Abstract
Paget's disease of bone is a common disorder which may affect one or many bones. Although many patients are asymptomatic, a variety of symptoms and complications may occur. Fortunately, effective pharmacologic therapy, primarily with potent bisphosphonates, is now available to treat patients with complications or symptoms. This review of Paget's disease of bone will include epidemiology and pathophysiology, complications and clinical findings, indications for treatment, and the drugs currently available to treat this condition.
Keywords: Paget's disease of bone, bisphosphonates, osteoclasts
Introduction
Paget's disease of bone (PDB), which is also known as osteitis deformans, was first described by Sir James Paget more than 130 years ago [Paget, 1877]. Paget's disease is a common focal skeletal disorder that may involve a single bone (monostotic) or multiple bones (polyos-totic). Although many PDB patients are asymptomatic, significant symptoms including bone pain, bone deformity, secondary arthritis, and neurologic problems occur in some patients. Treatment with calcitonin [Avramides, 1977; Singer, 1977] was developed in the 1970s and more recently very effective therapy with newer bisphosphonates [Silverman, 2008] has become available.
Epidemiology
PDB is the second most common bone remodeling disease after osteoporosis. This condition occurs in 1–2% of white adults older than 55 years [Ralston et al. 2008]. An analysis of archeological skeletons from Northern England (900—1850) [Rogers et al. 2002] found an overall prevalence of PDB of 2.1% in cases older than 40 years. The prevalence before 1500 was 1.7% and after 1500 was 3.1%. The prevalence of PDB increases with age and is slightly more common in men. PDB is rare under 25 years and unusual before 40 years of age [Siris and Roodman, 2008]. In a survey of 864 patients the mean age at diagnosis was 58 years [Siris, 1991].
The prevalence differs amongst various ethnic/geographic groups. This disease is most common in the United Kingdom and Western Europe but is also common in British immigrants to Australia, New Zealand, South Africa, and South America [Altman, 2002]. The disease is uncommon in African blacks, Scandinavia, China, Japan, Southeast Asia, and the Indian subcontinent [Altman, 2002]. Furthermore, there is evidence of decreasing incidence and severity of PDB in the United Kingdom [Cooper et al. 1999, 2006] and New Zealand [Doyle et al. 2002; Cundy et al. 2004; Cundy, 2006] over the past 25—30 years. The incidence of PDB does not appear to have clearly decreased in United States [Tiegs et al. 2000] or Spain [Guanabens et al. 2008]. In Italy, the incidence has remained fairly stable [Gennari et al. 2005], however, the severity of disease may have increased in Southern Italy during recent years [Rendina et al. 2006]. First-degree relatives of patients with PDB have an increased risk of PDB, particularly if the patient has an early age of diagnosis or deforming bone disease [Siris et al. 1991]. Family history is positive in approximately 15–30% of patients with PDB and first degree relatives of patients with PDB have about a sevenfold greater risk for the development of Paget's disease [Siris and Roodman, 2008]. Familial PDB also tends to be diagnosed at a younger age and involve more of the skeleton than sporadic disease [Seton et al. 2003].
These findings suggest that genetic and environmental factors are important in the development of this disease.
Pathophysiology
PDB is a chronic, progressive disorder involving one or more bones. Skeletal lesions of PDB are characterized by increased osteoclastic bone resorption, increased but somewhat disorganized bone formation, and increased vascularity of bone [Ralston et al. 2008]. The osteoclasts are increased in number and size and may contain more nuclei than normal. The nuclei may contain inclusion bodies that resemble viral particles [Roodman, 1996].
The initial lesion is believed to be a focal increase in osteoclastic bone resorption. This is followed by accelerated bone formation. Because of the accelerated bone turnover, new collagen fibers are not laid down in an orderly linear fashion but rather in a disorganized manner. The resultant bone is a mosaic of woven and lamellar bone [Siris and Roodman, 2008] that is mechanically insufficient and at increased risk for fracture or deformity.
PDB is considered to be a disease of the osteo-clasts [Wirfel et al. 1999]. Bone marrow and circulating osteoclast precursors demonstrate increased sensitivity to factors known to stimulate bone resorption such as 1,25 dihydroxy vitamin D and receptor activator of NF-kB ligand (RANKL) [Roodman and Windle, 2005]. Increased interleukin-6 (IL-6) expression and signaling may contribute to increased osteo-clastic activity [Roodman et al. 1992; Hoyland et al. 1994]. RANKL (which stimulates osteo-clastic differentiation) expression is increased in pagetic marrow cells [Menaa et al. 2000] and elevated levels of circulating RANKL were found recently in PDB patients [Martini et al. 2007]. Osteoblasts are increased in numbers at pagetic sites, however, they are morphologically normal and are not considered to be a primary pathophysiologic factor in PDB by most authorities [Singer et al. 2006].
Environmental factors
Several potential environmental factors have been associated with the development of PDB. Rural life and animal contacts are associated with a greater risk of PDB in Italy [Gennari et al. 2006] and Spain [Lopez-Abente et al. 1997] suggesting that animals may carry infectious agents. Viral infection has been suggested because the nuclear inclusion bodies in osteoclasts appear to represent viral nucleo-capsids [Mills and Singer, 1976]. Paramyxovirus, and in particular canine distemper virus and measles virus are the most extensively studied environmental agents, however controversy remains whether viruses play a role in the development of PDB. Some studies suggest an association between PDB and dog ownership [O'Driscoll and Anderson, 1985] and in particular dogs not vaccinated for canine distemper [Khan et al. 1996]. Other studies refute this association [Siris et al. 1990; Seton et al. 2003]. Another study found an association between prior dog and possibly prior cat ownership and PDB in patients younger than 60 years [Holdaway et al. 1990]. In situ hybridization (ISH) [Basle et al. 1986] and reverse transcrip-tase polymerase chain reaction (RT-PCR) [Reddy et al. 1995, 1996; Friedrichs et al. 2002] have suggested the presence of measles virus nucleocapsid sequences in pagetic osteo-clasts and mononuclear cells. Canine distemper virus RNA has also been found using ISH [Gordon et al. 1991] and RT-PCR in pagetic bone cells [Gordon et al. 1992; Mee et al. 1998]. Furthermore, transfection of measles virus nucleocapsid gene into osteoclast precursors produces a pagetic-like phenotype [Kurihara et al. 2000, 2006]. Infection of osteo-clast precursors with canine distemper virus induces osteoclastogenesis [Selby et al. 2006]. Addition of canine distemper virus to canine bone marrow cultures results in an increase in multinucleated osteoclast-like cell formation [Mee et al. 1995]. Other studies using RT-PCR, ISH, and immunocytochemistry do not find evidence of measles or canine distemper virus in bone biopsies, bone marrow, or peripheral blood mononuclear cells from PDB patients [Ralston et al. 1991; Birch et al. 1994; Helfrich et al. 2000; Ralston et al. 2007]. In a study of bone marrow cultures from patients with PDB, measles virus and canine distemper virus transcripts were not found [Ooi et al. 2000]. The reasons that some studies find viral transcripts and others do not are unclear, however, this difference does not appear to be due to assay sensitivity [Ralston et al. 2007]. The possibility of contamination as a cause for false positive results has been raised [Ralston et al. 2007]. Of note is that serologic evidence of canine distemper virus is absent in patients with PDB and PDB is rare in some regions where canine distemper virus is common [Cundy and Bolland, 2008]. The issue of whether or not viral infections are related to PDB is not resolved.
Other environmental exposures have been postulated to increase the risk of PDB. Arsenic used as pesticides in the cotton industry from 1917 to 1945 has been hypothesized to contribute to the high regional incidence of PDB in Lancashire, United Kingdom in 1974 and decline in this region by 1993 [Lever, 2002].
Genetic factors
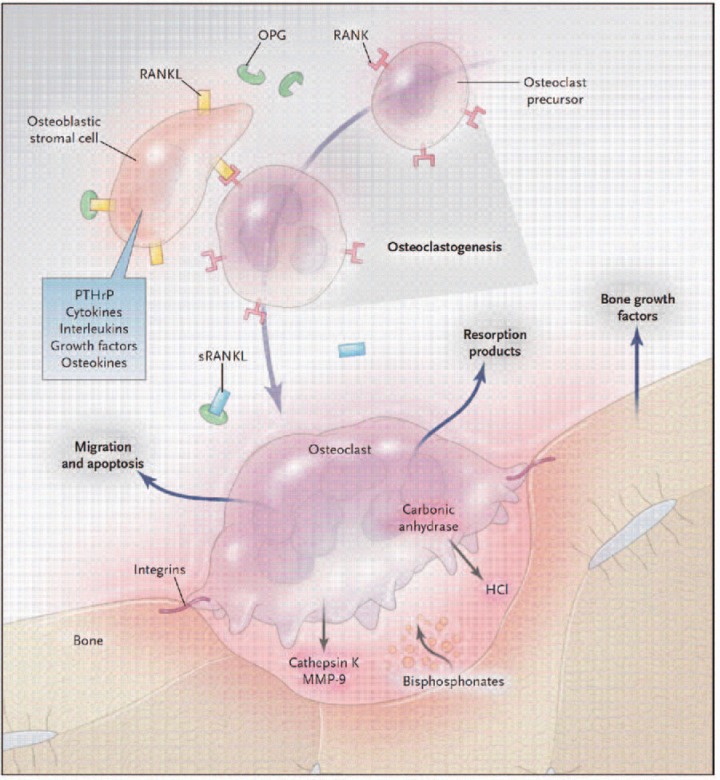
As mentioned above, patients with PDB often report a history of PDB in first-degree relatives. Before discussing the genetics of PDB and related disorders, is appropriate to review the RANK ligand/RANK/OPG system seen in Figure 1 [Deftos, 2005]. This system regulates osteoclast function [Boyle et al. 2003]. RANK (receptor activator of NF-kB) is present on osteoclast precursors. Rank ligand (RANKL), which is expressed in the marrow stroma and on osteo-blasts, binds RANK on osteoclast precursors promoting osteoclast proliferation and differentiation. Osteoprotegerin (OPG) is a decoy receptor which binds RANKL, thus preventing RANKL from binding RANK. OPG, therefore, inhibits osteoclast differentiation.
Figure 1.
Biology of the osteoclast in bone metabolism as a model for drug discovery. When bone is resorbed, growth factors in the bone matrix are released, often stimulating osteoclasts, which secrete resorption products into the circulation. Osteoblastic stromal (mesenchymal) cells and maturing osteoclasts express a soluble version of the osteokine-receptor activator of the nuclear factor KB ligand (sRANKL), its cell-bound receptor RANK, and osteoprotegerin (OPG), which sustain osteoclastogenesis. The stimulatory activity of RANKL and the inhibitory effects of OPG regulate osteolysis. Bisphosphonates concentrated under the osteoclasts inhibit resorptive function and promote osteoclast apoptosis. The incorporation of bisphosphonates into bone may increase resistance to resorption. Current therapies for hyperresorptive states can be accommodated by this model; the pathways outlined here suggest additional targets, for which therapies are under development: bone growth factor antagonists; guanine nucleotide exchange factors (GEFs), which inhibit cell migration; arginine—glycine—aspartic acid peptides, which act on integrins; carbonic anhy-drase inhibitors, which block the generation of hydrochloric acid; cathepsin K and matrix metalloproteinase 9 (MMP-9) inhibitors; and antibodies to parathyroid hormone-related protein (PTHrP) and RANKL. (With permission from Deftos LJ. (2005). Treatment of Paget's disease — taming the wild osteoclast. N Engt J Med 353: 872—875).
Juvenile PDB (also known as hereditary hyper-phosphatasia) is associated with inactivating mutations in OPG (TNFRSF11B) [Whyte et al. 2002]. Loss of this decoy receptor for RANK results in increased binding of RANK to RANKL and therefore increased osteoclastic differentiation/activity. OPG mutations not appear to be a common cause of classical Paget's disease, however, common polymorphisms of this gene may be associated with PDB in women [Daroszewska et al. 2004; Beyens et al. 2007].
Activating mutations of RANK (TNFRSF11A) cause familial expansile osteolysis [Hughes et al. 2000], expansile skeletal hyperphosphatasia [Whyte and Hughes, 2002], and early onset Paget's disease [Nakatsuka et al. 2003]. Mutations of RANK do not appear to be a common cause of classical PDB [Wuyts et al. 2001].
The genetics of classical PDB have been reviewed elsewhere [Lucas et al. 2006a; Ralston, 2008]. Genome wide-searches and linkage analysis have resulted in the discovery of several possible Paget's susceptibility genes (PDB1— PDB7).
These loci are PDB1 (chromosome 6) [Tilyard et al. 1982], PDB2 (chromosome 18q21) [Cody et al. 1997; Haslam et al. 1998], PDB3 (chromosome 5q35) [Laurin et al. 2001; Hocking et al. 2001], PDB4 (chromosome 5q31) [Laurin et al. 2001], PDB5 (chromosome 2p36) [Hocking et al. 2001], PDB6 (chromosome 10p13) [Hocking et al. 2001; Lucas et al. 2008], and PDB7 (chromosome 18q23) [Good et al. 2002]. Some of these loci may be false positives and others may represent genes that interact with other genes to cause or modify PDB [Ralston, 2008]. It has been suggested that the PDB7 locus may represent a gene that lowers the age of onset of PDB [Good et al. 2004]. Evaluation of patients with PDB3 (chromosome 5q35) mutations resulted in the discovery that the sequestosome 1 (SQSTM1) mutations are an important cause of PDB [Laurin et al. 2002; Hocking et al. 2002]. SQSTM1 gene mutations have been found in several populations [Lucas et al. 2006]. A meta-analysis found SQSTM1 gene mutations in 28.8% of familial and 6.1% of sporadic PDB patients [Rhodes et al. 2008]. SQSTM1 codes for p62 which ultimately plays a role in osteoclast regulation [Hiruma et al. 2008; Chamoux et al. 2009]. Recent studies have differed on whether somatic mutations of SQSTM1 are found in the affected bone of sporadic PDB patients. In a study of pagetic bone samples, none of 22 patients without germline SQSTM1 mutations had somatic SQSTM1 mutations [Matthews et al. 2009]. In another recent study, two of five patients without germline SQSTM1 mutations had somatic SQSTM1 mutations in pagetic bone and three of five pagetic osteosar-coma patients without germline SQSTM1 mutations had somatic SQSTM1 mutations in the tumor [Merchant et al. 2009]. Interestingly, patients in families with SQSTM1 mutations appear to have variable expressivity and incomplete penetrance of PDB and this mutation may predispose to rather than cause PDB [Leach et al. 2006]. In addition to SQSTM1, the evidence for PDB6 (chromosome 10p13) as a candidate gene, appears to remain the strongest [Lucas et al. 2008].
The rare syndrome of PDB inclusion body myo-pathy and frontotemporal dementia is caused by mutations in valosin-containing protein (VCP) [Watts et al. 2004; Kimonis and Watts, 2005; Watts et al. 2007; Viassolo et al. 2008]. Mutations in this gene do not appear to be the cause of common familial or sporadic PDB [Lucas et al. 2006a].
Clinical presentation, findings, and complications
Complications of PDB are listed in Table 1. PDB is often asymptomatic and is frequently discovered incidentally when an elevated serum alkaline phosphatase (SAP) is found on routine laboratory testing, or radiographically on x-rays performed for unrelated reasons. A recent population-based study from Olmsted County, Minnesota, USA [Wermers et al. 2008] found that 58% of the patients had symptoms at diagnosis. Seventy-two percent of these patients had polyostotic disease. Skeletal complications included bowing deformity in 7.6%, fracture of pagetic bone in 9.7%, and osteosarcoma in 0.4%. Osteoarthritis was present in 73%. Hearing loss was present in the 61%. Neurologic symptoms were uncommon. Overall survival was slightly better than predicted. PDB appears to have an adverse effect on some domains of quality of life [Gold et al. 1996; Langston et al. 2007].
Table 1.
Complications of Paget's disease of bone.
| Musculoskeletal |
| Bone pain |
| Bone deformity |
| Fractures |
| Osteoarthritis of joints adjacent to pagetic bone |
| Neurologic |
| Hearing loss |
| Headache |
| Cranial nerve deficits |
| Basilar invagination |
| Spinal stenosis |
| Spinal vascular steal syndrome |
| Peripheral neuropathies |
| Cardiovascular |
| Congestive heart failure |
| Calcification of the aortic valve |
| Conduction abnormalities |
| Vascular calcification |
| Endocardial calcification |
| Neoplastic |
| Sarcomas |
| Giant cell tumors |
| Miscellaneous |
| Peyronie's disease |
| Hypercalciuria |
| Hypercalcemia |
The most commonly involved bones include the pelvis, femur, spine, skull, and tibia [Siris, 1991]. The upper extremities, clavicles, ribs and scapulae are less frequently affected and the hands and feet are rarely involved [Siris, 1991]. This disease may involve one or more bones, however, it is unusual for a new bone to become involved after initial diagnosis [Cundy and Bolland, 2008]. PDB may be associated with osteoarthritis [Altman and Collins, 1980; Altman, 1999] in large joints such as the hip and knee, particularly when PDB involvement is adjacent to the joint or when bone deformity is present. Bone pain may be present and may be worse at night. Bone pain may be worse with weight-bearing if there is an osteolytic lesion [Whyte, 2006]. Bone enlargement or deformity may be present. There may be enlargement of the skull or frontal bossing and long bones may be bowed [Siris and Roodman, 2008]. When bowed, the femur and tibia typically bow anteriorly and laterally. Bowing of a femur or tibia can lead to pain due to gait abnormalities and arthritis [Siris and Roodman, 2008]. Painful fissure fractures may occur [Redden et al. 1981] and are more often on the convex surface of a bone. For example, fissure fractures of the femur are usually found laterally, unlike Looser zones of osteomalacia which are typically seen in the medial aspect of the proximal femur. Pain that increases dramatically may represent a fracture. Patients may note warmth of the skin over pagetic lesions due to increased vascularity. Dilated scalp veins may be present when there is skull involvement.
Neurologic complications of PDB have been reviewed elsewhere [Schmidek, 1977; Poncelet, 1999; McCloskey and Kanis, 2002; Rubin and Levin, 2009]. Some symptoms and neurologic complications may be related to skull involvement. Patients with skull involvement may have headaches [Siris and Roodman, 2008]. Cranial nerve deficits have been reported but appear to be rare. Optic atrophy, ophthalmoplegia, anosmia, trigeminal neuralgia, and facial palsy have been reported [Rubin and Levin, 2009]. Hearing loss may occur when the temporal bone is involved. Presbycusis and PDB affect similar populations and it may be difficult to differentiate presbycusis from pagetic hearing loss [Bone, 2006]. Pagetic hearing loss is sensori-neural which is greater at high pitches and characteristically accompanied by low-frequency ‘air-bone gap’ [Monsell, 2004]. The hearing loss appears to be caused by loss of bone density in the cochlear capsule [Monsell et al. 1999; Monsell, 2004]. Some patients may have tinnitus. Another neurologic complication resulting from skull involvement is basilar invagi-nation from softening of the skull base which may cause cause hydrocephalus with headache and dizziness. This basilar invagination can result in syringomyelia and compression of the brainstem. This neurologic syndrome is usually slowly progressive and symptoms may include ataxia, vertigo, tinnitus, dysphagia, and dysarthria [Rubin and Levin, 2009].
Spinal involvement may cause pain, neurologic symptoms, and even paralysis. These may be caused by a compression of the spinal cord or nerve roots as well as ischemia due to ‘vascular steal’ [Herzberg and Bayliss, 1980]. Peripheral neuropathies such as ulnar, median, sciatic, and posterior tibial have been reported [Rubin and Levin, 2009]. Neurologic symptoms may improve with calcitonin [Chen et al. 1979; Herzberg and Bayliss, 1980] or bisphosphonate [Wallace et al. 1995; Pane, 2007] therapy.
High output heart failure is rare but may occur with extensive PDB, particularly when underlying cardiac disease is present [Siris, 1991]. Decreased peripheral vascular resistance and increased stroke volume was found in echocar-diographic study of PDB [Morales-Piga et al. 2000]. Increased frequencies of aortic stenosis [Strickberger et al. 1987; Hultgren, 1998] and conduction abnormalities have been reported [Hultgren, 1998]. Increased calcification of the vasculature has also been reported [Laroche and Delmotte, 2005].
Osteosarcomas or other sarcomas (chondrosar-coma, fibrosarcoma) are the most serious complications of PDB. Although these sarcomas are more common than in age-matched controls, they occur in less than 1% of patients with PDB [Siris and Roodman, 2008]. Sarcomas may present with worsening pain, lytic lesion on x-ray, fracture, a new mass, or rising SAP [Hansen et al. 2006]. The most common bones involved in pagetic osteosarcoma include pelvis, femur, humerus, tibia, and skull [Hansen et al. 2006]. Interestingly, despite being frequently involved by PDB, osteosarcoma is uncommon in the pagetic spine [Hansen et al. 2006]. Patients with pagetic sarcomas have a poor prognosis and most patients die from local extension of disease or pulmonary metastases within 3 years [Siris and Roodman, 2008]. A recent study reviewing all cases of pagetic sarcoma at the Royal Orthopaedic Hospital in Birmingham, UK, since 1975 [Mangham et al. 2009] suggested a declining incidence of sarcoma in PDB to about 0.3%. The mean age at presentation of sarcoma after 1996 was 76.6 years compared to 70.4 years in patients presenting before 1996, suggesting age at presentation may be increasing. The median survival remained poor at 0.66 years.
Skeletal or extraskeletal benign giant cell tumors or osteoclastomas [Singer and Mills, 1993; Ziambaras et al. 1997] have also been reported and appear to be corticosteroid responsive. There appears to be geographic clustering with many of these patients being descendents of four residents of Avellino, Italy [Rendina et al. 2004]. Finally, Peyronie's disease appears to be associated with PDB [Lyles et al. 1997].
Laboratory findings
The serum calcium and phosphorus are usually normal. In untreated patients, the serum total and bone specific alkaline phosphatase (BSAP) levels are usually elevated [Whyte, 2006]. In one study, the total SAP was elevated in 78% of PDB patients and BSAP elevated in 84% of PDB patients [Alvarez et al. 1995]. The elevation in totalor BSAP reflects both the extentofthe disease and the associated increased osteoblastic activity. The SAP may be normal in patients with minimal skeletal involvement or monostotic PDB, inactive disease, or after specific antipagetic treatment. The BSAP may be useful in these circumstances or when coexisting liver disease results in elevation of the SAP. Other markers of bone formation and bone resorption may also be elevated [Alvarez et al. 2001] but are expensive and usually not needed. The serum uric acid is sometimes elevated [Siris and Roodman, 2008] but gout has not been proven to occur more frequently in patients with PDB than the general population [Siris and Roodman, 2008]. Although hypercalcemia may complicate extensive PDB during periods of immobilization or dehydration, hypercalcemia usually reflects a coexisting condition such as primary hyperparathyroidism (PHPT). The presence of hypercalcemia should prompt an evaluation for an underlying cause and should not be assumed to be related to PDB. Hypercalciuria may occur due to increased bone resorption particularly with immobilization [Kanis, 1991b]. It is probably prudent to measure the serum 25-hydroxy vitamin D level to exclude vitamin D deficiency as a contributing factor to an elevated SAP. Furthermore, vitamin D deficiency should always be corrected prior to initiating bisphospho-nate therapy. The serum parathyroid hormone level may be elevated in a significant percentage (∼15–20%) of patients with PDB [Siris et al. 1989, 1998]. In many of these patients, the elevation of parathyroid hormone is believed to represent secondary PHPT occurring at times of active pagetic bone formation. Secondary PHPT can also occur after suppression of bone resorption with bisphosphonate therapy [Siris et al. 1998]. Inadequate vitamin D stores and age-related declines in renal function could also contribute to secondary PHPT. PHPT may coexist with PDB, however, its prevalence is probably not more than expected [Gutteridge et al. 1999a]. Successful surgical treatment of PHPT may be followed by a significant decrease in SAP [Gutteridge et al. 1999a]. Because PHPT whether primary or secondary may have a negative impact on pagetic bone lesions, it is appropriate to treat secondary PHPT with adequate calcium and vitamin D and consider appropriate parathyroid surgery in the patient with coexistent PDB and PHPT [Brandi and Falchetti, 2006; Siris and Roodman, 2008].
Radiologic findings
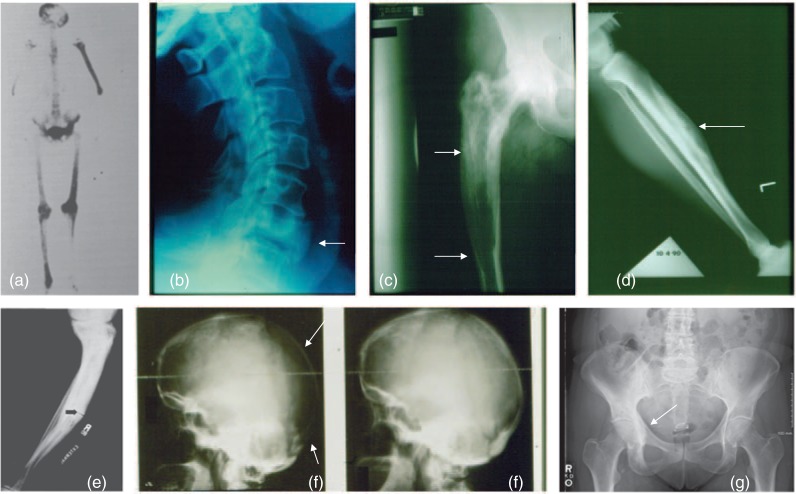
A total body bone scan or a radiographic skeletal survey is often performed to assess the extent of disease. I prefer radionuclide bone scanning followed by targeted X-ray imaging of the bones that demonstrate abnormal radionuclide uptake. The enhanced skeletal uptake of radionuclide reflects increased bone formation and increased blood flow and may detect PDB before X-ray findings can be appreciated. Alternatively, some lesions in which osteoblastic activity has become inactive will be negative on bone scanning despite findings of PDB on plain radiographs. Treatment of PDB may render the radionuclide scan normal. On plain radiographs the earliest lesions are lytic due to increase osteoclastic activity. Lytic lesions may progress at a rate of about 1 cm yearly [Whyte, 2006]. The advancing lytic lesion may appear like a blade of grass or ‘V-shaped lesion’ in long bones and osteoporosis circumscripta in the skull [Cushing and Bone, 2002]. In response to increased bone resorption, bone formation is increased, resulting in a picture of mixed lytic and sclerotic areas. Bony enlargement, cortical thickening, increased trabecular pattern, and deformity or bowing may be seen. Skull involvement typically begins with radiolucent areas (osteoporosis circumscripta) followed by osteoblastic activity resulting in enlargement of the skull and a classic ‘cotton wool’ appearance [Cushing and Bone, 2002]. Involved vertebrae may have coarsened trabecular patterns and are sometimes osteosclerotic (ivory vertebra). The posterior elements are usually involved [Cushing and Bone, 2002]. Compression deformities may occur [Cushing and Bone, 2002]. Anterior, posterior, or lateral enlargement of verterbral bodies may be seen [Kanis, 1991a]. Radiographic examples are shown in Figure 2.
Figure 2.
Radiographic appearance of Paget's disease of bone (PDB). (a) Bone scan in polyostotic PDB (with permission, courtesy of The Paget Foundation for Paget's Disease of Bone and Related Disorders). (b) Enlarged and osteosclerotic vertebral body. (c) Pagetic femur with osteosclerotic area proximal to a lytic front. (d) Lytic area in enlarged pagetic tibia. (e) Fissure fracture in a pagetic tibia (with permission, courtesy of The Paget Foundation for Paget's Disease of Bone and Related Disorders). (f) Lytic area in pagetic skull (osteoporosis circumscripta) with remineralization after aminobisphosphonate therapy. (g) PDB involving the right hemipelvis.
Nonspecific therapy
Acetaminophen, aspirin, or nonsteroidal anti-inflammatory drugs may be used for secondary degenerative joint disease [Siris and Roodman, 2008] and mild bone pain. Compensatory shoe lifts or orthotics may be useful in patients with bowed/shortened lower extremities and ambulation aids can be used as needed [Silverman, 2008]. Physical therapy is sometimes helpful.
Specific antipagetic drug treatment
Indications for specific antipagetic drug treatment
For the most part, indications are based on clinical experience rather than outcomes from clinical trials of significant size and duration. Clinically significant progressive deformity has been observed in untreated patients [Siris and Feldman, 1997]. Indications for treatment [Lyles et al. 2001; Siris et al. 2006; Silverman, 2008] are shown in Table 2 and include symptoms due to active PDB including bone pain at a pagetic site, fatigue fracture, headache from skull disease, or pain related to pagetic vertebrae. Neurologic symptoms due to spine or skull involvement are also indications for treatment. Patients with hearing loss due to temporal bone involvement should be treated in an attempt to prevent further hearing loss. Preoperative treatment is indicated in patients scheduled for elective surgery involving pagetic bone such as a total hip arthroplasty or tibial osteotomy in an attempt to decrease blood loss. Hypercalcemia related to immobilization in a patient with polyostotic PDB is another indication for treatment.
Table 2.
Indications for specific antipagetic therapy.
| Bone pain at a pagetic site |
| Neurologic symptoms |
| Pagetic hearing loss (treat to prevent further hearing loss) |
| Hypercalcemia |
| Before surgery on pagetic bone |
| High-output heart failure in a patient with extensive skeletal involvement |
| Prevention of progression/complications |
| Active Paget's disease of bone at sites such as skull, spine, weight-bearing long bones, adjacent to large joints, to prevent complications. This is controversial because it is not proven that treating such patients will prevent complications |
While treatment to prevent complications in asymptomatic patients seems reasonable, this remains an area of controversy. This includes treatment of patients with involvement adjacent to large joints to avoid arthritis, treatment of patients who may be at risk for bowing of long bones, treatment of patients with temporal bone involvement to avoid hearing loss, and treatment of patients with significant skull or spine involvement to avoid neurologic problems.
Therapeutic options for specific antipagetic Therapy
Table 3 lists the agents approved by the US Food and Drug Administration (FDA) for treatment of PDB. Specific antipagetic drugs inhibit osteoclastic activity. Salmon calcitonin has been available for many years. It is typically given a dose of 100IU subcutaneously daily which is associated with an approximately 50% reduction in SAP and relief of some symptoms [Siris and Roodman, 2008]. Improved bone pain, healing of osteolytic lesions, and improvement in neurologic complications have been reported. The maintenance dose is often subsequently decreased to 50—100IU three times weekly. This drug is less effective than the more potent bisphosphonates and resistance due to antibody formation against calcitonin may occur [Levy et al. 1988]. Side effects include nausea and flushing in some patients [Siris and Roodman, 2008]. Calcitonin is now used much less frequently than the more potent bisphosphonates and is primarily used in patients who cannot take or tolerate oral or intravenous (IV) bispho-sphonate therapy. Nasal spray calcitonin is not FDA approved for PDB.
Table 3.
Drugs approved by the FDA for treatment of Paget's disease of bone.
| Drug | Administration/dosage |
|---|---|
| Calcitonin (Miacalcin) | 50—100 units by sc injection daily or three times weekly for 6—18 months |
| Etidronate (Didronel) | 200—400 mg orally daily for 6 months; must be taken with 6—8 ounces of water on an empty stomach with no food, beverages, or medications for 2 hours before and after the dose; course should not exceed 6 months; repeat courses can be given after rest periods of 3—6 months. |
| Pamidronate (Aredia) | Approved regimen is 30 mg iv over 4 hours on 3 consecutive days. The drug is often used at 60 mg or 90 mg iv over 2—4 hours and repeated as clinically indicated. A single infusion is often effective in mild disease; two to three infusions may be required in more severe disease. |
| Alendronate (Fosamax) | 40 mg orally daily for 6 months. Must be taken on an empty stomach with 6—8 ounces of water in the morning. Patient should wait at least 30 minutes before eating food or drinking anything other than water or taking a medication. Patient should not lie down for at least 30 minutes. |
| Tiludronate (Skelid) | 400 mg orally daily for 3 months. Must be taken with 6—8 ounces of water on an empty stomach with no food, beverages, or medications for 2 hours before and after the dose. |
| Risedronate (Actonel) | 30 mg orally daily for 2 months. Must be taken on an empty stomach with 6—8 ounces of water in the morning. Patient should wait at least 30 minutes before eating food or drinking anything other than water or taking a medication. Patient should not lie down for at least 30 minutes. |
| Zoledronic acid (Reclast) | 5 mg iv over 15 minutes. The drug should not be used if the creatinine clearance is less than 35 ml/min. Patient should have adequate calcium and vitamin D to reduce the risk of hypocalcemia. |
* Clodronate is not available in the US, but is available in some countries and is given 400–1600 mg daily orally for 3—6 months or 300 mg iv daily for 5 days.
iv, intravenous; sc, subcutaneous.
Bisphosphonates are nonbiodegradable synthetic analogs of inorganic pyrophosphate. These drugs adhere to mineralized surfaces, inhibit osteoclastic bone resorption (Figure 1) and have very long skeletal half-lives [Licata, 2005]. These drugs are often divided into two classes; simple bispho-sphonates such as etidronate and tiludronate and the more potent nitrogen-containing bispho-sphonates such as pamidronate, alendronate, risedronate, and zoledronic acid (ZA) [Licata, 2005]. The simple bisphosphonates appear to inhibit osteoclastic function by forming toxic analogues of ATP in osteoclasts [Frith et al. 2001]. Etidronate has been available for many years for treatment of PDB. For PDB, etidronate is typically given as 400 mg daily for 6 months followed by 6 months without therapy. This treatment results in approximately 50% reductions in SAP and SAP will normalize in approximately one in six patients [Siris et al. 1996; Miller et al. 1999]. Higher doses are not recommended because of the risk of a mineralization defect [Gibbs et al. 1986]. Tiludronate is another ‘less potent’ bisphosphonate. It is given in a dosage of 400 mg daily for 3 months. In one study, 35% of PDB patients achieved a normal SAP and 72% of patients had a decrease in SAP of at least 50% [McClung et al. 1995] with this regimen. Tiludronate appears to be somewhat more effective than etidronate for treatment of PDB [Roux et al. 1995].
The more potent amino bisphosphonates include alendronate, risedronate, pamidronate, and ZA, and are the preferred drugs. The potency of nitrogen-containing bisphosphonates in inhibiting osteoclastic bone resorption may be related to their ability to inhibit farnesyl diphosphate synthase [Dunford et al. 2001]. Alendronate and risedronate are given orally while pamidro-nate and ZA are given intravenously. In the treatment of PDB, alendronate is given at a dosage of 40 mg daily for 6 months. In a trial comparing alendronate to etidronate in the treatment of PDB, 63% of the patients treated with alendro-nate had normalization of SAP compared to 17% with etidronate [Siris et al. 1996]. A recently published trial compared alendronate 40 mg by tablet daily for 6 months with alendronate 280 mg in oral buffered solution once weekly for 6 months. Both drugs were equally effective in lowering SAP, however, the 280 mg oral buffer solution was associated with more gastrointestinal symptoms including nausea, abdominal pain, and diarrhea [Hooper et al. 2009]. Risedronate is given in a dosage of 30 mg daily for 2 months to treat PDB. In a pivotal trial of risedronate, 73% of PDB patients treated with risedronate normalized SAP compared to 15% of patients receiving etidronate [Miller et al. 1999]. A statistically significant reduction in pain was seen in the risedro-nate but not the etidronate group [Miller et al. 1999]. In a study of 13 patients with severe PDB (mean SAP 17 times the upper normal limit (UNL)) [Singer et al. 1998], there was a 77% decrease in SAP after an 8 week course of rise-dronate 30 mg daily. Ten patients had a second course of risedronate resulting in a mean 87% decrease in SAP. Pamidronate is FDA approved for PDB in a dosage of 30 mg intravenously over 4 hours on three consecutive days, however, other regimens are more commonly used [Siris, 1994]. Patients with mild disease may be treated with a single 60—90 mg infusion while patients with more severe disease may receive multiple 90 mg infusions. In a prospective, nonrando-mized study [Tucci and Bontha, 2001] that evaluated the response of 80 PDB patients to IV pamidronate (180 mg over 3—6 weeks), the mean decrease in SAP was 63%. Normalization of SAP occurred in 86% of patients with a baseline SAP less than three times the UNL, 38% of patients with a baseline SAP three to six times the UNL, and 12% of patients with a baseline SAP more than six times the UNL [Tucci and Bontha, 2001]. In a study comparing oral risedronate (one or two courses of 30 mg daily for 8 weeks) to IV pamidronate (first course 30 mg three times; second course if needed 60 mg three times) in patients with acquired resistance to intravenous clodronate, 86% of risedronate-treated patients and 80% of pamidronate-treated patients achieved remission [Rendina et al. 2004]. Another study compared intravenous pamidronate to oral alendronate [Walsh et al. 2004]; 56% of patients treated with pamidronate and 86% of patients treated with alendronate achieved biochemical remission (normalization of SAP and urinary deoxypyridinoline to creati-nine ratio). Remission rates in previously untreated patients were similar with pamidronate and alendronate, however, in patients previously treated with pamidronate the remission rate was higher with alendronate. Zoledronic acid was FDA approved for PDB in 2007. It is given in a dosage of 5 mg IV over 15—30 minutes. A study of patients with a mean baseline SAP of about four times normal was done comparing a single dose of ZA 5 mg IV to risedronate 30 mg by mouth daily for 60 days. At 6 months 89% of the patients treated with ZA and 58% of those treated with risedronate had normalized SAP [Reid et al. 2005]. In this study, SF-36 testing revealed that the ZA group had greater improvement in physical functioning at 3 months and general health at 6 months as compared to the risedronate group. In an 18 month extension of this study in patients who had had a therapeutic response defined as a normalization of SAP or a 75% or greater reduction in SAP, the SAP increased from 6 months to 24 months in the risedronate group but was stable in the ZA group, suggesting that the biochemical effect of ZA appears to be more durable than risedronate [Hosking et al. 2007]. Improvement in osteolytic lesions has been reported with amino-bisphosphonate therapy [Thiebaud et al. 1988; Reid et al. 1996; Brown et al. 2000; Walsh et al. 2004].
Acquired resistance to the biochemical response from etidronate, clodronate, and pamidronate has been reported [Rendina et al. 2004; Papapoulos et al. 2006]. This may be defined as an increase in the nadir SAP with subsequent treatments, requirement of a higher dose to get an equivalent response, and shortening of the length of time the patient remains in remission after a new course [Papapoulos et al. 2006]. These patients may respond well to an alternate bisphosphonate [Gutteridge et al. 1999b; Joshua et al. 2003; Rendina et al. 2004; Papapoulos et al. 2006].
In summary, when specific antipagetic therapy is needed, the amino bisphosphonates (alendro-nate, risedronate, pamidronate, and ZA) are first line therapy and provide both oral and intravenous options. Intravenous therapy may be preferred in patients with significant upper gastrointestinal problems or esophageal disease, patients in whom compliance to oral therapy is problematic, patients in whom a rapid antiresorptive effect is desired (e.g. neurologic symptoms or the urgent need for surgery on pagetic bone), or by patient preference. ZA appears to be the most potent drug and with the most sustained therapeutic effect for at least 2 years [Hosking et al. 2007].
Adverse effects of bisphosphonates have been reviewed recently [Kennel and Drake, 2009]. The major side effects of the oral amino bispho-sphonates are esophageal and upper gastrointestinal symptoms [Kennel and Drake, 2009]. The most common side effect of intravenous pami-dronate and ZA is a flu-like syndrome which occurs within a few days of treatment. In the ZA pivotal osteoporosis trial, this acute phase reaction occurred in 31.6% of patients after the first infusion, 6.6% of patients after the second infusion, and 2.8% of patients after the third infusion [Black et al. 2007]. This side effect can occur with oral bisphosphonates but is much less common. Use of acetaminophen may be useful in preventing these symptoms.
Severe musculoskeletal pain, attributed to bisphosphonate therapy, which may resolve slowly or incompletely after bisphosphonate cessation has been reported [Wysowski and Chang, 2005]. All bisphosphonates list this potential side effect in their prescribing information and the FDA issued an alert about this issue in 2008 [US Food and Drug Adminstration, 2008a]. This complication appears to be rare.
Osteonecrosis of the jaw (ONJ) has been reported in patients treated with bisphosphonates [Woo et al. 2006]. Most of the cases have been in patients treated with high doses of intravenous bisphosphonates for malignancy and after tooth extraction [Woo et al. 2006]. This condition appears to be rare in patients treated with osteoporosis or PDB doses of bisphosphonates. ONJ has been estimated to occur in 1—10% of patients treated with IV bisphosphonates in cancer dosage regimens and about 1/10,000–< 1/100,000 patient years in osteoporosis doses [Khosla et al. 2007]. The true incidence of ONJ in the treatment of nonmalignant bone diseases is unknown. It is appropriate to stress the importance of good dental hygiene and regular dental care prior to bisphosphonate therapy. If dental surgery is needed, it would be reasonable to delay initiation of bisphosphonate therapy until after the patient has had surgery and has healed. If dental surgery is needed while the patient is on bisphosphonate therapy, the dentist should be made aware of the bisphosphonate therapy. There is no evidence that stopping the bisphosphonate would be protective.
Mild hypocalcemia may occur especially after IV bisphosphonates, however, clinically significant hypocalcemia is unusual [Black et al. 2007]. Severe hypocalcemia following bisphosphonate therapy for PDB has, however, been reported [Whitson et al. 2006]. Patients treated with bisphosphonates for PDB should receive adequate calcium and vitamin D. A total calcium intake from diet and supplements in the range of 1500mg daily is appropriate. A serum 25-hydroxy vitamin D level of greater than 30 ng/ml is considered adequate.
Atrial fibrillation has also been a concern with bisphosphonate therapy. In the HORIZON pivotal fracture trial for ZA, the treatment group had an increased incidence of serious atrial fibrillation [Black et al. 2007]. An analysis of the Fracture Intervention Trial (FIT) suggested increased atrial fibrillation with alendronate [Cummings et al. 2007] but another study did not confirm this finding [Sorensen et al. 2008]. An FDA statement released in November 2008 suggested that healthcare providers should not change their prescribing practices based on a possible association of bisphosphonate therapy with atrial fibrillation [US Food and Drug Adminstration, 2008b].
Twenty-three cases of esophageal cancer were recently reported in patients receiving alendro-nate from late 1995 to May 2008 by an FDA epidemiologist [Wysowski, 2009]. The median time of alendronate use to diagnosis was 2.1 years. There were also 31 patients from Europe and Japan with esophageal cancer who had received alendronate, risedronate, ibandronate, or etidronate [Wysowski, 2009]. Others have suggested that the incidence of esophageal cancer in bisphosphonate-treated patients is not greater than expected [Abrahamsen et al. 2009b; Solomon et al. 2009]. It is not clear whether esophageal cancer is increased patients receiving oral bisphosphonates or if certain populations of patients (e.g. pre-existing Barrett's esophagus) might be at greater risk from bisphosphonates. It seems prudent to avoid prescribing oral bisphosphonates to patients with Barrett's esophagus or other significant known esophageal disease [Kennel and Drake, 2009].
Atypical poorly-healing fractures with severe suppression of bone turnover as well as subtrochan-teric femoral fractures have been reported in patients on long-term bisphosphonate therapy [Odvina et al. 2005; Kwek et al. 2008a, 2008b; Lenart et al. 2008, 2009; Goh et al. 2007; Visekruna et al. 2008; Edwards et al. 2009]. In the case of subtrochanteric femoral fractures, fracture may be preceded by pain and a stress fracture. These stress fractures are typically in the lateral aspect of the proximal femur, are associated with cortical thickening at the fracture site and may be bilateral. Some of these features are reminiscent of insufficiency fractures of PDB. It is not clear whether these uncommon fractures are an unusual osteoporotic fracture or caused by long-term bisphosphonate treatment [Abrahamsen et al. 2009b].
Ocular inflammation (iritis, uveitis, conjunctivitis, episcleritis, and scleritis) are rare side effects that can be seen with amino bisphosphonates. If iritis occurs, the drug should be stopped and ophthalmologic consultation obtained [Kennel and Drake, 2009]. Although amino bisphos-phonates cannot be used in these patients, non-nitrogen-containing bisphosphonates such as etidronate or tiludronate can be used [Siris and Roodman, 2008]. Synovitis, which may recur with rechallenge, has also been reported with aminobisphosphonate therapy [Jones et al. 2008].
Gallium nitrate and plicamycin (previously known as mithramycin) have been used in the past for PDB but are more toxic and not specifically FDA approved for this indication. These drugs are currently rarely used for PDB and are not generally recommended for this condition.
Denosumab is a monoclonal antibody to RANKL which is currently being studied for osteoporosis [Miller, 2009]. By inhibiting RANKL binding to RANK, this drug inhibits osteoclastic activity. It is not known if this drug will be effective for treatment of PDB.
The therapeutic biochemical target for antipa-getic therapy is unclear. A 25% decrease in a biochemical marker such as SAP or BSAP may be considered to be a response [Selby et al. 2002]. Some authorities recommend normalization or near normalization of the SAP if this is possible [Siris, 1999; Papapoulos, 2002]. Patients are typically monitored with SAP or BSAP measurements every 3—6 months. Retreatment (at least 6 months after initial treatment) may be considered if the serum total or BSAP has increased 25% above the nadir value or if the patient develops recurrent symptoms [Siris, 1999]. Other authorities suggest retreatment only when patients have symptoms related to PDB as SAP levels appear to correlate poorly with quality of life [Langston et al. 2007]. Some patients with clinically significant and active PDB affecting small areas of the skeleton (e.g. monostotic disease of the tibia) may have normal biochemical markers of bone turnover. In this setting, repeat bone scanning may be useful in monitoring treatment.
Surgery
Surgery including fracture stabilization, corrective osteotomy and total joint arthroplasty is sometimes needed for patients with PDB [Parvizi et al. 2006]. Pagetic bone is very vascular [Rongstad et al. 1994] and it is recommended that patients receive specific antipagetic therapy prior to elective surgery on pagetic bone. Patients with PDB are at increased risk for heterotopic ossification after total hip arthroplasty and some authorities recommend preventive regimens with low dose radiation or pharmacologic agents in these patients. Patients with spinal involvement may have radiculopathy or myelopathy. This can be related to compressive spinal stenosis or ‘vascular steal’ resulting in reversible ischemia of the spinal cord. In this setting, aggressive antipagetic therapy is often effective, thus avoiding complicated surgery [Chen et al. 1979; Herzberg and Bayliss, 1980; Wallace et al. 1995; Bone, 2006]. When orthopedic surgery is needed on pagetic bone, it is best done by surgeons with previous experience in treating pagetic bone.
Patient followup
Patients who do not have indications for specific antipagetic therapy can be monitored with a yearly SAP or BSAP. Patients receiving active antipagetic therapy can be monitored with a SAP or BSAP every 3—6 months. It is reasonable to obtain radiographs of osteolytic lesions periodically, remembering that the lytic front typically advances approximately 1 cm annually.
Summary
PDB is a common condition, particularly in older persons of Northern European extraction. The incidence and severity of this condition may be decreasing. PDB is caused by accelerated osteoclastic bone resorption followed by increased bone formation in one or more bones resulting in skeletal lesions which can be osteolytic, mixed osteolytic/osteosclerotic, or osteosclerotic. Recently the SQSTM1 gene has been found to be one important cause of familial PDB. Symptoms and complications related to PDB can include bone pain, fracture, bone deformity, osteoarthri-tis, hearing loss, neurologic symptoms, cardiovascular complications, and, rarely, malignant transformation. Very effective medical therapy with potent aminobisphosphonates is available for patients requiring specific antipagetic treatment. Surgery is occasionally needed on pagetic bone and when possible should only be undertaken following effective medical therapy and by surgeons with significant experience treating this condition.
Additional information for patients and professionals
The Paget Foundation for Paget's Disease of Bone and Related Disorders, 120 Wall Street, Suite 1602, New York, NY 10005-4035, USA. Tel: +1 800 237 2438 or +1 212 509 5335, fax: +1 212 509 8492, www.paget.org, e-mail: PagetFdn@aol.com.
Acknowledgments
I would like to thank Virginia Wiatrowski for her secretarial assistance and Dr Fergus McKiernan for his thoughtful review of this manuscript.
Footnotes
The author discloses the following possible conflicts of interest. Past speaker for Merck, P&G, Novartis; past research grants from Merck; past consultant for Merck and P&G.
References
- Abrahamsen B., Eiken P., Eastell R. (2009a) Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: A register-based national cohort study. J Bone Miner Res 24: 1095–1102 [DOI] [PubMed] [Google Scholar]
- Abrahamsen B., Eiken P., Eastell R. (2009b) More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med 360: 1789. [DOI] [PubMed] [Google Scholar]
- Altman R.D. (1999) Arthritis in Paget's disease of bone. J Bone Miner Res 14(supplement 2): 85–87 [DOI] [PubMed] [Google Scholar]
- Altman R.D. (2002) Epidemiology of Paget's disease of bone. Clin Rev Bone Miner Metab 1: 99–102 [Google Scholar]
- Altman R.D., Collins B. (1980) Musculoskeletal manifestations of Paget's disease of bone. Arthritis Rheum 23: 1121–1127 [DOI] [PubMed] [Google Scholar]
- Alvarez L., Guanabens N., Peris P., Monegal A., Bedini J.L., Deulofeu R., et al. (1995) Discriminative value of biochemical markers of bone turnover in assessing the activity of Paget's disease. J Bone Miner Res 10: 458–465 [DOI] [PubMed] [Google Scholar]
- Alvarez L., Guanabens N., Peris P., Vidal S., Ros I., Monegal A., et al. (2001) Usefulness of biochemical markers of bone turnover in assessing response to treatment of Paget's disease. Bone 29: 447–452 [DOI] [PubMed] [Google Scholar]
- Avramides A. (1977) Salmon and porcine calcitonin treatment of Paget's disease of bone. Clin Orthop Rel Res 127: 78–85 [PubMed] [Google Scholar]
- Basle M.F., Fournier J.G., Rozenblatt S., Rebel A., Bouteille M. (1986) Measles virus RNA Detected in Paget's disease bone tissue by in situ hybridization. J Gen Virol 67: 907–913 [DOI] [PubMed] [Google Scholar]
- Beyens G., Daroszewska A., de Freitas F., Fransen E., Vanhoenacker F., Verbruggen L., et al. (2007) Identification of sex-specific associations between polymorphisms of the osteoprotegerin gene, TNFRSF1 IB, and Paget's disease of bone. J Bone Miner Res 22: 1062–1071 [DOI] [PubMed] [Google Scholar]
- Birch M.A., Taylor W., Fraser W.D., Ralston S.H., Hart C.A., Gallagher J.A. (1994) Absence of paramyxovirus RNA in cultures of Pagetic bone cells and in pagetic bone. J Bone Miner Res 9: 11–16 [DOI] [PubMed] [Google Scholar]
- Black D.M., Delmas P.D., Eastell R., Reid I.R., Boonen S., Cauley J.A., et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809–1822 [DOI] [PubMed] [Google Scholar]
- Bone H.G. (2006) Nonmalignant complications of Paget's disease. J Bone Miner Res 21(supplement 2): P64–P68 [DOI] [PubMed] [Google Scholar]
- Boyle W.J., Simonet W.S., Lacey D.L. (2003) Osteoclast differentiation and activation. Nature 423: 337–342 [DOI] [PubMed] [Google Scholar]
- Brandi M.L., Falchetti A. (2006) What is the relationship between Paget's disease of bone and hyperparathyroidism? J Bone Miner Res 21(supplement 2): P69–P74 [DOI] [PubMed] [Google Scholar]
- Brown J.P., Chines A.A., Myers W.R., Eusebio R.A., Ritter-Hrncirik C., Hayes C.W. (2000) Improvement of Pagetic bone lesions with risedronate treatment: A radiologic study. Bone 26: 263–267 [DOI] [PubMed] [Google Scholar]
- Chamoux E., Couture J., Bisson M., Morissette J., Brown J.P., Roux S. (2009) The p62 P392L mutation link to Paget's disease induces activation of human osteoclasts. Molec Endocrinol epub 9 July 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.R., Rhee R.S.C., Wallach S., Avramides A., Flores A. (1979) Neurologic disturbances in Paget disease of bone: Response to calcitonin. Neurology 29: 448–457 [DOI] [PubMed] [Google Scholar]
- Cody J.D., Singer F.R., Roodman G.D., Otterund B., Lewis T.B., Leppert M., et al. (1997) Genetic linkage of Paget disease of the bone to chromosome 18q. Am J Hum Genet 61: 1117–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Schafheutle K., Dennison E., Kellingray S., Guyer P., Barker D. (1999) The epidemiology of Paget's disease in Britain: Is the prevalence decreasing? J Bone Miner Res 14: 192–197 [DOI] [PubMed] [Google Scholar]
- Cooper C., Harvey N.C., Dennison E.M., van Staa T.P. (2006) Update on the epidemiology of Paget's disease of bone. J Bone Miner Res 21(supplement 2): P3–P8 [DOI] [PubMed] [Google Scholar]
- Cummings S.R., Schwartz A.V., Black D.M. (2007) Alendronate and atrial fibrillation. N Engl J Med 356: 1895–1896 [DOI] [PubMed] [Google Scholar]
- Cundy T. (2006) Is the prevalence of Paget's disease of bone decreasing? J Bone Miner Res 21(supplement 2): P9–P13 [DOI] [PubMed] [Google Scholar]
- Cundy T., Bolland M. (2008) Paget disease of bone. Trends Endocrinol Metab 19: 246–253 [DOI] [PubMed] [Google Scholar]
- Cundy H.R., Gamble G., Wattie D., Rutland M., Cundy T. (2004) Paget's disease of bone in New Zealand: Continued decline in disease severity. Calcif Tissue Int 75: 358–364 [DOI] [PubMed] [Google Scholar]
- Cushing F.R., Bone H.G. (2002) Radiographic diagnosis and laboratory evaluation of Paget's disease of bone. 1: 115–134 [Google Scholar]
- Daroszewska A., Hocking L.J., McGuigan F.E.A., Langdahl B., Stone M.D., Cundy T., et al. (2004) Susceptibility to Paget's disease of bone is influenced by a common polymorphic variant of osteoprotegerin. J Bone Miner Res 19: 1506–1511 [DOI] [PubMed] [Google Scholar]
- Deftos L.J. (2005) Treatment of Paget's disease — taming the wild osteoclast. N Engl J Med 353: 872–875 [DOI] [PubMed] [Google Scholar]
- Doyle T., Gunn J., Anderson G., Gill M., Cundy T. (2002) Paget's disease in New Zealand: Evidence for declining prevalence. Bone 31: 616–619 [DOI] [PubMed] [Google Scholar]
- Dunford J.E., Thompson K., Coxon F.P., Luckman S.P., Hahn F.M., Poulter CD, et al. (2001) Structure-activity relationships for inhibition of farne-syl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphospho-nates. J Pharm Exp Therap 296: 235–242 [PubMed] [Google Scholar]
- Edwards M.H., McCrae F.C., Young-Min S.A. (2009) Alendronate-related femoral diaphysis fracture — what should be done to predict and prevent subsequent fracture of the contralateral side? Osteoporosis Int. epub 27 June 2009. [DOI] [PubMed] [Google Scholar]
- Friedrichs W.E., Reddy S.V., Bruder J.M., Cundy T, Cornish J., Singer F.R., et al. (2002) Sequence analysis of measles virus nucleocapsid transcripts in patients with Paget's disease. J Bone Miner Res 17: 145–151 [DOI] [PubMed] [Google Scholar]
- Frith J.C., Monkkonen J., Auriola S., Monkkonen H., Rogers M.J. (2001) The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate. Arthritis Rheum 44: 2201–2210 [DOI] [PubMed] [Google Scholar]
- Gennari L., Di Stefano M., Merlotti D., Giordano N., Martini G., Tamone C., et al. (2005) Prevalence of Paget's disease of bone in Italy. J Bone Miner Res 20: 1845–1850 [DOI] [PubMed] [Google Scholar]
- Gennari L., Marlotti D., Martini G., Nuti R. (2006) Paget's disease of bone in Italy. J Bone Miner Res 21(supplement 2): P14–P21 [DOI] [PubMed] [Google Scholar]
- Gibbs C.J., Aaron J.E., Peacock M. (1986) Osteomalacia in Paget's disease treated with short term, high dose sodium etidronate. Br Med J 292: 1227–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S.K., Yang K.Y., Koh J.S.B., Wong M.K., Chua S.Y., Chua D.T.C., et al. (2007) Subtrochanteric insufficiency fractures in patients on alendronate therapy. J Bone Joint Surg 89-B: 349–353 [DOI] [PubMed] [Google Scholar]
- Gold D.T., Boisture J., Shipp K.M., Pieper C.F., Lyles K. (1996) Paget's disease of bone and quality of life. J Bone Miner Res 11: 1897–1903 [DOI] [PubMed] [Google Scholar]
- Good D.A., Busfield F., Fletcher B.H., Duffy D.L., Resting J.B., Anderson J., et al. (2002) Linkage of Paget disease of bone to a novel region on human chromosome 18q23. Am J Hum Genet 70: 517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D.A., Busfield F., Fletcher B.H., Lovelock P.K., Duffy D.L., Resting J.B., et al. (2004) Identification of SQSTM1 mutations in familial Paget's disease in Australian pedigrees. Bone 35: 277–282 [DOI] [PubMed] [Google Scholar]
- Gordon M.T., Anderson D.C., Sharpe P.T. (1991) Canine distemper virus localised in bone cells of patients with Paget's disease. Bone 12: 195–201 [DOI] [PubMed] [Google Scholar]
- Gordon M.T., Mee A.P., Anderson D.C., Sharpe P.T. (1992) Canine distemper virus transcripts sequenced from Pagetic bone. Bone Miner 19: 159–174 [DOI] [PubMed] [Google Scholar]
- Guanabens N., Garrido J., Gobbo M., Morales A. Piga, del Pino J., Torrijos A., et al. (2008) Prevalence of Paget's disease of bone in Spain. Bone 43: 1006–1009 [DOI] [PubMed] [Google Scholar]
- Gutteridge D.H., Gruber H.E., Rermode D.G., Worth G.R. (1999a) Thirty cases of concurrent Paget's disease and primary hyperparathyroidism: Sex distribution, histomorphometry, and prediction of the skeletal response to parathyroidectomy. Calcif Tissue Int 65: 427–435 [DOI] [PubMed] [Google Scholar]
- Gutteridge D.H., Ward L.C., Stewart G.O., Retallack R.W., Will R.R., Prince R.L., et al. (1999b) Paget's disease: Acquired resistance to one aminobi-sphosphonate with retained response to another. J Bone Miner Res 14(supplement 2): 79–84 [DOI] [PubMed] [Google Scholar]
- Hansen M.F., Seton M., Merchant A. (2006) Osteosarcoma in Paget's disease of bone. J Bone Miner Res 21(supplement 2): P58–P63 [DOI] [PubMed] [Google Scholar]
- Haslam S.I., Van Hul W, Morales-Piga A., Balemans W, San-Millan J.L., Nakatsuka R., et al. (1998) Paget's disease of bone: Evidence for a susceptibility locus on chromosome 18q and for genetic heterogeneity. J Bone Miner Res 13: 911–917 [DOI] [PubMed] [Google Scholar]
- Helfrich M.H., Hobson R.P., Grabowski P.S., Zurbriggen A., Cosby S.L., Dickson G.R., et al. (2000) A negative search for a paramyxoviral etiology of Paget's disease of bone: Molecular, immunological, and ultrastructural studies in U.R. patients. J Bone Miner Res 15: 2315–2329 [DOI] [PubMed] [Google Scholar]
- Herzberg L., Bayliss E. (1980) Spinal-cord syndrome due to non-compressive Paget's disease of bone: A spinal-artery steal phenomenon reversible with cal-citonin. Lancet 2(2184): 13–15 [DOI] [PubMed] [Google Scholar]
- Hiruma Y, Rurihara N., Subler M.A., Zhou H., Boykin C.S., Zhang H., et al. (2008) A SQSTMl/p62 mutation linked to Paget's disease increases the osteoclastogenic potential of the bone microenviron-ment. Hum Molec Genet 17: 3708–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking L.J., Herbert C.A., Nicholls R.R., Williams F., Bennett S.T., Cundy T., et al. (2001) Genomewide search in familial Paget disease of bone shows evidence of genetic heterogeneity with candidate loci on chromosomes 2q36, 10pl3, and 5q35. Am J Hum Genet 69: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking L.J., Lucas G.J.A., Daroszewska A., Mangion J., Olavesen M., Cundy T., et al. (2002) Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget's disease. Hum Mol Genet 11: 2735–2739 [DOI] [PubMed] [Google Scholar]
- Holdaway I.M., Ibbertson H.K., Wattie D., Scragg R., Graham P. (1990) Previous pet ownership and Paget's disease. Bone Miner 8: 53–58 [DOI] [PubMed] [Google Scholar]
- Hooper M., Faustino A., Reid I.R., Hosking D., Gilchrist N.L., Selby P., et al. (2009) Randomized, active-controlled study of once-weekly alendronate 280 mg high dose oral buffered solution for treatment of Paget's disease. Osteoporosis Int 20: 141–150 [DOI] [PubMed] [Google Scholar]
- Hosking D., Lyles K., Brown J.P., Fraser W.D., Miller P., Curiel M.D., et al. (2007) Long-term control of bone turnover in Paget's disease with zoledronic acid and risedronate. J Bone Miner Res 22: 142–148 [DOI] [PubMed] [Google Scholar]
- Hoyland J.A., Freemont A.J., Sharpe P.T. (1994) Interleukin-6, IL-6 receptor, and IL-6 nuclear factor gene expression in Paget's disease. J Bone Miner Res 9: 75–80 [DOI] [PubMed] [Google Scholar]
- Hughes A.E., Ralston S.H., Marken J., Bell C, MacPherson H., Wallace R.G.H., et al. (2000) Mutations in TNFRSF11A, affecting the signal pep-tide of RANK, cause familial expansile osteolysis. Nature Genetics 24: 45–48 [DOI] [PubMed] [Google Scholar]
- Hultgren H. (1998) Osteitis deformans (Paget's disease) and calcific disease of the heart valves. Am J Cardiol 81: 1461–1464 [DOI] [PubMed] [Google Scholar]
- Jones D.P.G., Savage R.L., Highton J. (2008) Alenodronate-induced synovitis. J Rheumatol 35: 537–538 [PubMed] [Google Scholar]
- Joshua F., Epstein M., Major G. (2003) Bisphosphonate resistance in Paget's disease of bone. Arthritis Rheum 48: 2321–2323 [DOI] [PubMed] [Google Scholar]
- Kanis J.A. (1991a) Radiological features. In: Kanis J.A. (ed.), Pathophysiology and treatment of Paget's disease of bone, Carolina Academic Press/Martin Dunitz, 41–88 [Google Scholar]
- Kanis J.A. (1991b) Biochemical and endocrine aspects of Paget's disease. In: Kanis J.A. (ed.), Pathophysiology and treatment of Paget's disease of bone, Carolina Academic Press/Martin Dunitz, 89–109 [Google Scholar]
- Kennel K.A., Drake M.T. (2009) Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin Proc 84: 632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.A., Brennan P., Newman J., Gray R.E.S., McCloskey E.V., Kanis J.A. (1996) Paget's disease of bone and unvaccinated dogs. Bone 19: 47–50 [DOI] [PubMed] [Google Scholar]
- Khosla S., Burr D., Cauley J., Dempster D.W., Ebeling PR, Felsenberg D., et al. (2007) Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 22: 1479–1491 [DOI] [PubMed] [Google Scholar]
- Kimonis V.E., Watts G.D.J. (2005) Autosomal dominant inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Alzheimer Dis Assoc Disord 19(supplement 1): S44–S47 [DOI] [PubMed] [Google Scholar]
- Kurihara N, Reddy S.V., Menaa C, Anderson D., Roodman G.D. (2000) Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J Clin Invest 105: 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N, Zhou H., Reddy S.V., Palacios V. Garcia, Subler M.A., Dempster D.W., et al. (2006) Experimental models of Paget's disease. J Bone Miner Res 21(supplement 2): P55–P57 [DOI] [PubMed] [Google Scholar]
- Kwek E.B.K., Koh J.S.B., Howe T.S. (2008a) More on atypical fractures of the femoral diaphysis. N Engl J Med 359: 316–317 [DOI] [PubMed] [Google Scholar]
- Kwek E.B.K., Goh S.K., Koh J.S.B., Png M.A., How T.S. (2008b) An emerging pattern of subtro-chanteric stress fractures: A long-term complication of alendronate therapy? Injury 39: 224–231 [DOI] [PubMed] [Google Scholar]
- Langston A.L., Campbell M.K., Fraser W.D., MacLennan G., Selby P., Ralston S.H., et al. (2007) Clinical determinants of quality of life in Paget's disease of bone. Calcif Tissue Int 80: 1–9 [DOI] [PubMed] [Google Scholar]
- Laroche M., Delmotte A. (2005) Increased arterial calcification in Paget's disease of bone. Calcif Tissue Int 11: 129–133 [DOI] [PubMed] [Google Scholar]
- Laurin N, Brown J.P., Lemainque A., Duchesne A., Huot D., Lacourciere Y., et al. (2001) Paget disease of bone: Mapping of two loci at 5135-qter and 5131. Am J Hum Genet 69: 528–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin N, Brown J.P., Morissette J., Raymond V. (2002) Recurrent mutation of the gene encoding sequestosome 1 (SQSTMl/p62) in Paget disease of bone. Am J Hum Genet 70: 1582–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach R.J., Singer F.R., Ench Y., Wisdom J.H., Pina D.S., Johnson-Pais T.L. (2006) Clinical and cellular phenotypes associated with sequestosome 1 (SQSTM1) mutations. J Bone Miner Res 21(supplement 2): P45–P50 [DOI] [PubMed] [Google Scholar]
- Lenart B.A., Lorich D.G., Lane J.M. (2008) Atypical fractures of the femoral diaphysis in postme-nopausal women taking alendronate. N Engl J Med 358: 1304–1305 [DOI] [PubMed] [Google Scholar]
- Lenart B.A., Neviaser A.S., Lyman S., Chang C.C., Edobor-Osula F., Steele B., et al. (2009) Association of low-energy femoral fractures with prolonged bisphosphonate use: A case control study. Osteoporosis Int 20: 1353–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever J.H. (2002) Paget's disease of bone in Lancashire and arsenic pesticide in cotton mill wastewater: A speculative hypothesis. Bone 31: 434–436 [DOI] [PubMed] [Google Scholar]
- Levy F., Muff R., Dotti-Sigrist S., Dambacher A., Fischer J.A. (1988) Formation of neutralizing antibodies during intranasal synthetic salmon calcito-nin treatment of Paget's disease. J Clin Endocrinol Metab 67: 541–545 [DOI] [PubMed] [Google Scholar]
- Licata A. (2005) Discovery, clinical development, and therapeutic uses of bisphosphonates. Ann Pharmacother 39: 668–677 [DOI] [PubMed] [Google Scholar]
- Lopez-Abente G., Morales-Piga A., Elena-Ibanez A., Rey-Rey J.S., Corres-Gonzalez J. (1997) Cattle, pets, and Paget's disease of bone. Epidemiology 8: 247–251 [DOI] [PubMed] [Google Scholar]
- Lucas G.J.A., Daroszewska A., Ralston S.H. (2006a) Contribution of genetic factors to the patho-genesis of Paget's disease of bone and related disorders. J Bone Miner Res 21(supplement 2): P31–P37 [DOI] [PubMed] [Google Scholar]
- Lucas G.J.A., Mehta S.G., Hocking L.J., Stewart T.L., Cundy T., Nicholson G.C., et al. (2006b) Evaluation of the role of valosin-containing protein in the pathogenesis of familial and sporadic Paget's disease of bone. Bone 38: 280–285 [DOI] [PubMed] [Google Scholar]
- Lucas G.J.A., Riches PL, Hocking L.J., Cundy T., Nicholson G.C., Walsh J.P., et al. (2008) Identification of a major locus for Paget's disease on chromosome 10pl3 in families of British descent. J Bone Miner Res 23: 58–63 [DOI] [PubMed] [Google Scholar]
- Lyles K.W., Gold D.T., Newton R.A., Parekh S., Shipp K.M., Pieper C.F., et al. (1997) Peyronie's disease is associated with Paget's disease of bone. J Bone Miner Res 12: 929–934 [DOI] [PubMed] [Google Scholar]
- Lyles K.W., Siris E.S., Singer F.R., Meunier P.J. (2001) A clinical approach to diagnosis and management of Paget's disease of bone. J Bone Miner Res 16: 1379–1387 [DOI] [PubMed] [Google Scholar]
- Mangham D.C., Davie M.W., Grimer R.J. (2009) Sarcoma arising in Paget's disease of bone: Declining incidence and increasing age at presentation. Bone 44: 431–436 [DOI] [PubMed] [Google Scholar]
- Martini G., Gennari L., Merlotti D., Salvadori S., Franci M.B., Campagna S., et al. (2007) Serum OPG and RANKL levels before and after intravenous bisphosphonate treatment in Paget's disease of bone. Bone 40: 457–463 [DOI] [PubMed] [Google Scholar]
- Matthews B.G., Naot D., Bava U., Callon K.E., Pitto R.P., McCowan S.A., et al. (2009) Absence of somatic SQSTM1 mutations in Paget's disease of bone. J Clin Endocrinol Metab 94: 691–694 [DOI] [PubMed] [Google Scholar]
- McCloskey E.V., Kanis J.A. (2002) Neurological complications of Paget's disease. Clin Rev Bone Miner Metab 1: 135–143 [Google Scholar]
- McClung M.R., Tou C.K.P., Goldstein N.H., Picot C. (1995) Tiludronate therapy for Paget's disease of bone. Bone 17: 493S–496S [DOI] [PubMed] [Google Scholar]
- Mee A.P., May C, Bennett D., Sharpe P.T. (1995) Generation of multinucleated osteoclast-like cells from canine bone marrow: Effects of canine distemper virus. Bone 17: 47–55 [DOI] [PubMed] [Google Scholar]
- Mee A.P., Dixon J.A., Hoyland J.A., Davies M., Selby P.L., Mawer E.B. (1998) Detection of canine distemper virus in 100% of Paget's disease samples by in situ-reverse transcriptase-polymerase chain reaction. Bone 23: 171–175 [DOI] [PubMed] [Google Scholar]
- Menaa C, Reddy S.V., Kurihara N., Maeda H., Anderson D., Cundy T., et al. (2000) Enhanced RANK ligand expression and responsivity of bone marrow cells in Paget's disease of bone. J Clin Invest 105: 1833–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant A., Smielewska M., Patel N, Akunowicz J.D., Saria E.A., Delaney J.D., et al. (2009) Somatic mutations in SQSTM1 detected in affected tissues from patients with sporadic Paget's disease of bone. J Bone Miner Res 24: 484–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P.D. (2009) Denosumab: Anti-RANKL antibody. Curr Osteoporos Rep 7: 18–22 [DOI] [PubMed] [Google Scholar]
- Miller P.D., Brown J.P., Siris E.S., Hoseyni M.S., Axelrod D.W., Bekker P.J., et al. (1999) A randomized, double-blind comparison of risedronate and etidronate in the treatment of Paget's disease of bone. Am J Med 106: 513–520 [DOI] [PubMed] [Google Scholar]
- Mills B.G., Singer F.R. (1976) Nuclear inclusions in Paget's disease of bone. Science 194: 201–202 [DOI] [PubMed] [Google Scholar]
- Monsell E.M. (2004) The mechanism of hearing loss in Paget's disease of bone. Laryngoscope 114: 598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell E.M., Cody D.D., Bone H.G., Divine G.W. (1999) Hearing loss as a complication of Paget's disease of bone. J Bone Miner Res 14(supplement 2): 92–95 [DOI] [PubMed] [Google Scholar]
- Morales-Piga A.A., Moya J.L., Bachiller F.J., Munoz-Malo M.T., Benavides J., Abraira V. (2000) Assessment of cardiac function by echocardio-graphy in Paget's disease of bone. Clin Exper Rheum 18: 31–37 [PubMed] [Google Scholar]
- Nakatsuka K., Nishizawa Y., Ralson S.H. (2003) Phenotypic characterization of early onset Paget's disease of bone caused by a 27-bp duplication in the TNFRSF11A gene. J Bone Miner Res 18: 1381–1385 [DOI] [PubMed] [Google Scholar]
- O'Driscoll J.B., Anderson D.C. (1985) Past pets and Paget's disease. Lancet 2(8461): 919–921 [DOI] [PubMed] [Google Scholar]
- Odvina C.V., Zerwekh J.E., Rao S., Maalouf N., Gottschalk F.A., Pak C.Y.C. (2005) Severely suppressed bone turnover: A potential complication of alendronate therapy. J Clin Endocrinol Metab 90: 1294–1301 [DOI] [PubMed] [Google Scholar]
- Ooi C.G., Walsh C.A., Gallagher J.A., Fraser W.D. (2000) Absence of measles virus and canine distemper virus transcripts in long-term bone marrow cultures from patients with Paget's disease of bone. Bone 27: 417–421 [DOI] [PubMed] [Google Scholar]
- Paget J. (1877) On a form of chronic inflammation of bone (osteitis deformans). Medico-Chir Trans 60: 29–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pane A. (2007) Paget disease manifesting with com-pressive sixth-nerve palsy that resolved with intravenous zoledronic acid therapy. Arch Ophthalmol 125: 1440–1441 [DOI] [PubMed] [Google Scholar]
- Papapoulos S.E. (2002) Pharmacological management of Paget's disease of bone. Clin Rev Bone Miner Metab 1: 149–157 [Google Scholar]
- Papapoulos S.E., Eckhoff E.M.W., Zwinderman A.H. (2006) Acquired resistance to bisphosphonates in Paget's disease of bone. J Bone Miner Res 21(supplement 2): P88–P91 [DOI] [PubMed] [Google Scholar]
- Parvizi J., Klein G.R., Sim F.H. (2006) Surgical management of Paget's disease of bone. J Bone Miner Res 21(supplement 2): P75–P82 [DOI] [PubMed] [Google Scholar]
- Poncelet A. (1999) The neurologic complications of Paget's disease. J Bone Miner Res 14(supplement 2): 88–91 [DOI] [PubMed] [Google Scholar]
- Ralston S.H. (2008) Pathogenesis of Paget's disease of bone. Bone 43: 819–825 [DOI] [PubMed] [Google Scholar]
- Ralston S.H., Digiovine F.S., Gallacher S.J., Boyle I.T., Duff G.W. (1991) Failure to detect para-myxovirus sequences in Paget's disease of bone using the polymerase chain reaction. J Bone Miner Res 6: 1243–1248 [DOI] [PubMed] [Google Scholar]
- Ralston S.H., Afzal M.A., Helfrich M.H., Fraser W.D., Gallagher J.A., Mee A., et al. (2007) Multicenter blinded analysis of RT-PCR detection methods for paramyxoviruses in relation to Paget's disease of bone. J Bone Miner Res 22: 569—-577. [DOI] [PubMed] [Google Scholar]
- Ralston S.H., Langston A.L., Reid I.R. (2008) Pathogenesis and management of Paget's disease of bone. Lancet 372: 155–163 [DOI] [PubMed] [Google Scholar]
- Redden J.F., Dixon J., Vennart W., Hosking D.J. (1981) Management of fissure fractures in Paget's disease. Int Orthop 5: 103–106 [DOI] [PubMed] [Google Scholar]
- Reddy S.V., Singer F.R., Roodman G.D. (1995) Bone marrow mononuclear cells from patients with Paget's disease contain measles virus nucleocapsid messenger ribonucleic acid that has mutations in a specific region of the sequence. J Clin Endocrinol Metab 80: 2108–2111 [DOI] [PubMed] [Google Scholar]
- Reddy S.V., Singer F.R., Mallette L., Roodman G.D. (1996) Detection of measles virus nucleocapsid transcripts in circulating blood cells from patients with Paget disease. J Bone Miner Res 11: 1602–1607 [DOI] [PubMed] [Google Scholar]
- Reid I.R., Nicholson G.C., Weinstein R.S., Hosking D.J., Cundy T., Kotowicz M.A., et al. (1996) Biochemical and radiologic improvement in Paget's disease of bone treated with alendronate: A randomized, placebo-controlled trial. Am J Med 17: 341–348 [DOI] [PubMed] [Google Scholar]
- Reid I.R., Miller P., Lyles K., Fraser W., Brown J.P., Saidi Y., et al. (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget's disease. N Engl J Med 353: 898–908 [DOI] [PubMed] [Google Scholar]
- Rendina D., Mossetti G., Soscia E., Sirignano C, Insabato L., Viceconti R., et al. (2002) Giant cell tumor and Paget's disease of bone in one family. Clin Orthop 421: 218–224 [DOI] [PubMed] [Google Scholar]
- Rendina D., Mossetti G., Viceconti R., Sorrentino M., Nunziata V. (2004) Risedronate and pamidro-nate treatment in the clinical management of patients with severe Paget's disease of bone and acquired resistance to bisphosphonates. Calcif Tissue Int 75: 189–196 [DOI] [PubMed] [Google Scholar]
- Rendina D., Gennari L., De Filippo G., Merlotti D., de Campora E., Fazioli F., et al. (2006) Evidence for increased clinical severity of familial and sporadic Paget's disease of bone in Campania, Southern Italy. J Bone Miner Res 21: 1828–1835 [DOI] [PubMed] [Google Scholar]
- Rhodes E.C., Johnson-Pais T.L., Singer F.R., Ankerts D.P., Bruder J.M., Wisdom J., et al. (2008) Sequestosome 1 (SQSTM1) mutations in Paget's disease of bone from the United States. Calcif Tissue Int 82: 271–277 [DOI] [PubMed] [Google Scholar]
- Rogers J., Jeffrey D.R., Watt I. (2002) Paget's disease in an archeological population. J Bone Miner Res 17: 1127–1134 [DOI] [PubMed] [Google Scholar]
- Rongstad K.M., Wheeler D.L., Enneking WF. (1994) A comparison of the amount of vascularity in Pagetic and normal human bone. Clin Orthop 306: 247–249 [PubMed] [Google Scholar]
- Roodman G.D. (1996) Paget's disease and osteoclast biology. Bone 19: 209–212 [DOI] [PubMed] [Google Scholar]
- Roodman G.D., Windle J.J. (2005) Paget disease of bone. J Clin Invest 115: 200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman G.D., Kurihara N., Ohsaki Y., Kukita A., Hosking D., Demulder A., et al. (1992) Interleukin 6: A potential autocrine/paracrine factor in Paget's disease of bone. J Clin Invest 89: 46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C., Gennari C, Farrerons J., Devogelaer J.P., Mulder H, Kruse H.P., et al. (1995) Comparative prospective, double-blind, multicenter study of the efficacy of tiludronate and etidronate in the treatment of Paget's disease of bone. Arthritis Rheum 38: 851–858 [DOI] [PubMed] [Google Scholar]
- Rubin D.J., Levin R.M. (2009) Neurologic complications of Paget disease of bone. Endocr Pract 15: 158–166 [DOI] [PubMed] [Google Scholar]
- Schmidek H.H. (1977) Neurologic and neurosurgical sequelae of Paget's disease of bone. Clin Orthop RelRes 127: 70–77 [PubMed] [Google Scholar]
- Selby P.L., Davie M.W.J., Ralston S.H., Stone M.D. (2002) Guidelines on the management of Paget's disease of bone. Bone 31: 366–373 [DOI] [PubMed] [Google Scholar]
- Selby P.L., Davies M., Mee A.P. (2006) Canine distemper virus induces human osteoclastogenesis through NF-KB and sequestosome 1/P62 activation. J Bone Miner Res 21: 1750–1756 [DOI] [PubMed] [Google Scholar]
- Seton M., Choi H.K., Hansen M.F., Sebaldt R.J., Cooper C. (2003) Analysis of environmental factors in familial versus sporadic Paget's disease of bone — the New England registry for Paget's disease of bone. J Bone Miner Res 18: 1519–1524 [DOI] [PubMed] [Google Scholar]
- Silverman S.L. (2008) Paget disease of bone: Therapeutic options. J Clin Rheumatol 14: 299–305 [DOI] [PubMed] [Google Scholar]
- Singer F.R. (1977) Human calcitonin treatment of Paget's disease of bone. Clin Orthop Rel Res 127: 86–93 [PubMed] [Google Scholar]
- Singer F.R., Mills B.G. (1993) Giant cell tumor arising in Paget's disease of bone. Clin Orthop 293: 293–301 [PubMed] [Google Scholar]
- Singer F.R., Clemens T.L., Eusebio R.A., Bekker P.J. (1998) Risedronate, a highly effective oral agent in the treatment of patients with severe Paget's disease. J Clin Endocrinol Metab 83: 1906–1910 [DOI] [PubMed] [Google Scholar]
- Singer F.R., Mills B.G., Gruber H.E., Windle J.J., Roodman G.D. (2006) Ultrastructure of bone cells in Paget's disease of bone. J Bone Miner Res 21(supplement 2): P51–P54 [DOI] [PubMed] [Google Scholar]
- Siris E. (1991) Indications for medical treatment of Paget's disease of bone. In: Singer F.R., Wallach S. (eds), Paget's disease of bone: clinical assessment, present and future therapy, Elsevier [Google Scholar]
- Siris E. (1994) Perspectives: A practical guide to the use of pamidronate in the treatment of Paget's disease. J Bone Miner Res 9: 303–304 [DOI] [PubMed] [Google Scholar]
- Siris E.S. (1999) Goals of treatment for Paget's disease of bone. J Bone Miner Res 14(supplement 2): 49–52 [DOI] [PubMed] [Google Scholar]
- Siris E., Feldman F. (1997) Clinical vignette: Natural history of untreated Paget's disease of the tibia. J Bone Miner Res 12: 691–692 [DOI] [PubMed] [Google Scholar]
- Siris E., Roodman G.D. (2008) Paget's disease of bone. In: Rosen C.J., Compston J.E., Lian J.B. (eds), Primer on the metabolic bone diseases and disorders of mineral metabolism, 7th edition American Society for Bone and Mineral Research, 335–343 [Google Scholar]
- Siris E., Clemens T.P., McMahon D., Gordon A., Jacobs T.P., Canfield R.E. (1989) Parathyroid function in Paget's disease of bone. J Bone Miner Res 4: 75–79 [DOI] [PubMed] [Google Scholar]
- Siris E., Kelsey J.L., Flaster E., Parker S. (1990) Paget's disease of bone and previous pet ownership in the United States: Dogs exonerated. Int J Epidemiol 19: 455–458 [DOI] [PubMed] [Google Scholar]
- Siris E.S., Ottman R., Flaster E., Kelsey J.L. (1991) Familial aggregation of Paget's disease of bone. J Bone Miner Res 6: 495–500 [DOI] [PubMed] [Google Scholar]
- Siris E., Weinstein R.S., Altman R., Conte J.M., Favus M., Lombardi A., et al. (1996) Comparative study of alendronate versus etidronate for the treatment of Paget's disease of bone. J Clin Endocrinol Metab 81: 961–967 [DOI] [PubMed] [Google Scholar]
- Siris E.S., Chines A.A., Altman R.D., Brown J.P., Johnston C.C., Jr, Lang R., et al. (1998) Risedronate in the treatment of Paget's disease of bone: An open label, multicenter study. J Bone Miner Res 13: 1032–1038 [DOI] [PubMed] [Google Scholar]
- Siris E.S., Lyles K.W., Singer F.R., Meunier P.J. (2006) Medical management of Paget's disease of bone: Indications for treatment and review of current therapies. J Bone Miner Res 21(supplement 2): P94–P98 [DOI] [PubMed] [Google Scholar]
- Solomon D., Patrick A., Brookhart M.A. (2009) More on reports of esophageal cancer with oral bisphosphonate use. N Engl J Med 360: 1789–1790 [PubMed] [Google Scholar]
- Sorensen H.T., Christensen S., Mehnert F., Pedersen L., Chapurlat R.D., Cummings S.R., et al. (2008) Use of bisphosphonates among women and risk of atrial fibrillation and flutter: Population base case-control study. BMJ 336: 813–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickberger S.A., Schulman S.P., Hutchins G.M. (1987) Association of Paget's disease of bone with calcific aortic valve disease. Am J Med 82: 953–956 [DOI] [PubMed] [Google Scholar]
- Thiebaud D., Jaeger P., Gobelet C, Jacquet A.F., Burckhardt P. (1988) A single infusion of the bisphosphonate AHPrBP (APD) as treatment of Paget's disease of bone. Am J Med 85: 207–212 [DOI] [PubMed] [Google Scholar]
- Tiegs R.D., Lohse CM, Wollan P.C., Melton L.J. (2000) Long-term trends in the incidence of Paget's disease of bone. Bone 27: 423–427 [DOI] [PubMed] [Google Scholar]
- Tilyard M.W., Gardner R.J.M., Milligan L., Cleary T.A., Stewart R.D.H. (1982) A probable linkage between familial Paget's disease and the HLA Loci*. Aust N. Z. J Med 12: 498–500 [DOI] [PubMed] [Google Scholar]
- Tucci J.R., Bontha S. (2001) Intravenously administered pamidronate in the treatment of Paget's disease of bone. Endocr Pract 7: 423–429 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (2008a) Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa).
- US Food and Drug Administration (2008b) Bisphosphonates marketed as Alendronate (Fosamax, Fosamax Plus D), Etidronate (Didronel), Ibandronate (Boniva), Pamidronate (Aredia), Risedronate (Actonel, Actonel w/Calcium), Tiludronate (Skelid), and Zoledronic acid (Reclast, Zometa).
- Viassolo V., Previtali S.C., Schiatti E., Magnani G., Minetti C, Zara F., et al. (2008) Inclusion body myopathy, Paget's disease of the bone and frontotem-poral dementia: Recurrence of the VCP R155 H mutation in an Italian family and implications for genetic counselling. Clin Genet 74: 54–60 [DOI] [PubMed] [Google Scholar]
- Visekruna M., Wilson D., McKiernan F.E. (2008) Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab 93: 2948–2952 [DOI] [PubMed] [Google Scholar]
- Wallace E., Wong J., Reid I.R. (1995) Pamidronate treatment of the neurologic sequelae of Pagetic spinal stenosis. Arch Intern Med 155: 1813–1815 [PubMed] [Google Scholar]
- Walsh J.P., Ward L.C., Stewart G.O., Will R.K., Criddle R.A., Prince R.L., et al. (2004) A randomized clinical trial comparing oral alendronate and intravenous pamidronate for the treatment of Paget's disease of bone. Bone 34: 747–754 [DOI] [PubMed] [Google Scholar]
- Watts G.D.J., Wymer J., Kovach M.J., Mehta S.G., Mumm S., Darvish D., et al. (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nature Genetics 36: 377–381 [DOI] [PubMed] [Google Scholar]
- Watts G.D.J., Thomasova D., Ramdeen S.K., Fulchiero E.C., Mehta S.G., Drachman D.A., et al. (2007) Novel VCP mutations in inclusion body myo-pathy associated with Paget disease of bone and fron-totemporal dementia. Clin Genet 72: 420–426 [DOI] [PubMed] [Google Scholar]
- Wermers R.A., Tiegs R.D., Atkinson E.J., Achenbach S.J., Melton L.J., III (2008) Morbidity and mortality associated with Paget's disease of bone: A population-based study. J Bone Miner Res 23: 819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson H.E., Lobaugh B., Lyles K.W. (2006) Severe hypocalcemia following bisphosphonate treatment in a patient with Paget's disease of bone. Bone 39: 954–958 [DOI] [PubMed] [Google Scholar]
- Whyte M.P. (2006) Paget's disease of bone. N Engl J Med 355: 593–600 [DOI] [PubMed] [Google Scholar]
- Wirfel K.L., Bruder J.M., Vardag A., Roodman G.D. (1999) Osteoclast function in healthy subjects and in patients with Paget's disease. Endocrinologist 9: 263–269 [Google Scholar]
- Woo S.B., Hellstein J.W., Kalmar J.R. (2006) Systematic review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 144: 753–761 [DOI] [PubMed] [Google Scholar]
- Wuyts W, Wesenbeeck L.V., Morales-Piga A., Ralston S., Hocking L., Vanhoenacker F., et al. (2001) Evaluation of the role of RANK and OPG genes in Paget's disease of bone. Bone 28: 104–107 [DOI] [PubMed] [Google Scholar]
- Wysowski D.K. (2009) Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med 360: 89–90 [DOI] [PubMed] [Google Scholar]
- Whyte M.P., Hughes A.E. (2002) Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J Bone Miner Res 17: 26–29 [DOI] [PubMed] [Google Scholar]
- Whyte M.P., Obrecht S.E., Finnegan P.M., Jones J.L., Podgornik M.N., McAlister WH, et al. (2002) Osteoprotegerin deficiency and juvenile Paget's disease. N Engl J Med 347: 175–184 [DOI] [PubMed] [Google Scholar]
- Wysowski D.K., Chang J.T. (2005) Alendronate and risedronate: Reports of severe bone, joint, and muscle pain. Arch Intern Med 165: 346–347 [DOI] [PubMed] [Google Scholar]
- Ziambaras K, Totty W.A., Teitelbaum S.L., Dierkes M., Whyte M.P. (1997) Extraskeletal osteoclastomas responsive to dexamethasone treatment in Paget bone disease. J Clin Endocrinol Metab 82: 3826–3834 [DOI] [PubMed] [Google Scholar]