Abstract
There is increasing interest in therapies that can be administered less frequently and/or avoid gastrointestinal irritation. The efficacy of once-yearly zoledronic acid (5 mg) in the treatment and prevention of osteoporosis has been evaluated in different patient populations. In the 3-year HORIZON-Pivotal Fracture Trial in postmenopausal women with osteoporosis, zoledronic acid reduced the risk of vertebral and hip fracture by 70% and 41%, respectively, versus placebo. The efficacy of zoledronic acid in preventing subsequent fracture in patients with a hip fracture was evaluated in the HORIZON-Recurrent Fracture Trial. New vertebral and nonvertebral fractures were significantly reduced by treatment initiated within 90 days of incident hip fracture, without evidence of delayed fracture healing. Data from a 1-year study show that a single zoledronic acid 5-mg infusion is superior to oral risedronate 5 mg/day for treatment and prevention of glucocorticoid-induced osteoporosis. Increases in bone mineral density and decreases in bone turnover markers were significantly greater with zoledronic acid than with risedronate. Two different treatment regimens of zoledronic acid were found to be more effective than placebo for prevention of bone loss in postmenopausal women and reducing markers of bone turnover after 2 years.
In conclusion, zoledronic acid 5 mg once-yearly infusion has demonstrated marked efficacy in the treatment and prevention of primary and secondary osteoporosis, with a combination of fracture risk reduction and prevention of bone loss at key sites. It is the only agent shown to reduce the incidence of fracture and mortality in patients with a previous low-trauma hip fracture.
Keywords: bone mineral density, glucocorticoid-induced osteoporosis, hip fractures, osteopenia, osteoporosis, postmenopausal, zoledronic acid
Introduction
Fractures occurring as a result of primary osteoporosis are an important cause of disability among postmenopausal women [Robbins et al. 2007], a sector of the population that is increasing in line with the aging of populations not only in the Western world, but also in Asia and Latin America. Furthermore, the prevalence of secondary osteoporosis is increasing, with the widespread clinical use of glucocorticoids for a variety of disorders, including autoimmune, pulmonary, and gastrointestinal diseases, malignancies, and for immunosuppression in the treatment of patients receiving organ transplants. Glucocorticoid-induced osteoporosis (GIO) is the most common form of secondary osteoporosis, and fractures have been reported in 30–50% of patients receiving chronic glucocorticoid therapy [Mazziotti et al 2007].
Bisphosphonate treatment leads to inhibition of osteoclast-mediated bone resorption and subsequent reduction in vertebral and nonvertebral fracture risk. As a result, the bisphosphonates have become widely accepted as effective, well-tolerated treatments for postmenopausal osteoporosis and GIO. The available bisphosphonates fall into two groups [Suzuki et al 2006]: non-nitrogen containing and nitrogen containing. The less-potent non-nitrogen-containing bisphosphonates (clodronate and etidronate) act by being incorporated into modified ATP, thereby blocking the energy supply to osteoclasts and triggering apoptotic cell death. The potent nitrogen-containing bisphosphonates (alendronate, ibandronate, risedronate and zoledronic acid) inactivate osteoclasts, by disrupting key cellular functions mediated by small GTPase signaling proteins. The potency of nitrogen-containing bisphosphonates is related to their ability to inhibit the enzyme farnesyl diphosphate synthase, with zoledronic acid showing the greatest potency and alendronate the lowest (zoledronic acid > risedronate > ibandronate > alendronate) [Nancollas et al. 2006].
As with any chronic condition, patient compliance and persistence with therapy are important factors in clinical efficacy [Silverman, 2006; Conte and Guarneri, 2004], and bisphosphonate treatment is no exception. Treatment of osteoporosis is complicated by the asymptomatic nature of this disease and the lack of options for patient self monitoring [Boonen et al. 2008c]. Moreover, poor compliance with oral dosing of bisphosphonates is further complicated by the need for pre-and postdose fasting, and posture requirements (patients taking oral bisphosphonates should not lie down for 30minutes [60minutes for ibandronate] after taking the tablet) [Boonen et al. 2008c]. Importantly, failure to adhere to daily oral bisphosphonate therapy has been implicated in smaller increases in bone density and higher fracture rates [Siris et al. 2006; Yood et al. 2003]. Although it might be expected that less-frequent dosing might improve compliance, the introduction of once-weekly oral dosing for alendronate and risedronate has had insufficient impact [Huybrechts et al. 2006; Lo et al. 2006; Cramer et al. 2005]. While medication possession ratios are improved with less-frequent dosing during the first year of therapy (69.2% versus 57.6% with once-weekly and once-daily dosing, respectively), compliance remains suboptimal. Even with weekly regimens, intermittent use is common, with 49.6% of women experiencing 60-day prescription gaps during the first year of therapy [Lo et al. 2006]. Overall, after 2 years of therapy, compliance levels appear to stabilize at around 40% with oral regimens [Siris et al. 2006].
Issues relating to compliance and persistence have fuelled a move to highly potent bisphosphonates that have a pharmacodynamic profile compatible with infrequent intravenous (iv) dosing, such as quarterly dosing with ibandronate and yearly dosing with zoledronic acid. In particular, zoledronic acid achieves therapeutic activity at micromolar concentrations [Conte and Guarneri, 2004]. Compared with other bisphosphonates, it has the highest kinetic binding affinity, with rapid uptake, lower desorption, higher reattachment after release from bone and lower diffusion within bone [Boonen et al. 2008c]. After dosing, a fraction of the zoledronic acid is reversibly taken up by the skeleton, and elimination of drug is mainly by renal excretion [Weiss et al. 2008]. Disposition of the drug in blood and noncalcified tissue is governed by extensive uptake into and slow release from bone. Together, these characteristics allow zoledronic acid to achieve prolonged therapeutic concentrations when given as a single annual infusion. A dose-finding study, for example, showed that a 4-mg infusion was as effective as more frequent administration in improving bone density and reducing bone resorption [Reid et al. 2002]. Furthermore, onset of activity with zoledronic acid is rapid. In a 24-week trial in post-menopausal women with low bone mineral density (BMD), a single 5-mg zoledronic acid infusion reduced bone resorption markers more rapidly than weekly oral alendronate (70 mg) [Saag et al. 2007a]. Importantly, once-yearly dosing with zoledronic acid offers a treatment option that provides greater convenience for patients and ensures treatment adherence.
This review presents the current efficacy and safety data for zoledronic acid in treatment of primary and secondary osteoporosis and prevention of fractures.
Postmenopausal osteoporosis
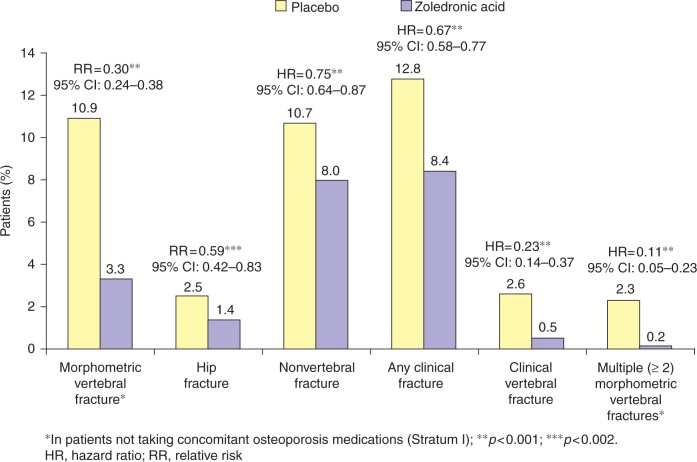
The ability of an annual administration of zoledronic acid (5 mg) to reduce the risk of vertebral, hip and other types of fracture has been demonstrated in a large, randomized, double-blind, placebo-controlled trial: the Health Outcomes and Reduced Incidence with Zoledronic acid ONce yearly (HORIZON) Pivotal Fracture Trial (PFT) [Black et al. 2007]. In this 3-year study, 3889 patients (mean age 73 years) were randomly assigned to receive a single 15-minute infusion of zoledronic acid (5mg) at baseline, 12months and 24 months, and 3876 were assigned to receive placebo. There were two strata, one with osteoporosis-treatment-naïve patients and, uniquely for a bisphosphonate clinical trial, one in which concomitant therapy with ‘usual’ osteoporosis therapies (e.g. hormone-replacement therapy, tibolone, selective estrogen receptor modulators, and calcitonin) were allowed. Patients were followed for 36 months. There were two coprimary endpoints, including new vertebral fracture (in patients not taking concomitant osteoporosis medications) and hip fracture (in all patients). Secondary endpoints included changes in BMD and bone turnover markers in the serum (C-terminal telopeptide [βbT-CTx], bone-specific alkaline phosphatase, N-terminal propeptide), as well as safety outcomes. Results from HORIZON-PFT showed that treatment with zoledronic acid reduced the risk of morphometric vertebral fracture by 70% during the 3-year study period compared with placebo [Black et al. 2007]. Fracture rates were 3.3% in patients receiving zoledronic acid versus 10.9% in the placebo group (relative risk [RR], 0.30; 95% confidence interval [CI], 0.24–0.38) (Figure 1). Zoledronic acid also reduced the risk of hip fracture by 41%, with fracture rates of 1.4% and 2.5% in the zoledronic acid and placebo groups, respectively (hazard ratio [HR], 0.59; 95% CI, 0.42–0.83) (Figure 1). In this study, all of the secondary endpoints were met; for example, nonvertebral fractures, clinical fractures and clinical vertebral fractures were reduced by 25%, 33%, and 77%, respectively (p < 0.001 for all comparisons) (Figure 1). Notably, the effect of zoledronic acid was independent of concomitant administration of other osteoporosis therapies, including hormone-replacement therapy, selective oestrogen-receptor modulators, calcitonin and tibolone [Reid et al. 2008]. The effect of zoledronic acid on nonvertebral fracture was evaluated further in a post-hoc analysis of the so-called ‘super six’ fracture sites (wrist, hip, pelvis, humerus, leg and clavicle) [Black et al. 2009]. The incidence of nonvertebral ‘super six’ fractures at 3 years in the zoledronic acid and placebo groups were 5.7% and 8.6%, respectively, equivalent to a 34% reduction in fracture risk (HR, 0.66; 95% CI, 0.55–0.78; p < 0.0001).
Figure 1.
Relative risk of fracture incidence in a 3-year study of once-yearly zoledronic acid [5mg] versus placebo in women with postmenopausal osteoporosis (HORIZON-PFT) [Black et al. 2007].
In HORIZON-PFT, zoledronic acid was also associated with a significant increase in BMD and a decrease in bone turnover markers [Black et al. 2007]. In the zoledronic-acid group, BMD increased significantly at the total hip (6.02%; 95% CI, 5.77–6.28), lumbar spine (6.71%; 95% CI, 5.69–7.74), and femoral neck (5.06%; 95% CI, 4.76–5.36) compared with placebo (p < 0.001 for all comparisons). All three biochemical markers of bone turnover decreased significantly in patients in the zoledronic acid group compared with those in the placebo group.
At 12 months, levels of serum βbT-CTx, bone-specific alkaline phosphatase and N-terminal propeptide were 59% (95% CI, 55–63), 30% (95% CI, 27–32) and 58% (95% CI, 55–60) lower, respectively, in the zoledronic acid group (p < 0.001 for all comparisons). Further analysis of 147 bone samples using microcomputed tomography (μCT) and histomorphometry confirmed the decrease of bone turnover produced by zoledronic acid [Recker et al. 2008]. Zoledronic acid induced a 63% median reduction of activation frequency (p < 0.0001), with reduced mineralizing surface and volume referent bone formation rate versus placebo, indicating reduced bone turnover. (μCT analysis also revealed that patients in the zoledronic-acid group had higher median trabecular bone volume (p = 0.020), higher trabecular numbers (p = 0.008) and decreased trabecular separation (p = 0.011) than those receiving placebo, indicating better preservation of trabecular structure. In a subgroup analysis of the HORIZON-PFT, reduction in fracture risk and treatment-by-factor interactions were evaluated across 14 determinants of response. Of these, nine were preplanned subgroups (age, BMD T-score and baseline vertebral fracture status, race, weight/body mass index, geographical region, prior bisphosphonate use, creatinine clearance, and baseline use of osteoporosis medications) and five were post hoc (hip BMD, smoking, height loss since age 25 years, fall in past 12 months, and daily activity). The analysis revealed that zoledronic acid significantly reduced the risk of vertebral fractures regardless of age group, creatinine clearance status and BMI (all p < 0.0001). Zoledronic acid had greater effects on vertebral fracture risk in younger women (adjusted odds ratio [OR], 0.18 [95% CI, 0.10–0.31] in the <70 years age group versus 0.37 [95% CI, 0.27–0.52] in ⩾75 years age group), overweight women (OR, 0.22 [95% CI, 0.15–0.31] in women with body mass index (BMI) ⩾25kg/m2 versus 0.38 [95% CI, 0.27–0.53] in those with BMI 19–24.9kg/m2), and women with better baseline renal function (OR, 0.21 [95% CI, 0.14–0.29] in women with creatinine clearance ⩾60mL/min versus 0.35 [95% CI, 0.25–0.48] in <60mL/min group). There were significant treatment-by-factor interactions (p ⩽ 0.05) with zoledronic acid with age, creatinine clearance and BMI [Eastell et al. 2009], indicating that zoledronic acid-treated subjects who were younger, more overweight, or with better creatinine clearance were significantly less likely to experience a vertebral fracture than placebo-treated subjects.
In patients previously receiving oral bisphosphonate therapy, studies have shown that they can be safely switched to annual zoledronic acid without compromising therapeutic efficacy. In 225 postmenopausal women with low BMD on alendronate who were randomized to oral weekly alendronate (70 mg) continuation (n = 112) or switched to zoledronic acid (n = 113), BMD values remained stable during the 12-month study in both groups, demonstrating maintenance of bone mass [McClung et al. 2007]. Mean bone turnover marker levels in patients switched to zoledronic acid were reduced from baseline after 3 months although, in contrast to the HORIZON-PFT, levels returned to baseline after 6 months, and increased thereafter, yet remained within the premenopausal range. The benefits of zoledronic acid on vertebral fractures in patients who had previously received other bisphosphonates compared with those who were bisphosphonate naïve were evaluated in a post-hoc analysis of the HORIZON-PFT [Lippuner et al. 2008]. Among bisphosphonate-naïve patients, the incidence of vertebral fracture over 3 years was 10.7% with placebo and 3.2% with zoledronic acid (RR, 0.29; 95% CI, 0.23–0.38; p < 0.0001). In patients with previous bisphosphonate experience, 12.1% of those receiving placebo had a vertebral fracture compared with 4.2% of those receiving zoledronic acid (RR, 0.35; 95% CI, 0.19–0.62; p < 0.0001).
Recent hip fracture patients
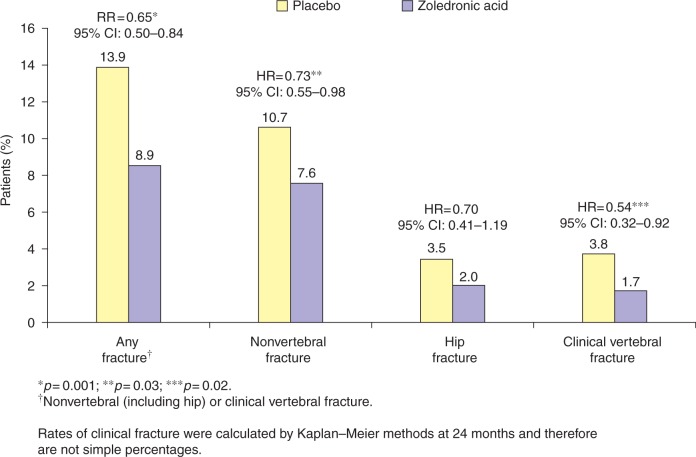
The previous occurrence of a hip fracture is an important risk factor for future osteoporotic fractures [Ryg, 2009; Colón-Emeric et al. 2003]. Zoledronic acid is the only osteoporosis therapy that has been evaluated to assess its efficacy in preventing new fractures following an incident hip fracture in the HORIZON-Recurrent Fracture Trial (RFT) [Lyles et al. 2007]. Men and women aged ≥ 50 years who had experienced a low-trauma hip fracture were randomized to an annual infusion of zoledronic acid (5 mg) or placebo, within 90 days of their original fracture. Overall, the rates of any new clinical fracture were just 8.6% in the zoledronic acid group, compared with 13.9% in the placebo group, demonstrating a 35% reduction in risk with zoledronic acid (p = 0.001) [Figure 2]. The respective rates of a new clinical vertebral fracture were 1.7% and 3.8% (p = 0.02), while those for new nonvertebral fractures were 7.6% and 10.7% (p = 0.03) [Figure 2] [Lyles et al. 2007]. When the secondary efficacy variables of total hip and femoral neck BMD were evaluated by gender, zoledronic acid was shown to significantly increase total hip BMD (Table 1) and femoral neck BMD at 36 months in men as well as in women [European Medicines Agency, 2008]. There was no evidence for delayed healing of the original fracture in either of the treatment groups. Interestingly, 101 of 1054 patients in the zoledronic acid group (9.6%) died compared with 141 of 1057 patients in the placebo group (13.3%), demonstrating a 28% reduction in risk of deaths from any cause in the zoledronic acid group (p = 0.01). The underlying cause of the difference in mortality between the two treatment arms is unknown [Lyles et al. 2007].
Figure 2.
Relative risk of new fracture occurrence in patients receiving once-yearly zoledronic acid (5 mg) or placebo within 90 days of a low-trauma hip fracture (HORIZON-RFT) [Lyles et al. 2007].
Table 1.
Between-treatment percentage change in total hip bone mineral density (BMD) in patients receiving once-yearly zoledronic acid (5mg) or placebo following a low-trauma hip fracture (HORIZON-RFT) [European Medicines Agency, 2008].
| Time point | Treatment | n | LS mean* | LS mean difference* (95% CI*) | p value* |
|---|---|---|---|---|---|
| Gender | |||||
| Month 12 | |||||
| Male | Zoledronic acid | 154 | 1.97 | 2.01 (0.67, 3.35) | 0.0032 |
| Placebo | 169 | −0.03 | |||
| Female | Zoledronic acid | 527 | 2.77 | 4.15 (3.40, 4.89) | <0.0001 |
| Placebo | 514 | −1.38 | |||
| Month 24 | |||||
| Male | Zoledronic acid | 85 | 3.59 | 3.81 (1.38, 6.23) | 0.0021 |
| Placebo | 100 | −0.22 | |||
| Female | Zoledronic acid | 320 | 4.95 | 5.88 (4.56, 7.20) | <0.0001 |
| Placebo | 300 | −0.93 | |||
| Month 36 | |||||
| Male | Zoledronic acid | 33 | 7.27 | 7.06 (3.12, 10.99) | 0.0005 |
| Placebo | 31 | 0.22 | |||
| Female | Zoledronic acid | 95 | 4.87 | 6.11 (3.82, 8.39) | <0.0001 |
| Placebo | 93 | −1.24 |
CI, confidence intervals; LS mean, least squares mean of the percent change from baseline; n, number of patients with evaluable measurements at both baseline and post-baseline visit as determined by efficacy window.
LS mean, LS mean difference, 95% CI and p values were calculated using a contrast from a threeway ANOVA model with treatment, region, sex and treatment-by-sex interaction in the model. Percentage change from baseline, 100x (post-baseline value — baseline value)/baseline value.
A prespecified secondary analysis and subsequent post-hoc analyses of HORIZON-RFT data showed that timing of therapy-influenced efficacy and suggested that 2 or more weeks after fracture repair is the optimal time for zoledronic acid infusion [Eriksen et al. 2009]. In total, 46% of patients were dosed within 6 weeks of surgical hip fracture repair, and 54% were dosed after. In all, patients dosed later than 6 weeks demonstrated greater increases in total hip and femoral neck BMD at 12 months than those dosed within 6 weeks. When an analysis was performed based on infusion time divided into 2 week intervals, at 12 months, there were significant increases in total hip BMD at all timepoints except for in those patients dosed at less than 2 weeks. There was also a consistent reduction in clinical fractures at 12 months regardless of timing of infusion, except for in the less-than-2-weeks subgroup. Zoledronic acid had no adverse effects on fracture healing, regardless of the timing of infusion. Based on these findings, it is recommended to give the zoledronic acid infusion 2 or more weeks after hip fracture repair in patients with a recent low-trauma hip fracture [Novartis Europharm Limited, 2008].
The benefits of zoledronic acid in elderly individuals were confirmed in an analysis of pooled data from HORIZON-PFT and HORIZON-RFT showing that the cohort of women aged over 75 years receiving zoledronic acid (n = 1961) had a reduced incidence of new fractures compared with those receiving placebo (n = 1926) [Boonen et al. 2009]. New clinical fracture incidence over 1 year was 4.1% with zoledronic acid versus 5.7% with placebo (HR, 0.72; 95% CI, 0.54–0.96; p = 0.026). The 3-year incidence of new clinical fractures was 10.8% and 16.6%, respectively (HR, 0.65; 95% CI, 0.54–0.78). Significant reductions in 3-year risk of clinical vertebral (p < 0.0001) and nonvertebral fractures (p = 0.0025) were also observed in elderly patients receiving zoledronic acid.
Prevention of bone loss
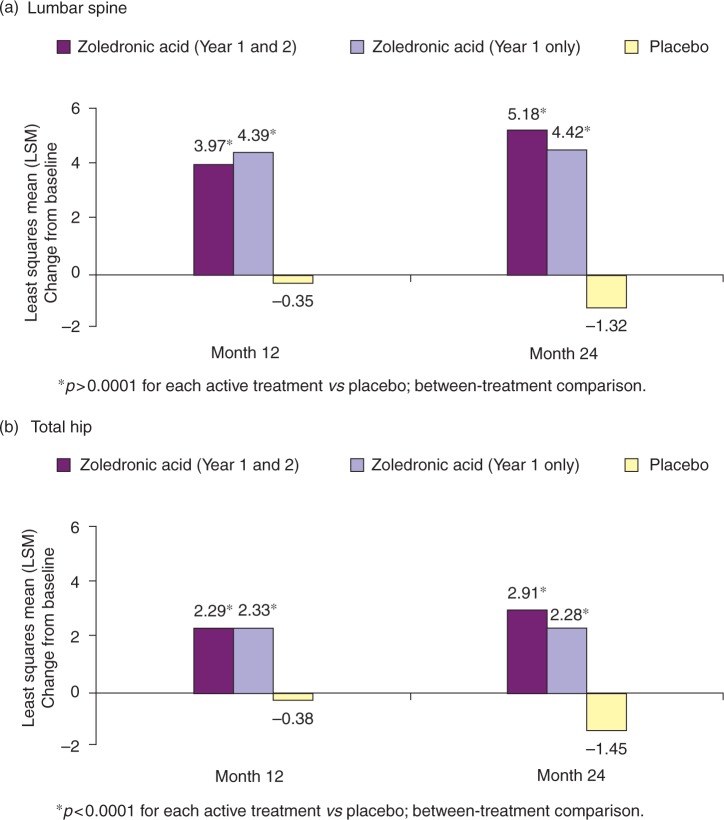
In addition to the fracture outcomes studies, zoledronic acid has also been evaluated to assess the role of preventing bone loss in postmenopausal women with low bone mass (or osteopenia). In a 2-year, randomized, double-blind, placebo-controlled trial, a single infusion of zoledronic acid (5 mg) given once yearly (n = 198) or once in 2 years (n = 181) was compared with placebo (n = 202) for prevention of bone loss in postmenopausal women aged 45 years or older with osteopenia [McClung et al. 2009]. The primary endpoint of the study was the percentage change from baseline to month 24 in lumbar spine BMD. Secondary efficacy endpoints included the percentage change in lumbar spine BMD at month 12 and in total hip, femoral neck, trochanter, and distal radius BMD at months 12 and 24, and changes in markers of bone turnover at months 1, 3, 6, 9, 12, 15, 18, and 24. After 2 years of treatment, both zoledronic acid regimens were more effective than placebo in improving lumbar spine BMD and improving BMD at all other measured sites (Table 2). Furthermore, superiority of zoledronic acid to placebo in terms of lumbar spine BMD and hip BMD was seen at all timepoints (months 12 and 24) [Figure 3]. Levels of β-CTX, N-terminal propeptide and bone-specific alkaline phosphatase decreased significantly from baseline in both zoledronic acid treatment groups relative to placebo at all timepoints (p < 0.0001). Following the second infusion, bone turnover marker levels decreased significantly in patients receiving zoledronic acid once in 2 years, but continued to increase slowly within the premenopausal reference range for those receiving once-yearly treatment. During the second year of the study, levels of all three bone turnover markers remained significantly reduced in the once-yearly zoledronic acid group compared with the once-in-2-years zoledronic acid and placebo groups at all time-points. These data demonstrate that although there are differences in biomarker levels between the zoledronic acid once-yearly and once-in-2-years dosing strategies, there is generally no difference in BMD outcomes. Therefore, in patients with low BMD (osteopenia) but not osteoporosis, it is recommended that a zoledronic acid 5-mg infusion is given once, and the patient followed up over time, with BMD and biomarker (if available) monitoring. Individual clinician judgment should be used to determine whether a second dose is necessary. However, if additional risk factors for osteoporosis are present, patients should be treated annually, as in patients with confirmed osteoporosis.
Table 2.
Between-treatment percentage change in bone mineral density in postmenopausal women with osteopenia receiving one of two zoledronic acid regimens or placebo for 24 months [McClung et al. 2009].
| Efficacy variable | ZOL 2×5 mg* n = 198 | ZOL 1×5 mg$ n = 181 | Placebo n = 202 |
|---|---|---|---|
| Lumbar spine BMD | |||
| LSM (SE) | 5.18 (0.272) | 4.42 (0.279) | −1.32 (0.268) |
| Total hip BMD | |||
| LSM (SE) | 2.91 (0.207) | 2.28 (0.216) | −1.45 (0.202) |
| Femoral neck BMD | |||
| LSM (SE) | 2.20 (0.294) | 1.64 (0.308) | −1.35 (0.288) |
| Trochanter BMD | |||
| LSM (SE) | 4.83 (0.278) | 4.16 (0.290) | −1.15 (0.272) |
| Distal radius BMD | |||
| LSM (SE) | −0.07 (0.255) | −0.18 (0.266) | −2.39 (0.248) |
ZOL 2×5 mg, zoledronic acid received at randomization and at month 12;
ZOL 1×5 mg, zoledronic acid received at randomization and placebo received at Month 12.
n, number of subjects; BMD, bone mineral density; LSM, least squares mean; SE, standard error.
Figure 3.
Percentage change in bone mineral density from baseline in postmenopausal women with osteopenia receiving one of two zoledronic acid regimens or placebo at 12 and 24months [McClung et al. 2009].
Glucocorticoid-induced osteoporosis
Bisphosphonate treatment is the current standard of care for GIO [Mok et al. 2008; Saag et al. 2007b]. Randomized clinical trials have demonstrated superiority of alendronate over the vitamin D3 analog alfacalcidol in the treatment of GIO [De Nijs et al. 2006], and risedronate is proven to be effective in both the prevention [Cohen et al. 1999] and the treatment [Reid et al. 2000] of the condition. Zoledronic acid and risedronate are approved by the US Food and Drug Administration (FDA) both for prevention and treatment of GIO, while alendronate is approved for the treatment of GIO.
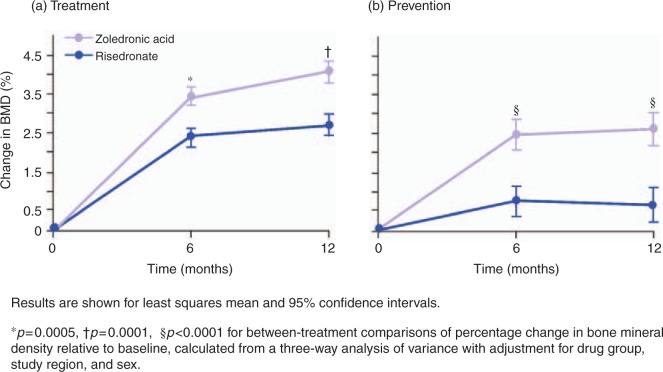
In a 1-year randomized, double-blind, double-dummy, non-inferiority trial, the efficacy of intravenous zoledronic acid was compared with that of daily oral risedronate for treatment and prevention of GIO [Reid et al. 2009]. At baseline, 833 patients were stratified by gender, and by duration of glucocorticoid therapy, with those receiving treatment for 3 months or less assigned to the ‘prevention’ category, and those having received more than 3 months of treatment assigned to the ‘treatment’ category. Patients received a single zoledronic acid (5 mg) infusion or oral risedronate 5mg/day for 12months. The primary endpoint was the percentage change from baseline in lumbar spine BMD, and secondary endpoints included BMD at other appendicular sites, changes in bone turnover marker levels, and safety outcomes. Results of the study showed that zoledronic acid was not only noninferior, but also superior to risedronate in increaseing lumbar spine BMD both in the prevention (zoledronic acid, 2.6%; risedronate, 0.6%; p < 0.0001) and treatment (zoledronic acid, 4.1%; risedronate, 2.7%; p = 0.0001) subpopulations at 12 months (Figure 4). Zoledronic acid was also significantly more effective than risedronate at 12 months in increasing femoral neck (treatment: 1.5 versus 0.4%, p = 0.005; prevention: 1.3 versus 0.0%, p = 0.005), trochanter (treatment: 2.0 versus 0.6%, p = 0.0005; prevention: 2.8 versus 0.5%, p < 0.0001), and total hip (treatment: 1.6 versus 0.4%, p < 0.0001; prevention: 1.5 versus 0.0%, p < 0.0001) BMD and reducing bone turnover markers (βbT-CTx and N-terminal propeptide; both p < 0.05in the prevention and treatment subgroups).
Figure 4.
Percentage change over time in bone mineral density at the lumbar spine in patients receiving oral risedronate 5 mg/day or a single zoledronic acid 5mg infusion for (a) treatment or (b) prevention of glucocorticoid-induced osteoporosis [Reid et al. 2009]. Reprinted with permission from The Lancet.
Rheumatoid arthritis-related bone loss
The efficacy of zoledronic acid in preventing erosions in bone has also been investigated in patients with rheumatoid arthritis, in whom osteoclast activity is central to the development of bone damage. In a proof-of-concept study, patients receiving methotrexate (7.5–20 mg/week) for early rheumatoid arthritis were randomized to receive either adjuvant zoledronic acid (5 mg) or placebo (given at baseline and week 13) [Jarrett et al. 2006]. Results showed that zoledronic acid produced a reduction of 61% in bone erosions in the hand and wrist (assessed by magnetic resonance imaging) versus placebo (mean±SD: 0.9 ± 1.6 versus 2.3 ± 3.1). The mean increase in the number of hand and wrist bones with erosions was significantly lower with zoledronic acid compared with placebo (0.3 ± 0.8 versus 1.4 ± 1.8; p = 0.029).
Hormone-ablative therapy-induced bone loss
Hormone-ablative therapies such as gonadotropin-releasing hormone (GnRH), luteinizing hormone-releasing hormone (LHRH) agonists, antiandrogens, and aromatase inhibitors (AIs) are often used in patients with early-stage breast cancer or high-risk prostate cancer to delay progression or prevent recurrence of the disease. However, these therapies can significantly affect estrogen or testosterone levels, leading to loss of bone mass, and zoledronic acid has again proven useful in these patient populations in preserving skeletal integrity [Brufsky, 2008; Pfeilschifter and Diel, 2000].
In the Austrian Breast and Colorectal Cancer Study Group trial, zoledronic acid was found to be effective in counteracting AI-induced bone loss (AIBL) in 401 premenopausal women with hormone-responsive breast cancer treated with adjuvant endocrine therapy (goserelin plus either anastrozole or tamoxifen). The addition of zoledronic acid (4mg every 6 months over 3 years) demonstrated inhibition of bone loss in the lumbar spine and trochanter and improvements in lumbar spine T-scores that were significantly greater than goserelin plus anastrozole alone (p < 0.0001; Figure 5) [Gnant et al. 2007]. Similar results were observed in the Zometa/Femara Adjuvant Synergy Trial (ZFAST), where in 602 postmenopausal women with stage I—IIIa estrogen – and/or progesterone-receptor-positive breast cancer receiving adjuvant letrozole therapy, zoledronic acid 4mg given every 6 months appeared to prevent AIBL [Brufsky, 2008].
Figure 5.
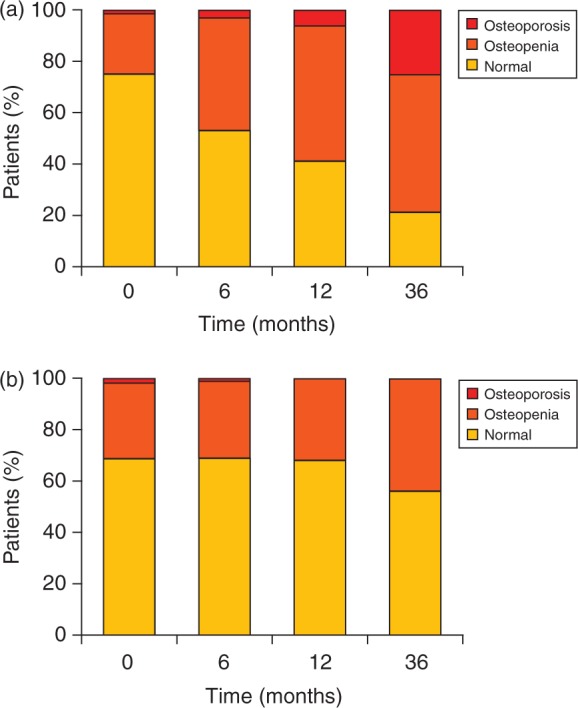
Premenopausal patients with hormone-responsive breast cancer treated with goserelin and anastrozole (a) without zoledronic acid or (b) with zoledronic acid. Graph shows the proportion of patients with normal bone mineral density, osteopenia, or osteoporosis in the lumbar spine [Gnant et al. 2007]. Reprinted with permission from the Journal of Clinical Oncology.
In men with non-metastatic prostate cancer receiving a GnRH agonist, zoledronic acid 4mg given once every 3 months for 1 year prevented androgen deprivation-induced bone loss at the hip and lumbar spine. Mean lumbar spine BMD was increased by 5.6% in the zoledronic acid group versus a 2.2% decrease in the placebo group (p < 0.001). Respective values at other measured sites were: femoral neck, 1.2% versus −2.1%; trochanter, 2.2% versus −2.7%; and total hip, 1.1% versus −2.8% (p < 0.001 for all) [Smith et al. 2003]. In a subsequent trial in men with nonmetastatic prostate cancer treated with a GnRH, zoledronic acid had similar effects on BMD [Michaelson et al. 2007].
Safety of intravenous bisphosphonate therapy
Zoledronic acid has been generally well tolerated in clinical trials conducted to date [Reid et al. 2009; Black et al. 2007; Devogelaer et al. 2007; Lyles et al. 2007; McClung et al. 2007]. The most common adverse events during treatment with zoledronic acid are pyrexia, influenza-like symptoms, myalgia, headache, and arthralgia, usually occurring within 3 days of infusion. These symptoms affect approximately 30% of patients at the first infusion and less than 7% at the second [Black et al. 2007]. Furthermore, there is evidence that patients can be switched safely from other bisphosphonates to zoledronic acid [McClung et al. 2007]. In patients switched from weekly oral alendronate (70 mg) to zoledronic acid, the overall incidence of adverse events was similar compared with those who continued alendronate (zoledronic acid, 86.7%; alendronate, 80.4%) [McClung et al. 2007], although headache occurred more commonly within the first 3 days after infusion with zoledronic acid (12.4%) than with alendronate (6.3%).
The renal safety of zoledronic acid in women with postmenopausal osteoporosis was investigated in a predefined renal safety cohort of HORIZON-PFT [Boonen et al. 2008b]. Short-term measurements (before and after each of the three annual infusions) of serum creatinine, estimated creatinine clearance and urinary protein were made in 5035 patients, and annual measurements over 3 years were taken in 7714 patients. Transient pre- to post-infusion rises in serum creatinine were seen in 31 patients receiving zoledronic acid and 10 receiving placebo. The increases resolved within 12 months in all except two patients who had received placebo. The results showed no cumulative renal toxicity with repeated infusions. The analysis also showed that the skeletal response to zoledronic acid was not affected by renal impairment, with histomorphometry, biomarkers of bone turnover, BMD and antifracture efficacy showing similar results in patients with creatinine clearance <60mL/min compared with those with higher creatinine clearance [Boonen et al. 2008a].
‘Real-world’ postmarketing data show that in the 17 months since approval (as of February 2009), the US FDA's Adverse Event Reporting System (AERS) received 24 evaluable cases of renal impairment and acute renal failure associated with use of zoledronic acid (Reclast®) for osteoporosis in postmenopausal women and men, Paget's disease of bone, and the prevention and treatment of GIO. Fourteen of the 24 patients had underlying medical conditions associated with risk of renal impairment or acute renal failure, or had been exposed to nephrotoxic drugs, in spite of label warnings regarding the use of zoledronic acid 5 mg in such patients. In 13 of the 24 cases, patients had documented transient increases in serum creatinine (median increase 4mg/dL) following drug infusion. Many patients improved following iv fluid administration or other supportive care. Three patients required haemodialysis. Seven patients died; four due to acute renal failure, although any association between zoledronic acid use and these deaths was deemed difficult to establish.
Based on—postmarketing reports, the Reclast® label was updated in March 2009 to include data on acute renal failure and to reinforce existing warnings, with recommendations to monitor serum creatinine in patients with pre-existing renal compromise or other risk factors, including concomitant nephrotoxic medications or diuretic therapy, before infusion [US Food and Drug Administration (FDA), 2009].
A notable adverse event that has been reported in cancer patients receiving more frequent dosing regimens of iv bisphosphonates is osteonecrosis of the jaw (ONJ). These reports have prompted intense scrutiny in other patient populations receiving bisphosphonates, to establish the incidence of this event in the absence of anticancer agents or corticosteroids [Rizzoli et al. 2008]. In HORIZON-PFT and HORIZON-RFT there were no instances of spontaneously reported ONJ in a total of 4929 women who received three (n = 3875) or one infusion (n = 1054) of zoledronic acid 5 mg [Grbic et al. 2008; Lyles et al. 2007]. A detailed analysis of safety data from HORIZON-PFT was carried out by an independent, blinded, expert adjudication committee, who assessed all maxillofacial adverse events [Grbic et al. 2008]. Their review showed that one patient in each group (zoledronic acid and placebo) had a lesion meeting a conservative ONJ criteria (exposed bone in the maxillofacial area that does not heal within 6 weeks after identification of the lesion). In both cases, the event resolved with appropriate care (antibiotic therapy, debridement or both). Thus, use of stringent diagnostic criteria and expert adjudication found no evidence for an increased risk of ONJ in patients receiving one to three annual infusions of zoledronic acid in a non-oncology population.
In HORIZON-PFT, serious atrial fibrillation occurred more frequently in the zoledronic acid group (50 patients [1.3%]) than in the placebo group (20 patients [0.5%]; p < 0.001) [Black et al. 2007]. These findings were not confirmed in the HORIZON-RFT study, which consisted of patients who were older with greater cardiovascular comorbidity, and who were therefore at greater risk for cardiac complications [Lyles et al. 2007]. The potential for a mechanistic association between bisphosphonate use and atrial fibrillation was subsequently investigated and published by the FDA, based on data from 19,687 bisphosphonate-treated patients and 18,358 placebo-treated patients who were followed for 6months to 3 years [US Food and Drug Administration (FDA), 2008]. The FDA concluded that there is no clear association between bisphosphonate use and atrial fibrillation, and advised that “healthcare professionals should not alter their prescribing patterns for bisphosphonates and patients should not stop taking their bisphosphonate medication”.
Patient preference for intravenous bisphosphonate therapy
Intravenous bisphosphonate therapy is generally preferred by patients to more frequently dosed regimens. In a 24-week, randomized, double-blind clinical trial that assessed the onset of action of a single 15-minute infusion of zoledronic acid 5mg (n = 69) compared to oral weekly alendronate 70mg (n = 59), at the end of the study, patients were asked to respond to four questions to determine their preference for the different treatment modalities [Saag et al. 2007a]. While still blinded to therapy, patients were asked which treatment was (1) more convenient, (2) more satisfying, (3) they would be more willing to take for a long period of time, and (4) was preferred. Overall, 66.4% of the patients who completed the questionnaire (n = 122) expressed a preference for a once-yearly iv infusion, compared with 19.7% who preferred a once-weekly pill. In total, 13.9% indicated that both treatment modalities were equal (Table 3). Similarly, in a 1-year, randomized, double-blind clinical trial of postmenopausal women with low BMD on alendronate who were randomized to oral weekly alendronate 70mg continuation (n = 112) or switched to zoledronic acid (n = 113), 78.7% of patients preferred a once-yearly i.v. infusion [McClung et al. 2007] (Table 3).
Table 3.
Patient preference for yearly intravenous versus weekly oral treatment regimens.
| Patient preference questionnaire (% of responders) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Once-yearly iv | Once-weekly pill | Both are equal | |||||||
| Saag et al. 2007a | McClung et al. 2007 | Reid et al. 2009 | Saag et al. 2007a | McClung et al. 2007 | Reid et al. 2009 | Saag et al. 2007a | McClung et al. 2007 | Reid et al. 2009 | |
| More convenient | 66.4 | 79.2 | 81.0 | 15.6 | 7.2 | 9.0 | 18.0 | 13.6 | 10.0 |
| More satisfying | 59.8 | NA | 78.0 | 18.9 | NA | 8.0 | 20.5 | NA | 14.0 |
| Better fits lifestyle | NA | 72.4 | NA | NA | 8.1 | NA | NA | 19.5 | NA |
| More willing to take for a long time | 68.0 | 71.5 | 84.0 | 15.6 | 9.0 | 9.0 | 16.4 | 19.5 | 7.0 |
| Overall preference | 66.4 | 78.7 | NP | 19.7 | 9.0 | NP | 13.9 | 11.8 | NP |
NA, not applicable; NP, results not presented.
Patient preference was also assessed at the end of the 1-year randomized, double-blind, noninferiority trial of intravenous zoledronic acid versus daily oral risedronate for the treatment and prevention of GIO [Reid et al. 2009]. Most patients (n = 785/833) stated a preference and, of these, 81% preferred the intravenous preparation for convenience, and 78% for satisfaction (Table 3).
Conclusions
Oral bisphosphonates are considered the standard of care for most patients initiating therapy for the management of osteoporosis; however, efficacy can be limited by poor patient compliance, both in the short and long term. This disappointing aspect of oral treatment has fuelled the investigation of more convenient and efficacious dosing regimens, most notably once-yearly administration of zoledronic acid, which has a short infusion time (15 minutes) and a favourable pharmacological profile (rapid uptake, low desorption, high reattachment after release from bone and low diffusion within bone).
Large, randomized, double-blind clinical trials have shown that zoledronic acid is the most effective antiresorptive in reducing fracture risk and prevention of subsequent fractures, as well as demonstrating robust efficacy in a number of allied osteoporosis indications. Importantly, patients can be switched safely from alendronate to zoledronic acid with no loss of efficacy, and the majority of patients stated that they preferred once-yearly iv infusion to weekly oral treatment.
In conclusion, zoledronic acid 5 mg once-yearly infusion has demonstrated marked efficacy in the treatment and prevention of primary and secondary osteoporosis across a broad spectrum of patients, with a combination of fracture risk reduction and prevention of bone loss at key sites, persistent efficacy and good compliance. It is the only agent shown to reduce the incidence of fracture and mortality in patients with a previous low-trauma hip fracture. These efficacy benefits are balanced with a generally well tolerated and manageable safety profile.
Acknowledgements
In would like to acknowledge Ms Sola Neunie for the editorial support she provided during the preparation of this manuscript.
This article was funded by Novartis Pharmaceuticals.
Footnotes
René Rizzoli has served in the speaker bureau or advisory boards for Amgen, Danone, Eli Lilly, Merck Sharp & Dohme, Novartis, Nycomed Roche-Glaxo-Smith-Klein and Servier.
References
- Black D.M., Delmas P.D., Eastell R., Reid I.R., Boonen S., Cauley J. A., et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809–1822 [DOI] [PubMed] [Google Scholar]
- Black D.M., Eastell R., Cosman F., Man Z., Bucci-Rechtweg C., Mesenbrink P. (2009) Effect of once-yearly zoledronic acid 5 mg on ‘super six’ non-vertebral fractures. Bone 44(Suppl 2): S429 [Google Scholar]
- Boonen S., Black D., Sellmeyer D., Eriksen E.F., Bone H., Skag A. (2008a) The skeletal response to zoledronic acid is not affected by renal impairment: results from the HORIZON-PFT study. Ann Rheum Dis 67(Suppl II): 402 [Google Scholar]
- Boonen S., Sellmeyer D.E., Lippuner K., Orlov-Morozov A., Abrams K., Mesenbrink P., et al. (2008b) Renal safety of annual zoledronic acid infusions in osteoporotic postmenopausal women. Kidney Int 74: 641–648 [DOI] [PubMed] [Google Scholar]
- Boonen S., Vanderschueren D., Venken K., Milisen K., Delforge M., Haentjens P. (2008c) Recent developments in the management of postmenopausal osteoporosis with bisphosphonates: enhanced efficacy by enhanced compliance. J Intern Med 264: 315–332 [DOI] [PubMed] [Google Scholar]
- Boonen S., Black D.M., Colón-Emeric C., Delmas P., Eastell R., Magaziner J., et al. (2009) Annual treatment with zoledronic acid continues to be effective in old age. Osteoporos Int 20(Suppl 1): S5-S22 [Google Scholar]
- Brufsky A.M. (2008) Cancer treatment-induced bone loss: pathophysiology and clinical perspectives. Oncologist 13: 187–195 [DOI] [PubMed] [Google Scholar]
- Cohen S., Levy R.M., Keller M., Boling E., Emkey R. D., Greenwald M., et al. (1999) Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 42: 2309–2318 [DOI] [PubMed] [Google Scholar]
- Colón-Emeric C., Kuchibhatla M., Pieper C., Hawkes W., Fredman L., Magaziner J., et al. (2003) The contribution of hip fracture to risk of subsequent fractures: data from two longitudinal studies. Osteoporos Int 14: 879–883 [DOI] [PubMed] [Google Scholar]
- Conte P., Guarneri V. (2004) Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 9(Suppl 4): 28–37 [DOI] [PubMed] [Google Scholar]
- Cramer J.A., Amonkar M.M., Hebborn A., Altman R. (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21: 1453–1460 [DOI] [PubMed] [Google Scholar]
- De Nijs R.N., Jacobs J.W., Lems W.F., Laan R.F., Algra A., Huisman A. M., et al. (2006) Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med 355: 675–684 [DOI] [PubMed] [Google Scholar]
- Devogelaer J.P., Brown J.P., Burckhardt P., Meunier P.J., Goemaere S., Lippuner K., et al. (2007) Zoledronic acid efficacy and safety over five years in postmenopausal osteoporosis. Osteoporos Int 18: 1211–1218 [DOI] [PubMed] [Google Scholar]
- Eastell R., Black D.M., Boonen S., Adami S., Felsenberg D., Lippuner K., et al. (2009) Effect of once-yearly zoledronic acid 5 mg on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab 94: 3215–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen E.F., Lyles K.W., Colón-Emeric C.S., Pieper C.F., Magaziner J. S., Adachi J. D., et al. (2009) Antifracture efficacy and reduction of mortality in relation to timing of first dose of zoledronic acid after hip fracture. J Bone Miner Res 24: 1308–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency Assessment report for Aclasta, Type II variation. European Medicines Agency. EMEA/H/C/000595/II/0016. (2008) Available at: www.emea.europa.eu/humandocs/PDFs/EPAR/aclasta/Aclasta-H-535-II-16-AR.pdf Accessed 12 June 2009.
- Gnant M.F., Mlineritsch B., Luschin-Ebengreuth G., Grampp S., Kaessmann H., Schmid M., et al. (2007) Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 25: 820–828 [DOI] [PubMed] [Google Scholar]
- Grbic J.T., Landesberg R., Lin S.Q., Mesenbrink P., Reid I. R., Leung P. C., et al. (2008) Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the health outcomes and reduced incidence with zoledronic acid once yearly pivotal fracture trial. J Am Dent Assoc 139: 32–40 [DOI] [PubMed] [Google Scholar]
- Huybrechts K.F., Ishak K.J., Caro J.J. (2006) Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone 38: 922–928 [DOI] [PubMed] [Google Scholar]
- Jarrett S.J., Conaghan P.G., Sloan V.S., Papanastasiou P., Ortmann C. E., O'Connor, et al. (2006) Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum 54: 1410–1414 [DOI] [PubMed] [Google Scholar]
- Lippuner K., Eastell R., Reid D.M., Bengtsson C., Recknor C., Ish-Shalom S. (2008) Effect of previous bisphosphonate use on response to zoledronic acid: results from the HORIZON-PFT study. Ann Rheum Dis 67(Suppl II): 57 [Google Scholar]
- Lo J.C., Pressman A.R., Omar M.A., Ettinger B. (2006) Persistence with weekly alendronate therapy among postmenopausal women. Osteoporos Int 17: 922–928 [DOI] [PubMed] [Google Scholar]
- Lyles K.W., Colón-Emeric C.S., Magaziner J.S., Adachi J.D., Pieper C. F., Mautalen C., et al. (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357: 1799–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotti G., Giustina A., Canalis E., Bilezikian J.P. (2007) Glucocorticoid-induced osteoporosis: clinical and therapeutic aspects. Arq Bras Endocrinol Metabol 51: 1404–1412 [DOI] [PubMed] [Google Scholar]
- McClung M., Recker R., Miller P., Fiske D., Minkoff J., Kriegman A., et al. (2007) Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 41: 122–128 [DOI] [PubMed] [Google Scholar]
- McClung M.R., Miller P., Recknor C., Bucci-Rechtweg C., Yu S., Benhamou C.L. Efficacy and safety of zoledronic acid 5 mg in the prevention of osteoporosis in postmenopausal women with osteopenia: The HORIZON Prevention Study. (2009) Osteoporos Int 20(Suppl 2): S191–S22919291345 [Google Scholar]
- Michaelson M.D., Kaufman D.S., Lee H., McGovern F.J., Kantoff P. W., Fallon M. A., et al. (2007) Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol 25: 1038–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok C.C., Tong K.H., To C.H., Siu Y.P., Ma K.M. (2008) Risedronate for prevention of bone mineral density loss in patients receiving high-dose glucocorticoids: a randomized double-blind placebocontrolled trial. Osteoporos Int 19: 357–364 [DOI] [PubMed] [Google Scholar]
- Nancollas G.H., Tang R., Phipps R.J., Henneman Z., Guide S., Wu W., et al. (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38: 617–627 [DOI] [PubMed] [Google Scholar]
- Novartis Europharm Limited (2008) Aclasta summary of product characteristicsNovartis Europharm Limited: Horsham, United Kingdom [Google Scholar]
- Pfeilschifter J., Diel I.J. (2000) Osteoporosis due to cancer treatment: pathogenesis and management. J Clin Oncol 18: 1570–1593 [DOI] [PubMed] [Google Scholar]
- Recker R.R., Delmas P.D., Halse J., Reid I.R., Boonen S., Garcia-Hernandez P.A., et al. (2008) Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. J Bone Miner Res 23: 6–16 [DOI] [PubMed] [Google Scholar]
- Reid D.M., Hughes R.A., Laan R.F., Sacco-Gibson N.A., Wenderoth D. H., Adami S., et al. (2000) Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-induced Osteoporosis Treatment Study. J Bone Miner Res 15: 1006–1013 [DOI] [PubMed] [Google Scholar]
- Reid I.R., Brown J.P., Burckhardt P., Horowitz Z., Richardson P., Trechsel U., et al. (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346: 653–661 [DOI] [PubMed] [Google Scholar]
- Reid D.M., Delmas P.D., Bone H., Skag A., Giannini S., Lippuner K., et al. (2008) Zoledronic acid reduces fractures and increases bone mineral density with and without concomitant osteoporosis therapy: results from the HORIZON-PFT study. Ann Rheum Dis 67(Suppl II): 635 [Google Scholar]
- Reid D.M., Devogelaer J.P., Saag K., Roux C., Lau C. S., Reginster J. Y., et al. (2009) Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 373: 1253–1263 [DOI] [PubMed] [Google Scholar]
- Rizzoli R., Burlet N., Cahall D., Delmas P.D., Eriksen E. F., Felsenberg D., et al. (2008) Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone 42: 841–847 [DOI] [PubMed] [Google Scholar]
- Robbins J., Aragaki A.K., Kooperberg C., Watts N., Wactawski-Wende J., Jackson R.D., et al. (2007) Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA 298: 2389–2398 [DOI] [PubMed] [Google Scholar]
- Ryg J., Rejnmark L., Overgaard S., Brixen K., Vestergaard P. (2009) Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977–2001. J Bone Miner Res 24: 1299–1307 [DOI] [PubMed] [Google Scholar]
- Saag K., Lindsay R., Kriegman A., Beamer E., Zhou W. (2007a) A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone 40: 1238–1243 [DOI] [PubMed] [Google Scholar]
- Saag K.G., Shane E., Boonen S., Marin F., Donley D. W., Taylor K.A., et al. (2007b) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357: 2028–2039 [DOI] [PubMed] [Google Scholar]
- Silverman S. (2006) Adherence to medications for the treatment of osteoporosis. Rheum Dis Clin North Am 32: 721–731 [DOI] [PubMed] [Google Scholar]
- Siris E.S., Harris S.T., Rosen C.J., Barr C.E., Arvesen J. N., Abbott T. A., et al. (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81: 1013–1022 [DOI] [PubMed] [Google Scholar]
- Smith M.R., Eastham J., Gleason D.M., Shasha D., Tchekmedyian S., Zinner N. (2003) Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 169: 2008–2012 [DOI] [PubMed] [Google Scholar]
- Suzuki K., Takeyama S., Sakai Y., Yamada S., Shinoda H. (2006) Current topics in pharmacological research on bone metabolism: inhibitory effects of bisphosphonates on the differentiation and activity of osteoclasts. J Pharmacol Sci 100: 189–194 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (FDA) (2008) Update of safety review follow-up to the October 1, 2007 early communication about the ongoing safety review of bisphosphonates.
- US Food and Drug Administration (FDA) (2009) Drug Safety Newsletter. Volume 2, Number 2 Available at: www.fda.gov/Drugs/DrugSafety/DsrugSafetyNewsletter/default.htm Accessed 31 July 2009 [Google Scholar]
- Weiss H.M., Pfaar U., Schweitzer A., Wiegand H., Skerjanec A., Schran H. (2008) Biodistribution and plasma protein binding of zoledronic acid. Drug Metab Dispos 36: 2043–2049 [DOI] [PubMed] [Google Scholar]
- Yood R.A., Emani S., Reed J.I., Lewis B.E., Charpentier M., Lydick E. (2003) Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int 14: 965–968 [DOI] [PubMed] [Google Scholar]