Abstract
In osteoarthritis (OA), the alterations in joint tissues are numerous and involve morphological, biochemical and metabolic changes and an upregulation of the inflammatory pathways. The focus of this article is a brief narrative review of the effects of diacerein, an antirheumatic drug from the anthraquinone chemical class, and its active metabolite, rhein, on the factors that participate in the complex interaction between OA tissues and cells leading to the progression of joint structural changes.
Keywords: cartilage, diacerein, osteoarthritis, rhein, subchondral bone, synovial membrane
Introduction
Osteoarthritis (OA) is a condition that represents a pathological imbalance in the degradative and reparative processes of the articular tissues. Although we still do not completely understand what initiates the degeneration of the articular tissues, significant progress has been made with respect to the pathogenesis of the disease. There is now evidence of a global cross-talk between the joint tissues, with the diffusion of catabolic factors from the synovial membrane and subchondral bone to the cartilage.
Although OA is characterized by a degeneration of articular cartilage, at the clinical stage of the disease this is accompanied by changes in the synovial membrane where an inflammatory reaction is often observed [Martel-Pelletier et al. 2005]. In addition, a complex relationship between the subchondral bone and the cartilage is currently regarded as a major pathophysiological factor in the progression of OA. Indeed, some continuity between subchondral bone and cartilage in OA has been demonstrated, which suggests a cross-talk between these tissues [Martel-Pelletier et al. 2007].
One hypothesis regarding the pathological development of OA at the clinical stage of the disease can be summarized as follows [Martel-Pelletier et al. 2005]. The cartilage matrix is first broken down by proteolytic enzymes. Matrix fragments are released into the fluid, which can promote inflammation in the synovial membrane.
The inflammation of the membrane, through the synthesis of mediators, creates a vicious circle, in which the cartilage matrix is further degraded, subsequently provoking more inflammation. Several soluble mediators have been identified in articular tissues from arthritic diseases and studies have shown that inflammation in knee OA is primarily due to the presence of the cytokine interleukin-1b (IL-1b). Thus, IL-1b plays a fundamental role in the pathophysiology of OA, in which its catabolic effects are multiple: this cytokine is able to stimulate its own production, to increase the synthesis of catabolic factors as well as chondrocyte apoptosis, and to decrease some of the cartilage macromolecule synthesis. Therefore, targeting this cytokine and related factors is of great importance in therapeutic approaches to OA.
Diacerein/rhein
The current therapies for OA, including the non-steroidal anti-inflammatory drugs (NSAIDs), although effective against the disease symptoms, are palliative and do not stop the disease progression. There are, however, promising agents and compounds that have been shown to reduce the severity of the disease as well as the symptoms. Among them is diacerein, a drug belonging to the anthraquinone chemical class that is employed in the treatment of OA.
This article is a brief review of how its mechanism of action differs from that of a classic NSAID. Contrary to a classic NSAID that targets cyclo-oxygenase (COX)-2, an enzyme responsible for prostaglandin production, diacerein is known to act on the IL-1b system.
Pharmacokinetics of diacerein/rhein
Diacerein in the body is entirely converted into rhein before reaching the systemic circulation. Rhein is either eliminated by the renal route or conjugated in the liver to rhein glucuronide and rhein sulfate. In turn, these metabolites are mainly eliminated by the kidneys [Nicolas et al. 1998]. Data also showed that the pharmacokinetics of diacerein are about the same in young].healthy volunteers and elderly people, both after a single dose of 50 mg or twice daily for a total dose of 100 mg or 150 mg [Nicolas et al. 1998; Fedeli, 1988; Petitjean et al. 1991]. Pharmacokinetic studies of diacerein performed on healthy volunteers revealed that the plasma peak concentration of rhein after an oral administration was 10−5M [Nicolas et al. 1998; Spencer and Wilde, 1997]. Moreover, a further study [Segré, 1988, reported in Sanchez et al. 2003], showed that following daily administration of oral diacerein at 50 mg every 12 hours for 1 month, rhein reaches the synovial fluid at concentrations of 10−6–10−5M.
In in vitro studies, the concentrations most used varied between 10−7M and 10−4M. The majority of the studies employed concentrations around The biological activation of cells by cytokines is 10−5 M, which is in the higher range reached in the synovial fluid. However, as treatment with diacerein is characterized by a slow onset of action, with a maximal clinical effect being reached after a few months (about 3 months), the concentrations Utilized for in vitro studies thus mimic the effect observed in vivo attained after months of treatment.
Effects on cartilage and synovial membrane cells
IL-1B (Figure 1). Evaluation of the effects of diacerein and its active metabolite, rhein, on the production of IL-1B in human OA synovial membrane and cartilage showed that both drugs significantly decreased the synthesis of this cytokine (Table 1) [Martel-Pelletier et al. 1998]
Figure 1.
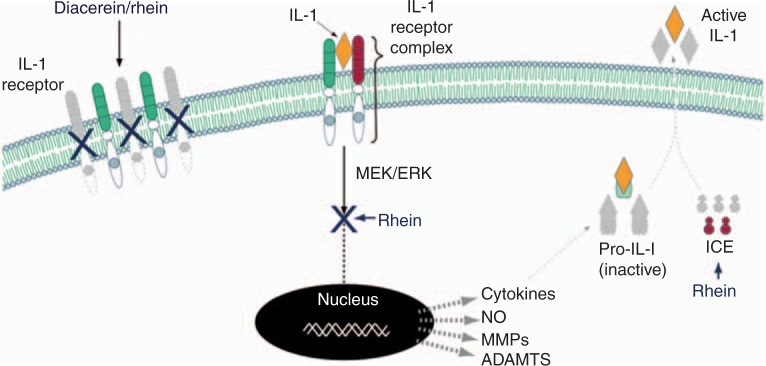
Effect of diacerein/rhein on the IL-1 system. Diacerein/rhein reduces the level of IL-1 receptors leading to fewer receptors to form heterodimer complexes. Following the association of IL-1 with its specific cell surface receptor complex, there is an activation of downstream signalling pathways involving some MAP kinases. In OA articular tissues, rhein has been shown to reduce numerous genes including cytokines, nitric oxide (NO), metalloproteases (MMPs), a disintegrin and metalloproteinase domain with thrombospondin motifs (ADAMTS), etc., through the inhibition of the MEK/ERK intracellular cascade. IL-1B is produced as a precursor which is cleaved at the cell membrane by the IL-1 converting enzyme (ICE) which releases IL-1B as an active cytokine into the extracellular matrix. Rhein reduces the production of ICE leading to a reduction in IL-1B activation. The grey color indicates that lesser amounts of the factors are produced. The figure is from and reproduced with permission of TRB Chemedica International. MEK = mitogen-activated protein kinase (MAP kinase), ERK = extracellular-signal-regulated kinase.
Table 1.
Summary of the effects of diacerein/rhein on articular joint tissues/cells.
| Tissues/cells | Effects |
|---|---|
| Cartilage/chondrocytes and synovial membrane/synoviocytes | ↓IL-1β system (IL-1β, ICE, IL-1RI) |
| ↓IL-1β -induced MMP-3, collagenase, ADAMTS-4, ADAMTS-5, NO, iNOS | |
| ↑ IL-1β -induced PGE2, COX-2 | |
| ↓IL-1β -inhibition of collagen, proteoglycans | |
| Subchondral bone | ↓MMP-13 |
| Osteoblasts | ↓ Vitamin D3-induced osteocalcin |
| ↓uPA | |
| ↑PGE2/COX-2 | |
| Osteoclasts | ↓MMP-13, cathepsin K |
| ↓Survival, differentiation |
ADAMTS, disintegrin and metalloproteinase domain with thrombospondin motifs; COX-2, cyclooxygenase-2; ICE, IL-1 converting enzyme; IL-1β, interleukin-1β IL-1RI, IL-1 receptor type I; iNOS, inducible nitric oxide synthase; MMP, metalloprotease; NO, nitric oxide; PGE2, prostaglandin E2; uPA, urokinase-type plasminogen activator.
IL-1b is synthesized in the cell as a biologically inactive precursor, which requires a proteolytic cleavage to permit the activation of the cytokine and the exiting of the cell. This is achieved through a highly selective protease, the IL-1-converting enzyme (ICE), also named caspase-1. Hence, the action of ICE on IL-1p appears to be a key limiting factor for the secretion and activity of this cytokine. ICE has been shown to be expressed and synthesized by both human synovial membrane and cartilage and its levels are elevated during the OA process [Saha et al. 1999]. Diacerein and rhein markedly and significantly decreased ICE production in cartilage [Moldovan et al. 2000].
The biological activation of cells by cytokines is mediated through an association with specific cell surface receptors. For IL-1B, this occurs through binding to two types of specific membrane receptors, types I and II; type I was shown to be responsible for mediating the signal. The levels of this receptor type were also found to be markedly increased in OA chondrocytes and synovial fibroblasts, thus potentializing the effect of IL-1B activity [Sadouk et al. 1995; Martel-Pelletier et al. 1992; McCollum et al. 1991]. Investigation of the effects of diacerein and rhein on the binding and receptor levels in human OA chondrocytes showed that, at therapeutic concentrations, the drugs significantly inhibited the IL-1 binding level. Analysis of the competitive binding experiments revealed that both treated and untreated OA chondrocytes had similar IL-1 binding affinities, but that the receptor density, or number of receptors, was significantly reduced by both drugs [Martel-Pelletier et al. 1998].
Degradative enzymes
Further investigations were then directed to major catabolic pathways induced by IL-1B involved in the OA pathological process. In cartilage, the enzymatic matrix breakdown is a key feature in the progression of the disease. The loosening of the collagen network as well as the alterations in the aggrecan (proteoglycan) result from an increase in the amount of enzymes belonging to the MMP (metallo-proteases) and ADAMTS (disintegrin and metallo-proteinase domain with thrombospondin motifs) families. In regard to collagen degradation, three collagenases, MMP-1, MMP-8, and MMP-13, have been identified in humans, with high production levels found in OA. MMP-1 and MMP-13 are the major enzymes that account for collagen type II degradation in pathological cartilage. Moreover, it has been shown that in OA MMP-13 is produced during the remodelling phase, not only in the cartilage but also in the subchondral bone. Stromelysin-1 or MMP-3 is also considered an important enzyme in cartilage matrix turnover as, in addition to cleaving the proteoglycans, it is implicated in the enzymatic cascade responsible for the activation of proMMP-1. With regard to the proteoglycans, also found in OA articular tissues are aggrecan fragments with a proteolytic cleavage at the Glu373-Ala374 bond of the interglobular domain, between the G1 and G2 domains. The enzymes responsible for such cleavage belong to a subgroup of the ADAM family, the ADAMTS, and are named aggrecanases. Two such enzymes have been reported to be present in cartilage, ADAMTS-4 and ADAMTS-5. Recent studies in mice demonstrated that of the two aggrecanases, ADAMTS-5 is the predominant one involved in the OA degradative process [Stanton et al. 2005; Glasson et al. 2005]; however, in humans this still needs to be confirmed.
Data showed that diacerein and rhein significantly inhibited the IL-1β-stimulated MMP-3 and collagenase activity [Alvarez-Soria et al. 2008; Legendre et al. 2007; Sanchez et al. 2003; Tamura and Ohmori, 2001; Martel-Pelletier et al. 1998]. On the ADAMTS, both drugs decreased the IL-1β-stimulated ADAMTS-4 and ADAMTS-5 and a marked inhibition was found with rhein [Legendre et al. 2007].
Nitric oxide
Nitric oxide (NO) is produced through the activity of inducible nitric oxide synthase (iNOS) and is a major catabolic factor involved in the pathophysiology of OA. IL-1β is a very potent stimulator of NO. In OA, NO is involved in the promotion of cartilage catabolism and reduction in anabolism via a number of mechanisms. In brief, this factor reduces cartilage macromolecular synthesis and increases MMP activity, COX-2/prostaglandin E2 (PGE2) production, and apoptosis. Both diacerein and rhein treatments markedly and significantly decreased IL-1b-induced NO production [Sanchez et al. 2003; Pelletier et al. 1998]. Interestingly, in one experiment [Pelletier et al. 1998], in which an NSAID was used as comparator, data revealed that only a slight inhibition was obtained for the NSAID, and that occurred at a high concentration. This finding, among others, illustrates that these two classes of drugs have different mechanisms of action. Additional experiments also showed that diacerein and rhein reduced both the expression and production levels of iNOS [Pelletier et al. 1998].
Prostaglandin E2 and cyclooxygenase-2
The effect of diacerein was also investigated on PGE2 and on COX, as the latter is involved in one of the key steps in the synthesis of this prostanoid. As is well known, the spontaneous synthesis of PGE2 or COX-2 in chondrocytes is low and their production is markedly increased following IL-1β treatment. In contrast to the effects of NSAIDs that reduce PGE2 and COX-2, rhein and diacerein upregulate PGE2 and COX-2 production [Sanchez et al. 2003; Pelletier et al. 1998]. These data correlate with other studies done with diacerein on other cell types [Alvarez-Soria et al. 2008; Pomarelli et al. 1980]. Although such elevation could appear detrimental in the context of OA, it is of interest that a metabolite of COX-2, 15-deoxy PGJ2 (15d-PGJ2, displays anti-inflammatory properties. 15d-PGJ2 is a ligand of a nuclear receptor that exerts its effects possibly through binding to the peroxisome proliferator-activated receptor γ (PPARγ). The PPARs are a family of ligand-activated transcription factors, which, following ligand binding, heterodimerize with the retinoic X receptor (RXR) [Fahmi et al. 2002a]. This complex binds to PPAR-responsive elements (PPREs) in the promoter regions of target genes, thus inducing anti-inflammatory effects. Therefore, increasing COX-2 levels could lead to the formation of this metabolite, which would activate the PPARγ and abrogate the IL-1β-induced production of catabolic factors. Treatment of human chondrocytes with 15d-PGJ2 resulted in the inhibition of IL-1β-induced NO and MMP-13 as well as proteoglycan degradation [Fahmi et al. 2001, 2002a, 2002b; Bordji et al. 2000]. Moreover, this PPARY ligand also completely inhibited the effects of two other pro-inflammatory cytokines, tumor necrosis factor (TNF)-a and IL-17, on these cells. Similarly, PPARg activators suppressed IL-1b-induced MMP-1 expression and production in human OA synovial fibroblasts [Fahmi et al. 2002c] and IL-1β and TNF-a expression in rheumatoid synovial fibroblasts [Ji et al. 2001]. In rat synovial fibroblasts, which also express PPARg, 15d-PGJ2 dose-dependently prevented lipopolysaccharide (LPS)-induced iNOS, COX-2, IL-1 and TNF-a expression [Simonin et al. 2002]. The protective effect of PPARγ activators has also been demonstrated in several animal models of arthritis including a guinea pig model of OA [Kobayashi et al. 2005]. Data showed that diacerein and rhein increased the activation of PPARγ (authors' personal data). Thus, the effect of increasing COX-2 might not be as damaging as it might seem, as the increased level could lead to the formation of a metabolite that would, in turn, activate a nuclear receptor with anticatabolic effects.
Apoptosis
The role of chondrocyte death by apoptosis in cartilage degradation is likely an important local factor contributing to the loss of matrix, and it has been reported that apoptosis is implicated in the loss of chondrocytes in OA [Blanco et al. 1998]. The involvement of the caspase cascade in cell death by apoptosis is well documented, and the caspases-3, −8, and −9 are the primary enzymes involved in cell apoptosis. These enzymes induce cell death by a number of mechanisms, including DNA fragmentation and inactivation of the proteins that protect cells against apoptosis. Data showed that rhein at a physiological concentration did not affect caspases-3/7, but at a concentration of about 10 times the physiological concentration, it induced a marked decrease in the activity of these enzymes in both synovial fibroblasts and chondrocytes [Legendre et al. 2009]. However, these authors also showed that DNA fragmentation was not induced by rhein at any of the concentrations used.
Cartilage matrix macromolecules
Data showed that diacerein and rhein decreased the inhibitory effect of IL-1 on the synthesis of collagen and proteoglycans [Domagala et al. 2006; Sanchez et al. 2003; Pujol et al. 2000; Yaron et al. 1999]. It was further suggested that this may occur through the stimulation of transforming growth factor (TGF)-β1 expression [Felisaz et al. 1999], as diacerein counteracts the IL-1β downregulation of matrix synthesis [Redini et al. 1988].
Effects on subchondral bone
Investigations were also performed on the effects of diacerein on subchondral bone. It is currently suggested that alterations in the subchondral bone may be more intimately related to the OA process and are not merely a consequence of the disease. Indeed, although cartilage degradation characterizes OA, there is evidence that the remodelling of subchondral bone is a contributing factor. Interestingly, although it was originally thought that the calcified cartilage layer was an impenetrable structure, in OA the presence of channels and microcracks between the subchondral region and the uncalcified cartilage has been demonstrated as well as vascularization in the subchondral bone [Sokoloff, 1993], which could favour the diffusion of factors from the subchondral bone region to the basal layer of cartilage and be responsible for the cartilage remodelling in the deep zone. In addition, recent work indicates that biological and morphological disturbances occur in this tissue very early on in the OA process, which may contribute to the initial events of the pathological process. However, human OA subchondral bone osteoblasts demonstrate an altered metabolic activity or phenotype compared to normal, in which elevated levels of the bone markers alkaline phosphatase and osteocalcin, and enzymes including the urokinase/plasmin system and MMP-13, for example, are found [Massicotte et al. 2002; Hilal et al. 1998, 1999]. Moreover, data also demonstrated that human OA subchondral bone, although showing sclerosis at a later stage, undergoes phases of bone resorption [Kwan Tat et al. 2008], and emerging data indicate a generalized undermineralization of OA subchondral bone [Couchourel et al. 2009]. Thus, therapeutic strategies aimed at modifying the metabolism of subchondral bone may be indicated in the treatment of OA.
Evaluation of the effects of diacerein and rhein on OA subchondral bone (Table 1) osteoblasts revealed that on the cell biomarkers, these drugs dose-dependently inhibited vitamin D3-induced osteocalcin release [Pelletier et al. 2001]. This is interesting, as abnormally elevated osteocalcin levels have been observed in the subchondral bone of OA patients, and osteocalcin is believed to be involved in the local modulation of bone formation. Specifically, osteocalcin retards bone formation/mineralization. As OA subchondral bone is undermineralized, reducing osteocalcin would favour a more mineralized tissue. Of the metabolic factors, the production of urokinase-type plasminogen activator (uPA) by osteoblasts is inhibited by these drugs [Pelletier et al. 2001]. This reduction in uPA activity could retard bone formation by preventing the release of trapped growth factors, thus preventing further sclerosis. Moreover, the production of MMP-13 in this tissue is also inhibited by diacerein and rhein [Boileau et al. 2008]. This is important as MMP-13 acts directly to resorb bone; therefore, reducing the MMP-13 level would contribute to curbing bone resorption.
In bone biology, osteoblasts and osteoclasts contribute either alone or in combination to the remodelling process. The disturbance between the activities of these two cells is suggested to be responsible for the development of an altered bone metabolism. Investigation of diacerein and rhein on some parameters of the osteoclasts revealed that both drugs reduced not only MMP-13 but also cathepsin K [Boileau et al. 2008]. These data are important because, in osteoclasts, MMP-13 is known to work in conjunction with cathepsin K in the induction of bone resorption; therefore, the reduction in activity of these two enzymes would impact the balance between bone resorption and formation. Exploration of the effect of these drugs on osteoclast survival and differentiation showed that they effectively block not only the survival of mature osteoclasts but also the differentiation and proliferation of pre-osteoclasts into mature osteoclasts, the final effect being a reduction in the number of osteoclasts. Although further studies are needed to fully elucidate the precise mechanism of action of diacerein and rhein on osteoclasts, it may be related to their effect on PGE2, the levels of which, as mentioned above, are increased by these drugs in many cell types including human subchondral bone osteoblasts [Pelletier et al. 2001]. Hence, it has been reported that high PGE2 levels inhibited bone resorption and that human OA subchondral bone osteoblasts expressing low levels of PGE2 enhanced the formation of osteoclasts, whereas those expressing higher levels did not [Kwan Tat et al. 2008].
Effects on signalling pathways
The intracellular mechanisms by which these drugs exert their effect appear to occur through the down-regulation of the activation of some MAP kinases. Although other signalling pathways have been found for the different cells including the activation of c-Jun N-terminal kinase (JNK) on chondrocytes [Legendre et al. 2007; Martin et al. 2003] and p38 on osteoblasts [Boileau et al. 2008], it appears that on all articular cells, rhein reduces the catabolic pathways of OA through inhibition of MEK/ERK signalling [Boileau et al. 2008; Legendre et al. 2007; Domagala et al. 2006; Martin et al. 2003].
In vivo effects on the OA process in animal models and human clinical studies
The effect of diacerein was also studied in vivo in OA animal models, and data from studies on different animal models concur with those obtained in vitro with human articular cells. Indeed, in OA animal models, the drug has been shown to decrease the disease severity as well as the collagenase levels in the cartilage of dogs and rabbits [Brandt, 2006; Smith et al. 1999; Brandt et al. 1997; Mazieres et al. 1993, 1996], IL-1β and the loss of hydroxyproline and proteoglycans in mouse cartilage [Colville-Nash, 2002; Moore et al. 1997, 1998], iNOS and apoptosis in dog cartilage [Pelletier et al. 2003], and subchondral bone remodelling in sheep and rats [Tamura et al. 2002; Hwa et al. 2001; Ghosh et al. 1998].
Moreover, the conclusion of a meta-analysis [Rintelen et al. 2006] and data from a Cochrane review [Fidelix et al. 2006] indicate that oral diacerein demonstrated a good safety profile, was associated with significant improvement in symptoms of patients with hip and knee OA, had similar efficacy to NSAIDs but with a carry-over effect once treatment was stopped, and reduced NSAID consumption. In addition, an in vivo study in humans with hip OA showed that this drug demonstrates a structure-modifying effect [Dougados et al. 2001].
Conclusion
The data from basic research both in vitro and in vivo in animals provide evidence that diacerein treatment could impact the abnormal metabolism of OA articular tissues and cells by reducing the major catabolic processes, with a coherent body of evidence of its effect in clinical studies. Importantly, although an in vivo structure-modifying effect has been shown in humans with hip OA [Dougados et al. 2001], studies on the tissue structure need to be done in knee OA.
Even though such studies in knee OA have been impaired due to the imaging tools (X-ray) being unsatisfactory and having significant limitations, in recent years important advances have been made in the quantitative assessment of global structural changes in knee OA with the use of magnetic resonance imaging (MRI) to assess cartilage volume and thickness as well as synovial membrane and subchondral bone lesions [Raynauld et al. 2003, 2004, 2006, 2008a, 2008b; Pelletier et al. 2007, 2008; Berthiaume et al. 2005]. Such technology allows the analysis of OA disease progression over time and reduces the number of patients needed in clinical trials, improves retention of these patients, and reduces the overall costs and the length of clinical trials.
In conclusion, the current pharmacological management of OA is based primarily on the use of analgesics, NSAIDs, and antiCOX-2s. Although studies have confirmed the efficacy of NSAIDs and antiCOX-2s as symptomatic treatments for OA, these drugs have not proven to positively affect the natural course of OA in humans. The development of pharmacological agents capable of modifying the OA disease process is now crucial. In this context, basic research has shown that diacerein is an attractive candidate.
Acknowledgements
The authors thank Virginia Wallis for her assistance with the manuscript preparation.
Footnotes
JMP and JPP have received grants from Laboratoires Negma-Lerads, Toussus-le-Noble, France and from TRB Chemedica International S.A., Geneva, Switzerland, as well as being speakers for these two companies at international and local congresses and meetings.
References
- Alvarez-Soria M.A., Herrero-Beaumont G., Sanchez-Pernaute O., Bellido M., Largo R. (2008) Diacerein has a weak effect on the catabolic pathway of human osteoarthritis synovial fibroblast–comparison to its effects on osteoarthritic chondrocytes. Rheumatology (Oxford) 47: 627–633 [DOI] [PubMed] [Google Scholar]
- Berthiaume M.J., Raynauld J.P., Martel-Pelletier J., Labonté F., Beaudoin G., Bloch D. A., et al. (2005) Meniscal tear and extrusion are strongly associated with the progression of knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis 64: 556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco F.J., Guitian R., Vazquez-Martul E., de Toro F.J., Galdo F. (1998) Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum 41: 284–289 [DOI] [PubMed] [Google Scholar]
- Boileau C., Tat S.K., Pelletier J.P., Cheng S., Martel-Pelletier J. (2008) Diacerein inhibits the synthesis of resorptive enzymes and reduces osteoclastic differentiation/survival in osteoarthritic subchondral bone: a possible mechanism for a protective effect against subchondral bone remodelling. Arthritis Res Ther 10: R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordji K., Grillasca J.P., Gouze J.N., Magdalou J., Schohn H., Keller J. M., et al. (2000) Evidence for the presence of peroxisome proliferator-activated receptor (PPAR) alpha and gamma and retinoid Z receptor in cartilage. PPARgamma activation modulates the effects of interleukin-lbeta on rat chondrocytes. J Biol Chem 275: 12243–12250 [DOI] [PubMed] [Google Scholar]
- Brandt K.D. (2006) Studies in animal models of osteoarthritis as predictors of a structure-modifying effect of diacerhein in humans with osteoarthritis. Biorheology 43: 589–594 [PubMed] [Google Scholar]
- Brandt K.D., Smith G., Kang S.Y., Myers S., O'Connor B., Albrecht M. (1997) Effects of diacerhein in an accelerated canine model of osteoarthritis. Osteoarthritis Cartilage 5: 438–449 [DOI] [PubMed] [Google Scholar]
- Colville-Nash P.R. (2002) Comparison of the pharmacologic effect of diacerein and a selective COX-2 inhibitor in the mouse induced-granuloma model. Presse Med 31: 4S16–4S17 (abstract). [PubMed] [Google Scholar]
- Couchourel D., Aubry I., Delalandre A., Lavigne M., Martel-Pelletier J., Pelletier J.-P., et al. (2009) Altered mineralization of human osteoarthritic osteoblasts is due to abnormal collagen type 1 production. Arthritis Rheum 60: 1438–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagala F., Martin G., Bogdanowicz P., Ficheux H., Pujol J.P. (2006) Inhibition of interleukin-1beta-induced activation of MEK/ERK pathway and DNA binding of NF-kappaB and AP-1: potential mechanism for Diacerein effects in osteoarthritis. Biorheology 43: 577–587 [PubMed] [Google Scholar]
- Dougados M., Nguyen M., Berdah L., Mazieres B., Vignon E., Lequesne M. (2001) Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the Chondromodulating Effect of Diacerein in OA of the Hip. Arthritis Rheum 44: 2539–2547 [DOI] [PubMed] [Google Scholar]
- Fahmi H., Di Battista J.A., Pelletier J.P., Mineau F., Ranger P., Martel-Pelletier J. (2001) Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-1beta-induced nitric oxide and matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum 44: 595–607 [DOI] [PubMed] [Google Scholar]
- Fahmi H., Pelletier J.P., Martel-Pelletier J. (2002a) PPARgamma ligands as modulators of inflammatory and catabolic responses on arthritis. An overview. J Rheumatol 29: 3–14 [PubMed] [Google Scholar]
- Fahmi H., Pelletier J.P., Mineau F., Martel-Pelletier J. (2002b) 15d-PGJ(2) is acting as a ‘dual agent’ on the regulation of COX-2 expression in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 10: 845–848 [DOI] [PubMed] [Google Scholar]
- Fahmi H., Pelletier J.P., Di Battista J.A., Cheung H.S., Fernandes J., Martel-Pelletier J. (2002c) Peroxisome proliferator-activated receptor gamma acitvators inhibit MMP-1 production in human synovial fibroblasts by reducing the activity of the activator protein 1. Osteoarthritis Cartilage 10: 100–108 [DOI] [PubMed] [Google Scholar]
- Fedeli S. (1988) Livelli plasmatici di Diacerina nell'huomo anziano dopo somministrazioni repetute del farmaco a posologie diverse. Laboratoires NEGMA-PROTER. Study report, July 1988. [Google Scholar]
- Felisaz N., Boumediene K., Ghayor C., Herrouin J.F., Bogdanowicz P., Galerra P., et al. (1999) Stimulating effect of diacerein on TGF-betal and beta2 expression in articular chondrocytes cultured with and without interleukin-1. Osteoarthritis Cartilage 7: 255–264 [DOI] [PubMed] [Google Scholar]
- Fidelix T.S., Soares B.G., Trevisani V.F. (2006) Diacerein for osteoarthritis. Cochrane Database Syst Rev CD005117. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Xu A., Hwa S.Y., Burkhardt D., Little C. (1998) Evaluation of the effects of diacerhein in the sheep model of arthritis. Rev Prat 48: S24-S30 [PubMed] [Google Scholar]
- Glasson S.S., Askew R., Sheppard B., Carito B., Blanchet T., Ma H. L., et al. (2005) Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434: 644–648 [DOI] [PubMed] [Google Scholar]
- Hilal G., Martel-Pelletier J., Pelletier J.P., Ranger P., Lajeunesse D. (1998) Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheum 41: 891–899 [DOI] [PubMed] [Google Scholar]
- Hilal G., Martel-Pelletier J., Pelletier J.P., Duval N., Lajeunesse D. (1999) Abnormal regulation of urokinase plasminogen activator by insulin-like growth factor 1 in human osteoarfhritic subchondral osteoblasts. Arthritis Rheum 42: 2112–2122 [DOI] [PubMed] [Google Scholar]
- Hwa S.Y., Burkhardt D., Little C., Ghosh P. (2001) The effects of orally administered diacerein on cartilage and subchondral bone in an ovine model of osteoarthritis. J Rheumatol 28: 825–834 [PubMed] [Google Scholar]
- Ji J.D., Cheon H., Jun J.B., Choi S.J., Kim Y. R., Lee Y. H., et al. (2001) Effects of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) on the expression of inflammatory cytokines and apoptosis induction in rheumatoid synovial fibroblasts and monocytes. J Autoimmun 17: 215–221 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Notoya K., Naito T., Unno S., Nakamura A., Martel-Pelletier J., et al. (2005) Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, reduces the progression of experimental osteoarthritis in guinea pigs. Arthritis Rheum 52: 479–487 [DOI] [PubMed] [Google Scholar]
- Kwan S. Tat, Pelletier J.P., Lajeunesse D., Fahmi H., Lavigne M., Martel-Pelletier J. (2008) The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappaB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells. Clin Exp Rheumatol 26: 295–304 [PMC free article] [PubMed] [Google Scholar]
- Legendre F., Bogdanowicz P., Martin G., Domagala F., Leclercq S., Pujol J. P., et al. (2007) Rhein, a diacerhein-derived metabolite, modulates the expression of matrix degrading enzymes and the cell proliferation of articular chondrocytes by inhibiting ERK and JNK-AP-1 dependent pathways. Clin Exp Rheumatol 25: 546–555 [PubMed] [Google Scholar]
- Legendre F., Heuze A., Boukerrouche K., Leclercq S., Boumediene K., Galera P., et al. (2009) Rhein, the metabolite of diacerhein, reduces the proliferation of osteoarthritic chondrocytes and synoviocytes without inducing apoptosis. Scand J Rheumatol 38: 104–111 [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., McCollum R., Di Battista J.A., Faure M.P., Chin J. A., Fournier S., et al. (1992) The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes. Identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum 35: 530–540 [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Mineau F., Jolicoeur F.C., Cloutier J.M., Pelletier J.P. (1998) In vitro effects of diacerhein and rhein on IL-1 and TNF-alpha systems in human osteoarthritic tissues. J Rheumatol 25: 753–762 [PubMed] [Google Scholar]
- Martel-Pelletier J., Lajeunesse D., Pelletier J.P. (2005) Etiopathogenesis of osteoarthritis, In: Koopman W.J., Moreland L.W. (eds), Arthritis & allied conditions. A textbook of rheumatology, Lippincott, Williams & Wilkins: Baltimore, pp. 2199–2226 [Google Scholar]
- Martel-Pelletier J., Lajeunesse D., Reboul P., Pelletier J.P. (2007) The role of subchondral bone in osteoarthritis, In: Sharma L., Berenbaum F. (eds), Osteoarthritis: a companion to rheumatology, MosbyElsevier: Philadelphia, pp. 15–32 [Google Scholar]
- Martin G., Bogdanowicz P., Domagala F., Ficheux H., Pujol J.P. (2003) Rhein inhibits interleukin-1 beta-induced activation of MEK/ERK pathway and DNA binding of NF-kappa B and AP-1 in chondrocytes cultured in hypoxia: a potential mechanism for its disease-modifying effect in osteoarthritis. Inflammation 27: 233–246 [DOI] [PubMed] [Google Scholar]
- Massicotte F., Lajeunesse D., Benderdour M., Pelletier J.-P., Hilal G., Duval N., et al. (2002) Can altered production of interleukin 1β, interleukin-6, transforming growth factor-β and prostaglandin E2 by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthritis Cartilage 10: 491–500 [DOI] [PubMed] [Google Scholar]
- Mazieres B., Berdah L., Thiechart M., Viguier G. (1993) Diacetylrhein on a postcontusion model of experimental osteoarthritis in the rabbit. Rev Rhum Ed Fr 60: 77S–81S [PubMed] [Google Scholar]
- Mazieres B., Blanckaert A., Thiechart M., Viguier G. (1996) Diacetylrhein administrated ‘curatively’ in an experimental model of post-contusion osteoarthritis in rabbits. Rev Prat 46: S42-S45 (abstract). [PubMed] [Google Scholar]
- McCollum R., Martel-Pelletier J., Di Battista J.A., Pelletier J.P. (1991) Regulation of interleukin 1 receptors in human articular chondrocytes. J Rheumatol 18: 85–88 [PubMed] [Google Scholar]
- Moldovan F., Pelletier J.P., Jolicoeur F.C., Cloutier J.M., Martel-Pelletier J. (2000) Diacerhein and rhein reduce the ICE-induced IL-lbeta and IL-18 activation in human osteoarthritic cartilage. Osteoarthritis Cartilage 8: 186–196 [DOI] [PubMed] [Google Scholar]
- Moore A.R., Greenslade K.J., Alam C.A., Willoughby D.A. (1997) Effects of diacerhein on cytokine determinations in a model of cartilage degradation induced by granuloma in mice. Rev Prat 47: S24-S26 (abstract). [PubMed] [Google Scholar]
- Moore A.R., Greenslade K.J., Alam C.A., Willoughby D.A. (1998) Effects of diacerhein on granuloma induced cartilage breakdown in the mouse. Osteoarthritis Cartilage 6: 19–23 [DOI] [PubMed] [Google Scholar]
- Nicolas P., Tod M., Padoin C., Petitjean O. (1998) Clinical pharmacokinetics of diacerein. Clin Pharmacokinet 35: 347–359 [DOI] [PubMed] [Google Scholar]
- Petitjean O., Tod M., Louchahi K. (1991) Étude de la pharmacocinétique de l'ART 50® en administration aigüe orale a la dose de 50 mg chez le volontaire sain âgé de 61–70 ans et de plus de 70 ans, et en administration réitérée a la dose de 50 mg × 2/jour chez le volontaire âgé de plus de 70 ans. Latoratoires NEGMA, Study PC/ART 9013N. Study report, April 1991. [Google Scholar]
- Pelletier J.P., Mineau F., Fernandes J.C., Duval N., Martel-Pelletier J. (1998) Diacerhein and rhein reduce the interleukin 1 beta stimulated inducible nitric oxide synthesis level and activity while stimulating cyclooxygenase-2 synthesis in human osteoarthritic chondrocytes. J Rheumatol 25: 2417–2424 [PubMed] [Google Scholar]
- Pelletier J.P., Lajeunesse D., Reboul P., Mineau F., Fernandes J. C., Sabouret P., et al. (2001) Diacerein reduces the excess synthesis of bone remodeling factors by human osteoblast cells from osteoarthritic subchondral bone. J Rheumatol 28: 814–824 [PubMed] [Google Scholar]
- Pelletier J.P., Mineau F., Boileau C., Martel-Pelletier J. (2003) Diacerein reduces the level of cartilage chondrocyte DNA fragmentation and death in experimental dog osteoarthritic cartilage at the same time that it inhibits caspase-3 and inducible nitric oxide synthase. Clin Exp Rheumatol 21: 171–177 [PubMed] [Google Scholar]
- Pelletier J.P., Raynauld J.P., Berthiaume M.J., Abram F., Choquette D., Haraoui B., et al. (2007) Risk factors associated with the loss of cartilage volume on weight bearing areas in knee osteoarthritis patients assessed by quantitative MRI: a longitudinal study. Arthritis Res Ther 9: R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J.-P., Raynauld J.-P., Abram F., Haraoui B., Choquette D., Martel-Pelletier J. (2008) A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage 16: S8-S13 [DOI] [PubMed] [Google Scholar]
- Pomarelli P., Berti M., Gatti M.T., Mosconi P. (1980) A non-steroidal anti-inflammatory drug that stimulates prostaglandin release. Farmaco 35: 836–842 [PubMed] [Google Scholar]
- Pujol J.P., Felisaz N., Boumediene K., Ghayor C., Herrouin J. F., Bogdanowicz P., et al. (2000) Effects of diacerein on biosynthesis activities of chondrocytes in culture. Biorheology 37: 177–184 [PubMed] [Google Scholar]
- Raynauld J.P., Kauffmann C., Beaudoin G., Berthiaume M.J., de Guise J.A., Bloch D.A., et al. (2003) Reliability of a quantification imaging system using magnetic resonance images to measure cartilage thickness and volume in human normal and osteoarthritic knees. Osteoarthritis Cartilage 11: 351–360 [DOI] [PubMed] [Google Scholar]
- Raynauld J.-P., Martel-Pelletier J., Abram J., Pelletier J.-P. (2008a) Use of quantitative magnetic resonance imaging (qMRI) in the cross-sectional and longitudinal evaluation of structural changes in knee osteoarthritis (OA) patients, In: Reid D.M., Miller C.G., Baburaj K. (eds), Clinical trials in rheumatoid arthritis and osteoarthritis, Springer-Verlag: London [Google Scholar]
- Raynauld J.-P., Martel-Pelletier J., Berthiaume M.-J., Abram F., Choquette D., Haraoui B., et al. (2008b) Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann Rheum Dis 67: 683–688 [DOI] [PubMed] [Google Scholar]
- Raynauld J.P., Martel-Pelletier J., Berthiaume M.J., Beaudoin G., Choquette D., Haraoui B., et al. (2006) Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther 8: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynauld J.P., Martel-Pelletier J., Berthiaume M.J., Labonté F., Beaudoin G., de Guise J.A., et al. (2004) Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum 50: 476–487 [DOI] [PubMed] [Google Scholar]
- Redini F., Galera P., Mauviel A., Loyau G., Pujol J.P. (1988) Transforming growth factor beta stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett 234: 172–176 [DOI] [PubMed] [Google Scholar]
- Rintelen B., Neumann K., Leeb BF. (2006) A meta-analysis of controlled clinical studies with diacerein in the treatment of osteoarthritis. Arch Intern Med 166: 1899–1906 [DOI] [PubMed] [Google Scholar]
- Sadouk M., Pelletier J.P., Tardif G., Kiansa K., Cloutier J.M., Martel-Pelletier J. (1995) Human synovial fibroblasts coexpress interleukin-1 receptor type I and type II mRNA: the increased level of the interleukin-1 receptor in osteoarthritic cells is related to an increased level of the type I receptor. Lab Invest 73: 347–355 [PubMed] [Google Scholar]
- Saha N., Moldovan F., Tardif G., Pelletier J.P., Cloutier J.M., Martel-Pelletier J. (1999) Interleukin-1beta-converting enzyme/Caspase-1 in human osteoarthritic tissues: localization and role in the maturation of IL-1beta and IL-18. Arthritis Rheum 42: 1577–1587 [DOI] [PubMed] [Google Scholar]
- Sanchez C., Mathy-Hartert M., Deberg M.A., Ficheux H., Reginster J.Y., Henrotin Y.E. (2003) Effects of rhein on human articular chondrocytes in alginate beads. Biochem Pharmacol 65: 377–388 [DOI] [PubMed] [Google Scholar]
- Segré G. (1988) Étude pilote du passage de la rhéine dans le liquide synovial. Laboratoires NEGMA, Study 4A29. Study report, July 1988. [Google Scholar]
- Simonin M.A., Bordji K., Boyault S., Bianchi A., Gouze E., Becuwe P., et al. (2002) PPAR-gamma ligands modulate effects of LPS in stimulated rat synovial fibroblasts. Am J Physiol Cell Physiol 282: C125-C133 [DOI] [PubMed] [Google Scholar]
- Smith G.N., Jr, Myers S.L., Brandt K.D., Mickler E.A., Albrecht M. (1999) Diacerhein treatment reduces the severity of osteoarthritis in the canine cruciate-deficiency model of osteoarthritis. Arthritis Rheum 42: 545–554 [DOI] [PubMed] [Google Scholar]
- Sokoloff L. (1993) Microcracks in the calcified layer of articular cartilage. Arch Pathol Lab Med 117: 191–195 [PubMed] [Google Scholar]
- Spencer CM, Wilde M.I. (1997) Diacerein. Drugs 53: 98–108 [DOI] [PubMed] [Google Scholar]
- Stanton H., Rogerson F.M., East C.J., Golub S.B., Lawlor K. E., Meeker C.T., et al. (2005) ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434: 648–652 [DOI] [PubMed] [Google Scholar]
- Tamura T., Ohmori K. (2001) Rhein, an active metabolite of diacerein, suppresses the interleukin-1alpha-induced proteoglycan degradation in cultured rabbit articular chondrocytes. Jpn J Pharmacol 85: 101–104 [DOI] [PubMed] [Google Scholar]
- Tamura T., Shirai T., Kosaka N., Ohmori K., Takafumi N. Tamura. (2002) Pharmacological studies ofdiacerein in animal models of inflammation, arthritis and bone resoption. Eur J Pharmacol 448: 81–87 [DOI] [PubMed] [Google Scholar]
- Yaron M., Shirazi I., Yaron I. (1999) Anti-interleukin-1 effects of diacerein and rhein in human ostearthritic synoarthritic synovial tissue and cartilage tures. Osteoarthrists Cartilage 7: 272–280 [DOI] [PubMed] [Google Scholar]


