Abstract
The etiology of hip fractures is multifactorial and includes bone and fall-related factors. Low bone mineral density (BMD) and BMD-related and BMD-independent geometric components of bone strength, evaluated by hip strength analysis (HSA) and finite element analysis analyses on dual-energy X-ray absorptiometry (DXA) images, and ultrasound parameters are related to the presence and incidence of hip fracture. In addition, clinical risk factors contribute to the risk of hip fractures, independent of BMD. They are included in the fracture risk assessment tool (FRAX) case finding algorithm to estimate in the individual patient the 10-year risk of hip fracture, with and without BMD. Fall risks are not included in FRAX, but are included in other case finding tools, such as the Garvan algorithm, to predict the 5- and 10-year hip fracture risk. Hormones, cytokines, growth factors, markers of bone resorption and genetic background have been related to hip fracture risk. Vitamin D deficiency is endemic worldwide and low serum levels of 25-hydroxyvitamin D [25(OH)D] predict hip fracture risk. In the context of hip fracture prevention calculation of absolute fracture risk using clinical risks, BMD, bone geometry and fall-related risks is feasible, but needs further refinement by integrating bone and fall-related risk factors into a single case finding algorithm for clinical use.
Keywords: bone mineral density (BMD), bone geometry, fall risks, fracture risk assessment tool (FRAX), Garvan fracture risk calculator, genetic background, hip fracture risk
Introduction
The life-time risk of hip fracture for a white woman of 50 years of age is about 15%, equivalent to the risk of developing breast cancer [Sambrook and Cooper, 2006], but varies between populations [Ismail et al. 2002]. The incidence of hip fractures increases exponentially with advancing age, but the age-adjusted incidence is decreasing in developed countries, but not in some developing countries [Brauer et al. 2009]. Hip fractures incur significant costs and cause considerable disability and morbidity [Burge et al. 2007]. After a hip fracture, the risk of mortality and subsequent fracture is increased and is highest within the first years after a fracture [Bliuc et al. 2009; Geel Van et al. 2009; Ryg et al. 2009; Center et al. 2007; Helden Van et al. 2006].
The etiology of hip fractures is multifactorial, including bone and fall-related risk factors (Figure 1). We reviewed the literature on risk factors that predict hip fractures, with special attention to studies that analyzed bone mineral density (BMD), structural characteristics of the hip, clinical bone and fall-related risks, hormones, cytokines, bone markers, genetic background and its combinations.
Figure 1.
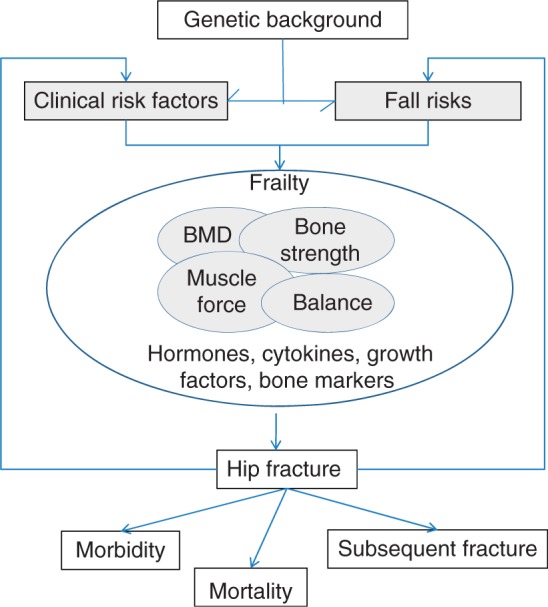
Multifactorial etiology and consequences of hip fracture.
Bone mineral density and hip fracture risk
Many studies have demonstrated that low bone mineral density (BMD) is a risk factor for hip fractures. In a large meta-analysis of prospective cohort studies the relative risk for hip fractures was 2.6 [95% confidence interval (CI): 2.0–3.5] per standard deviation (SD) of decrease in BMD [Marshall et al. 1996]. This meta-analysis also confirmed earlier studies that site-specific measurements of BMD in the hip are better predictors of hip fracture than measurements at other skeletal sites [Stone et al. 2003; Marshall et al. 1996; Melton et al. 1993]. In the Study of Osteoporotic Fractures (SOF), during >8 years follow up, femoral neck (FN) BMD was a better predictor of hip fracture risk [relative risk (RR): 2.37, CI: 2.12–2.66)] than spine BMD (RR: 1.49, CI: 1.34–1.65) [Stone et al. 2003]. In the same study, the proportion of hip fractures attributable to osteoporosis was 0.28 in patients with a total hip BMD t-score <-2.5 and 0.51 when t-score < −1.5. In the Dubbo Osteoporosis Epidemiology Study (DOES) study, during a follow up of 14 years, the RR per SD decrease in FN BMD was 3.6 (2.6–4.5), and was lower but still significant after correction for age, sex and fall risks (RR: 2.8, CI: 2.3–3.4) [Nguyen et al. 2005].
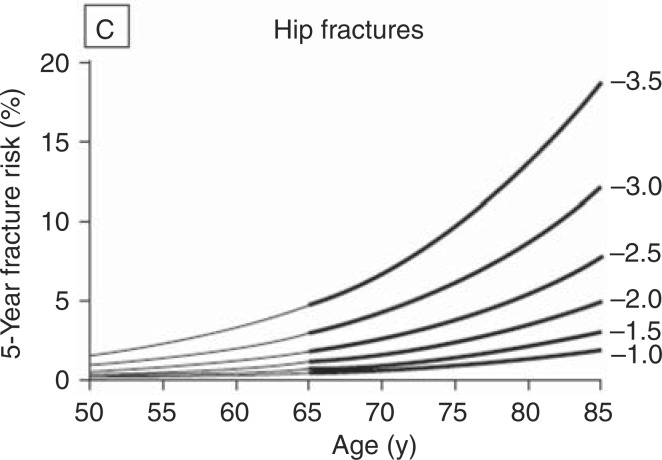
The relation between BMD and hip fracture risk is strongly dependent on age (Figure 2). The 5-year hip fracture risk is <5% at any BMD below the age of 65 years, but varies between <5% at a FN t-score of >-2.0 to 20% at a FN t-score of — 3.5 in elderly women [Bates et al. 2002]. On the other hand, the lifetime risk for hip fracture at any age between 50 and 80 years is 10% at a FN t-score of 0, around 33% at a FN t-score of −2.5 and 41–49% at a FN t-score of −3.5 [Bates et al. 2002].
Figure 2.
Risk of hip fractures based on age and on FN BMD during 5 years of follow-up in the Study of Osteoporotic Fractures (SOF) (Bates et al. 2002).
About half of hip fractures occur in women who are not osteoporotic by BMD testing. The prevalence of osteoporosis in patients with a hip fracture has been reported in several studies. In the Epidemiology of Osteoporosis (EPIDOS) study, 48% of hip fracture patients had FN t-score <-2.5 [Schott et al. 1998]. In the SOF study 46% of patients with hip fracture had a total hip t-score of <-2.5 [Black et al. 2001]. In the zoledronate hip trial, which included women and women with a recent hip fracture in patients who were ambulatory before fracture and had an expected life expectancy of >6 months in the opinion of the investigator, 41% had osteoporosis in the FN [Lyles et al. 2007].
In a short term study of 3 years, bone loss in the hip was related to fracture risk [Bruyere et al. 2009]. In some studies the risk of pertrochanteric and cervical hip fractures were studied separately. In a small retrospective case-control study, trochanteric fractures were related to low BMD, but not cervical fractures [Karlsson et al. 1993].
Hip geometry and hip fracture risk
In clinical practice, measurement of bone mineral density is used as a surrogate marker for the measurement of bone strength. As mentioned, however, there is a large overlap of BMD between hip fracture patients and controls and only 40–50% of patients with a hip fracture do have osteoporosis, indicating that BMD only partially reflects hip fracture risk. Bone strength is determined by many components including BMD, bone architecture (geometry), microarchitecture (trabecular number, thickness, perforation, and connectivity), cortical porosity, matrix properties, tissue mineralization density, crystal characteristics and damage accumulation and repair [Bouxsein, 2008; Lewiecki and Borges, 2006].
Hip structural (or strength) analysis (HSA) refers to the methodology used to calculate bone strength based on measurement of geometric characteristics in the proximal femur [Beck, 2003; Beck et al. 2000]. With HSA, measurements or estimates are obtained of the mineralized cortical bone surface cross-sectional area (CSA), the cross-s ectional moment of inertia (CSMI), the section modulus (Z), the buckling ratio (BR) and cortical thickness (Figure 3). HSA has been mostly applied on images generated by dual-energy X-ray absorptiometry (DXA), but also by CT and MRI. HSA has been further analysed using finite element analysis (FEA).
Figure 3.
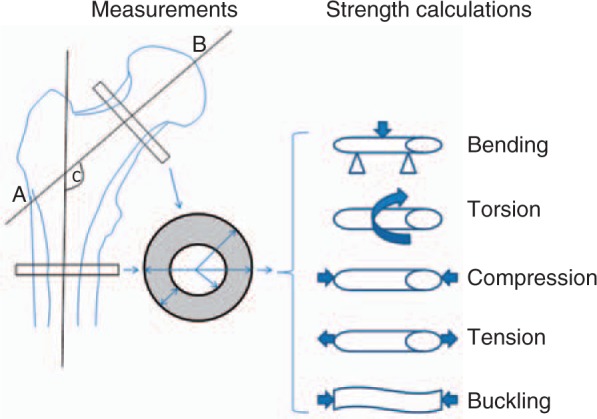
Measurements and biomechanical strength calculations from hip geometry. AB = hip axis length; C = femoral neck angle.
Hip strength analysis using dual-energy X-ray absorptiometry (DXA)
The HSA methodology has been described in detail by Beck et al. (Figure 3)[Beck, 2003; Beck et al. 2000]. This method uses the DXA scan image of the proximal femur to extract data on cross-sectional geometry in specific locations. The software program analyzes cross-sectional areas of bone in three regions of interest (ROIs) at which the various geometric parameters are calculated. The HSA RIO's include the narrowneck (NN) (the narrowest portion of the FN), the intertrochanteric (IT) region (a bisector of the intersection of the FN and shaft axes) and the shaft region. The selected regions are 5mm in width. The NN and IT regions contain cortical and trabecular bone, whereas the shaft region is considered to contain only cortical bone.
The CSA (mm2) is the surface area of bone in the cross-section excluding soft tissue voids. After the centroid has been located, CSMI (cm4) is computed as the integral weighted by the square of distance from the centroid. The CSMI reflects how the mass is distributed, which affects strength in bending, but the CSMI itself is not a measure of strength. Z is a physical property of a section and is inversely related to the maximum bending stress in the section, making it an index of the strength of the section (section modulus). Z is determined by the CSMI, divided by the centroidal distance or distance from the neutral axis to the outermost edge of the section, which in the case of bone, is the subperiosteal surface. The buckling ratio is the ratio of the outer radius, Ro to the cortical thickness. It is thought that with ratios >10, a precipitous loss of strength may occur with local buckling [Young, 1989]. Additionally advanced hip analysis (AHA) is reported, which provides the following structural parameters: hip axis length (HAL, mm), distance from base of greater trochanter to inner pelvic rim and femoral strength index (FSI, unitless), which is the ratio of estimated compressive yield strength of FN to expected compressive stress of a fall on the greater trochanter adjusted for the patiennt's age, height, and weight [Leslie et al. 2009; Faulkner et al. 2006].
At present there are several studies addressing the role of hip geometry in relation to the risk of hip fracture. In cross-sectional studies, lower CSMI, Z, HAL and FSI were modestly associated with hip fracture, independent of BMD [Faulkner et al. 2006; Ahlborg et al. 2005]. Several prospective studies are available on calculated strength and hip fracture risk. In the Rotterdam study cohort predictive models for hip fracture were better with BMD than with HSA-derived section modulus. Buckling ratio was a better predictor of hip fracture than section modulus, but the predictive ability of the buckling ratio was essentially identical to that of BMD and did not offer additional predictive value [Rivadeneira et al. 2007]. In the population-based prospective study of osteoporotic fractures, the geometric parameters derived from HSA provided an assessment of hip fracture risk that exceeded what was obtained with areal hip BMD, though average cortical thickness, average buckling ratio, and CSA plus subperiosteal diameter were equivalent. Only the FN angle remained a significant predictor after correction for age and BMD [Kaptoge et al. 2008]. Leslie et al. recently reported hip DXA measurements in 30,953 women aged ≥50 years with 270 incident hip fractures and 1347 non-hip osteoporotic fractures during 3.7 years of observation. Scans were reprocessed to derive parameters of hip bone geometry. HAL and SI made a small but statistically significant contribution to hip fracture prediction that was independent of age and BMD measurement [Leslie et al. 2009].
Structural engineering models (SEMs) integrate density and geometric information embedded in DXA scans with the applied force. This enables the evaluation of stress throughout the bone and therefore is of potential value in predicting hip fractures. Indeed, several SEMs have been developed that show superior accuracy in the determination of the bone strength over BMD alone [Testi et al. 1999, 2002; Beck et al. 1998]. In a recently published retrospective study by Yang et al, 51 postmenopausal women (age 58–81 years) who had sustained a recent low-trauma hip fracture were compared with 153 age-, height-, and weight-matched controls using HAS and SEM derived parameters [Yang et al. 2003]. A finite element model was generated to calculate stress within the bone when falling sideways. The finite element model was significantly better than total hip BMD alone and similar to the total hip BMD plus bone width in discriminating all hip fracture and FN fracture. No index was better than total hip BMD for discriminating trochanteric fractures. It was concluded that the finite element model has the potential to replace hip BMD in discriminating hip fractures [Yang et al. 2003].
In a study using FEA in 1000 femurs, the impact of an oblique fall to the side was simulated, a scenario known to account for a large proportion of hip fractures in the elderly. Cortical bone in the proximal femur appeared to be a crucial differentiator [Bryan et al. 2009].
From the currently available HSA data, it appears that the proximal femur remodels by redistributing bone mass as compensation for the declining mass in order to preserve strength in bending. This compensation though is insufficient to completely preserve strength and may ultimately result in excessive cortical thinning. This is captured in the BR, a condition that cannot be derived from BMD alone. It is also clear that the HSA structural parameters are highly correlated with BMD and although predictive of fracture risk, they are not currently better predictors of fracture risk. The major limitations of HSA with DXA primarily reflect limitations imposed by the two-dimensional nature of DXA. Despite these limitations important information is to be gained in terms of the biomechanics and pathophysiology of hip fracture through the study of hip geometry. DXA-based structural engineering models of the proximal femur may be additionally useful in predicting hip fractures, but its clinical implications need further study.
Only limited studies on HSA made a distinction between trochanteric and cervical hip fractures. In the nested case-control study from the EPIDOS cohort who sustained a hip fracture, CSMI correlated with trochanteric, but not with cervical hip fractures, and not any more after adjustment for FN BMD [Szulc et al. 2006]. In a small retrospective case controlled study the risk of trochanteric fractures could be discriminated based on a BMD t-score <-2.5 criterion, whereas cervical fracture cases would remain under-diagnosed if solely using this criterion. Instead, geometrical risk factors were able to discriminate cervical fracture cases even among individuals with t-score >-2.5 [Pulkkinen et al. 2009]. For cervical and trochanteric fractures combined, BMD and geometric measures independently contributed to hip fracture discrimination. The findings need to be confirmed with larger prospective studies.
Other bone measuring techniques
Quantitative ultrasound
Quantitative ultrasound (US) is yet another method to measure characteristics of bone strength and density. Broad band ultrasound attenuation (BUA) and speed of sound (SOS) are related to fracture risk, independent of BMD [Stewart and Hannan, 2000]. In women around the time of the menopause US predicted osteoporotic fractures as well as, and independent of, BMD [Stewart and Hannan, 2000]. Calcaneal quantitative US (QUS) methods have shown predictive power for hip fracture approaching the performance of femoral DXA. A decrease by 1 SD of the QUS parameters corresponded to an increase of the hip fracture risk from 2.2 to 2.6 with two heel QUS devices [Krieg et al. 2006]. Risk gradients did not differ significantly among the variables of the two heel QUS devices. Phalanges QUS device was not predictive of hip fracture risk [Krieg et al. 2006].
Femur Ultrasound Scanner (FemUS) with QUS measurements at the proximal femur with a specially developed prototype device was shown to be feasible and showed a good performance for hip fracture discrimination in a small pilot study comparing fracture patients to controls, but needs further validation [Barkmann et al. 2009].
Quantitative computer tomography
Quantitative computer tomography (QCT) has been used for delineating cortical and trabecular bone regions in the hip to calculate bone strength with FEA (Figure 4). Using custom code (ON Diagnostics, Berkeley, CA, USA), the QCT images were processed and converted into finite element models using 1.5-mm-sided, cube-shaped, eight-noded brick elements, from which bone strength was calculated by simulating a fall to the side (Figure 4) [Keaveny and Bouxsein, 2008]. When the ratio of the applied impact force (Φ) to the bone strength, is greater than a critical value—the biomechanical fracture threshold—fracture should occur [Lewiecki et al. 2009]. No prospective data are available for hip fracture prediction. Of interest, significant changes in calculated hip strength were found with bisphosphonates, parathyroid hormone (PTH) and its concomitant and subsequent combinations [Keaveny and Bouxsein, 2008; Krug et al. 2005].
Figure 4.
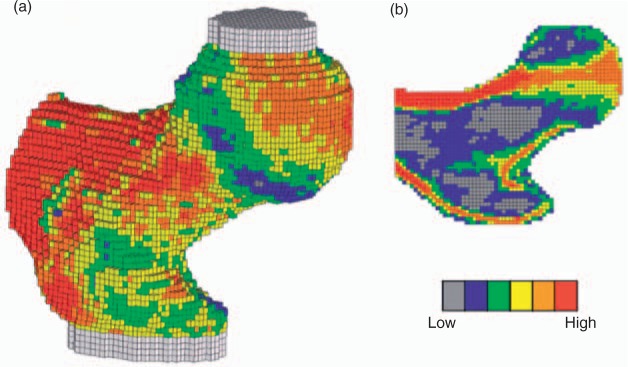
Typical finite element [FE] model, showing 3D [a] and 2D sectional [b] views. The color coding shows the spatial variation of material strength assigned to the individual finite elements. The bone was oriented in a typical sideways fall configuration and shear-free loads were applied vertically through the molds covering the femoral head and greater trochanter, whereas moment and torque and axial force [but not shear force] restraint were applied to the distal end, just below the lesser trochanter [Keaveny et al. 2008).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) enables evaluation of the trabecular networks, but the small dimensions of trabeculae (∼100 μ) require very high imaging resolutions. The resolution of the images limits the application of 3D structural analysis in the hip. Only limited data are available on structural hip measurements [Pulkkinen et al. 2008] and cortical shell geometry of the femur [WHO]. The potential of MRI as a means of imaging proximal femur structure, requires improvements in technique and resolution enhancements [Pulkkinen et al. 2008].
Plain radiography of the hip
Computerized analysis of the trabecular structure has been examined for its relation to bone strength in the hip. Structural parameters of trabecular bone and bone geometry predicted in vitro failure loads of the proximal femur with similar accuracy as DXA, when using appropriate image analysis technology [Kanis et al. 2008], but no prospective data are available.
Clinical risk factors for hip fracture
The fracture risk assessment tool
The fracture risk assessment tool (FRAX) case finding algorithm has been developed to predict the 10-year risk of major and hip fractures based on clinical risk factors, with and without BMD [National Osteoporosis Foundation, 2008]. Included risk factors are age, sex, BMI, personal and family history of fracture, smoking, alcohol intake, chronic glucocorticoid use, rheumatoid arthritis and other causes of secondary osteoporosis and FN BMD (Table 1). FRAX can be used online in daily practice to calculate the individual 10-year absolute risk of hip fracture, with and without including BMD. FRAX can also be used to decide when to measure BMD and in whom to start drug therapy. Using FRAX, diagnostic and therapeutic decisions can be based on thresholds, which are available in recently upgraded osteoporosis guidelines in the UK and US [Garvan Medical Research Institute; Saag and Geuens, 2009; Dargent Molina et al. 1996]. These thresholds are based on cost-effectiveness calculations and at levels of subsequent fracture risk after a first fracture.
Table 1.
Comparison between the FRAX and Garvan algorithms for risk factors and predicted fracture risk (http://www.shef.ac.uk/FRAX; http://www.garvan.org.au/promotions/bone-fracture-risk/calculator/).
| Garvan nomogram | FRAX™ algorithm |
|---|---|
| Risk factors | Risk factors |
| Age | Age |
| Sex | Sex |
| Femoral neck BMD* | Femoral neck BMD |
| Body weight* | Body weight |
| History of prior fractures since age 50 years$ | History of prior fractures |
| History of falls in the previous 12 months | Height |
| Parental history of hip fracture | |
| Current smoking | |
| Chronic glucocorticoid use‡ | |
| Rheumatoid arthritis | |
| Secondary osteoporosis | |
| Alcohol (three or more units per day) | |
| Predicted fractures (5- and 10-year probability) | Predicted fractures (10-year probability) |
| Hip | Hip |
| Clinical spine | Spine |
| Wrist | Wrist |
| Humerus | Humerus |
| Distal femur | |
| Proximal tibia/fibula | |
| Distal tibia/fibula | |
| Patella | |
| Pelvis | |
| Rib | |
| Sternum | |
| Hands and feet (excluding digits) |
BMD, bone mineral density.
Either bone mineral density or body weight is used in the nomogram;
Excluding major trauma fractures
Past or present exposure to prednisone equivalent dose of 5mg or more for more than 3 months.
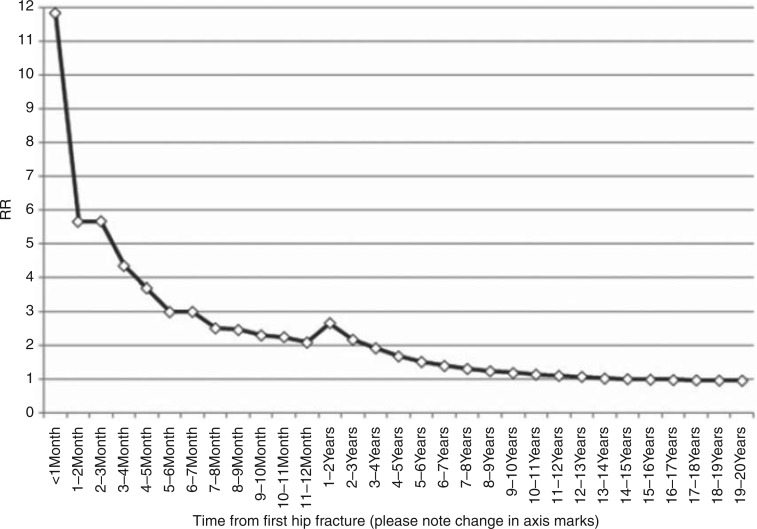
However, FRAX has several shortcomings. In the context of predicting hip fracture, a shortcoming is the lack of implementation of fall risks (discussed below). Another shortcoming is that FRAX does not take into account the clustering of fractures in time, ie the risk of subsequent fractures after a first fracture is highest during the first years after a fracture, although the increased risk persists over longer time, be it at a lower level than immediately after a fracture. This has been documented for repeat vertebral, non-vertebral and all clinical fractures and recently also for repeat hip fractures [Ryg et al. 2009; van Geel et al. 2009; Center et al. 2007; van Helden et al. 2006]. Ryg et al. reported clustering of repeat hip fractures in a nation-wide registrar in Denmark including nearly 170,000 first and nearly 28,000 second hip fractures (Figure 5) [Ryg et al. 2009].
Figure 5.
Relative risk (RR) of second hip fracture compared with the expected risk in an age- and sex-matched normal population in selected time intervals after the first hip fracture (Ryg et al. 2009).
Neither does FRAX take into account the dose of some risk factors, such as the daily and cumulative dose of glucocorticoids or the previous fracture load in terms of number and severity of previous vertebral and non-vertebral fractures [WHO]. The Garvan algorithm includes the number of recent falls and the number of previous fractures (Table 1) [Garvan Medical Research Institute].
Fall risks and the risk of hip fracture
The risk of hip fracture is related to the presence of fall risks. This has been documented in many prospective studies (Table 2).
Table 2.
Fall-related risks and the risk for hip fracture, independent of BMD (13, 50–62).
| Postural instability and/or quadriceps weakness |
|---|
| History of falls |
| Fall to the side |
| Self-reported health |
| Self-reported physical activity |
| Impaired cognition |
| Slower walking speed |
| Type II diabetes mellitus |
| Parkinson's disease |
| Poor vision, depth perception |
| Lack of exercise in the last year |
| Frailty |
| Muscle composition |
BMD, bone mineral density.
During a follow up of 1.9 years, hip fracture risk was related to gait speed, tandem walk and poor vision, independent of age and FN BMD [Ensrud et al. 2007; Robbins et al. 2007]. During a follow up of 14 years, postural instability and/or quadriceps weakness and a history of falls predicted hip fracture, independent of age, low BMD and prior fracture [Nguyen et al. 2005].
In the Women's Health Initiative (WHI) study, 11 factors predicted hip fracture within 5 years, of which two were related to fall risk (self-reported health and self-reported physical activity), independent of age, weight, height, race/ethnicity, history of fracture after age 54 years, parental hip fracture, current smoking, current corticosteroid use, and treated diabetes [Sandhu et al. 2009].
During a follow up of 10 years in the SOF study, impaired cognition, slower walking speed, Parkinson's disease and depth perception predicted hip fracture, independent of older age, previous self-reported fracture after age 50, maternal history of hip fracture after age 50, greater height at age 25, nulliparity, type II diabetes mellitus (each independently predicted a 1.2- to 1.8-fold increase in hip fracture risk), whereas each SD (0.13 g/cm2) decrease in hip BMD was independently associated with a 1.8-fold increase in risk [Greenspan et al. 1998].
The Garvan fracture risk calculator
The Garvan fracture risk calculator is yet another tool that is available online to calculate the risk of osteoporotic and hip fracture (Table 1) [Garvan Medical Research Institute]. The Garvan algorithm differs from FRAX in several aspects (Table 1). It includes age (>60 years), sex and BMD (or body weight in the absence of BMD). In contrast to FRAX, it takes into account a history of recent falls (1, 2, and >2) and the number of previous fractures (1, 2, and >2), but does not include other risks included in FRAX. It also predicts more types of fractures than FRAX.
As a result, calculations of 10-year hip fracture risk differ between the two algorithms (Table 3). FRAX underestimates hip fracture risk in patients with two or more fractures and with two or more recent falls. On the other hand, Garvan underestimates fracture risk in women with a parent history of hip fracture and in women with secondary osteoporosis. In spite of these differences, both approaches were reasonably accurate in women [Sandhu et al. 2009].
Table 3.
Ten-year riskof hip fracture (%) according to the FRAX and Garvan algorithm in women of 80 years old, with a weight of 70kg and a height of 170 cm according to the presence of specified risk factors.
| FRAX | Garvan | ||
|---|---|---|---|
| No other risks | 7.4 | 6.7 | |
| Recent fall | 1 | 7.4 | 9.4 |
| 2 | 7.4 | 13.3 | |
| >2 | 7.4 | 18.5 | |
| Fracture histoy | 1 | 11.0 | 14.4 |
| 2 | 11.0 | 29.6 | |
| >2 | 11.0 | 54.6 | |
| 1 Recent fall + 1 fracture | 11.0 | 20.0 | |
| 3 Recent falls +3 fractures | 11.0 | 90.3 | |
| Parent with hip fracture | 23.0 | 6.7 | |
| Secondary osteoporosis | 13.0 | 6.7 |
FRAX, fracture risk assessment tool.
Fall orientation
Fall orientation is a determinant of hip fracture risk. Among frail elderly nursing home fallers, a fall to the side is an independent risk factor for hip fracture [Ensrud et al. 2007]. Patients who suffered hip fractures were more likely to have fallen sideways or straight down (odds ratio 3.3; 95%CI: 2.0–5.6) than women who fell without a fracture [Cummings et al. 1995].
Frailty and hip fractures
Although there is no generally accepted definition of frailty, in most studies frailty includes a combination of weight loss, fatigue, low physical activity, walking speed and muscle strength [Rolland et al. 2008]. Frailty has been linked with osteoporosis and hip fracture risk [Rolland et al. 2008]. During a follow up of 9 years, frailty is a risk factor for falls (OR: 1.4). The risk for hip fractures (HR: 1.4), non-vertebral fractures (HR: 1.3) and mortality (HR: 1.8) were significantly increased after correction for age, FN BMD, history of falls and fracture, BMI and several medical conditions [Lang et al. 2009].
Weight loss is a risk factor for hip fractures, and weight gain is protective [Wainwright et al. 2005].
One of the major changes in body composition with age is sarcopenia, which is associated with lower BMD, higher risk of falls and decreased physical functioning, which is related to hip fracture risk [Rolland et al. 2008]. Decreased thigh muscle Hounsfield Units, a measure of fatty infiltration of muscle, is associated with increased risk of hip fracture, and appears to account for the association between reduced muscle strength, physical performance and muscle mass with risk of hip fracture. This characteristic captures a physical characteristic of muscle tissue which may have importance in hip fracture etiology [Robbins et al. 2005].
The role of cognitive decline and depression on hip fracture risk is less clear [Ensrud et al. 2007].
Hip fracture risk in non-osteoporotic patients
Among non-osteoporotic participants, several characteristics increased fracture risk, including advancing age, lack of exercise in the last year, reduced visual contrast sensitivity, falls in the last year, prevalent vertebral fracture, and lower total hip BMD [Center and Eisman, 2008].
In the EPIDOS study, during a follow up of 3 years, grip strength and coordination were related to hip fracture risk in non-osteoporotic women [Rivadeneira et al. 2009].
Genetic factors
The genetic background is an important independent determinant for various aspects of bone structure and fracture risk. Osteoporosis is now widely accepted as being multifactorial with several genes involved, each having a small to moderate effect on various parameters affecting bone physiology and risk of fracture. Gene—gene interactions and gene—environment interactions potentially increase this complexity [Rivadeneira et al. 2006]. The heritability factor (H2) of hip fractures is high (H2: 0.48), and even higher than for other fractures (H2: 0.27) [Rivadeneira et al. 2006]. The clinical importance of genetic background is reflected in the inclusion of family history of hip fracture in the parents as an independent risk factor for fractures in FRAX [Saag and Geusens, 2009; National Osteoporosis Foundation, 2008].
Genetic polymorphisms have been related to BMD, hip geometry and osteoporotic fractures, but data on the prediction of hip fracture are scarce. Twenty loci have been related to hip BMD. The many single-nucleotide polymorphisms (SNPs) associated with BMD map to genes in signaling pathways with relevance to bone metabolism and highlight the complex genetic architecture that underlies osteoporosis and variation in BMD [Dong et al. 2009]. HSA was correlated with ESR2 haplotype 1 homozygote women [thinner cortices, increased neck width, and higher bone instability (buckling ratios)) [Medici et al. 2006]. Similar patterns of interaction were observed for BMD, cortical thickness, bone strength (section modulus), and instability (buckling ratio) [Medici et al. 2006]. The RANKL gene was related to compression strength index (CSI), independent of BMD and non-BMD components [Richards et al. 2008].
Several variants in candidate genes have been shown to influence independently an individual's genetic susceptibility to fracture, and examples include the genes for the vitamin D receptor (VDR), collagen1 (COL1A1), the estrogen receptor (ESR1), insulin-like growth factor (IGF-1), sclerostin (SOST), and lipoprotien-receptor-related protein (LRP5, and LRP6) [Rivadeneira et al. 2009], but not the bone morphogenic protein (BMP2) gene [Richards et al. 2009]. In a genome-wide association study TNFRSF11B (osteoprotegerin) gene LRP5 (lipoprotein-receptor-related protein) gene variants of key biological proteins increased the risk of osteoporosis and osteoporotic fracture [Styrkarsdottir et al. 2008]. SNPs from the LRP5, SOST, SPP1, and TNFRSF11A loci were significantly associated with fracture risk; odds ratios ranged from 1.13 to 1.43 per allele. These effects on fracture were partially independent of BMD at SPP1 and SOST [Moffett et al. 2005].
Common sequence variants that are consistently associated with BMD and with low-trauma fractures have been found in three populations of European descent (RANKL, OPG, ESR1 and other genes of unknown function) [Garnero et al. 1996]. Although these variants alone were not considered clinically useful in the prediction of risk to the individual person, they provide insight into the biochemical pathways underlying osteoporosis.
The A allele of TNF-alfa was associated with a 22% decrease in the risk of hip fracture per copy, independent of BMD, or bone strength indices [Chapurlat et al. 2000]. The incorporation of COL1A1 genotypes improved the risk reclassification by 4% for hip fracture beyond age, BMD, prior fracture, and fall [Rivadeneira et al. 2006].
However, specific genetic markers for hip fracture risk are not available for routine use in clinical practice.
Bone markers, hormones, cytokines, growth factors and hip fracture risk
Bone markers and hip fracture risk
Hip fracture risk has been found to be related with bone markers, such as urinary deoxypyridi-noline CTX, NTX [Saito et al. 2006]. In the prospective EPIDOS study during 3 years serum CTX1 was related to hip fracture risk [Rivadeneira et al. 2009; Szulc et al. 1993]. Other documented markers for hip fracture risk include serum undercarboxylated osteocalcin and the degree of mineralization-related collagen cross-linking in the FN cancellous bone in cases of hip fracture and controls [Cauley et al. 2008; Garnero et al. 2007; Vergnaud et al. 1997].
Hormones and hip fracture risk
Vitamin D
In a nested case-control study, low serum 25-hydroxyvitamin D [25(OH)D] concentrations were associated with a higher risk for hip fracture [Lee et al. 2008]. Women with the lowest 25(OH) D concentrations (≤47.5nmol/l) had a higher fracture risk than did those with the highest concentrations (≥70.7nmol/l) (adjusted odds ratio, 1.71 [CI, 1.05–2.79]), and the risk increased statistically significantly across quartiles of serum 25(OH) D concentration (p for trend = 0.016). This association was independent of number of falls, physical function, frailty, renal function, and sex-steroid hormone levels and seemed to be partially mediated by bone resorption. However, no relation between serum levels of 25(OH)D and hip fracture risk was found in the Os des FEmmes de LYon (OFELY) study, in which only few women had severe vitamin D deficiency [Abdallah et al. 2005].
Sex hormones
High serum sex hormone binding globulin (SHBG) is associated with an increased risk of subsequent hip fracture and high endogenous testosterone with a decreased risk, independent of each other, serum estradiol concentration, and other putative risk factors. But endogenous estradiol has no independent association with hip fracture [Boonen et al. 2002].
Cytokines, growth factors and hip fracture risk
Elderly women with hip fractures exhibit an increased RANKL/OPG mRNA content of iliac bone. This is associated with increased fracture susceptibility, which is not in itself explained by low BMD [Abdallah et al. 2005]. Frailty has been related to serum levels of testosterone, estrogens, IGF-1, growth hormone (GH), vitamin D and pro-inflammatory cytokines [Ensrud et al. 2007]. The exposure to stimulatory and inhibitory components of the IGF system is different between FN and trochanteric fractures [Cummings et al. 1998].
Consequences for hip fracture prevention
The multifactorial etiology of hip fracture makes it attractive to consider hip fracture prevention in a multidisciplinary approach, involving prevention of bone and fall-related risks. However, the evidence of hip fracture prevention is limited to treatment with drugs that affect bone metabolism and with calcium and vitamin D supplements.
Primary analyses of the pivotal randomized controlled trials with hip fracture prevention as a primary or secondary endpoint indicate that alendronate [Cummings et al. 1998; Black et al. 1996, 2000], risedronate [McClung et al. 2001], zoledronate [Black et al. 2007] and denosumab [Cummings et al. 2009] reduce the risk of hip fractures, and, in post hoc analyses, also strontium ranelate [Reginster et al. 2005, 2007].
However, prevention of hip fractures with these drugs has only been demonstrated in well-selected patients at high risk. In the only trial with hip fracture prevention as a primary end-point, risedronate decreased the risk of hip fractures in the total group of patients older than 70 years by 30%, by 40% in patients with a t-score <-2.5 and by 60% in patients with vertebral fracture at baseline, but not in patients mainly selected on the base of fall risks without proven low BMD [McClung et al. 2001].
Alendronate reduced the risk of hip fractures in patients (55–81 years old) with a prevalent vertebral fracture and in patients with a prevalent vertebral fracture or a FN t-score <-2.5, but not in patients without demonstrated low BMD or vertebral fracture [Cummings et al. 1998; Black et al. 1996, 2000]
Zoledronate reduced the risk of hip fracture by 41% in patients between 65 and 89 years of age with a t-score < −2.5 without a prevalent vertebral fracture or < −1.5 if they had a vertebral fracture [Black et al. 2007].
Denosumab reduced the risk of hip fracture by 40% in patients between 60 and 90 years old with a t-score < −2.5 [Cummings et al. 2009].
Strontium ranelate did not prevent hip fractures in the primary analysis in patients older than 70 years with a t-score < −2.5, but, in a post hoc analysis performed at the request of EMEA, reduced the risk of hip fractures in postmenopausal women older than 74 years with a t-score < −2.4 (-36%), but not in women <74 years old with one additional fracture risk factor (a history of osteoporotic fracture after menopause, residence in a retirement home, frequent falls, or a maternal history of osteoporotic fractures of the hip, spine, or wrist) [Reginster et al. 2005].
Thus, these drugs reduce the risk of hip fractures in patients with a clearly demonstrated bone-related fracture risk (low BMD and/or prevalent vertebral fracture), but not in the absence of documented bone-related risks or when selected on the basis of fall risks alone or in younger age groups in spite of clinical risk factors.
The mechanisms of action of inhibiting high bone turnover include a decrease in bone turnover and an increase in BMD, with preservation of bone microarchitecture, increased mineralization and geometrical changes in the hip that contribute to bone strength [Lewiecki and Borges, 2006]. Strontium ranelate increases BMD (up to 30% after 8 years) [Reginster et al. 2009] and parameters of bone microarchitecture in the forearm [Ammann et al. 2007], with only limited effects on markers of bone turnover [Meunier et al. 2004].
Although fall prevention strategies have been shown to reduce the risk of falls, none indicate that fall prevention on its own can reduce the risk of fracture, including hip fracture [Chang et al. 2004].
In the context of hip fracture prevention, vitamin D and calcium deserve special attention. Vitamin D deficiency is endemic worldwide [Kuchuk et al. 2009] and calcium intake in elderly and in fracture patients is low [Helden Van et al. 2008]. Hip fracture prevention has been demonstrated with calcium and vitamin D supplements in elderly institutionalized women who were deficient in calcium and vitamin D at baseline [Deluca, 2008], but not in other studies in ambulatory elderly women with less vitamin D deficiency than in the Chapuy study (below) and using a lower dose (400 IU/day) [Lips et al. 1996]. No other data are available on hip fracture prevention with calcium and vitamin D supplements. Vitamin D has a wide spectrum of actions [Deluca, 2008; Bischoff Ferrari et al. 2005], including on the skeleton (calcium absorption, bone mineralization), muscles, blood vessels and the immune system. Vitamin D supplements had only limited effects on BMD [Chapuy et al. 1992], but reduced the risk of falls with doses of 700–800IU of vitamin D3/day [Bischoff Ferrari et al. 2004]. Hip fracture prevention by calcium and vitamin D supplements is therefore probably the result of a combined action of calcium and vitamin D supplements on calcium homeostasis and muscle strength.
Further studies will be needed to study the effect of combined treatment of calcium, vitamin D, bone-directed drugs and fall prevention to reduce the risk of hip fractures and to identify the risk factors that would contribute to select women for such treatment.
Conclusions
The etiology of hip fractures is multifactorial. Low BMD, bone strength parameters, clinical bone and fall-related risk factors, hormones, cytokines, growth factors, bone markers and genetic background all contribute to hip fracture risk (Figure 1). Prediction of hip fracture can thus be enhanced by combining these predictors, which is possible in daily practice by evaluation of the presence of clinical risk factors, measuring BMD and evaluating the risk of falls. Calculation of absolute fracture risk integrating clinical risks, BMD, bone geometry and fall-related risks is possible, but needs further refinement by integrating bone and fall-related risk factors into a single case finding algorithm for clinical use. Hip fracture prevention is possible using drugs in high risk patients. In how far hip fracture prevention programs should also take into account prevention of falls for prevention of hip fractures remains to be proven for efficacy.
Footnotes
None declared.
References
- Abdallah B.M., Stilgren L.S., Nissen N., Kassem M., Jorgensen H.R., Abrahamsen B. (2005) Increased RANKL/OPG mRNA ratio in iliac bone biopsies from women with hip fractures. Calcif Tissue Int 76: 90–97 [DOI] [PubMed] [Google Scholar]
- Ahlborg H.G., Nguyen N.D., Nguyen T.V., Center J.R., Eisman J.A. (2005) Contribution of hip strength indices to hip fracture risk in elderly men and women. J Bone Miner Res 20: 1820–1827 [DOI] [PubMed] [Google Scholar]
- Ammann P., Badoud I., Barraud S., Dayer R., Rizzoli R. (2007) Strontium ranelate treatment improves trabecular and cortical intrinsic bone tissue quality, a determinant of bone strength. J Bone Miner Res 22: 1419–1425 [DOI] [PubMed] [Google Scholar]
- Barkmann R., Dencks S., Laugier P., Padilla F., Brixen K., Ryg J., et al. (2009) Femur ultrasound (FemuUS)-first clinical results on hip fracture discrimination and estimation of femoral BMD. Osteoporos Int: Epub 20 Aug 2009. [DOI] [PubMed] [Google Scholar]
- Bates D.W., Black D.M., Cummings S.R. (2002) Clinical use of bone densitometry: clinical applications. JAMA 288: 1898–1900 [DOI] [PubMed] [Google Scholar]
- Beck T. (2003) Measuring the structural strength of bones with dual-energy x-ray absorptiometry: principles, technical limitations, and future possibilities. Osteoporos Int 14(Suppl 5): S81–88 [DOI] [PubMed] [Google Scholar]
- Beck TJ, Mourtada F.A., Ruff C. B., Scott W.W., Jr, Kao G. (1998) Experimental testing of a DEXA-derived curved beam model of the proximal femur. J Orthop Res 16: 394–398 [DOI] [PubMed] [Google Scholar]
- Beck TJ, Looker A.C., Ruff C.B., Sievanen H., Wahner H.W. (2000) Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey Dual-Energy X-Ray Absorptiometry Data. J Bone Miner Res 15: 2297–2304 [DOI] [PubMed] [Google Scholar]
- Ferrari H.A. Bischoff, Hughes B. Dawson, Willett W.C., Staehelin H.B., Bazemore M.G., Zee R. Y., et al. (2004) Effect of vitamin D on falls: a meta-analysis. JAMA 291: 1999–2006 [DOI] [PubMed] [Google Scholar]
- Ferrari H.A. Bischoff, Willett W.C., Wong J.B., Giovannucci E., Dietrich T, Hughes B. Dawson. (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293: 2257–2264 [DOI] [PubMed] [Google Scholar]
- Black D.M., Cummings S.R., Karpf D.B., Cauley J.A., Thompson D. E., Nevitt M. C., et al. (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348: 1535–1541 [DOI] [PubMed] [Google Scholar]
- Black D.M., Thompson D.E., Bauer D.C., Ensrud K., Musliner T., Hochberg M. C., et al. (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. Fit Research Group. J Clin Endocrinol Metab 85: 4118–4124 [DOI] [PubMed] [Google Scholar]
- Black D.M., Steinbuch M., Palermo L., Dargent-Molina P., Lindsay R., Hoseyni M. S., et al. (2001) An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int 12: 519–528 [DOI] [PubMed] [Google Scholar]
- Black D.M., Delmas P.D., Eastell R., Reid I.R., Boonen S., Cauley J. A., et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809–1822 [DOI] [PubMed] [Google Scholar]
- Bliuc D., Nguyen N.D., Milch V.E., Nguyen T.V., Eisman J.A., Center J.R. (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301: 513–521 [DOI] [PubMed] [Google Scholar]
- Boonen S., Rosen C., Bouillon R., Sommer A., McKay M., Rosen D., et al. (2002) Musculoskeletal effects of the recombinant human IGF-I/IGF binding protein-3 complex in osteoporotic patients with proximal femoral fracture: a double-blind, placebo-controlled pilot study. J Clin Endocrinol Metab 87: 1593–1599 [DOI] [PubMed] [Google Scholar]
- Bouxsein M.L. (2008) Technology insight: noninvasive assessment of bone strength in osteoporosis. Nat Clin Pract Rheumatol 4: 310–318 [DOI] [PubMed] [Google Scholar]
- Brauer C.A., Perraillon M. Coca, Cutler D.M., Rosen A.B. (2009) Incidence and mortality of hip fractures in the United States. JAMA 302: 1573–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyere O., Varela A.R., Adami S., Detilleux J., Rabenda V., Hiligsmann M., et al. (2009) Loss of hip bone mineral density over time is associated with spine and hip fracture incidence in osteoporotic postmenopausal women. Eur J Epidemiol: Epub 29 Aug 2009. [DOI] [PubMed] [Google Scholar]
- Bryan R., Nair P.B., Taylor M. (2009) Use of a statistical model of the whole femur in a large scale, multi-model study of femoral neck fracture risk. J Biomech 42: 2171–2176 [DOI] [PubMed] [Google Scholar]
- Burge R., Hughes B. Dawson, Solomon D.H., Wong J.B., King A., Tosteson A. (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22: 465–475 [DOI] [PubMed] [Google Scholar]
- Cauley J.A., Lacroix A.Z., Wu L., Horwitz M., Danielson M. E., Bauer D. C., et al. (2008) Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 149: 242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center J.R., Eisman J.A. (2008) Genetics of osteoporosis, In: Miller P., Papapoulos S. (eds). ASBMR's primer on the metabolic bone diseases and disorders of mineral metabolism, Vol. 7, American Society for Bone and Mineral Research: Washington [Google Scholar]
- Center J.R., Bliuc D., Nguyen TV, Eisman J.A. (2007) Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 297: 387–394 [DOI] [PubMed] [Google Scholar]
- Chang J.T., Morton S.C., Rubenstein L.Z., Mojica W.A., Maglione M., Suttorp M. J., et al. (2004) Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trials. BMJ 328: 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapurlat R.D., Garnero P., Breart G., Meunier P.J., Delmas P.D. (2000) Serum type I collagen breakdown product (serum CTX) predicts hip fracture risk in elderly women: the EPIDOS Study. Bone 27: 283–286 [DOI] [PubMed] [Google Scholar]
- Chapuy M.C., Arlot M.E., Duboeuf F., Brun J., Crouzet B., Arnaud S., et al. (1992) Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327: 1637–1642 [DOI] [PubMed] [Google Scholar]
- Cummings S.R., Nevitt M.C., Browner W.S., Stone K, Fox KM, Ensrud K.E., et al. (1995) Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 332: 767–773 [DOI] [PubMed] [Google Scholar]
- Cummings S.R., Black D.M., Thompson D.E., Applegate W.B., Connor E. Baart, Musliner T.A., et al. (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280: 2077–2082 [DOI] [PubMed] [Google Scholar]
- Cummings S.R., San Martin J., Mcclung M.R., Siris E.S., Eastell R., Reid I. R., et al. (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361: 756–765 [DOI] [PubMed] [Google Scholar]
- Molina P. Dargent, Favier F., Grandjean H., Baudoin C., Schott A. M., Hausherr E., et al. (1996) Fall-related factors and risk of hip fracture: the EPIDOS Prospective Study. Lancet 348: 145–149 [DOI] [PubMed] [Google Scholar]
- Deluca H.F. (2008) Evolution of our understanding of vitamin D. Nutr Rev 66: S73–87 [DOI] [PubMed] [Google Scholar]
- Dong S.S., Liu X.G., Chen Y., Guo Y., Wang L., Zhao J., et al. (2009) Association analyses of RANKL/RANK/OPG gene polymorphisms with femoral neck compression strength index variation in caucasians. Calcif Tissue Int 85: 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud K.E., Ewing S.K., Taylor B.C., Fink H.A., Stone K. L., Cauley J. A., et al. (2007) Frailty and risk of falls, fracture, and mortality in older women: the Study of Osteoporotic Fractures. J Gerontol A Biol Sci Med Sci 62: 744–751 [DOI] [PubMed] [Google Scholar]
- Faulkner KG, Wacker WK, Barden H.S., Simonelli C., Burke P.K., Ragi S., et al. (2006) Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporos Int 17: 593–599 [DOI] [PubMed] [Google Scholar]
- Garnero P., Hausherr E., Chapuy M.C., Marcelli C., Grandjean H., Muller C., et al. (1996) Markers of bone resorption predict hip fracture in elderly women: the Epidos Prospective Study. J Bone Miner Res 11: 1531–1538 [DOI] [PubMed] [Google Scholar]
- Garnero P., Munoz F., Rendu E. Sornay, Delmas P.D. (2007) Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY Study. Bone 40: 716–722 [DOI] [PubMed] [Google Scholar]
- Garvan Medical Research Institute, O.B.B.P. Fracture Risk Calculator.
- Geel Van T.A.C.M., Van Helden S., Geusens P.P., Winkens B., Dinant G.J. (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68: 99–102: Epub 2008 Aug 2003. [DOI] [PubMed] [Google Scholar]
- Greenspan S.L., Myers E.R., Kiel D.P., Parker R.A., Hayes W.C., Resnick N.M. (1998) Fall direction, bone mineral density, and function: risk factors for hip fracture in frail nursing home elderly. Am J Med 104: 539–545 [DOI] [PubMed] [Google Scholar]
- Helden Van S., Cals J., Kessels F., Brink P., Dinant G.J., Geusens P. (2006) Risk of new clinical fractures within 2 years following a fracture. Osteoporos Int 17: 348–354 [DOI] [PubMed] [Google Scholar]
- Helden Van S., Geel Van A.C.M., Geusens P.P., Kessels A., Nieuwenhuijzen A.C. Kruseman, Brink P.R. (2008) Bone and fall-related fracture risks in women and men with a recent clinical fracture. J Bone Joint Surg Am 90: 241–248 [DOI] [PubMed] [Google Scholar]
- Ismail A.A., Pye S.R., Cockerill W.C., Lunt M., Silman A. J., Reeve J., et al. (2002) Incidence of limb fracture across Europe: results from the European Prospective Osteoporosis Study (EPOS). Osteoporos Int 13: 565–571 [DOI] [PubMed] [Google Scholar]
- Kanis J.A., McCloskey E.V., Johansson H., Strom O., Borgstrom F., Oden A. (2008) Case finding for the management of osteoporosis with FRAX– assessment and intervention thresholds for the UK. Osteoporos Int 19: 1395–1408 [DOI] [PubMed] [Google Scholar]
- Kaptoge S., Beck T.J., Reeve J., Stone K.L., Hillier T. A., Cauley J. A., et al. (2008) Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res 23: 1892–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M.K., Johnell O., Nilsson B.E., Sernbo I., Obrant K.J. (1993) Bone mineral mass in hip fracture patients. Bone 14: 161–165 [DOI] [PubMed] [Google Scholar]
- Keaveny T.M., Bouxsein M.L. (2008) Theoretical implications of the biomechanical fracture threshold. J Bone Miner Res 23: 1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaveny T.M., Hoffmann P.F., Singh M., Palermo L., Bilezikian J. P., Greenspan S. L., et al. (2008) Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res. 23(12): 1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M.A., Cornuz J., Ruffieux C., Van Melle G., Buche D., Dambacher MA, et al. (2006) Prediction of hip fracture risk by quantitative ultrasound in more than 7000 Swiss women > or =70 years of age: comparison of three technologically different bone ultrasound devices in the SEMOF study. J Bone Miner Res 21: 1457–1463 [DOI] [PubMed] [Google Scholar]
- Krug R., Banerjee S., Han E.T., Newitt D.C., Link T.M., Majumdar S. (2005) Feasibility of in vivo structural analysis of high-resolution magnetic resonance images of the proximal femur. Osteoporos Int 16: 1307–1314 [DOI] [PubMed] [Google Scholar]
- Kuchuk N.O., Van Schoor N.M., Pluijm S.M., Chines A., Lips P. (2009) Vitamin D status, parathyroid function, bone turnover, and bmd in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res 24: 693–701 [DOI] [PubMed] [Google Scholar]
- Lang T.F., Cauley J., Tylavsky F., Bauer D., Cummings S., Harris T. for the Health ABC Study (2009) Computed tomography measurement soft high muscle cross-sectional area and attenuation coefficient predict hip fracture: the Health, Agingand Body Composition Study. J Bone Miner Res: Epub 4 Aug 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Lacroix A.Z., Wu L., Cauley J.A., Jackson R. D., Kooperberg C., et al. (2008) Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. J Clin Endocrinol Metab 93: 1796–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie W.D., Pahlavan P.S., Tsang J.F., Lix L.M. (2009) Prediction of hip and other osteoporotic fractures from hip geometry in a large clinical cohort. Osteoporos Int 20: 1767–1774 [DOI] [PubMed] [Google Scholar]
- Lewiecki E.M., Borges J.L. (2006) Bone density testing in clinical practice. Arq Bras Endocrinol Metabol 50: 586–595 [DOI] [PubMed] [Google Scholar]
- Lewiecki E.M., Keaveny T.M., Kopperdahl D.L., Genant H.K., Engelke K, Fuerst T., et al. (2009) Once-monthly oral ibandronate improves biomechanical determinants of bone strength in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 94: 171–180 [DOI] [PubMed] [Google Scholar]
- Lips P., Graafmans W.C., Ooms M.E., Bezemer P.D., Bouter L.M. (1996) Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med 124: 400–406 [DOI] [PubMed] [Google Scholar]
- Lyles K.W., Emeric C.S. Colon, Magaziner J.S., Adachi J.D., Pieper C.F., Mautalen C., et al. (2007) Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med 357: nihpa40967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D., Johnell O., Wedel H. (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312: 1254–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung M.R., Geusens P., Miller P.D., Zippel H., Bensen W. G., Roux C., et al. (2001) Effect of rise-dronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344: 333–340 [DOI] [PubMed] [Google Scholar]
- Medici M., Van Meurs J.B., Rivadeneira F., Zhao H., Arp P. P., Hofman A., et al. (2006) BMP-2 gene polymorphisms and osteoporosis: the Rotterdam Study. J Bone Miner Res 21: 845–854 [DOI] [PubMed] [Google Scholar]
- Melton L.J., 3rd, Atkinson E.J., O'Fallon W.M., Wahner H.W., Riggs B.L. (1993) Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res 8: 1227–1233 [DOI] [PubMed] [Google Scholar]
- Meunier P.J., Roux C., Seeman E., Ortolani S., Badurski J. E., Spector T. D., et al. (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350: 459–468 [DOI] [PubMed] [Google Scholar]
- Moffett S.P., Zmuda J.M., Oakley J.I., Beck T.J., Cauley J. A., Stone K. L., et al. (2005) Tumor necrosis factor-alpha polymorphism, bone strength phenotypes, and the risk of fracture in older women. J Clin Endocrinol Metab 90: 3491–3497 [DOI] [PubMed] [Google Scholar]
- National Osteoporosis Foundation (2008) Clinician's guide to prevention and treatment of osteoporosis, Vol. 2009, National Osteoporosis Foundation: Washington [Google Scholar]
- Nevitt M.C., Cummings S.R. (1993) Type of fall and risk of hip and wrist fractures: the study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 41(11): 1226–1234 [DOI] [PubMed] [Google Scholar]
- Nguyen N.D., Pongchaiyakul C., Center J.R., Eisman J.A., Nguyen TV. (2005) Identification of high-risk individuals for hip fracture: a 14-year prospective study. J Bone Mineral Res 20: 1921–1928, http://www.garvan.org.au/promotions/bone-fracture-risk/calculator/ [DOI] [PubMed] [Google Scholar]
- Pulkkinen P., Jamsa T, Lochmuller E.M., Kuhn V., Nieminen M.T., Eckstein F. (2008) Experimental hip fracture load can be predicted from plain radiography by combined analysis of trabecular bone structure and bone geometry. Osteoporos Int 19: 547–558 [DOI] [PubMed] [Google Scholar]
- Pulkkinen P., Partanen J., Jalovaara P., Jamsa T. (2009) BMD T-score discriminates trochanteric fractures from unfractured controls, whereas geometry discriminates cervical fracture cases from unfractured controls of similar BMD. Osteoporos Int: Epub 26 Sept 2009. [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Seeman E., De Vernejoul M.C., Adami S., Compston J., Phenekos C., et al. (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) Study. J Clin Endocrinol Metab 90: 2816–2822 [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Malaise O., Neuprez A., Bruyere O. (2007) Strontium ranelate in the prevention of osteoporotic fractures. Int J Clin Pract 61: 324–328 [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Bruyere O., Sawicki A., Varela A. Roces, Fardellone P., Roberts A., et al. (2009) Long-term treatment of postmenopausal osteoporosis with strontium ranelate: results at 8 years. Bone 45: 1059–1064 [DOI] [PubMed] [Google Scholar]
- Richards J.B., Rivadeneira F., Inouye M., Pastinen T.M., Soranzo N., Wilson S. G., et al. (2008) Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371: 1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.B., Kavvoura F.K., Rivadeneira F., Styrkarsdottir U., Estrada K., Halldorsson B.V., et al. (2009) Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 151: 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadeneira F., Van Meurs J.B., Kant J., Zillikens M.C., Stolk L., Beck T. J., et al. (2006) Estrogen receptor beta (ESR2) polymorphisms in interaction with estrogen receptor alpha (ESR1) and insulin-like growth factor I (IGF1) variants influence the risk of fracture in postmenopausal women. J Bone Miner Res 21: 1443–1456 [DOI] [PubMed] [Google Scholar]
- Rivadeneira F., Zillikens M.C., De Laet C.E., Hofman A., Uitterlinden A. G., Beck T. J., et al. (2007) Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res 22: 1781–1790 [DOI] [PubMed] [Google Scholar]
- Rivadeneira F., Styrkarsdottir U., Estrada K., Halldorsson B.V., Hsu Y. H., Richards J. B., et al. (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41: 1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J.A., Schott A.M., Garnero P., Delmas P.D., Hans D., Meunier P.J. (2005) Risk factors for hip fracture in women with high BMD: EPIDOS Study. Osteoporos Int 16: 149–154 [DOI] [PubMed] [Google Scholar]
- Robbins J., Aragaki A.K., Kooperberg C., Watts N., Wactawski J. Wende, Jackson R.D., et al. (2007) Factors associated with 5-year risk of hip fracture in postmenopausal women. JAMA 298: 2389–2398 [DOI] [PubMed] [Google Scholar]
- Rolland Y., Van Kan G. Abellan, Benetos A., Blain H., Bonnefoy M., Chassagne P., et al. (2008) Frailty, osteoporosis and hip fracture: causes, consequences and therapeutic perspectives. J Nutr Health Aging 12: 335–346 [DOI] [PubMed] [Google Scholar]
- Ryg J., Rejnmark L., Overgaard S., Brixen K., Vestergaard P. (2009) Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977–2001. J Bone Miner Res 24: 1299–1307 [DOI] [PubMed] [Google Scholar]
- Saag KG, Geusens P. (2009) Progress in osteoporosis and fracture prevention: focus on postmenopausal women. Arthritis Res Ther 11: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M., Fujii K., Marumo K. (2006) Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int 79: 160–168 [DOI] [PubMed] [Google Scholar]
- Sambrook P., Cooper C. (2006) Osteoporosis. Lancet 367: 2010–2018 [DOI] [PubMed] [Google Scholar]
- Sandhu S.K., Nguyen N.D., Center J.R., Pocock N.A., Eisman J.A., Nguyen TV. (2009) Prognosis of fracture: evaluation of predictive accuracy of the FRAX algorithm and Garvan nomogram. Osteoporos Int: Epub 25 Jul 2009. [DOI] [PubMed] [Google Scholar]
- Schott A.M., Cormier C., Hans D., Favier F., Hausherr E., Dargent-Molina P., et al. (1998) How hip and whole-body bone mineral density predict hip fracture in elderly women: the EPIDOS Prospective Study. Osteoporos Int 8: 247–254 [DOI] [PubMed] [Google Scholar]
- Stewart A.D., Hannan J. (2000) Total and regional bone density in male runners, cyclists, and controls. Med Sci Sports Exerc 32: 1373–1377 [DOI] [PubMed] [Google Scholar]
- Stone K., Seeley D., Lui L., Cauley J., Ensrud K. E., Browner W., et al. (2003) BMD at multiple sites and risk of fracture of multiple types: long-term fracture prediciton. J Bone Miner Res 18: 12–18 [DOI] [PubMed] [Google Scholar]
- Styrkarsdottir U., Halldorsson B.V., Gretarsdottir S., Gudbjartsson D.F., Walters G. B., Ingvarsson T., et al. (2008) Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358: 2355–2365 [DOI] [PubMed] [Google Scholar]
- Szulc P., Chapuy M.C., Meunier P.J., Delmas P.D. (1993) Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest 91: 1769–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc P., Duboeuf F., Schott A. M., Molina P. Dargent, Meunier P.J., Delmas P.D. (2006) Structural determinants of hip fracture in elderly women: re-analysis of the data from the EPIDOS Study. Osteoporos Int 17: 231–236 [DOI] [PubMed] [Google Scholar]
- Testi D., Viceconti M., Baruffaldi F., Cappello A. (1999) Risk of fracture in elderly patients: a newpredictive index based on bone mineral density and finite element analysis. Comput Methods Programs Biomed 60: 23–33 [DOI] [PubMed] [Google Scholar]
- Testi D., Viceconti M., Cappello A., Gnudi S. (2002) Prediction of hip fracture can be significantly improved by a single biomedical indicator. Ann Biomed Eng 30: 801–807 [DOI] [PubMed] [Google Scholar]
- van Geel T.A., van Helden S., Geusens P.P., Winkens B., Dinant G.J. (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis. 68(1): 99–102 [DOI] [PubMed] [Google Scholar]
- van Helden S., Cals J., Kessels F., Brink P., Dinant G.J., Geusens P. (2006) Risk of new clinical fractures within 2 years following a fracture. Osteoporos Int. 17(3): 348–354 [DOI] [PubMed] [Google Scholar]
- Vergnaud P., Garnero P., Meunier P.J., Breart G., Kamihagi K., Delmas P.D. (1997) Undercarboxylated osteocalcin measured with a specific immunoas-say predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab 82: 719–724 [DOI] [PubMed] [Google Scholar]
- Wainwright S.A., Marshall L.M., Ensrud K.E., Cauley J.A., Black D. M., Hillier T. A., et al. (2005) Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 90: 2787–2793 [DOI] [PubMed] [Google Scholar]
- WHO FRAX WHO Fracture Risk Assessment Tool, Web version 3.0 ed
- Yang Q., Khoury M.J., Friedman J.M., Flanders WD. (2003) On the use of population attributable fraction to determine sample size for case-control studies of gene-environment interaction. Epidemiology 14: 161–167 [DOI] [PubMed] [Google Scholar]
- Young WC. (1989) Elastic stability formulas for stress and strain, In: Young WC, Budynas R.G., Roark R.J. (eds), Roark's formulas for stress and strain, 6th edn, McGraw-Hill: New York [Google Scholar]