Abstract
Objective: To investigate how different types of meniscal tears predispose to different patterns of meniscal position in subjects with and without symptomatic knee osteoarthritis (OA). Methods: A cross-sectional analysis of 161 women participating in an observational study to evaluate knee OA progression was performed using baseline MRI data. Meniscal morphologic features were scored in three separate locations. Meniscal position measures were determined for extrusion and proportion of coverage. Analysis was performed using multiple linear regression models treating each tear type as an individual variable with a binary response.
Results: Complex tears, cysts and maceration of the medial meniscus were associated with more medial (p=0.0004, p=0.004, p <0.0001, respectively) and anterior extrusion (p =0.03, p=0.03, p<0.0001, respectively) than normal menisci. Horizontal tears of the lateral meniscus had more lateral (p=0.005) and anterior extrusion (p<0.0001) than normal menisci. Anterior and body tears of the medial meniscus were associated with more anterior extrusion (p=0.0006, p=0.01, respectively), whereas meniscal body tears alone had more medial extrusion than normal menisci (p= 0.0002). Meniscal body tears of the lateral meniscus had more lateral extrusion than normal menisci (p=0.01).
Conclusion: Anterior horn and meniscal body tears and the more severe macerated and complex tear types predisposed to more medial meniscal extrusion. Laterally, only meniscal body and horizontal tears significantly affected extrusion, potentially reflecting a lower overall prevalence of lateral meniscal tears. These results may have important implications in identifying tear types associated with more meniscal dysfunction, with the ultimate goal of identifying those at greatest risk for knee OA progression.
Keywords: knee, magnetic resonance imaging, meniscal extrusion, meniscal position, meniscal subluxation, meniscal tear, osteoarthritis
Introduction
The menisci play an important role in knee bio-mechanics providing shock absorption, load distribution, and joint stabilization [Jones et al. 1996; Seedhom et al. 1974]. Alteration in these functions secondary to meniscal damage and subsequent meniscectomy leads to increased contact stresses and resultant osteoarthritis (OA) of the knee [McNicholas et al. 2000; Roos et al. 1998; Allen et al. 1984; Johnson et al. 1974; Tapper and Hoover, 1969]. Meniscal tears and changes in meniscal position (also termed extrusion or subluxation) were recently found to be strongly associated with the progression of symptomatic knee OA [Hunter et al. 2006; Berthiaume et al. 2005]. These studies highlight a critical role of the meniscus in determining load distribution within the knee and its predisposition to disease progression. Further evaluation of the Boston Osteoarthritis of the Knee Study (BOKS) cohort demonstrated that degenerative meniscal tears are related to the extent of abnormality of meniscal position [Hunter et al. 2006].
A retrospective review of knee magnetic resonance imaging (MRI) data previously revealed that the extent of meniscal extrusion is associated with the type of meniscal abnormality [Costa et al. 2004]. This study was limited however by its retrospective nature, the absence of normal menisci as a reference standard, and analysis involving the medial meniscus alone with extrusion data restricted to one MRI plane. Given the critical importance of the meniscus to joint function, further elucidation of the relation between specific meniscal tears and position could identify tears which are most concerning in terms of predisposing to progression of knee OA. Identification of a tear type with increased risk for abnormal position may provide predictive indication of a knee at greater risk for rapid progression.
The purpose of this study was to investigate the relationship between meniscal tears and meniscal position and, more specifically, how different types of tears and their location predispose to different patterns of meniscal position in participants with symptomatic knee OA and an age- and sex-matched sample without OA.
Methods
Study sample
All study participants were women at least 40 years of age. The recruitment of subjects has been described in detail elsewhere [Eckstein et al. 2008]. Briefly, 180 individuals at 7 clinical centers were recruited through hospital advertisements, clinics, print media, patient lists, volunteer contacts, and previous studies to participate in a longitudinal 24-month observational study to evaluate OA progression. Standing anterior-posterior (AP) knee radiographs were obtained at each site to establish a baseline level of knee OA using the Kellgren and Lawrence (K/L) grading scale [Peterfy et al. 2003]. Nineteen participants were not included in the analysis secondary to self-withdrawal, failure to comply with follow up, protocol violation, or motion artifact during MRI acquisition. A total of 161 subjects remained and were included in the final analysis. The subjects were divided into two groups, those who were symptomatic and had radiographic OA (K/L grade 2 and 3) and control participants who were asymptomatic and without definite radio-graphic OA (K/L grade < 1). There was a significant difference in body mass index (BMI) between OA and control subjects (p< 0.00001). Symptoms were assessed using the Western Ontario and McMaster Universities (WOMAC) osteoarthritis index for pain, stiffness, and function and the Visual Analog Scale (VAS) for pain in the study knee. Inclusion criteria for the OA group were frequent symptoms, mild to moderate radiographic OA in the medial compartment, a BMI <30, and a medial tibiofemoral joint space width <2mm as detected on a posterior—anterior (PA) Lyon-Schuss radiographic view of the knee. In patients with bilateral radiographic knee OA, the study knee was defined as the knee that was more symptomatic. If identical pain scores existed, the knee with more advanced radiographic changes was chosen. If both radiographic severity and pain scores were identical, the knee in the subject's dominant leg (e.g. the leg used to kick a ball or to start to run) was selected as the study knee. Inclusion criteria for control subjects were complete absence of bilateral knee symptoms, K/L grade <1 in either knee, and a BMI < 28. The knee of the dominant leg was chosen as the study knee in these participants. Exclusion criteria for both groups were any prior history of knee ligamentous or meniscal injury or surgical history of partial or complete meniscectomy.
After the enrollment process was completed, a central reader blinded to the initial K/L grade assignments re-read each radiograph for standardization of the K/L grade status. If the grades differed, a third reader was called upon to adjudicate. The intrareader reproducibility (determined using 30 radiographs with K/L grades from 0 to 3) revealed an intraclass correlation coefficient of 0.91 and a kappa of 0.66. The control group was established from 95 participants classified as having K/L grade 0 and four classified as having K/L grade 1. A total of 62 subjects displayed mild to moderate radiographic OA (31 each having K/L grade 2 and K/L grade 3) and were placed into the OA group. All participants were allowed to take standard of care pain medications including acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase (COX)-2 inhibitors, and oral corticosteroids. No medications known to alter the progression rate of OA were allowed, nor were intra-articular corticosteroid or hyaluronic acid injections permitted in the study knee. Glucosamine or chondroitin was allowed if participants had been on stable therapy for the past 3 months (13 in each group). The study was conducted in compliance with local institutional review boards, informed consent regulations, the International Conference on Harmonization Good Clinical Practices Guidelines, and the Declaration of Helsinki.
MRI measurements
Siemens Magnetom Trio 3.0-T magnets (Siemens AG, Erlangen, Germany) were used at three of the seven study sites, whereas Signa Excite/Genesis 3.0 T MRI long bore magnets and short bore magnets (GE Healthcare Technologies, Waukesha, WI) were equally divided among the remaining sites. Birdcage CP coils (Transmit/Receive) with a ‘split top’ design were manufactured for the project (Clinical MR Solutions, Brookfield, WI) and used at all sites. Double oblique coronal spoiled gradient recalled acquisitions at steady state (SPGR) with selective water excitation were acquired at an in-plane spatial resolution of 0.31 mm × 0.31 mm and a slice thickness of 1.0 mm (TE = 7.2—8.5 ms, TR=16—17 ms, flip angle =12°, bandwidth = 31.25 kHz [GE] or 130 Hz/pixel [Siemens], matrix size = 512 × 512). A fat-suppressed 2D dual echo fast spin-echo (FSE) sequence was acquired in the sagittal plane with an in-plane resolution of 0.63 mm × 0.63 mm and a slice thickness of 3.0 mm (TE = 9/39ms, TR= 2700—3600, flip angle =170°, bandwidth = 0.32 kHz [GE] or 199 Hz/pixel [Siemens], echo-train length =6 [GE] or 3 [Siemens], matrix size = 256 × 256). All MRIs were performed with the study participants lying in a supine position with knees in extension.
Meniscal tears were assessed using a comprehensive scoring method, the Boston—Leeds Osteoarthritis Knee Score (BLOKS). This scoring system is applicable for use in conjunction with conventional MRI techniques [Hunter et al. 2008].
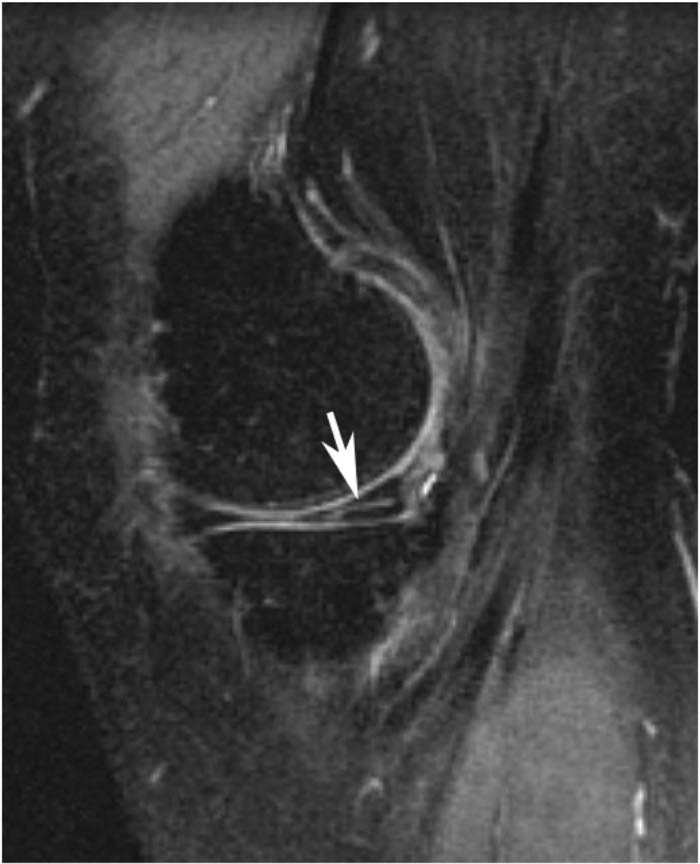
Meniscal morphology was scored for the body and anterior and posterior horns on both medial and lateral menisci. The body was scored using coronal sequences and the anterior and posterior horns were scored using sagittal MRI sequences. In the BLOKS system meniscal tears, which are defined as high signal within the meniscus extending to an articular surface, or meniscal maceration were scored as present or absent in these six regions. Vertical tears (encompassing radial and longitudinal tears extending to both femoral and tibial surfaces), horizontal tears (defined as horizontal tears with a slightly oblique course extending out through the inferior surface of the meniscus as shown in Figure 1), complex tears (defined by high signal, that extends to two surfaces and at least three points), intrameniscal signal and meniscal maceration (defined by loss of overall normal morphological appearance with increased diffuse signal in the meniscal tissue) were included in the analysis. Meniscal root tear were excluded from the analysis as none were identified in either the medial or lateral menisci.
Figure 1.
Sagittal fat-suppressed T2-weighted MRI showing an undersurface horizontal oblique tear of the posterior horn of the medial meniscus (arrow).
MRI images were read in pairs by two musculo-skeletal radiologists. The interobserver reliability (weighted kappa value with 95% confidence intervals) for reading meniscal tears data was 0.51 (0.24—0.78) and 0.79 (0.40—1.00), respectively.
Meniscal position measures to the nearest millimeter in both the medial and lateral compartments were determined for subluxation (both anterior and in the coronal plane), meniscal height, and meniscal covering and uncovering of the tibial plateau using coronal MRIs and eFilm Workstation software. Images in which the medial tibial spine volume was maximal were selected for all readings on coronal sequences. The reference point for measuring the extent of subluxation, tibial width, and amount of coverage was the edge of the tibial plateau without osteophytes. Proportion of coverage was calculated as meniscal covering divided by the sum of meniscal covering and meniscal uncovering. Sagittal images were used to assess anterior subluxation of both the medial and lateral menisci. Interobserver reliability (ICC values) for reading the measures of meniscal position ranged from 0.86 to 0.93.
Statistical analysis
With the possibility of multiple tear types existing for a single subject, tear types were treated as individual variables with a binary response. Using normal menisci as a reference, six tear types were identified and treated as individual variables. The analysis was performed using multiple linear regression models to assess whether different types of tears and their locations predisposed to different patterns of meniscal position. All statistical analyses were performed using SAS software, (SAS Institute Inc, Cary, NC, release 9.0).
Results
The demographics of the study sample are presented in Table 1. A total of 161 female subjects (99 controls and 62 with radiographic OA) were recruited. They were similar in age (56.1 ±8.8 years and 57.1 ±8.1 years, respectively), but differed with respect to BMI (24.9±4.8 and 36.9±5.3, respectively) and K/L grade of radiographic OA severity (95 K/L grade 0 and 4 K/L grade 1 for the controls, and 31 each K/L grade 2 and 3 for the OA subjects). The prevalence of any medial meniscal abnormality was similar between groups, 57% in the control group compared with 59% in the OA group, mostly representing increased intrameniscal signal changes in both, but with an additional increased prevalence of meniscal maceration in the OA group. Lateral meniscal abnormality was less frequent to a similar degree in both groups, 16% for the controls compared with 23% in the OA group, also mostly representing intrameniscal signal changes.
Table 1.
Demographics of 161 study participants at baseline.
| Control N = 99 | OA N = 62 | |
|---|---|---|
| Age, mean ± SD, years | 56.1 ± 8.8 | 57.1 ± 8.1 |
| Sex, % female | 100 | 100 |
| BMI, mean ± SD, kg/m2 | 24.9 ± 4.8 | 36.9 ± 5.3 |
| Kellgren and Lawrence grade | ||
| 0 | 95 | 0 |
| 1 | 4 | 0 |
| 2 | 0 | 31 |
| 3 | 0 | 31 |
| Medial meniscal abnormality, % (n) | 57% (57) | 59% (36) |
| Horizontal tear | 10% (10) | 8.2% (5) |
| Vertical tear | 1% (1) | 1.6% (1) |
| Complex tear | 2% (2) | 1.6% (1) |
| Meniscal cyst | 1% (1) | 6.6% (4) |
| Maceration | 3% (3) | 18% (11) |
| Intrameniscal signal (no tear) | 48% (48) | 42.6% (26) |
| Medial meniscal abnormality region, % (n) | ||
| Anterior | 6% (6) | 8.2% (5) |
| Body | 15% (15) | 27.9% (17) |
| Posterior | 57% (57) | 54.1% (33) |
| Lateral meniscal abnormality, % (n) | 16% (16) | 23% (14) |
| Horizontal tear | 3% (3) | 1.6% (1) |
| Vertical tear | 0 (0) | 3.3% (2) |
| Complex tear | 1% (1) | 0 (0) |
| Meniscal cyst | 1% (1) | 0 (0) |
| Maceration | 0 (0) | 1.6% (1) |
| Intrameniscal signal (no tear) | 15% (15) | 23.0% (14) |
| Lateral meniscal abnormality region, % (n) | ||
| Anterior | 15% (15) | 18% (11) |
| Body | 8% (8) | 9.8% (6) |
| Posterior | 8% (8) | 11.5% (7) |
BMI, body mass index; OA, osteoarthritis.
As demonstrated in Table 2, complex tears, meniscal cysts and meniscal maceration were associated with more medial extrusion than normal menisci (p = 0.0004, p = 0.004, and p< 0.0001, respectively). Likewise, complex tears, meniscal cysts, and maceration were significantly associated with more anterior extrusion than normal menisci (p = 0.03, p = 0.03, and p< 0.0001, respectively). Maceration alone significantly predicted a lower proportion of coverage than normal menisci (p< 0.0001).
Table 2.
Relation of tear type to meniscal position of the medial meniscus at baseline adjusted for osteoarthritis and body mass index effects.
| Medial extrusion (mm), Coefficient (SE) | Anterior extrusion (mm), Coefficient (SE) | Proportion of coveragea, % Coefficient (SE) | |
|---|---|---|---|
| Horizontal tear (n = 5) | 0.008 (0.04) | 0.02 (0.05) | 0.43 (2.25) |
| Vertical tear (n = 1) | −0.04 (0.09 | 0.10 (0.12) | 2.46 (5.27) |
| Complex tear (n = 1) | 0.27 (0.07)* | 0.23 (0.10)* | −4.95 (4.49) |
| Meniscal cyst (n = 4) | 0.14 (0.06)* | 0.16 (0.09) | −0.78 (3.72) |
| Maceration (n = 11) | 0.21 (0.04)* | 0.22 (0.05)* | −7.63 (2.19)* |
| Signal (n = 26) | 0.02 (0.02) | 0.03 (0.04) | 1.27 (1.20) |
| Mean value in normal menisci (n = 68) | 0.360 | 0.453 | 13.90 |
Indicates significance α = 0.05.
Proportion of coverage = meniscal covering/meniscal covering + meniscal uncovering
Types of tears of the lateral meniscus were similarly associated with meniscal malposition as seen in Table 3. Subjects with horizontal tears had more lateral and anterior extrusion than normal menisci (p = 0.005 and p<0.0001, respectively). Horizontal tears also predicted a significantly lower proportion of coverage than normal menisci (p = 0.04).
Table 3.
Relation of tear type to meniscal position of the lateral meniscus at baseline adjusted for osteoarthritis and body mass index effects.
| Lateral extrusion (mm), Coefficient (SE) | Anterior extrusion (mm), Coefficient (SE) | Proportion of coveragea, % Coefficient (SE) | |
|---|---|---|---|
| Horizontal tear (n = 1) | 0.18 (0.06)* | 0.28 (0.06) | −8.06 (3.69)* |
| Vertical tear (n = 2) | −0.06 (0.08) | −0.07 (0.09) | 0.82 (5.20) |
| Maceration (n = 1) | −0.02 (0.11) | 0.11 (0.12) | −8.06 (7.22) |
| Signal (n = 14) | −0.002 (0.02) | −0.001 (0.03) | 3.27 (1.57)* |
| Mean value in normal menisci (n = 131) | 0.19 | 0.04 | 19.35 |
| Complex tearb (n = 1) | |||
| Meniscal cystb | 0.30 | 0.60 | 25.0 |
Indicates significance α = 0.05
Proportion of coverage = meniscal covering/meniscal covering + meniscal uncovering.
Meniscal abnormalities occurring in a single subject not included in the model to prevent bias.
The relationship between tear location and meniscal position in the medial compartment is shown in Table 4. Subjects with meniscal body tears had significantly more medial extrusion than subjects with normal menisci (p = 0.0002). Significantly more anterior extrusion was present in subjects with anterior horn and meniscal body tears than in those with normal menisci (p =0.0006 and p = 0.01, respectively). Furthermore, subjects with meniscal body tears had a significantly lower proportion of coverage than those with normal menisci (p = 0.04).
Table 4.
Relation between tear location and meniscal position of the medial meniscus at baseline adjusted for osteoarthritis and body mass index effects.
| Medial extrusion (mm), Coefficient (SE) | Anterior extrusion (mm), Coefficient (SE) | Proportion of coveragea, % Coefficient (SE) | |
|---|---|---|---|
| Anterior (n = 5) | −0.031 (0.043) | 0.186 (0.056)* | 0.585 (2.461) |
| Body (n = 17) | 0.089 (0.029)* | 0.077 (0.038)* | −2.066 (1.661) |
| Posterior (n = 33) | 0.031 (0.023) | 0.029 (0.029) | 1.514 (1.299) |
| Mean value in normal menisci (n = 68) | 0.362 | 0.456 | 13.32 |
Indicates significance α = 0.05
Proportion of coverage = meniscal covering/meniscal covering + meniscal uncovering.
Table 5 reveals the relationship between tear location and meniscal position in the lateral compartment. Subjects with a tear of the meniscal body had significantly more lateral extrusion than subjects with normal menisci (p = 0.01). None of the tear locations significantly predicted anterior extrusion or proportion of coverage of the lateral meniscus. The lower prevalence of lateral meniscal abnormalities may limit the meaningfulness of these findings.
Table 5.
Relation between tear location and meniscal position of the lateral meniscus at baseline adjusted for osteoarthritis and body mass index effects.
| Lateral extrusion (mm), Coefficient (SE) | Anterior extrusion (mm), Coefficient (SE) | Proportion of coveragea, % Coefficient (SE) | |
|---|---|---|---|
| Anterior (n = 11) | −0.019 (0.032) | −0.007 (0.034) | −0.506 (2.002) |
| Body (n = 6) | 0.096 (0.037)* | 0.072 (0.040) | 3.733 (2.350) |
| Posterior (n = 7) | −0.040 (0.040) | 0.049 (0.043) | 1.925 (2.548) |
| Mean value in normal menisci (n = 131) | 0.19 | 0.04 | 19.26 |
Indicates significance α = 0.05
Proportion of coverage = meniscal covering/meniscal covering + meniscal uncovering.
Discussion
This study has demonstrated a significant association between meniscal tears and meniscal position. More severe types of tears predisposed to greater meniscal extrusion. The most common types of meniscal pathology represented here were macerated, horizontal tears and menisci with abnormal intrasubstance signal. The extent of extrusion mirrored the amount of damage related to the tears. An explanation of these findings may be provided in part through the micro-structural composition of the meniscus. The greater the disruption in the architectural framework of the meniscus as seen with severe tears, the less tensile stiffness of the collagen fiber bundles, and the more radial displacement that can be expected with weight bearing, especially in a population with an elevated BMI.
The strength of the meniscus is derived from an intricately woven pattern of collagen fibrils that maintain shape and structure when axially-loaded [Bullough et al. 1970]. Hoop strain, or the circumferential extension of the meniscus to resist radial displacement with an axial load, is altered with different types of meniscal tears [Jones et al. 1996]. In this study, meniscal maceration and complex tears were significantly associated with meniscal extrusion. This finding parallels an earlier report which showed that complex tears were strongly associated with major extrusion identified as < 3mm [Costa et al. 2004]. Our analysis also found that intrameniscal cysts contributed to extrusion, possibly a result of increased separation and cleavage of collagen fibrils with microcyst formation stretching fibers in a radial direction causing subluxation [Hajek et al. 1987].
A further finding in our series revealed that horizontal tears were significantly associated with anterior and lateral extrusion of the lateral meniscus. Horizontal tears, also known as horizontal cleavage lesions, were first described by Smillie as degenerative tears in the horizontal plane of the meniscus [Smillie, 1978] and have been shown to occur more commonly in middle-aged or older patients [Poehling et al. 1990]. Thus, it is reasonable to conclude that the complex, degenerative-type tears in our population of middle-aged, obese females with knee OA caused a disruption in meniscal architecture severe enough to result in a breakdown of hoop stress and subsequent extrusion. In contrast to this finding, horizontal tears as identified by Costa and colleagues were not associated with meniscal extrusion [Costa et al. 2004]. This discrepancy may be explained by their relatively small numbers of horizontal tears (n = 2) and their exclusion of data collection for the lateral meniscus.
Tear location, including anterior horn and meniscal body, was significantly associated with meniscal position of the medial meniscus. The finding that these tear locations significantly predicted extrusion may be related to the increased mobility of anterior horns compared with posterior horns [Vedi et al. 1999] which is in keeping with normal tibiofemoral kinematics. Movement of the anterior horns is required to maintain congruency of the joint surface while cam-shaped femoral condyles move on the tibia during flexion. Although a similar statistically significant result was not described in the study by Costa and colleagues, a strong trend is suggested for anterior and meniscal body tears predicting extrusion given their relatively low p-values (0.072 and 0.051, respectively) which further validates our data [Costa et al. 2004]. In our study posterior horn tears were not significantly associated with extrusion of the medial meniscus, although the highest percentage of abnormalities was found in the posterior horn, 54% compared with 28% and 8% for the meniscal body and anterior horn, respectively. This data is in keeping with the view that the relative immobility of the posterior part of the medial meniscus may account for the frequency with which it is torn. The finding that only meniscal body tears significantly predicted extrusion of the lateral meniscus may reflect a lower overall prevalence of lateral meniscal tears.
Meniscal tears and tear location not only have an influence on extrusion, they also have a profound effect on cartilage loss which leads to the progression of symptomatic knee OA [Hunter et al. 2006; Berthiaume et al. 2005]. Given that the meniscus plays a critical role in shock absorption and load distribution, the absence of a functional meniscus and the resultant alteration in these mechanical factors leads to increased stress concentration which promotes osteoarthritic changes [Ihn et al. 1993]. A nonfunctional meniscus mimics the findings after a complete or subtotal meniscectomy which have been shown to result in rapid OA progression [McNicholas et al. 2000; Roos et al. 1998; Allen et al. 1984; Johnson et al. 1974; Tapper and Hoover, 1969]. Therefore, it is reasonable to hypothesize that complex tears, meniscal maceration, intrameniscal cysts, and degenerative horizontal tears which lead to increased meniscal extrusion predispose to a more rapidly progressive knee OA. Indeed degenerative meniscal tears have been associated with worse long-term prognosis than longitudinal tears [Englund et al. 2003].
Some limitations of the research design include a study sample consisting solely of middle-aged, obese women. One could argue that the data obtained may not be accurately extrapolated to men or young, athletic individuals with normal body mass indices. Further investigation may be necessary to extend our results to other populations. In addition, obesity itself is a confounding factor which could be responsible for the increased meniscal extrusion observed in the symptomatic OA group compared with controls. A further limitation is that MR images were acquired with the subjects in a supine, non-weightbearing position with knees in extension. The measurements of extrusion are likely to be an underestimation of what would be expected in axially-loaded or flexed knees, which would alter our results. Another limitation of the study design involves the use of sagittal fat-suppressed dual echo FSE sequences alone for meniscal evaluation. Although most meniscal tears can be detected on sagittal images, a coronal intermediate-weighted FSE sequence may have provided better detection and characterization of meniscal tears. An additional method of assessment, such as the use of arthroscopy, was not performed to validate the relationship of tear type and position to extrusion and is recognized as another limitation of our study. Furthermore, the limited number of lateral meniscal tears detected in our study sample may have contributed to the paucity of significant results in our comparison of tear location and position of the lateral meniscus.
In conclusion, this study highlights that more severe, degenerative-type meniscal tears likely predisposed to more extrusion. Tear positions, including anterior horn and meniscal body, also significantly influenced meniscal extrusion. It is therefore plausible that degenerative meniscal pathology including meniscal maceration, intra-meniscal cysts, and complex and horizontal tears, with increased risk of extrusion, may predispose to OA progression. With a growing interest in meniscal preservation through repair techniques, transplantation, and tissue-engineering strategies, cognizant of the abnormal mechanics which may need correction prior to surgery, these results may have important implications for arthroscopists in guiding their management of meniscal tears with the goal of slowing the progression of knee OA.
Acknowledgments
We would like to thank the participants and staff of the Pfizer A9001140 Osteoarthritis Study. We are grateful to the dedicated group of study coordinators whose skills were essential in assuring the successful conduct of this study: Manal Al-Suqi, Emily Brown, Janie Burchett, Sandra Chapman, Wandra Davis, Eugene Dunkle, Susan Federmann, Kristen Fredley, Donna Gilmore, Joyce Goggins, Sasha Goldberg, Robert P. Marquis, Thelma Munoz, Bruce Niles, Norine Hall, Scott Squires, and Kim Tally. We would also like to express our thanks to the dedicated MRI technologists, the Duke Image Analysis Laboratory staff: Maureen Ainslie, April Davis, Allison Fowlkes, Mark Ward and Scott White, the Pfizer A9001140 Team: Lydia Brunstetter, Peggy Coyle, Yevgenia Davidoff, Charles Packard, Ann Remmers, Mark Tengowski, Jeff Evelhoch (now at Amgen, Thousand Oaks, CA) and John Kotyk (now at Washington University, St Louis, MI). Kenneth Brandt is to be thanked for adjudicating the radiographic readings. We would also like to thank all of the A9001140 site investigators: Deb Burstein, Julia Crim, Gary Hutchins, Chris Jackson, Virginia Byers Kraus, Nancy Lane, Thomas M. Link, Sharmila Majumdar, Steve Mazzuca, Prasad Pottumarthi, Thomas Schnitzer, Mihra Taljanovic, and Berchman Vaz.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Michel D. Crema and Monica D. Marra are shareholders of BICL, LLC. Ali Guermazi is President of BICL, LLC; Stockholder of Synarc, Inc; Grant Recipient of GE Healthcare; Scientific Adviser to Merck Serono, Stryker, Facet Solutions and Genzyme. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
References
- Allen P.R., Denham R.A., Swan A.V. (1984) Late degenerative changes after meniscectomy: factors affecting the knee after operation. J Bone Joint Surg Br 66: 666–671 [DOI] [PubMed] [Google Scholar]
- Berthiaume M.J., Raynauld J.P., Martel-Pelletier J., Labonte F., Beaudoin G., Bloch D.A., et al. (2005) Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis 64: 556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullough P.G., Munuera L., Murphy J., Weinstein A.M. (1970) The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br 52: 564–567 [PubMed] [Google Scholar]
- Costa C.R., Morrison W.B., Carrino J.A. (2004) Medial meniscal extrusion on knee MRI: is extent associated with severity of degeneration or type of tear? AJR Am J Roentgenol 183: 17–23 [DOI] [PubMed] [Google Scholar]
- Eckstein F., Buck R.J., Burstein D., Charles C, Crim J., Hudelmaier M., et al. (2008) Precision of 3.0 Tesla quantitative magnetic resonance imaging of cartilage morphology in a multicenter clinical trial. Ann Rheum Dis 67: 1683–1688 [DOI] [PubMed] [Google Scholar]
- Englund M., Roos E.M., Lohmander L.S. (2003) Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen year followup of meniscectomy with matched controls. Arthritis Rheum 48: 2178–2187 [DOI] [PubMed] [Google Scholar]
- Hajek P.C., Gylys-Morin V.M., Baker L.L., Sortoris D.J., Haghighi P., Resnick D. (1987) The high signal intensity of the knee: magnetic resonance evaluation and in vivo correlation. Invest Radial 22: 883–890 [DOI] [PubMed] [Google Scholar]
- Hunter D.J., Lo G.H., Gale D., Grainger A.J., Guermazi A., Conaghan P.G. (2008) The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score). Ann Rheum Dis 67: 206–211 [DOI] [PubMed] [Google Scholar]
- Hunter D.J., Zhang Y.Q., Niu J.B., Tu X., Amin S., Clancy M., et al. (2006) The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum 54: 795–801 [DOI] [PubMed] [Google Scholar]
- Ihn J.C., Kim S.J., Park I.H. (1993) In vitro study of contact area and pressure distribution in the human knee after partial and total meniscectomy. Int Orthop 17: 214–218 [DOI] [PubMed] [Google Scholar]
- Johnson R.J., Kettelkamp D.B., Clark W., Leaverton P. (1974) Factors effecting late results after meniscectomy. J Bone Joint Surg Am 56: 719–729 [PubMed] [Google Scholar]
- Jones R.S., Keene G.C., Learmonth D.J., Bickerstaff D., Nawana N.S., Costi J.J., et al. (1996) Direct measurement of hoop strains in the intact and torn human medial meniscus. Clin Biomech 11: 295–300 [DOI] [PubMed] [Google Scholar]
- McNicholas M.J., Rowley D.I., McGurty D., Adalberth T, Abdon P., Lindstrand A., et al. (2000) Total meniscectomy in adolescence: a thirty-year followup. J Bone Joint Surg Br 82: 217–221 [PubMed] [Google Scholar]
- Peterfy C, Li J., Zaim S., Duryea J., Lynch J., Miaux Y., et al. (2003) Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radial 32: 128–132 [DOI] [PubMed] [Google Scholar]
- Poehling G.G., Ruch D.S., Chabon S.J. (1990) The landscape of meniscal injuries. Clin Sports Med 9: 539–549 [PubMed] [Google Scholar]
- Roos H., Lauren M., Adalberth T, Roos E.M., Jonsson K., Lohmander L.S. (1998) Knee osteoarthritis after meniscectomy: prevalence of radio-graphic changes after twenty-one years, compared with matched controls. Arthritis Rheum 41: 687–693 [DOI] [PubMed] [Google Scholar]
- Seedhom B.B., Dowson D., Wright V. (1974) Proceedings: Functions of the menisci–a preliminary study. Ann Rheum Dis 33: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie I.S. (1978) Surgical pathology of the menisci, In: Smillie I. S. (ed.). Injuries of the Knee Joint, 5th ed. Churchill Livingstone: Edinburgh [Google Scholar]
- Tapper E.M., Hoover N.W. (1969) Late results after meniscectomy. J Bone Joint Surg Am 51: 517–526 [PubMed] [Google Scholar]
- Vedi V., Williams A., Tennant S.J., Spouse E., Hunt D.M., Gedroyc WM. (1999) Meniscal movement. An in-vivo study using dynamic MRI. J Bone Joint Surg. [DOI] [PubMed] [Google Scholar]