active vitamin D sterols | 14 (2.4%) | 17 (2.9%) | 12 (2.1%) | 28 (4.8%) | 29 (5.0%) | 0.01 |
| Phosphate binders | 37 (6.3%) | 34 (5.8%) | 38 (6.5%) | 50 (8.5%) | 58 (9.9%) | 0.04 |
| Alkali supplements | 18 (3.1%) | 10 (1.7%) | 11 (1.9%) | 16 (2.7%) | 19 (3.2%) | 0.33 |
Column percent does not total 100 due to participant non-response
GFR = glomerular filtration rate; ACE inhibitors = angiotensin converting enzyme inhibitors; ARB = angiotensin receptor blocker
Higher percent plant protein was strongly associated with other dietary intake variables including higher percentage of calories from carbohydrate, lower percentage of calories from protein, fat, and saturated fat, and lower intake of total calories, sodium and phosphate. Adjustment for total energy intake substantially attenuated the difference across quintiles for many correlated nutrients, such as sodium and phosphate (Table 2).
Table 2.
Macro- and micro-nutrient intakes by quintiles of percentage of total protein intake from plant sources
| Quintiles of Percent Plant Protein | P-value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Characteristic | <24% | 24–29% | 30–35% | 36–44% | >44% | |
| Mean ± SD or n (%) | (n=587) | (n=588) | (n=588) | (n=587) | (n=588) | |
| Dietary measures from DHQ | ||||||
|
| ||||||
| Total energy (kcal) | 1909 ± 893 | 1891 ± 797 | 1843 ± 804 | 1837 ± 854 | 1713 ± 763 | <0.001 |
| % kcal from carbohydrate | 43 ± 9 | 48 ± 9 | 50 ± 9 | 53 ± 9 | 58 ± 11 | <0.001 |
| % kcal from protein | 19 ± 4 | 16 ± 3 | 15 ± 3 | 15 ± 3 | 13 ± 3 | <0.001 |
| % kcal from fat | 37 ± 7 | 36 ± 7 | 35 ± 7 | 33 ± 8 | 30 ± 9 | <0.001 |
| % kcal from saturated fat | 12 ± 3 | 11 ± 3 | 11 ± 3 | 10 ± 3 | 8 ± 3 | <0.001 |
| Sodium intake (mg) | 3127 ± 1519 | 3054 ± 1416 | 2930 ± 1359 | 2892 ± 1469 | 2507 ± 1255 | <0.001 |
| Potassium intake (mg) | 2964 ± 1417 | 3034 ± 1281 | 2985 ± 1311 | 3133 ± 1410 | 3003 ± 1411 | 0.24 |
| Phosphate intake (mg) | 1289 ± 621 | 1224 ± 534 | 1142± 495 | 1129 ± 534 | 991 ± 439 | <0.001 |
| Sodium intake (mg/1000 kcal) | 1659 ± 348 | 1624 ± 320 | 1605 ± 311 | 1584 ± 325 | 1492 ± 408 | <0.001 |
| Potassium intake (mg/1000 kcal) | 1609 ±441 | 1650 ± 403 | 1668 ± 423 | 1773 ± 482 | 1817 ± 545 | <0.001 |
| Phosphate intake (mg/1000 kcal) | 690 ± 158 | 655 ± 127 | 632 ± 131 | 624 ± 130 | 589 ± 132 | <0.001 |
|
| ||||||
| Dietary biomarkers (unadjusted) | ||||||
|
| ||||||
| 24 hour urinary sodium (mg) | 4107 ± 1942 | 3863 ± 1887 | 3703 ± 1772 | 3638 ± 1581 | 3422 ± 1662 | <0.001 |
| 24 hour urinary potassium (mg) | 2303 ± 1198 | 2187 ± 998 | 2129 ± 966 | 2251 ± 1052 | 2164 ± 1004 | 0.04 |
| 24 hour urinary phosphate (mg) | 849±370 | 812 ±364 | 768 ±347 | 752 ±318 | 697 ±331 | <0.001 |
|
| ||||||
| Dietary biomarkers (adjusted for energy intake) | ||||||
|
| ||||||
| 24 hour urinary sodium (mg) | 3774 ± 321 | 3767 ± 287 | 3750 ± 289 | 3748 ± 308 | 3703 ± 275 | <0.001 |
| 24 hour urinary potassium (mg) | 2218 ±131 | 2215 ± 117 | 2208 ± 118 | 2207 ± 125 | 2189 ± 112 | <0.001 |
| 24 hour urinary phosphate (mg) | 781 ± 65 | 780 ± 58 | 776 ± 59 | 776 ± 62 | 767 ± 56 | <0.001 |
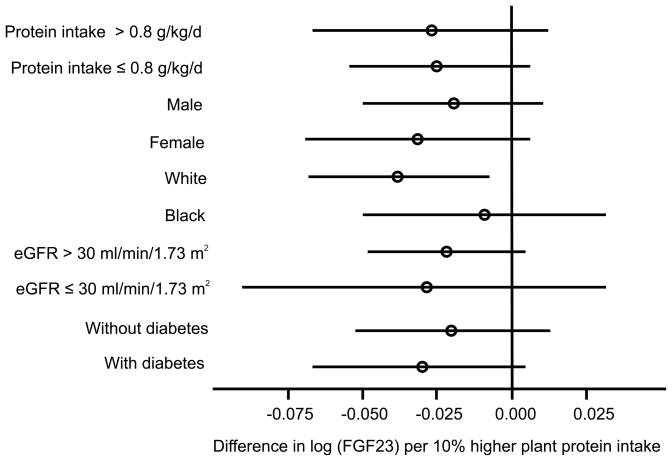
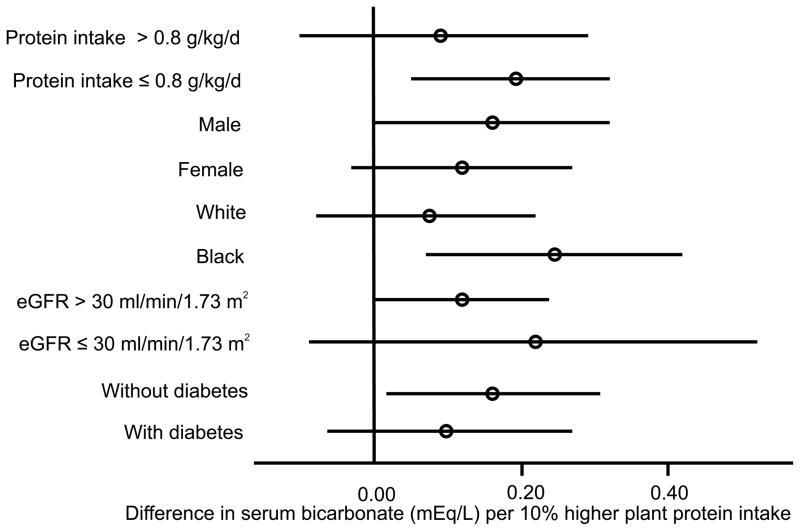
In unadjusted analyses, higher percent plant protein were strongly associated with 24 hour urinary phosphate (Table 3; p<0.001). Percent plant protein was no longer associated with 24 hour urinary phosphate after adjustment for demographics, total energy intake and total protein intake (p=0.24). Higher percent plant protein was not associated with serum phosphate, FGF23, or PTH in univariate analyses (Table 3). There was a marginal graded association between higher percent plant protein and higher serum bicarbonate (p=0.07). Table 4 presents associations between percent plant protein and metabolic parameters after adjustment for age, sex, race, diabetes, body mass index, eGFR, income, smoking, total energy intake, total protein intake, 24 hour urinary sodium, use of ACE inhibitors/ARBs, and use of diuretics. Higher percent plant protein intake was associated with lower FGF23 (p=0.05) and higher serum bicarbonate (p=0.01), but not with serum phosphate or PTH (p=0.9 and 0.5, respectively). The associations of percent plant protein with FGF23 (Figure 1) and serum bicarbonate (Figure 2) did not differ by diabetes status, sex, race, CKD stage (2 and 3 vs. 4 and 5) or total dietary protein intake (≤ 0.8 g/kg/d vs. >0.8 g/kg/d) (p-interaction > 0.10 for each). Results were similar in a sensitivity analysis excluding participants on alkali supplements, phosphate binders and active vitamin D sterols (n=2608; supplemental table 1), although the association between percent plant protein and FGF23 was no longer significant (p=0.07) due to a loss of statistical power. There was no association of higher percent plant protein with adverse metabolic consequences such as higher serum potassium (p=0.2), lower serum albumin (p=0.2) or lower hemoglobin (p=0.3).
Table 3.
Unadjusted mean values (± standard deviation) of metabolic parameters by quintiles of percent plant protein
| 1 | 2 | 3 | 4 | 5 | p-trend | |
|---|---|---|---|---|---|---|
| <24% | 24–29% | 30–35% | 36–44% | >44% | ||
| (n=587) | (n=588) | (n=588) | (n=587) | (n=588) | ||
| Serum phosphate (mg/dL) | 3.67 ± 0.66 | 3.73 ± 0.64 | 3.69 ± 0.65 | 3.69 ± 0.61 | 3.69 ± 0.66 | 0.69 |
| FGF23 (RU/mL)† | 138 (92, 239) | 144 (95, 247) | 143 (94, 224) | 139 (95, 232) | 134 (88, 215) | 0.60 |
| 24 urinary phosphate (mg) | 849 ± 369.96 | 812 ± 363.71 | 768 ± 347.17 | 752 ± 318.22 | 697 ± 331.06 | <0.001 |
| iPTH (pg/mL)† | 53 (34, 87) | 54 (33, 90) | 51 (33, 83) | 53 (34, 84) | 52 (35, 84) | 0.83 |
| Serum bicarbonate (mEq/L) | 24.47 ± 3.17 | 24.50 ± 3.05 | 24.50 ± 3.07 | 24.85 ± 3.22 | 24.82 ± 3.19 | 0.07 |
Median (interquartile range)
FGF23 = fibroblast growth factor 23; iPTH = intact parathyroid hormone
Table 4.
Adjusted* difference (95% confidence interval) in metabolic parameters by quintiles of percent plant protein intake
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Continuous per 10% increase in % plant protein | p-value‡ | |
|---|---|---|---|---|---|---|---|
| Serum phosphate (mg/dL) | Ref | 0.048 (−0.021, 0.117) | 0.021 (−0.051, 0.092) | 0.010 (−0.063, 0.083) | 0.018 (−0.061, 0.096) | −0.002 (−0.023, 0.020) | 0.89 |
| Log FGF23 (RU/mL) | Ref | −0.005 (−0.082, 0.072) | −0.001 (−0.080, 0.078) | −0.027 (−0.108, 0.054) | −0.057 (−0.144, 0.029) | −0.024 (−0.047, −0.000) | 0.05 |
| 24 urinary phosphate (mg) | Ref | 11.12 (−20.95, 43.18) | −10.90 (−43.96, 22.16) | −4.80 (−38.67, 29.07) | −5.45 (−41.64, 30.73) | −4.31 (−14.15, 5.54) | 0.39 |
| Log iPTH (pg/mL) | Ref | −0.003 (−0.074, 0.068) | −0.024 (−0.097, 0.050) | −0.005 (−0.079, 0.070) | 0.016 (−0.064, 0.096) | 0.008 (−0.014, 0.030) | 0.46 |
| Serum bicarbonate (mEq/L) | Ref | 0.013 (−0.341, 0.368) | 0.033 (−0.332, 0.399) | 0.230 (−0.144, 0.605) | 0.297 (−0.103, 0.698) | 0.141 (0.032, 0.251) | 0.01 |
all models adjusted for age, sex, race, diabetes, body mass index, eGFR, income, smoking, total energy, total protein, 24 hour urinary sodium, use of ACE inhibitors/ARBs, use of diuretics
p<0.05
p-value from continuous model
FGF23 = fibroblast growth factor 23; iPTH = intact parathyroid hormone
Figure 1.
Forest plot of difference in log fibroblast growth factor 23 (log FGF23) per 10% higher percentage of protein from plant sources across patient subgroups. Circles represent point estimate and bars represent 95% confidence intervals.
Figure 2.
Forest plot of difference in serum bicarbonate per 10% higher percentage of protein from plant sources across patient subgroups. Circles represent point estimate and bars represent 95% confidence intervals.
Discussion
In this multicenter, multi-ethnic study of participants across the full spectrum of CKD, we found that a greater percentage of dietary protein intake from plant sources was associated with better metabolic parameters, including higher serum bicarbonate and lower FGF23. FGF23 is a circulating hormone which increases the fractional excretion of phosphate in the urine and inhibits 1-alpha hydroxylase activity, thereby maintaining phosphate homeostasis in the setting of decreased GFR 18, 19. Circulating levels of FGF23 become elevated earlier in CKD than serum phosphate, suggesting it may be a more sensitive biomarker of abnormal phosphate metabolism and a possible inciting factor in the development of secondary hyperparathyroidism 20–22. Critically, recent observational studies report that FGF23 is among the most potent risk factors for death among patients with CKD, making it an attractive target for preventive strategies in this patient population 23.
Previous physiologic studies have demonstrated that changes in FGF23 can be induced by altering phosphate intake 24–28. Importantly, many of these studies utilized inorganic phosphate supplements, a highly bioavailable source of phosphate, to induce phosphate loading. This may have resulted in differences in bioavailable phosphate between feeding periods which are more dramatic than differences between whole food diets. Other studies which have modulated phosphate intake using whole food diets have shown conflicting results 29, 30. Recently, a feeding study in 9 patients with CKD demonstrated that consuming a vegetarian diet compared with a meat based diet for 7 days lowered FGF23 12. Our results build on these prior findings by observing that even if not strictly vegetarian, higher intake of plant-based compared with animal-based protein is associated with lower FGF23. Consistent with these prior experimental studies, our findings suggest that a diet with greater predominance of plant-based protein may lower FGF23, a potent risk factor for mortality in CKD.
Although we observed that the percentage of plant protein intake was associated with FGF23 levels, we did not observe an association with serum phosphate. Similar results have been seen in some feeding studies demonstrating that low phosphate diets can lower FGF23 without affecting serum phosphate 24. This is likely due to tight physiologic regulation of serum phosphate by FGF23 and potentially other phosphotonins 31. In this study, urinary phosphate excretion was strongly associated with percent plant protein in univariate analyses, but not after adjustment for energy and total protein intake. Given our hypothesis that bioavailable phosphate is lower on a plant-based diet, this finding was unexpected. In this study we used the DHQ to ascertain habitual dietary intake over one year. In contrast, 24 hour urinary phosphate represents dietary intake over a period of days which may be only weakly correlated with habitual intake due to day to day variation in diet. Differences in the period of assessment using a food frequency questionnaire (i.e. long term habitual intake) versus a urinary biomarker (i.e. short term intake) may explain the lack of robust association between 24 hour urinary phosphate and plant protein intake.
Another key finding of this study was the association between greater percentage of protein from plant sources and higher serum bicarbonate levels. Previous observational studies have shown that serum bicarbonate levels associate with the nonvolatile acid load of the diet (i.e. net endogenous acid production) which is determined in part by total dietary protein intake 32, 2, 33. This study builds on those findings and suggests that a higher intake of protein from plant, compared with animal sources, may further raise serum bicarbonate, presumably due to the lower sulfate content of plant-based proteins 14. This finding has clear clinical relevance in CKD given recent observational and experimental studies demonstrating that higher serum bicarbonate levels may lower risk for CKD progression and mortality 34–38.
In addition to its potential benefits on phosphate and acid-base homeostatsis, a higher intake of plant-based foods has theoretical risks in patients with kidney disease due to a high potassium content 39 and inhibition of iron absorption by phytic acid 40. In this study we did not observe associations between a higher intake of protein from plant sources and serum potassium or hemoglobin. It is important to note that we did not have information on the use of iron supplements, or indices of iron stores, such as ferritin or transferrin saturation, making it difficult to fully exclude an adverse association with iron absorption. We also did not observe an association between a greater percentage of protein from plant sources and lower serum albumin, a serologic marker of malnutrition.
Although we noted associations between higher relative intake of plant-based protein and more favorable levels of both FGF23 and serum bicarbonate, we acknowledge that the magnitude of the associations observed in this study were small. We believe the true associations between these factors may be underestimated in this study due to the relatively crude nature of dietary assessment in large population-based studies, and the use of only single measures of outcome variables, which may vary over time. Food frequency questionnaires are known to underestimate true dietary protein intake and correlate moderately with gold standard measures of dietary protein, such as weighed dietary records and dietary biomarkers 41. The CRIC study did not include a dietary validation, therefore we were unable to calibrate for measurement error, as frequently performed in studies of dietary intake 42. In addition, the observed interquartile range of percentage of protein from plant sources was 26% to 41%, values comparable to previously reported studies 43, 44. Within the range of the observed data, the magnitude of these associations was relatively small, however the magnitude of effect may be much larger if our study population had included more participants with a greater predominance of plant-based protein (i.e. 90% protein from plant sources).
Our study had a number of limitations to consider. The food frequency questionnaire that we used in this study, has not been validated in a population with CKD. Additionally, the method we used to allocate protein sources has not been previously validated. We considered protein from a variety of plant sources together in this study, although intestinal phosphate absorption and nonvolatile acid load may differ between different types of plant foods, such as soy and grains. The biological quality of protein also differs between plants and is generally lower among plant protein compared with animal protein45. We did not observe an association between plant protein intake and the nutritional marker, serum albumin, however we cannot conclude from this study that there is no adverse impact of a plant-based diet on nutritional status in CKD and this area warrants further investigation. Finally, this is an observational, cross-sectional study, and it limits our ability to make causal inferences about the dietary pattern and the more favorable metabolic profile.
Despite these limitations, this study has many strengths including a large, diverse population of patients across the spectrum of CKD. We used standardized questionnaires for the assessment of habitual dietary intake from which we derived our key exposure variable, as well as several critical confounding variables including total energy intake and total protein intake. Additionally, the study includes comprehensive assessment of other confounding variables including comorbidity, medication usage and other dietary biomarkers (i.e. 24 hour urinary sodium).
In conclusion, we have observed lower levels of FGF23 and higher levels of serum bicarbonate among patients with CKD consuming a greater percentage of dietary protein from plant sources. These relationships were similar across subgroups defined by severity of CKD, diabetic status, race and level of overall protein intake. To our knowledge, this is the first study to document these associations across a range of observed dietary intakes and in a large, diverse patient population. This study suggests that serum bicarbonate and FGF23, both risk factors for morbidity and mortality in CKD, are potentially modifiable by dietary strategies which favor plant-based protein sources.
Supplementary Material
Practical Application.
We observed that patients with chronic kidney disease consuming a higher percentage of protein from plant sources had lower levels of fibroblast growth factor 23 and higher serum bicarbonate. Our results indicate that a diet based on plant foods may have metabolic benefits in patients with chronic kidney disease, but the safety of this dietary pattern and impact on nutritional status still need to be determined.
Acknowledgments
Support:
We would like to acknowledge the time and commitment of the participants, investigators and staff of the CRIC study. The CRIC Study is supported by cooperative agreement project grants 5U01DK060990, 5U01DK060984, 5U01DK06102, 5U01DK061021,5U01DK061028, 5U01DK60980, 5U01DK060963, and 5U01DK060902 from the National Institute of Diabetes and Digestive and Kidney Diseases and by grants UL1RR024134, UL1RR025005, M01RR16500, UL1RR024989, M01RR000042, UL1RR024986, UL1RR029879, RR05096, and UL1RR024131 from the National Institutes of Health.
JJS was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant 5KL2RR025006 from the National Center for Research Resources, a component of the NIH and NIH Roadmap for Medical Research, as well as the National Kidney Foundation of Maryland. CAMA was supported by K01 HL092595-02 from the National Heart Lung and Blood Institute.
Footnotes
Financial Disclosure Declaration:
The authors have no relevant financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman AN. High-protein diets: Potential effects on the kidney in renal health and disease. Am J Kidney Dis. 2004;44(6):950–962. doi: 10.1053/j.ajkd.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Gennari FJ, Hood VL, Greene T, Wang X, Levey AS. Effect of Dietary Protein Intake on Serum Total CO2 Concentration in Chronic Kidney Disease: Modification of Diet in Renal Disease Study Findings. Clin J Am Soc Nephrol. 2006;1(1):52–57. doi: 10.2215/CJN.00060505. [DOI] [PubMed] [Google Scholar]
- 3.Cianciaruso B, Pota A, Pisani A, et al. Metabolic effects of two low protein diets in chronic kidney disease stage 4–5--a randomized controlled trial. Nephrol Dial Transplant. 2008;23(2):636–644. doi: 10.1093/ndt/gfm576. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Greene T, Sarnak MJ, et al. Effect of Dietary Protein Restriction on the Progression of Kidney Disease: Long-Term Follow-Up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2006;48(6):879–888. doi: 10.1053/j.ajkd.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Greene T, Beck GJ, et al. Dietary Protein Restriction and the Progression of Chronic Renal Disease: What Have All of the Results of the MDRD Study Shown? J Am Soc Nephrol. 1999;10(11):2426–2439. doi: 10.1681/ASN.V10112426. [DOI] [PubMed] [Google Scholar]
- 6.Pan Y, Guo LL, Jin HM. Low-protein diet for diabetic nephropathy: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88(3):660–666. doi: 10.1093/ajcn/88.3.660. [DOI] [PubMed] [Google Scholar]
- 7.Pedrini MT, Levey AS, Lau J, Chalmers* TC, Wang PH. The Effect of Dietary Protein Restriction on the Progression of Diabetic and Nondiabetic Renal Diseases: A Meta-Analysis. Ann Intern Med. 1996;124(7):627–632. doi: 10.7326/0003-4819-124-7-199604010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ikizler TA, Greene JH, Wingard RL, Parker RA, Hakim RM. Spontaneous dietary protein intake during progression of chronic renal failure. J Am Soc Nephrol. 1995;6(5):1386–1391. doi: 10.1681/ASN.V651386. [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009;90(2):407–414. doi: 10.3945/ajcn.2008.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikizler TA. Dietary Protein Restriction in CKD: The Debate Continues. Am J Kidney Dis. 2009;53(2):189–191. doi: 10.1053/j.ajkd.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Moe SM, Chen NX, Seifert MF, et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2008;75(2):176–184. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian Compared with Meat Dietary Protein Source and Phosphorus Homeostasis in Chronic Kidney Disease. Clin J Am Soc Nephrol. 2011;6(2):257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, et al. Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease. Clin J Am Soc Nephrol. 2010;5(3):519–530. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 14.Remer T, Manz F. Potential Renal Acid Load of Foods and its Influence on Urine pH. J Am Diet Assoc. 1995;95(7):791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 15.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subar AF, Thompson FE, Kipnis V, et al. Comparative Validation of the Block, Willett, and National Cancer Institute Food Frequency Questionnaires: The Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44(2):250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Gupta A, Quarles LD. Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr Opin Nephrol Hypertens. 2007;16(4):329–335. doi: 10.1097/MNH.0b013e3281ca6ffd. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast Growth Factor-23 Mitigates Hyperphosphatemia but Accentuates Calcitriol Deficiency in Chronic Kidney Disease. J Am Soc Nephrol. 2005;16(7):2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 21.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 Is a Potent Regulator of Vitamin D Metabolism and Phosphate Homeostasis. J Bone Miner Res. 2004;19(3):429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 22.Evenepoel P, Meijers B, Viaene L, et al. Fibroblast Growth Factor-23 in Early Chronic Kidney Disease: Additional Support in Favor of a Phosphate-Centric Paradigm for the Pathogenesis of Secondary Hyperparathyroidism. Clin J Am Soc Nephrol. 5(7):1268–1276. doi: 10.2215/CJN.08241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isakova T, Xie H, Yang W, et al. Fibroblast Growth Factor 23 and Risks of Mortality and End-Stage Renal Disease in Patients With Chronic Kidney Disease. JAMA. 305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoniucci DM, Yamashita T, Portale AA. Dietary Phosphorus Regulates Serum Fibroblast Growth Factor-23 Concentrations in Healthy Men. J Clin Endocrinol Metab. 2006;91(8):3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 25.Burnett S, Gunawardene S, Bringhurst F, Jüppner H, Lee H, Finkelstein J. Regulation of C-Terminal and Intact FGF-23 by Dietary Phosphate in Men and Women. J Bone Miner Res. 2006;21(8):1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari SL, Bonjour J-P, Rizzoli R. Fibroblast Growth Factor-23 Relationship to Dietary Phosphate and Renal Phosphate Handling in Healthy Young Men. J Clin Endocrinol Metab. 2005;90(3):1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 27.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 28.Nishida Y, Taketani Y, Yamanaka-Okumura H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70(12):2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 29.Vervloet MG, van Ittersum FJ, Buttler RM, Heijboer AC, Blankenstein MA, ter Wee PM. Effects of Dietary Phosphate and Calcium Intake on Fibroblast Growth Factor-23. Clin J Am Soc Nephrol. 2011;6(2):383–389. doi: 10.2215/CJN.04730510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isakova T, Gutierrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26(2):584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. 2003;285(1):E1–9. doi: 10.1152/ajpendo.00016.2003. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983;24(5):670–680. doi: 10.1038/ki.1983.210. [DOI] [PubMed] [Google Scholar]
- 33.Scialla JJ, Appel LJ, Astor BC, et al. Estimated Net Endogenous Acid Production and Serum Bicarbonate in African Americans with Chronic Kidney Disease. Clin J Am Soc Nephrol. 2011;6(7):1526–1532. doi: 10.2215/CJN.00150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum Bicarbonate Levels and the Progression of Kidney Disease: A Cohort Study. Am J Kidney Dis. 2009;54(2):270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate Supplementation Slows Progression of CKD and Improves Nutritional Status. J Am Soc Nephrol. 2009;20(9):2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadola L, Noboa O, Marquez MN, et al. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int. 2004;65(4):1224–1230. doi: 10.1111/j.1523-1755.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- 37.Nath K, Hostetter M, Hostetter T. Pathophysiology of chronic tubulo-interstitial disease in rats: Interactions of dietary acid load, ammonia, and complement component-C3. J Clin Invest. 1985;76(2):667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2010;79(3):356–362. doi: 10.1038/ki.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch AA, Fransen H, Jenab M, et al. Variation in intakes of calcium, phosphorus, magnesium, iron and potassium in 10 countries in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr. 63(S4):S101–S121. doi: 10.1038/ejcn.2009.77. [DOI] [PubMed] [Google Scholar]
- 40.Hallberg L, Rossander L, Skanberg AB. Phytates and the inhibitory effect of bran on iron absorption in man. Am J Clin Nutr. 1987;45(5):988–996. doi: 10.1093/ajcn/45.5.988. [DOI] [PubMed] [Google Scholar]
- 41.Bingham SA, Cassidy A, Cole TJ, et al. Validation of weighed records and other methods of dietary assessment using the 24 h urine nitrogen technique and other biological markers. Br J Nutr. 1995;73(04):531–550. doi: 10.1079/bjn19950057. [DOI] [PubMed] [Google Scholar]
- 42.Kaaks R, Riboli E, van Staveren W. Calibration of dietary intake measurements in prospective cohort studies. Am J Epidemiol. 1995;142(5):548– 556. doi: 10.1093/oxfordjournals.aje.a117673. [DOI] [PubMed] [Google Scholar]
- 43.Halkjar J, Olsen A, Bjerregaard LJ, et al. Intake of total, animal and plant proteins, and their food sources in 10 countries in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr. 2009;63(S4):S16–S36. doi: 10.1038/ejcn.2009.73. [DOI] [PubMed] [Google Scholar]
- 44.Smit E, Nieto FJ, Crespo CJ, Mitchell P. Estimates of Animal and Plant Protein Intake in US Adults: Results from the Third National Health and Nutrition Examination Survey, 1988–1991. J Am Diet Assoc. 1999;99(7):813–820. doi: 10.1016/S0002-8223(99)00193-5. [DOI] [PubMed] [Google Scholar]
- 45.National Research Council. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: The National Academies Press; 2005. Protein and Amino Acids; pp. 589–768. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.