Abstract
Osteoarthritis (OA) is the most common form of arthritis worldwide yet there is still a lack of effective treatments for this condition. Increasingly, attention has turned to the role of the synovium in OA as it is now recognized, in part from the use of modern imaging techniques, that synovitis is both common and associated with pain. This offers a target for treatment, for both symptom and potential structure modification. In this review we discuss the evidence for histological and imaging-detected synovitis and the current role of antisynovial therapies in OA.
Keywords: osteoarthritis, synovitis, histology, magnetic resonance imaging, ultrasound
Introduction
Osteoarthritis (OA) is the most prevalent form of arthritis worldwide, a major cause of joint pain and disability and the most common reason for total hip and knee replacement. There are over 8 million people in the United Kingdom living with OA and a 2003 survey of almost 2000 people with OA found that 81% are in constant pain or are limited in their scope to perform everyday tasks. It is estimated that almost half of the adult population of the USA will have symptomatic knee OA by the age of 85, with the highest risk among those that are obese [Murphy et al. 2008]. OA also has huge economic implications due to an increasing number of joint replacements, increasing hospital charges and an ageing population [Kim, 2008]. In 2004 the USA national bill for hospital charges for hip/knee replacements was US$26 billion and, if the current trend persists, it is estimated that 600,000 hip replacements and 1.4 million knee replacements will be performed in the US in 2015 [Kim, 2008].
Despite this, there is still a real lack of safe and effective treatments for OA, barring surgery and acetaminophen, and fzurther treatments are desperately required. Over recent years, attention has turned to the importance of synovitis in OA, although OA is not traditionally considered a classical inflammatory arthropathy, due to the relative lack of neutrophils in the synovial fluid and the lack of systemic manifestations of inflammation. OA symptoms however frequently include joint pain, swelling and stiffness, suggestive of at least local inflammation [Pelletier et al. 2001]. It is now recognized that synovitis is common in OA, both in early and late OA and this offers a potential target for treatment, both for symptom and potential structure modification.
Abnormalities in the osteoarthritis synovium
The gold standard for the diagnosis of synovitis is histology. The normal synovium is composed of 1—4 layers of cells which merge on their deep surface with a zone of loosely arranged fibrocollagenous tissue containing adipocytes, fibro-blasts, mast cells and macrophages. The synovial membrane has an abundant blood and nerve supply running throughout the loose fibrocollagenous tissue.
Biopsies from the synovium of OA knees (taken at arthroscopy for knee pain or at joint replacement) have demonstrated several key changes in the synovium, which although more pronounced in advanced OA, are present from the earliest stages of the OA process [Loeuille et al. 2005; Smithed al. 1997; Myers et al. 1990]. These synovial abnormalities include:
thickening of the lining layer;
increased vascularity;
inflammatory cell infiltration.
Synovial membrane histology in classical inflammatory arthritides such as rheumatoid arthritis (RA) is characterized by a wide heterogeneity. OA synovium also displays this spectrum of changes, although there is a lesser degree of inflammation than in RA. The OA spectrum ranges from marked hyperplasia of the lining layer, with a dense cellular infiltrate composed largely of lymphocytes and monocytes, through to a synovial membrane which is thickened by fibrotic tissue [Haraoui et al. 1991]. Surface fibrin deposition and fibrosis within the synovium is common in OA, particularly in the later stages [Loeuille et al. 2005].
Direct comparison of the synovium between 12 OA subjects and 18 RA subjects at time of total knee replacement has shown more hyperplasia of the lining cell layer and cellular infiltrate in severe RA (treated with steroids and methotrexate) than in OA. However, in milder RA (subjects treated with nonsteroidal anti-inflammatory drugs [NSAIDs] only) the histological changes are similar to those seen in OA [Haraoui et al. 1991].
The synovitis seen in OA knees tends to be diffuse and is generally not localized to areas of chondral defects, although an association has been reported between chondral defects and associated synovitis in the medial tibiofemoral compartment of the knee [Ayral et al. 2005; Loeuille et al. 2005]. Interestingly, work by Blom and colleagues demonstrated that in an OA mice model, using an MMP3-knockout model, macrophage activation in the synovium is essential for cartilage damage via the production of matrix metalloproteinases (MMPs), suggesting that inflammation within the synovium may be pivotal for cartilage damage [Blom et al. 2007].
How common is histological synovitis in osteoarthritis?
There is increasing evidence that synovitis is common in OA joints, particularly when the disease has been present for some time.
Microscopic changes in early osteoarthritis
A study of 29 people with knee pain and arthroscopic evidence of OA (chondropathy) but with no or minimal radiographic changes of OA demonstrated that over half have synovitis histologically, as defined by proliferation of the lining cells and increased mononuclear cell infiltration [Myers et al. 1990]. Work by Benito and colleagues in a smaller group of 25 patients demonstrated that early OA patients (normal XR with arthroscopic chondropathy) have a higher level of macrophage infiltration in the synovium, more blood vessel proliferation and higher markers of vascular proliferation than late OA patients (at the time of joint replacement) [Benito et al. 2005].
Microscopic changes in late osteoarthritis
A larger study of 104 subjects fulfilling American College of Rheumatology (ACR) criteria for OA collected synovial samples at time of total knee or hip replacement, and demonstrated extensive synovitis, as assessed by a semiquantitative score. Severe synovial inflammation was shown in 31%, with only 7 out of the 104 patients having no evidence of synovitis. Synovial inflammation was not confined to patients with extensive radiographic joint damage or end-stage disease. Lymphoid aggregates (usually suggestive of a classic inflammatory arthritis such as rheumatoid) were noted in the severely inflamed synovial samples [Haywood et al. 2003].
Smith and colleagues studied 36 patients with knee pain and a normal knee radiograph and 27 with severe knee OA needing joint replacement. Considerable variation in the synovial lining thickness of the synovial lining layer was seen, with some overlap between normal knees and early OA, however the scores for synovial cellularity, vascularity and inflammation were markedly increased in the OA synovium compared with normal knees, and with OA synovium at time of joint replacement showing the highest degree of change. Lymphoid aggregates in the synovium were not seen in normal tissue and only rarely in early OA, but were present in a third of severe OA synovial membranes, often resembling the synovial membranes of RA [Smith et al. 1997].
Cells and cytokines in the osteoarthritis synovium
Infiltration of the synovium with activated B cells and T lymphocytes and overexpression of proinflammatory mediators is common in both early and late OA [Benito et al. 2005]. Infiltration with B cells has been demonstrated in the synovium of half of OA knees in a group of 41 subjects fulfilling ACR criteria for knee OA, who presented with knee pain and/or joint swelling [Da et al. 2007]. Interestingly, work by Benito and colleagues demonstrated that patients with early OA have a higher level of mononuclear cell infiltration in the synovium compared with late OA, and higher expression of inflammatory mediators, including interleukin (IL)-1, tumour necrosis factor-alpha (TNF-x), vascular endothelial growth factor (VEGF) and intercellular adhesion molecules [Benito et al. 2005].
Smith and colleagues detected the production of IL-x and b and TNF-x in a sample of 63 OA synovial membranes, taken at time of total joint replacement (late OA) and at time of arthroscopy for knee pain (OA diagnosis based on chondropathy). Levels of IL-1 and TNF were elevated in both early and late OA, however these cytokines increase in production with a statistically significant increase in moderate and severe OA (p< 0.05). This increase in cytokines is thought to be due to an increase in lining layer cells of the synovium [Smith et al. 1997].
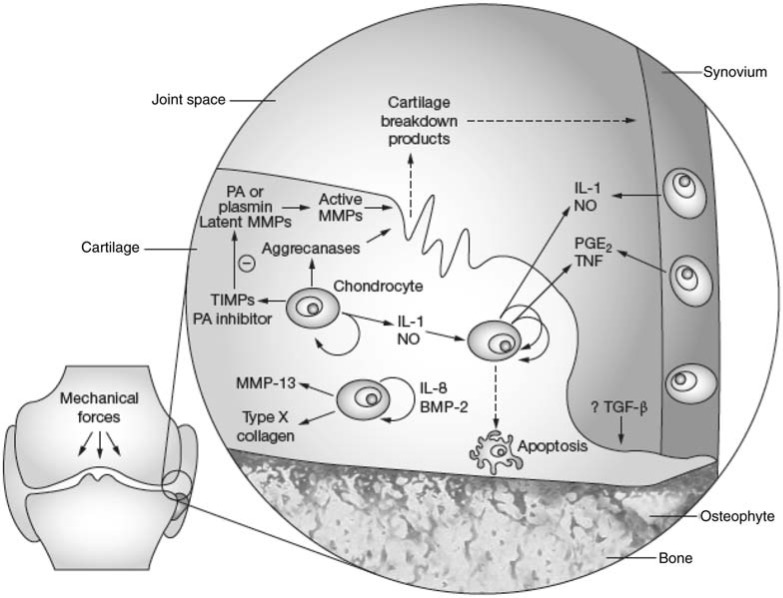
There is also evidence for T-cell activation and the production of Th1 cytokines (for example, interferon-g) in OA, with T cells and T-cell infiltrates seen in the synovial membrane [Sakkas and Platsoucas, 2007]. There is excessive production of cytokines and growth factors by the inflamed synovium and activated chondrocytes and these are thought to play a key part in the pathophys-iology of OA [Goldring, 2001; Pelletier et al. 2001; Smith et al. 1997]. These factors may be initially produced by the synovial membrane and diffuse into the cartilage via the synovial fluid [Pelletier et al. 2001] (Figure 1).
Figure 1.
Schematic representation of key pathological events and potential targets for disease modification in osteoarthritis. Mediators that represent potential therapeutic targets have been identified in both synovial tissue and cartilage. Less well identified are targets derived from bone. BMP, bone morphogenic protein; IL, interleukin; MMP, matrix metalloproteinase; NO, nitric oxide; PA, plasminogen activator; PGE2, prostaglandin E3; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinase; TNF, tumour necrosis factor. (Adapted by permission from Macmillan Publishers Ltd: [Nature Clinical Practice Rheumatology] (Abramson et al. 2006), copyright (2006).)
Studies in vitro and in vivo have demonstrated that IL-1 and TNF-x are the predominant pro-inflammatory and catabolic cytokines involved in the initiation and progression of articular cartilage destruction in OA [Goldring, 2001]. These cytokines stimulate chondrocytes to produce chemicals such as MMPs, which degrade matrix proteins and collagen. Chondrocytes then produce further IL-1, which then further stimulates MMP production. In human OA samples, chondrocytes produce more TNF-x and TNF-x convertase enzyme than normal cartilage and express higher quantities of the p55 TNF-x receptor, suggesting that OA cartilage may be more susceptible to damage from TNF-x than normal cartilage [Fernandes et al. 2002]. The increased levels of catabolic enzymes, prostaglandins, nitric oxide (NO) and other markers in OA fluids and tissues appear to be related to elevated levels of IL-1 and TNF-x [Goldring, 2001]. IL-6 is also upregulated during synovial inflammation and is produced by synovial cells, osteoblasts and chondrocytes and can be detected in synovial fluid samples taken from OA joints [Bonnet and Walsh, 2005].
Angiogenesis in the osteoarthritis synovium
Synovial and osteochondral angiogenesis are also features of OA and markers of angiogenesis in the synovium have been associated with histological synovitis, suggesting that angiogenesis may contribute to chronic synovitis [Walsh et al. 2007]. More recent work has concentrated on the potential role of VEGF in the OA synovium. VEGF is a potent stimulator of angiogenesis and macrophages and mast cells, present in abundance in chronically inflamed OA synovium, have been shown to produce VEGF [Bonnet and Walsh, 2005]. The authors hypothesize that production of VEGF by synovial macrophages is a possible molecular mechanism which exacerbates synovial angiogenesis and inflammation in OA [Haywood et al. 2003], although the exact regulation of angiogenesis in an OA joint is not yet completely understood [Ashraf and Walsh, 2008].
In summary, the synovium in OA joints is abnormal, even from the earliest clinical stages, with production of inflammatory cytokines IL-1, IL-6, TNF-x and VEGF, infiltration of mononu-clear cells, thickening of the synovial lining layer and fibrosis.
Modern imaging and osteoarthritis synovitis
Magnetic resonance imaging
MRI has been invaluable in improving our understanding of the role of the synovium in OA. Quantitative MRI markers of synovitis include synovial membrane thickness (commonly performed using segmentation and image analysis of individual MR slices), synovial fluid volume (also using segmentation techniques) and the rate of synovial enhancement after intravenous (IV) injection of contrast agent such as gadolinium-DTPA (diethylenetriamine pentaacetic acid). It has recently been demonstrated that volume acquisition of synovitis may also be combined with the rate of enhancement after IV contrast injection [Loeuille et al. 2009].
Intravenous contrast agents usually incorporate the heavy metal gadolinium, which distributes rapidly to vascular tissues. Inflamed (and therefore vascular) synovium is enhanced, with the signal intensity increasing in proportion to the concentration of gadolinium. It has been demonstrated that synovitis can be accurately quantified without using contrast [Pelletier et al. 2008] and recent concerns over the potential toxicity of gadolinium contrast in those with severe renal impairment means this area warrants further development. The use of IV contrast in MRI allows clear differentiation between synovitis and effusion in large joints, which may be more difficult to differentiate on noncontrast imaging, although ultrasound can differentiate between synovitis and effusion.
The frequency of synovitis in OA knees has been evaluated with MRI. Fifty two people with ACR criteria knee OA and a control group of 40 normal knees were imaged using noncontrast MRI to assess synovial thickening. Synovitis (as determined by synovial thickening) was observed in 73% of OA knees compared with 0% of the control group. Synovitis was also noted to be more likely with increasing K/L grade [Fernandez-Madrid et al. 1994]. This synovial thickening seen on MRI has been confirmed as histological synovitis in a small study by the same authors, of nine people, using arthroscopic sampling of the areas of MRI detected synovial thickening [Fernandez-Madrid et al. 1995]. A further study confirmed that MR detected synovial changes are confirmed as histo-logical synovitis [Loeuille et al. 2005]. This study assessed 39 people with knee OA with both non-contrast MRI and arthroscopy to assess the syno-vium macroscopically and take over 100 biopsy samples for microscopic analysis. The grade of MR synovial thickening correlated well with the degree of macroscopic synovitis seen at arthroscopy (r=0.58) and also with the degree of synovial changes seen microscopically (r = 0.41, p<0.0001). The authors also noted that the distribution of synovitis was diffuse, with no statistical difference seen between those people with marked chondral changes and those with few chondral changes, suggesting again that synovitis is present from the earliest stages of OA and is not related purely to areas of cartilage damage [Loeuille et al. 2005]. Further work, in a study of 15 subjects, demonstrated that synovial membrane which has a high rate of enhancement on MR imaging after administration of IV contrast was significantly associated with severe microscopic synovial vascular congestion [Loeuille et al. 2009].
Recent imaging studies have demonstrated an even higher frequency of imaging-detected synovitis in painful OA. A MRI study assessed 87 moderately symptomatic people meeting ACR criteria for knee OA using 1.5-T MRI. Pre- and post-gadolinium sequences of a single knee were evaluated for semiquantitative synovitis scores at nine intraarticular sites. Distribution of synovitis was extensive with 86% of subjects having synovitis at six or more sites [Conaghan, 2006].
Having established that MR can accurately detect synovial thickening and that this is confirmed as histological synovitis, it is important to understand the relationship between synovitis and symptoms. Three recent studies have demonstrated the relationship between synovitis in the knee and pain. Hill and colleagues evaluated the association of effusions, popliteal cysts, and synovial thickening with knee symptoms in 381 older persons with both knee pain and radio-graphic OA, 52 with no knee pain and radio-graphic OA, and 25 with neither pain nor radiographic changes [Hill et al. 2001]. All underwent MRI of one knee without the use of IV contrast. The authors noted that without IV contrast, synovitis may be underestimated and they attempted to distinguish between effusion and synovitis on MR images by oversampling knees with no or small effusions. Synovial thickening was measured as present or absent in three intra-articular areas by a trained reader, with a kappa for intra-observer reproducibility of 0.77. There was a significant association (p = 0.006) between synovitis and pain severity in those with knee pain and radiographic OA, after adjustment for radiographic change, BMI, age, sex and size of effusion. The mean pain score for those subjects with synovial thickening was 47mm on a pain visual analogue scale (VAS), compared with a mean score of 28mm for those without synovial thickening. There was also a significant increase (p< 0.001) in the frequency of both effusions (moderate or large) in the painful knees (54%) compared with those without pain (15%). Among those with small (grade 1) or no knee (grade 0) effusion, those with knee pain had a prevalence of synovial thickening of 73.6% compared with 21.4% of those without knee pain (p< 0.001, chi-squared) [Hill et al. 2001].
Further work by the same authors assessed the temporal relationship between synovitis and pain [Hill et al. 2007] in 270 subjects, all of whom had knee pain and radiographic OA, using MRI (without IV contrast) at baseline, 15 and 30 months. Synovitis was assessed at three sites using a semiquantitative score 0—3. Synovitis scoring was validated by comparison of synovitis scores of identical images with and without gadolinium contrast. Synovitis scores were identical in 13/20 cases, and underestimated in the non-contrast cases. Pain was assessed using a VAS for knee pain in the previous week. There was a significant correlation between change in total synovitis score and change in pain VAS score (p< 0.001, r=0.21) [Hill et al. 2007].
A recent, large MRI study of 454 people (48% women, mean age 59) with OA knee, used contrast-enhanced MRI to assess the presence of synovitis. Synovitis was demonstrated in 80%. Moreover, the presence of extensive synovitis was associated with an adjusted odds ratio for severe knee pain of 9.2 (3.2—26.3) [Baker et al. 2010].
Ultrasound
Ultrasound can also be used to detect synovitis with much greater sensitivity than clinical examination. As with MRI, most studies have assessed knee OA, although hip OA and more recently, the role of ultrasound in hand OA have also been examined [Keen et al. 2008a].
A large EULAR study of 600 people with knee OA demonstrated synovial hypertrophy or effusion in 46% [D'Agostino et al. 2005]. Synovial hypertrophy was defined as synovial thickening of ≤4mm and effusion recorded as present or absent based on the depth of fluid ≤4mm or ≤ 4mm in the suprapatellar recess. A further large cohort of 106 people aged between 35 and 55 assessed the ultrasound changes in early knee OA. All subjects had ≤3 months knee pain but the majority (87%) had either a normal radiograph but clinical features of OA, or mild radiographic OA (K/L grade 1) only. A third had synovial thickening (defined as ≤2mm) and 27 had a suprapatellar effusion [Kumm et al. 2009].
A smaller study using ultrasound assessed 41 people with OA knee and demonstrated the presence of synovitis (as demonstrated by synovial hypertrophy in the superior and lateral recesses) in 59% [Song et al. 2007] using a definition of synovial thickening of ‘any degree of synovial thickening’, rather than the stricter definition in the EULAR study. Using contrast-enhanced ultrasound increased this detection rate to 95% (on assessment of the superior recess). Unlike Hill and colleagues' MRI work, this study did not find an association between VAS pain and degree of synovitis, although the numbers in the study were small [Song et al. 2008].
Few studies have examined the OA hand using sensitive imaging techniques; however, the role of ultrasound in painful hand OA has recently been assessed. Thirty six subjects with painful hand OA underwent ultrasound imaging, which demonstrated that 46% had greyscale synovitis at baseline [Keen et al. 2008b]. Ultrasound also demonstrated that painful hand joints were significantly more likely to have synovitis than non-painful hand joints (plt0.001), however the extent of changes in individual joints did not correlate with the degree of symptoms [Keen et al. 2008b].
Studies which use ultrasound to assess synovitis in the OA hip generally assess the response to treatment, such as intra-articular steroid. Studies have suggested that synovitis is detected in 59% of painful OA hips referred for intraarticular steroid [Robinson et al. 2007]. A reproducible, semi-quantitative scoring system for assessing OA changes in the hip joint, including synovitis, has been suggested [Qvistgaard et al. 2006b].
Using imaging to assess therapies
Imaging may be used to evaluate the effectiveness of therapies. A recent study demonstrated a reduction in ultrasound-detected synovial hypertrophy in 75% of recipients of intraarticular steroid for painful hip OA, although it should be noted that all subjects were selected on the basis of ultrasound-detected synovitis prior to receiving intra-articular steroid [Micu et al. 2010]. Ultrasound has also been used to assess synovial changes in hand OA after treatment with intramuscular steroid [Keen et al. 2010]. MRI may be used pre- and post-treatment to detect changes in synovial volume and enhancement although much of the current evidence is in subjects with the classic inflammatory arthritides [Clunie et al. 1999; Ostergaard et al. 1995].
Synovitis and osteoarthritis structural progression
There is certainly a pathophysiological rationale for synovitis being important in cartilage degradation, as discussed above. There is also evidence from imaging studies to suggest that synovitis has an important role in structural degradation of the OA joint. Ayral and colleagues assessed the importance of synovitis on structural progression in the medial tibial femoral joint in a large study of 422 subjects with knee OA. All underwent arthroscopy at baseline and 1 year and the primary outcome was the change in the arthro-scopic chondropathy score. Synovial abnormalities were reported in 50% and subjects that had an ‘inflammatory’ appearance to synovium (21%) had an odds ratio (OR) for progression of the chondropathy score of 3.11 (1.07—5.69) [Ayral et al. 2005]. An MR study of 347 knees with minimal baseline cartilage damage has also demonstrated that the presence of synovitis or effusion was associated with an increased risk of fast cartilage loss, OR 3.36 (0.91—12.4) [Roemer et al. 2009]. Higher synovial volumes have also been shown to correlate with other measures of worsening OA, such as K/L score and joint space narrowing, in a small cohort of 44 subjects with knee OA who underwent contrast-enhanced MRI [Krasnokutsky, 2007].
A large, multicentre EULAR prospective study followed over 500 subjects with knee OA for 3 years following a baseline ultrasound examination. The primary endpoint of the study was a knee joint replacement. The multivariate analysis demonstrated that the presence of a joint effusion at baseline was a significant predictor of joint replacement at 3 years (hazard ratio [HR] 2.63) [Conaghan et al. 2010].
There is also evidence that raised systemic inflammatory markers have a role in future joint damage. C-reactive protein (CRP) levels (indicative of inflammation) are modestly but significantly increased in women with early knee OA and higher CRP levels predict those whose disease will progress radiographically over a period of 4 years, even after adjustment for weight, age and knee pain or injury [Spector et al. 1997]. Hyaluronic acid (HA) levels have been noted to be elevated twofold in people with OA compared with a control group and plasma HA levels correlated with an objective functional capacity score [Goldberg et al. 1991]. It has also been suggested that serum HA levels may predict radiographic progression at 5 years, although poor sensitivity and specificity means this is not currently of clinical use [Sharif et al. 2000].
A recent longitudinal study of over 900 women has demonstrated that plasma IL-6 levels are significantly elevated (even after adjustment for age and BMI) in women who have radiographic OA than women without OA. Plasma IL-6 levels have recently been shown to be an independent predictor of radiographic knee OA progression over 15 years [Livshits et al. 2009].
Osteoarthritis analgesic therapies and synovitis
Nonsteroidal anti-inflammatory drugs
Randomized controlled trials have demonstrated that NSAIDs are efficacious at reducing pain in OA [Zhang et al. 2008]. This efficacy is thought to be due to an anti-inflammatory, or anti-synovial, effect. NSAIDs have been shown to reduce both joint pain and the size of effusion on MRI, in a small group of patients with knee OA [Brandt et al. 2006] and a large study of 200 people with painful and radiographic knee OA has demonstrated that ibuprofen reduces the markers of cartilage and synovium metabolism (including CTX-II) during an acute flare of painful knee OA [Gineyts et al. 2004].
Corticosteroids
Corticosteroids, in particular those given intra-articularly, are frequently used in the treatment of OA, and it is presumed that the corticosteroid mechanism of pain reduction is via an effect on the synovium [Jones and Doherty, 1996]. Corticosteroids inhibit the production of pro-inflammatory chemicals interleukins 1 and 6 and TNF-x as well as decreasing the expression of COX-2. Steroids also inhibit the generation, proliferation and activation of T cells, which have been shown to infiltrate the synovium in OA joints.
There is good evidence for the short-term effectiveness (up to 4 weeks) in recipients of intraarticular IA steroid to the knee joint [Arden et al. 2008; Bellamy et al. 2006; Gossec and Dougados, 2004]; those with a knee joint effusion demonstrating a better response [Arden et al. 2008]. There are a number of studies confirming the effectiveness of intra-articular steroid for the painful OA hip [Lambert et al. 2007; Robinson et al. 2007; Qvistgaard et al. 2006a; Margules, 2001].
There is a lack of randomized controlled trials assessing the use of intra-articular steroid in the hand, particularly to the first carpometacarpal (CMC) joint, for which there is anecdotal evidence [Dieppe, 1991]. The only randomized controlled trial of intra-articular steroid to the first CMC joint noted no significant difference between steroid and placebo although the study was terminated early due to difficulty in recruitment [Meenagh et al. 2004].
Recently an observational open-label study has confirmed the effectiveness of intramuscular steroid for the treatment of painful hand OA. Thirty six patients with confirmed and symptomatic hand OA were given an intramuscular 120mg methyl prednisolone injection. Ultrasound imaging assessed the degree of synovitis before and after steroid. A significant proportion (67%) of patients had an improvement in symptoms at 4 weeks, with 45% having a sustained reduction in pain at 12 weeks [Keen et al. 2010] and there was a trend for responders to have higher baseline levels of synovitis.
One study has looked at the use of oral steroids in hand OA, using a novel drug (CRx-102) combining oral prednisolone (3mg) and dipyridamole, used to potentiate the action of the steroid [Kvien et al. 2008]. This 6-week randomized, placebo-controlled study of 83 people with painful hand OA had a primary outcome as the reduction in hand pain from baseline to 6 weeks, using the validated AUSCAN questionnaire. This study demonstrated a significant reduction in the 48-hour joint pain score at 6 weeks (p = 0.02). This may suggest a role for oral steroids in symptomatic relief of hand OA although it should be noted that this study did not evaluate steroid alone versus placebo.
Disease-modifying antirheumatic drugs in osteoarthritis
There is limited published evidence for the use of any disease-modifying antirheumatic drugs (DMARDs) in OA. Gold and hydroxychloro-quine have been shown to reduce NO production in chondrocyte culture and OA cartilage, suggesting they may have an anti-inflammatory mechanism in OA [Vuolteenaho et al. 2005]. Although there is very limited published evidence for the use of hydroxychloroquine in OA [Bryant et al. 1995], there is much anecdotal evidence that hydroxychloroquine may help sub-groups of patients.
Animal studies in OA have suggested a possible role for methotrexate in reducing cartilage damage in a rabbit model [Neidel et al. 1998; Mannoni et al. 1993].
There are two small studies of the use of methotrexate in human OA. The first, a placebo-controlled study of 89 patients used a small dose of 7.5mg methotrexate for painful knee OA and did not demonstrate any reduction in pain at 4 months [Holanda, 2007]. Subjects taking methotrexate required less analgesia (paracetamol) than the placebo group. The second used 10mg methotrexate weekly for 2 months, for painful hand OA, and demonstrated a significant improvement in pain at 2 months [Pavelka, 2006].
Anti-TNFs in osteoarthritis
There are few publications regarding the use of anti-TNF agents in OA. A larger, well-conducted study of 160 patients evaluated the effects of a single injection of intra-articular anakinra (an IL-1 receptor antagonist) to the symptomatic OA knee. No difference was seen between anakinra and placebo, although there was a trend to an improvement in pain score in the treatment group at 4 weeks, compared with placebo [Chevalier et al. 2009].
There is limited evidence for the potential effectiveness of systemic adalimumab and for intraarticular infliximab in small studies of painful hand OA [Fioravanti et al. 2009; Magnano et al. 2007].
Summary
Synovitis is very common in the OA joint and has been associated both with symptoms and with structural progression. Many of the current effective treatments used for OA have an antisynovial effect but further large-scale clinical trials are needed to confirm the role of antisynovial agents in symptom and structure modification.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
None declared.
References
- Abramson S.B., Attur M., Yazici Y. (2006) Prospects for disease modification in osteoarthritis. Nat Clin Pract Rheumatol 2: 304–312 [DOI] [PubMed] [Google Scholar]
- Arden N.K., Reading I.C., Jordan K.M., Thomas L., Platten H., Hassan A., et al. (2008) A randomised controlled trial of tidal irrigation vs corticosteroid injection in knee osteoarthritis: the KIVIS Study. Osteoarthritis Cartilage 16: 733–739 [DOI] [PubMed] [Google Scholar]
- Ashraf S., Walsh D.A. (2008) Angiogenesis in osteoarthritis. Curr Opin Rheumatol 20: 573–580 [DOI] [PubMed] [Google Scholar]
- Ayral X., Pickering E.H., Woodworth T.G., Mackillop N., Dougados M. (2005) Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis–results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13: 361–367 [DOI] [PubMed] [Google Scholar]
- Baker K., Grainger A., Niu J., Clancy M., Guermazi A., Crema M., et al. (2010) Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy N., Campbell J., Robinson V., Gee T., Bourne R., Wells G. (2006) Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2: CD005328. [DOI] [PubMed] [Google Scholar]
- Benito M.J., Veale D.J., FitzGerald O., van den Berg W.B., Bresnihan B. (2005) Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 64: 1263–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom A.B., van Lent P.L., Libregts S., Holthuysen A.E., van der Kraan P.M., van Rooijen N., et al. (2007) Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum 56: 147–157 [DOI] [PubMed] [Google Scholar]
- Bonnet C.S., Walsh D.A. (2005) Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 44: 7–16 [DOI] [PubMed] [Google Scholar]
- Brandt K.D., Mazzuca S.A., Buckwalter K.A. (2006) Acetaminophen, like conventional NSAIDs, may reduce synovitis in osteoarthritic knees. Rheumatology (Oxford) 45: 1389–1394 [DOI] [PubMed] [Google Scholar]
- Bryant L.R., des Rosier K.F., Carpenter M.T. (1995) Hydroxychloroquine in the treatment of erosive osteoarthritis. J Rheumatol 22: 1527–1531 [PubMed] [Google Scholar]
- Chevalier X., Goupille P., Beaulieu A.D., Burch F.X., Bensen W.G., Conrozier T., et al. (2009) Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 61: 344–352 [DOI] [PubMed] [Google Scholar]
- Clunie G.P., Wilkinson I.D., Lui D., Hall-Craggs M.A., Paley M.N., Edwards J.C., et al. (1999) Changes in articular synovial lining volume measured by magnetic resonance in a randomized, double-blind, controlled trial of intra-articular samarium-153 particulate hydroxyapatite for chronic knee synovitis. Rheumatology (Oxford) 38: 113–117 [DOI] [PubMed] [Google Scholar]
- Conaghan P. (2006) A MRI Study of the Extent of “Gold Standard”-Evaluated Synovitis and its Relationship to Pain in Osteoarthritis of the Knee. American College of Rheumatology: Abstract No 2094. [Google Scholar]
- Conaghan P.G., D'Agostino M.A., Le Bars M., Baron G., Schmidely N., Wakefield R., et al. (2010) Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: results from a large, 3-year, prospective EULAR study. Ann Rheum Dis 69: 644–647 [DOI] [PubMed] [Google Scholar]
- D'Agostino M.A., Conaghan P., Le Bars M., Baron G., Grassi W., Martin-Mola E., et al. (2005) EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis 64: 1703–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da R.R., Qin Y., Baeten D., Zhang Y. (2007) B cell clonal expansion and somatic hypermutation of Ig variable heavy chain genes in the synovial membrane of patients with osteoarthritis. J Immunol 178: 557–565 [DOI] [PubMed] [Google Scholar]
- Dieppe P.A. (1991) Are intra-articular steroid injections useful for the treatment of the osteoarthritis joint? Br J Rheumatol 30: 199. [DOI] [PubMed] [Google Scholar]
- Fernandes J.C, Martel-Pelletier J., Pelletier J.P. (2002) The role of cytokines in osteoarthritis pathophysiology. Biorheology 39: 237–246 [PubMed] [Google Scholar]
- Fernandez-Madrid F., Karvonen R.L., Teitge R.A., Miller P.R., Negendank W.G. (1994) MR features of osteoarthritis of the knee. Magn Reson Imaging 12: 703–709 [DOI] [PubMed] [Google Scholar]
- Fernandez-Madrid F., Karvonen R.L., Teitge R.A., Miller P.R., An T., Negendank W.G. (1995) Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging 13: 177–183 [DOI] [PubMed] [Google Scholar]
- Fioravanti A., Fabbroni M., Cerase A., Galeazzi M. (2009) Treatment of erosive osteoarthritis of the hands by intraarticular infliximab injections: a pilot study. Rheumatol Int 29: 961–965 [DOI] [PubMed] [Google Scholar]
- Gineyts E., Mo J.A., Ko A., Henriksen D.B., Curtis S.P., Gertz B.J., et al. (2004) Effects of ibuprofen on molecular markers of cartilage and synovium turnover in patients with knee osteoarthritis. Ann Rheum Dis 63: 857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R.L., Huff J.P., Lenz M.E., Glickman P., Katz R., Thonar E.J. (1991) Elevated plasma levels of hyaluronate in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 34: 799–807 [DOI] [PubMed] [Google Scholar]
- Goldring M.B. (2001) Anticytokine therapy for osteoarthritis. Expert Opin Biol Ther 1: 817–829 [DOI] [PubMed] [Google Scholar]
- Gossec L., Dougados M. (2004) Intra-articular treatments in osteoarthritis: from the symptomatic to the structure modifying. Ann Rheum Dis 63: 478–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraoui B., Pelletier J.P., Cloutier J.M., Faure M.P, Martel-Pelletier J. (1991) Synovial membrane histology and immunopathology in rheumatoid arthritis and osteoarthritis. In vivo effects of antirheumatic drugs. Arthritis Rheum 34: 153–163 [DOI] [PubMed] [Google Scholar]
- Haywood L., McWilliams D.F., Pearson C.I., Gill S.E., Ganesan A., Wilson D., et al. (2003) Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum 48: 2173–2177 [DOI] [PubMed] [Google Scholar]
- Hill C.L., Gale D.G., Chaisson C.E., Skinner K, Kazis L., Gale M.E., et al. (2001) Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol 28: 1330–1337 [PubMed] [Google Scholar]
- Hill C.L., Hunter D.J., Niu J., Clancy M., Guermazi A., Genant H., et al. (2007) Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 66: 1599–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda D. (2007) Baxia dose de methotrexate comparado a placebo em osteoartrite de joelho. Rev Bras Reumatol 47: 334–340 [Google Scholar]
- Jones A., Doherty M. (1996) Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis 55: 829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen H.I., Lavie F., Wakefield R.J., D'Agostino M.A., Hammer H.B., Hensor E., et al. (2008a) The development of a preliminary ultrasonographic scoring system for features of hand osteoarthritis. Ann Rheum Dis 67: 651–655 [DOI] [PubMed] [Google Scholar]
- Keen H.I., Wakefield R.J., Grainger A.J., Hensor E.M., Emery P., Conaghan P.G. (2008b) An ultrasonographic study of osteoarthritis of the hand: synovitis and its relationship to structural pathology and symptoms. Arthritis Rheum 59: 1756–1763 [DOI] [PubMed] [Google Scholar]
- Keen H.I., Wakefield R.J., Hensor E.M., Emery P., Conaghan P.G. (2010) Response of symptoms and synovitis to intra-muscular methylprednisolone in osteoarthritis of the hand: an ultrasonographic study. Rheumatology (Oxford) 49: 1093–1100 [DOI] [PubMed] [Google Scholar]
- Kim S. (2008) Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum 59: 481–488 [DOI] [PubMed] [Google Scholar]
- Krasnokutsky S., Samuels J., Attur M., Regatte R. (2007) Synovial but not cartilage volumes on MRI predict severity of knee OA. Osteoarthritis Cartilage 15(Suppl C): C28 [Google Scholar]
- Kumm J., Tamm A., Lintrop M. (2009) Association between ultrasonographic findings and bone/cartilage biomarkers in patients with early-stage knee osteoarthritis. Calcif Tissue Int 85: 514–522 [DOI] [PubMed] [Google Scholar]
- Kvien T.K., Fjeld E., Slatkowsky-Christensen B., Nichols M.j, Zhang Y., Proven A., et al. (2008) Efficacy and safety of a novel synergistic drug candidate, CRx-102, in hand osteoarthritis. Ann Rheum Dis 67: 942–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert R.G., Hutchings E.J., Grace M.G., Jhangri G.S., Conner-Spady B., Maksymowych W.P. (2007) Steroid injection for osteoarthritis of the hip: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 56: 2278–2287 [DOI] [PubMed] [Google Scholar]
- Livshits G., Zhai G., Hart D.J., Kato B.S., Wang H., Williams F.M., et al. (2009) Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum 60: 2037–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeuille D., Chary-Valckenaere I., Champigneulle J., Rat A.C., Toussaint F., Pinzano-Watrin A., et al. (2005) Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum 52: 3492–3501 [DOI] [PubMed] [Google Scholar]
- Loeuille D., Rat A.C., Goebel J.C., Champigneulle J., Blum A., Netter P., et al. (2009) Magnetic resonance imaging in osteoarthritis: which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage 17: 1186–1192 [DOI] [PubMed] [Google Scholar]
- Magnano M.D., Chakravarty E.F., Broudy C, Chung L., Kelman A., Hillygus J., et al. (2007) A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol 34: 1323–1327 [PubMed] [Google Scholar]
- Mannoni A., Altman R.D., Muniz O.E., Serni U., Dean D.D. (1993) The effects of methotrexate on normal and osteoarthritic lapine articular cartilage. J Rheumatol 20(5): 849–855 [PubMed] [Google Scholar]
- Margules K.R. (2001) Fluoroscopically directed steroid instillation in the treatment of hip osteoarthritis: safety and efficacy in 510 cases. Arthritis Rheum 44: 2449–2450; author reply 2455–2446. [DOI] [PubMed] [Google Scholar]
- Meenagh G.K., Patton J., Kynes C., Wright G.D. (2004) A randomised controlled trial of intra-articular corticosteroid injection of the carpometacarpal joint of the thumb in osteoarthritis. Ann Rheum Dis 63: 1260–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu M.C., Bogdan G.D., Fodor D. (2010) Steroid injection for hip osteoarthritis: efficacy under ultrasound guidance. Rheumatology (Oxford), in press. [DOI] [PubMed] [Google Scholar]
- Murphy L., Schwartz T.A., Helmick C.G., Renner J.B., Tudor G., Koch G., et al. (2008) Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum 59: 1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S.L., Brandt K.D., Ehlich J.W, Braunstein E.M., Shelbourne K.D., Heck D.A., et al. (1990) Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol 17: 1662–1669 [PubMed] [Google Scholar]
- Neidel J., Schroers B., Sintermann F. (1998) The effects of high-dose methotrexate on the development of cartilage lesions in a lapine model of osteoarthrosis. Arch Orthop Trauma Surg 117: 265–269 [DOI] [PubMed] [Google Scholar]
- Ostergaard M., Stoltenberg M., Gideon P., Wieslander S., Sonne-Holm S., Kryger P., et al. (1995) Effect of intraarticular osmic acid on synovial membrane volume and inflammation, determined by magnetic resonance imaging. Scand J Rheumatol 24: 5–12 [DOI] [PubMed] [Google Scholar]
- Pavelka K. (2006) Methotrexate in the treatment of erosive OA of the hands. Ann Rheum Dis 65(Suppl 2): 402 [Google Scholar]
- Pelletier J.P., Martel-Pelletier J., Abramson S.B. (2001) Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 44: 1237–1247 [DOI] [PubMed] [Google Scholar]
- Pelletier J.P., Raynauld J.P., Abram F., Haraoui B., Choquette D., Martel-Pelletier J. (2008) A new non-invasive method to assess synovitis severity in relation to symptoms and cartilage volume loss in knee osteoarthritis patients using MRI. Osteoarthritis Cartilage 16(Suppl 3): S8–S13 [DOI] [PubMed] [Google Scholar]
- Qvistgaard E., Christensen R., Torp-Pedersen S., Bliddal H. (2006a) Intra-articular treatment of hip osteoarthritis: a randomized trial of hyaluronic acid, corticosteroid, and isotonic saline. Osteoarthritis Cartilage 14: 163–170 [DOI] [PubMed] [Google Scholar]
- Qvistgaard E., Torp-Pedersen S., Christensen R., Bliddal H. (2006b) Reproducibility and inter-reader agreement of a scoring system for ultrasound evaluation of hip osteoarthritis. Ann Rheum Dis 65: 1613–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P., Keenan A.M., Conaghan P.G. (2007) Clinical effectiveness and dose response of image-guided intraarticular corticosteroid injection for hip osteoarthritis. Rheumatology (Oxford) 46: 285–291 [DOI] [PubMed] [Google Scholar]
- Roemer F.W, Zhang Y., Niu J., Lynch J.A., Crema M.D., Marra M.D., et al. (2009) Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology 252: 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkas L.I., Platsoucas CD. (2007) The role of T cells in the pathogenesis of osteoarthritis. Arthritis Rheum 56: 409–424 [DOI] [PubMed] [Google Scholar]
- Sharif M., Shepstone L., Elson C.J., Dieppe P.A., Kirwan J.R. (2000) Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis 59: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.D., Triantafillou S., Parker A., Youssef P.P., Coleman M. (1997) Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 24: 365–371 [PubMed] [Google Scholar]
- Song I.H., Althoff C.E., Hermann K.G., Scheel A.K., Knetsch T., Schoenharting M., et al. (2008) Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann Rheum Dis 67: 19–25 [DOI] [PubMed] [Google Scholar]
- Spector T.D., Hart D.J., Nandra D., Doyle D.V., Mackillop N., Gallimore J.R., et al. (1997) Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum 40: 723–727 [DOI] [PubMed] [Google Scholar]
- Vuolteenaho K., Kujala P., Moilanen T., Moilanen E. (2005) Aurothiomalate and hydroxychloroquine inhibit nitric oxide production in chondrocytes and in human osteoarthritic cartilage. Scand J Rheumatol 34: 475–479 [DOI] [PubMed] [Google Scholar]
- Walsh D.A., Bonnet C.S., Turner E.L., Wilson D., Situ M., McWilliams D.F. (2007) Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage 15: 743–751 [DOI] [PubMed] [Google Scholar]
- Zhang W., Moskowitz R.W, Nuki G., Abramson S., Altman R.D., Arden N., et al. (2008) OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16: 137–162 [DOI] [PubMed] [Google Scholar]