Abstract
Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase commonly known as statins are widely used for treating hypercholesterolemia. However, there is much evidence to suggest that statins may have other properties in addition to their cholesterol-lowering effect. In particular, statins may neutralize post-translational prenylation of vitally important regulatory small GTPases, which are involved in several processes such as tissue fibrosis, cell maturation, apoptosis, immune cell maturation, and immune response. The beneficial effect of statins has been reported in animal and in vitro models as well as in some clinical studies. As they have an acceptable safety profile, statins may be considered, in selected cases, as a valuable concomitant therapy in the treatment of rheumatic and autoimmune disorders.
Keywords: statins, connective tissue diseases, rheumatology, pleiotropic effect
Introduction
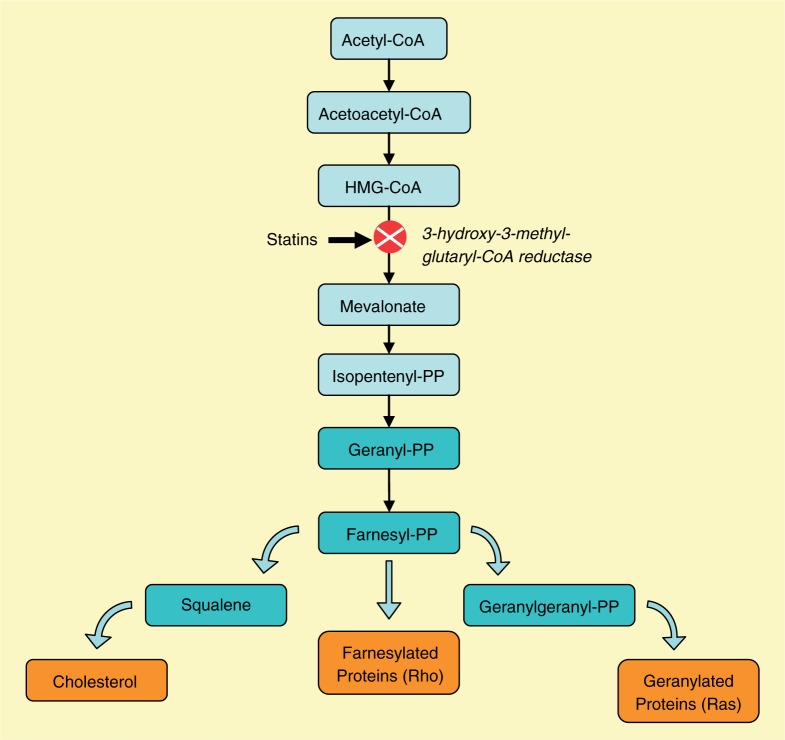
Statins, 3-hydroxymethyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, belong to a category of drugs that can inhibit a key enzyme in the pathway of cholesterol synthesis [Vaughan et al. 2000; Corsini et al. 1998]. They exert their biological effects by inhibiting the conversion of HMG-CoA to L-mevalonate (Figure 1). As a result, stimulation of low-density lipoprotein (LDL) receptor expression at the cell surface occurs, as the cholesterol level is the main negative regulator of LDL expression on hepatic cells. Increased cholesterol uptake occurs with LDL receptor expression, leading to a further reduction of blood LDL cholesterol. This mechanism was thought to be the main mode of action of statins. Marked reduction of LDL cholesterol was until recently believed to halt the progression of atherosclerosis. Despite the direct association between reduction of cholesterol level and improvement of clinical course in patients with cardiovascular events, not all beneficial effects can be explained by the inhibition of cholesterol synthesis only. It is often observed that in many cases the therapeutic effects of statins precede a reduction in lipid levels. This excludes reduction of LDL cholesterol as the main mechanism of action. In particular, inflammation and immune response, which are often present in patients with atherosclerosis, may be sufficiently reduced as a result of statins therapy. This suggests that properties of the drugs other than their lipid-lowering properties have to be involved [Pierre-Paul and Gahtan, 2003]. The observation that the benefits of statins which occur just after the beginning of therapy are not restricted to patients with hyper-cholesterolemia, and also exceed those of other lipid-lowering drugs despite a similar lipid effect, was the background to seeking another mechanism through which statins may provide the pharmacologic effect. Considerable experimental and clinical evidence has shown that statins have effects of significant biological importance beyond their cholesterol-lowering properties.
Figure 1.
Mevalonate biosynthetic pathway. HMG-CoA, 3-hydroxy-3-methyl-glutaryl coenzyme A; PP, pyrophosphate.
Pleiotropic effects of statins
These diverse properties, which are separate from their lipid-lowering potential, are sometimes called pleiotropic effect (from the Greek: pleio meaning many, tropos meaning manner). The term is a little controversial, since it is difficult to differentiate in vivo the effects that are independent of cholesterol lowering, as the decreased cholesterol level itself may produce many biological consequences. It is of particular importance that statins may exert a biological effect on the immune system. This may explain why the beneficial effect of statins is present just after the start of therapy and often precedes the reduction in cholesterol level. Perhaps what is more important is that the immunomodulatory effect of the drug might be used in the treatment of many conditions where a dysregulated immune response is responsible for causing the inflammation.
Inhibition of an early step in the cholesterol bio-synthetic pathway resulted not only in a diminution in cholesterol synthesis, but also decreased other intermediate metabolites downstream including 15-carbon farnesyl pyrophosphate and 20-carbon geranylgeranyl pyrophosphate. These isoprenoids attract much attention since they are involved in the post-translational isoprenylation of intracellular signaling molecules [Liao, 2002; Zhang and Casey, 1996]. Almost 100 different proteins may undergo prenylation, but importantly among these proteins is that approximately 40 small proteins belong to a small GTPase superfamily [Takai et al. 2001; Cohen et al. 2000]. GTPases are intercellular switches that are involved in many cellular processes, including apoptosis, gene expression, regulation of cytoskeleton actin, membrane trafficking, and migration [Colicelli, 2004; Etienne-Manneville and Hall, 2002]. The Rho and Rho-like proteins of the GTPases regulate the formation of the adhesion complex, as well as a number of inflammatory pathways, including mitogen-activated protein (MAP) kinase cascades [Braga, 2002; Mackay and Hall, 1998]. Data from experimental studies suggest that statins may inhibit inflammation mainly by the suppression of Rho family protein activity [Liao, 2002]. The GTPases that belong to the Ras family proteins are involved in signal transduction from growth factor receptors but also play a role in MAP kinase regulation. Evidence from laboratory studies showed that there are various ways of activating Ras and Rho superfamily molecules. Rho proteins are typically geranylated, whereas activation of the Ras family requires farnesylation [Colicelli, 2004].
Small molecular weight G proteins of the Ras superfamily (Ras, Rac, and Rho) are also involved in the regulation of growth, cell-to-cell interaction and apoptosis [Comparato et al. 2001; Glomset and Farnsworth, 1994]. Isoprenylation of small G proteins is essential for those proteins to achieve new regulatory functions [Corsini et al. 1993]. Equilibrium between the activities of these proteins provides a strong positive stimulus on the endothelium and promotes vasorelaxation [Barandier et al. 2003]. The influence of cardiovascular risk factors on the cascade of small G proteins results in the activation of the small RhoA protein and Rho kinase (ROCK), leading to the destabilization of nRNA for endothelial nitric oxide (eNOS) synthase [Eto et al. 2001]. At the same time a strong positive regulatory effect on prepro-endothelin-1 (prepro-ET-1) synthesis can be observed. It provides an imbalance between NO synthesis and overproduction of ET-1. As a result vasoconstriction of the vessel is observed. Endothelial and muscle smooth cells also express another small G protein Rac1, which is the major component of NAD(P)H oxidase that acts as the source of free radical species in many pathological conditions. Free radical species can interact with NO and decrease its bioavailability leading to endothelial dysfunction [Barandier et al. 2003]. Another biological function of RhoA and ROCK proteins is the regulation of endothelial barrier dysfunction by increasing phosphorylation of the myosin light chain. RhoA also plays a role in signal transduction to muscle and non-muscle myosin II [Somlyo and Somlyo, 2000]. All these events eventually lead to contraction of the blood vessel and promote the development of atherosclerotic plaque. Statins may interfere with isoprenylation of the small G protein and block their pathophysiological function. It has been suggested that this is one of the main mechanisms that explains the pleiotropic properties of HMG-CoA reductase inhibitors [Takemoto and Liao, 2001].
Lessons derived from experimental models suggest that statins upregulate eNOS expression and inhibit smooth muscle proliferation through the inhibition of the Rho-ROCK pathway. The effect is enhanced by the simultaneous inhibition of Ras. Animal models showed that Ras plays a significant role in the formation of atherosclerotic plaques, which has been demonstrated in apolipoprotein knock-out mice [George et al. 2002].
Immunomodulatory effects of statins
Many studies have shown that statins may possess immunomodulatory properties that might be of particular benefit in the treatment of autoimmune disorders. Statins may inhibit nuclear factor KB resulting in the inhibition of endothelial activation [Vaughan and Gotto, 2004; Meroni and Tremoli, 2003]. Statins regulate the class II transactivator, which reduces induction by interferon gamma (IFNγ) expression of major histocompatibility complex (MHC) class II molecules on antigen-presenting cells (APCs). MHC molecule expression on the surface of APCs is required for antigen presentation, thus the antigen is recognized by T cells in the context of MHC II antigens. Decreased expression results in antigen tolerance and downregulation of the T-cell response [Sadeghi et al. 2001; Kwak et al. 2000]. Interaction with the process of MHC II presentation contributes to the shift in the T cell from the Th1 response to the protective Th2 response [Lawman et al. 2004; Aktas et al. 2003].
Taking into account endothelium involvement, which is almost universal among connective tissue diseases, the role of statins is to repair the endothelium damage. Statins downregulate expression of the adhesion molecules on endothelial cells as well as reducing the levels of circulating soluble intravascular cell adhesion molecule-1 (ICAM-1), and E- and P-selectins [van Haelst et al. 2001; Romano et al. 2000a]. Recently the role of the integrin lymphocyte function-associated antigen-1 (LFA-1 CD11a—CD18) on lymphocytes has been debated. This integrin binds to the immunoglobulin superfamily molecule ICAM-1 and this is a key interaction that facilitates both adhesion/migration and costimulation. Some statins have shown the ability to block LFA-1/ICAM-1 interaction through direct binding to the so-called lovastatin binding site (L-site on the extracellular domain of LFA-1). As a result of this process inhibition both lymphocyte adhesion and costimulation occurs [Watts et al. 2005]. LFA-1, a β2 integrin, is critical in the development of inflammatory arthritis in the B/BxN serum transfer mouse model of arthritis. When statins (except pravastatin, which does not bind to the L-site) bind to LFA-1, they cause allosteric changes in the integrin structure preventing ICAM-1 binding [Watts et al. 2005; Weitz-Schmidt et al. 2001]. Another major player in leukocyte endothelium interaction is monocyte chemotactic protein-1 (MCP-1), a main chemoattractant for monocytes and T lymphocytes. MCP-1 is produced by human endothelial cells after stimulation by C- reactive protein, interleukin 1β (Il-1β), or lipopolysaccharide. Simvastatin inhibits de novo synthesis of MCP-1 in endothelial cells [Weitz-Schmidt et al. 2001]. Simvastatin and also lovastatin decreases synthesis of MCP-1 in human peripheral monocytes [Han et al. 2005; Romano et al. 2000a].
Role of statins in atherosclerosis
Statins not only reduce morbidity and mortality in coronary heart disease but also prolong life span in patients with hyper- and normocholesterolemia. The most recent evidence suggests that the action of statins is at least partially independent of the lipid-lowering properties of HMG-CoA reductase inhibitors. It may be speculated that not only the reduction in cholesterol synthesis but also the inhibition of lipid oxidation are the mechanisms through which statins may halt the progression of atherosclerosis. Atherogenesis and plaque formation begin with the accumulation of LDL cholesterol in the endothelial compartment of the blood vessel followed by its oxidation. Oxidated lipoproteins accumulate within the endothelial space and are the main target for monocytes and macrophages. Enzymes released from the monocytes/macro-phages break down the fibrous cap at the top of the plaque and this leads to rupture [Navab et al. 1996; Henney et al. 1991]. Statins possess the ability to stabilize atherosclerotic plaques. Several clinical studies have shown the potential of statins to decrease the evolution rate of atherosclerotic lesions towards rupture. One of the possible mechanisms involved in this process is the suppression by statins of cyclooxygenase-2 and microsomal prostaglandin synthase-1. As a result plaque stabilization occurs, which prevents rupture and subsequent thrombus formation, the main mechanism leading to acute myocardial infarction [Mezzetti, 2005; Cipollone et al. 2003].
Platelet activation and aggregation are a key step in thrombus formation; several factors are released upon their activation which generate a further amplification of the process [Daniel et al. 1999; Fuster et al. 1992]. Platelets can be activated by different stimuli including thrombin, adenosine diphosphate (ADP), and pro-inflammatory cytokines (including Il-1 and IFNγ) [Schror, 1995]. Platelets are also an important source of thromboxan A2, one of the most potent vasoconstrictors in the body. Statins, acting on the Rho-dependent pathway, may minimize platelet aggregation and thromboxan release through the partial inhibition of ADP and adenosine-5'-triphosphate (ATP) release from activated platelets and by exerting antioxidant effects [Kaneider et al. 2002].
Fibrinogen, an important factor in coagulation, exerts its pro-atherosclerotic action by stimulation of the proliferation and migration of smooth cells. These processes are realized by affecting the permeability of the vascular endothelium and function of the platelets and monocytes/macrophages as well as exerting pro-inflammatory and procoagulatory effects [Heinrich and Assmann, 1995; Warkentin, 1995]. Results obtained from the studies indicate that the effect of statins on the level of fibrinogen is relatively mild [Rosenson et al. 2001; Song and White, 2001]. However, in patients suffering from connective tissue diseases the level of fibrin-ogen may reflect the level of inflammation. This is why fibrinogen is well known as an acute phase marker and its level is raised significantly during the course of inflammation [Krysiak et al. 2003]. Statins may act in this area by cooling the inflammation following a decrease in fibrinogen level.
Another potent coagulation factor that is found in atherosclerotic plaques is the tissue factor (TF) [Rosenson and Tangney 1998]. It is synthesized by damaged or stimulated monocytes, macro-phages, and smooth muscle and endothelial cells. After binding to activated factor VII, the TF promotes coagulation [Dahlback, 2000]. Statins reduce the level and activity of the TF in human monocyte cultures activated, but also nonactivated, by inflammatory stimuli [Ferro et al. 2000; Colli et al. 1997]. This effect is not only restricted to mononuclear cells but can also be seen in the smooth muscle cells of the artery wall as well as in endothelial cells [Camera et al. 2002; Eto et al. 2002].
Several studies have addressed the influence of statins on thrombin formation. Particular emphasis was put on thrombin generation parameters such as thrombin fragment (F1+2), fibrinopeptide A, and thrombin-antithrombin III complexes [Krysiak et al. 2003]. According to data obtained from studies, simvastatin inhibits conversion of prothrombin to thrombin [Musial et al. 2001]. In addition, in ex vivo studies, simvastatin decreased thrombin generation and the total activity of the enzyme in patients with a significantly high cholesterol level [Szczeklik et al. 1999]. Statins were also shown to inhibit platelet-dependent formation of thrombin. The presumed mechanism of this activity is the improvement of the impaired interaction between platelets and coagulation factors, since platelet activity does not change during treatment [Aoki et al. 1997].
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disorder of unknown origin that serves as a prototype for other autoimmune disease. It affects mainly young people, especially women, which is a population not at risk of atherosclerosis development. Many years ago, the acute course of lupus with lupus nephropathy, hematological complication, and infection attracted the attention of physicians. Improvement of the management of lupus contributes significantly to improvement in prognosis and prolongation of life span. However, many years after the onset of lupus, survivors of the acute lupus phase face the problem of unexpected premature cardiovascular incidents.
The premature atherosclerosis in young lupus patients was first described by Urowitz and colleagues [Urowitz et al. 1976]. They coined the term ‘bimodal mortality’ and showed that the first peak of mortality was caused by the acute phase of disease, but the second was attributed to the fatal complication of atherosclerosis. The same conclusions came from the study of Bulkey and Roberts who in series of autopsies of young lupus female patients found significant atherosclerotic lesions in at least one coronary artery in the majority of cases [Bulkley and Roberts, 1975]. These findings were substantiated by subsequent studies that additionally showed that the incidence of myocardial infarction was five times higher in lupus patients than in the general population. Moreover, the specific target population for the disease was a group of young women in which an age-specific incidence that increased by a factor of as much as 50 was observed [Manzi et al. 1997; Johnsson et al. 1989].
The mechanism of accelerated atherosclerosis in lupus patients is not completely understood. It is speculated that atherosclerosis in SLE is the result of a combination of traditional risk factors, treatment, and inflammation [Esdaile et al. 2001; Petri, 2000]. However, the traditional risk factors fail to explain fully the process of accelerated atherosclerosis that suggests a main role of the inflammatory process in the beginning and progression of atherosclerotic lesions among patients with SLE. Moreover, the role of corticosteroid therapy and subsequent development of atherosclerosis in SLE patients is still subject to controversy and the results obtained from studies are contradictory [Roman et al. 2001]. Since the traditional risk factors and corticosteroid use are unable to explain fully the burden of accelerated atherosclerosis, SLE alone (also other connective tissue disorders) should be recognized as a novel independent risk factor.
Since the work of Ross, who explained that atherosclerosis is an inflammatory disease, we now better understand the mechanism of atherosclerosis in connective tissue diseases [Ross, 1999]. In recent years this new understanding of the pathogenesis of atherosclerosis has included insights into the role of inflammatory mediators in cardiovascular disease, and characterization of immunologic phenomena that are present in coronary vascular disease but may also be found in classic autoimmune diseases [Mustafa et al. 2000; Liuzzo et al. 1999; Cristea et al. 1986]. This contributes to the recognition of atherosclerosis in SLE as a chronic inflammatory disease characterized by circulating auto-antibodies, activated T and B lymphocytes, immune complexes, and pro-inflammatory cytokines. The level of inflammation in the course of SLE is high enough to start and progress atherosclerosis in patients.
Evidence derived from experimental models has shown that statins may deeply interfere with the immunological system leading to a reduction in SLE activity. In spite of the fact that SLE is not a typical Th1-mediated autoimmune disease, upregulation of MHC class II molecules, and increased production of IFNg are involved in the pathogenesis and progression of the disease. Statins can ameliorate IFNg-induced overexpression of MHC molecules class II on most APCs, but have no influence on MHC class I [Kwak et al. 2001, 2000]. This finding may be of particular importance as many data suggest that overexpression of MHC class II molecules is a key mechanism in autoimmune disorders [Botazzo et al. 1983]. Reduced expression of MHC is responsible for the impaired formation of the immunological synapse between the T cell-receptor complex and MHC. This ultimately leads to impaired antigen recognition and finally to antigen tolerance. Moreover, interaction with the process of MHC II presentation contributes to the shift of the T cell from the Th1 response to the protective Th2 response [Hakamada-Taguchi et al. 2003].
The term ‘protective response’ in the case of lupus is controversial, since this response leads to increased B-cell reactivity and auto-antibody production. Indeed the presence of antinuclear antibodies and lupus-like syndromes has been reported in patients on statins therapy [Hanson and Bossingham, 1998]. A question thus arises regarding the utility of statins in this setting. However, the immunomodulatory potential of statins may also be explained not by the switch to a Th2 response but by their ability to activate T regulatory cells (Tregs). Tregs are a unique population of CD4+ cells that suppress autoreactivity and are critical regulators of immune tolerance [Baecher-Allan et al. 2001]. Much data suggests that in many autoimmune disorders including lupus the Treg population is reduced or functionally suppressed [Valencia et al. 2007; Liu et al. 2004]. In animal models simvastatin can mediate the induction of mouse Fox3p+ Tregs that may stabilize equilibrium between activated and regulatory T cells [Kim et al. 2010].
Endothelium involvement in the course of SLE is almost universal and contributes significantly to the development of atherosclerosis. Statins also work in this area. The understanding of endothelium activation as an initial step in its dysfunction and damage lead to a search for potential targets for statins. The expression of endothelial cell adhesion molecules that reflects endothelial activation is strongly modulated by the use of statins. In particular, inhibition of RHO and RHO-mediated gene expression by statins leads to downregulation of P- and E-selectins [Romano et al. 2000b]. Statins may also interfere with second signal transmission, since they are able to decrease expression of the CD80/86 molecule on APCs (mainly on B cells). Costimulation is essential to evoke the full activation of T cells; in the absence of a strong costimulation signal, T cells may develop immune tolerance and subsequent lack of activity [Youssef et al. 2002]. In this model treatment with atorvastatin stopped the progression of lupus in lupus-prone New Zeland F1 mice [Lawman et al. 2004].
These promising results unfortunately have not been translated into clinical practice. So far only a few trials have been designed to verify the immunomodulatory properties of statins in humans. The study of Abud-Mendoza and colleagues in patients with refractory SLE and lupus nephropathy may serve as an example where a good therapeutic response has been observed [Abud-Mendoza et al. 2003]. These results should, however, be interpreted with caution as the group of patients comprised only eight subjects and it was not a controlled trial. In another study, 4 weeks of treatment with simvastatin in patients with SLE resulted in a marked suppression of the tumor necrosis factor (TNF)-a level and decreased lupus activity on the systemic lupus erythematosus disease activity index scale [Kotyla et al. 2006].
The influence of statins on SLE is not only restricted to disease activity but also comprises their effect on vessel function. Ferreira and colleagues reported improvement in arterial vasodilation in an 8-week controlled trial where flow-mediated dilatation was increased in SLE patients [Ferreira et al. 2007].
It should be mentioned that probably not all statins provide a pleiotropic effect in patients with connective tissue disease. The absence of immunomodulatory properties in pravastatin has been proven in the study of Costenbader and colleagues where two dose of pravastatin failed to affect lupus activity and had no influence on C-reactive protein reduction [Costenbader et al. 2007]. This indicates that pravastatin may differ from other statins with regard to pleotropic properties [Kotyla, 2009].
The lack of anti-inflammatory effect was also shown in a study by de Kruif and collegues where rosuvastatin was tested in a group of 15 SLE patients with mild disease [de Kruif et al. 2009]. It is speculated that, in this particular trial, the effect of statins may have been ameliorated by concomitant steroid use and statin-induced apoptosis, which in turn activated the immune response or the shifting from the Th1 to Th2 response resulting in B-cell hyperactivity and overproduction of auto-antibodies [de Kruif et al. 2009].
As shown in the above cited study, the pleiotropic effect does not seem to be a class effect of statins. Various compounds may differ from each other with regard to immunomodulatory/immunosuppression properties. Currently we do not know the main mechanism of immunomodulation. Some statins have the ability to bind to the L-site on the LFA-1 and inhibit the LFA-1— ICAM-1 interaction [Watts et al. 2005]. Contradictory results obtained from the study where various statins were used may be explained by the inability of pravastatin and rosuvastatin to bind to the L-site.
Understanding of the new role of statins in the treatment of SLE should result from the recognition of SLE as a complex systemic disease with late complications arising from the cardiovascular system. HMG-CoA reductase inhibitors have proven their efficacy in the treatment of atherosclerosis in a population not affected by connective tissue diseases and showed noncholes-terol-lowering properties, so it may be reasonable to use this group of compounds in the primary and secondary prevention of cardiovascular diseases among patients with SLE. It is clear that statins will never substitute glucocorticosteroids and immunosuppressants in the treatment of SLE, but having a neutral, possible beneficial influence on immune response, HMG-CoA reductase inhibitors should always be considered as a valuable concomitant therapy in SLE patients.
Rheumatoid arthritis
Epidemiologic studies in rheumatoid arthritis have consistently found that subjects with rheumatoid arthritis (RA) are at increased risk of cardiovascular disease, and this burden cannot be easily explained by the traditional risk factors alone. The mechanism underlying the high frequency of cardiovascular morbidity that occurs in patients is not fully understood. Research carried out over last decade has demonstrated, however, that similar to patients with other connective tissue diseases, RA is a cardiovascular risk factor. This is due to the fact that RA is an autoimmune disorder involving various types of inflammation that shares many similarities with atherosclerosis. Obviously inflammation present in RA patients is not restricted to the joints and synovial membrane but has a systemic character and is also present at the level of blood vessels. Vessel involvement is the same as in SLE patients and comprises several steps in vessel dysfunction and damage. An early step in vessel damage is endothelium dysfunction. This process is mainly driven by increased levels of circulating Il-6, TNF-α and C-reactive protein, which by working together ultimately lead to the inhibition of endothelium-dependent vasodilation, arterial stiffness, and subclinical atherosclerosis [Esteve et al. 2007; Mahmud and Feely, 2005]. Systemic inflammation also leads to deep changes in many physiological processes such as impairment of vascular injury repair, insulin resistance, hypercoagulable state, elevated homocysteine levels, and upregulation of immunocompetent cells. More importantly, endothelial damage and subclinical arterial stiffness, the first steps in atherosclerosis, often precede RA. The results from a study may serve as an example where the risk of myocardial infarction was particularly high within the 2 years before formal diagnosis of RA was made [Maradit-Kremers et al. 2005]. Patients with RA may differ from patients withnio symptoms of connective tissues disease. Compared with patients suffering from atherosclerosis, low body mass in RA patients is linked to a higher risk of a serious cardiovascular event, mainly due to the influence of inflammatory cytokines that possess catabolic properties. The results regarding the lipid profile in RA patients in general gave contradictory results. Indeed the routine test used for the detection of lipid profile abnormalities usually returns as normal. On the other hand, inflammation is known to alter lipoprotein metabolism in a manner that promotes atherogenicity [Libby et al. 2002].
Prevention of atherosclerosis in RA patients can be easily achieved with statins, but again the question arises of the additional, anti-inflammatory or immunomodulatory activities of the drugs. The results of a collagen-induced arthritis study that represents an animal model of RA, offers support for the thesis that statins may be useful in RA patients. In this study simvastatin significantly suppressed articular inflammation and decreased pro-inflammatory cytokine synthesis, IFNg, TNF-a, and Il-12 [Leung et al. 2003]. Similar results were reported by Lin and colleagues who examined the influence of simva-statin on peripherial monocytes from patients with active RA. Inhibition of the Rho pathway in this study resulted in the suppression of Toll-like receptor-2 activation and subsequent reduction of cytokines synthesis [Lin et al. 2010].
Suppression of inflammation could be also observed in vivo, in patients with refractory RA, where administration of the HMG-CoA reductase inhibitor simvastatin contributed to the reduction in number of swollen joints and improvement in self-assessed disease activity [Abud-Mendoza et al. 2003; Kanda et al. 2002]. This effect was confirmed in a large double-blind, randomized, placebo-controlled trial with atorvastatin (TARA study). According to the data obtained from the study, atorvastatin has a significant impact on disease activity and reduces the markers of inflammation [McCarey et al. 2004]. In another study, Yokota and colleagues reported the inhibition of production of IL-6, IL-8, and TNF-a in fibroblast-like synoviocytes from patients with RA [Yokota et al. 2006]. Two years later the same authors suggested a biphasic mode of action of simvastatin on fibro-blast-like synovioctes. They postulated that reduction of cytokine synthesis is observed in lower doses of simvastatin since higher doses predispose the fibroblast-like synovioctes to apopto-sis [Yokota et al. 2008].
Systemic sclerosis (scleroderma)
Systemic sclerosis is a connective tissue disease characterized by immune alterations that often correlate with organ injury. The main feature of the disease is the thickening and hardening of skin due to uncontrolled fibroblast proliferation and collagen synthesis. Although the skin is the most visible problem, it is internal organ involvement that is responsible for the reduction in life span and the development of serious complications. The accumulation of collagen fibers in almost all organs and the vascular bed contributes significantly to the presence of internal organ and vessel injury.
Lung involvement can be observed in most connective tissue diseases, however pulmonary manifestation in systemic sclerosis attracts much attention from physicians. It may occur as lung fibrosis and pulmonary arterial hypertension. In the course of systemic sclerosis lung fibrosis reflects profibrotic activity in the body rather than local inflammation. In scleroderma patients the attention on lung fibrosis has shifted from chronic inflammation to aberrant wound repair mechanisms with special emphasis on the interplay of fibroblasts and alveolar epithelial cells. As a consequence the therapeutic approach has changed from traditional anti-inflammatory drugs to those that may possess the ability to modify the fibrotic process. Unfortunately at present there is a lack of such agents that may dramatically change the course of fibrosis in systemic sclerosis. Theoretically fibrosis may be halted/reversed by increased apoptosis of the fibroblasts. In some laboratory models lovastatin has been shown to induce apoptosis of the fibroblasts from normal and fibrotic lungs. The process was associated with inhibition of the Ras signaling pathway, which explained the mode of action of statins in this model [Tan et al. 1999]. Apoptosis of the fibroblasts is not the only mechanism through which statins may affect fibroblast function.
The main player in fibrosis, that is characteristic of systemic sclerosis, is transforming growth factor beta (TGF-b), the growth factor involved in such processes as embryonic development immune responses, and regulation of tissue repair after injury [Clark and Cooker, 1998]. One of the most important effects of TGF-b is the stimulation of synthesis of the extracellular matrix proteins. As a result, increased production of collagens and other matrix proteins, such as fibronectin, can be observed [Varga et al. 1987]. The systemic sclerosis fibroblasts expressed increased levels of TGF-b receptors on their surface. This may explain increased collagen production by fibroblasts. The TGF-β also contributes to the imbalance between collagen synthesis and collagen-degrading metalloproteinases. As a result, collagen overproduction is seen. The process is augmented by stimulation of the production of protease inhibitors that prevents the breakdown of the extracellular matrix [Postlethwaite, 1995]. Data on statins influence on TGF-β synthesis gave contradictory results; some studies suggest that statins have the ability to block TGF-β synthesis, while others suggest they do not [Li et al. 2005; Porreca et al. 2002]. On the other hand, reduction of TGF-b may potentially be dangerous for the blood vessels. Some data indicate that TGF-β may exert a protective role in atherosclerosis development [Ryan et al. 2003]. Beneficial effects observed in vessels after statins therapy suggest that statins may use other signaling pathways increasing TGF-β levels but neutralizing its activity at more advanced stages of signaling. TGF-β is a potent inducer of connective tissue growth factor (CTGF), which is a fibroblast mitogen and promoter of collagen deposition and has been implicated in the pathogenesis of tissue fibrosis [Frazie et al. 1996]. Laboratory data showed simvastatin inhibited TGF-b-stimulated CTGF activity through a Rho-dependent mechanism [Watts and Spiteri, 2004]. This mechanism may explain why antifibrotic activity is observed in spite of the statins' potential to induce TGF-β synthesis. Similar results, such as the reduction in fibroblast proliferation, could be observed after inhibition of cyclin D1 gene expression on fibroblasts that is mediated through the RhoA signaling pathway. This finding is of special pathophysiological importance, as cyclin D1 is a critical regulator in the progression of the cell cycle and cyclin gene, and its product is upregulated in idiopathic pulmonary fibrosis-derived lung fibroblasts. Simvastatin, an established Rho molecule inhibitor, significantly reduced cyclin D1 mRNA and protein synthesis as well as suppressing cell proliferation [Watts et al. 2006].
Another potential mechanism of statin action is the inhibition of vascular smooth muscle synthesis of thrombospondin-1, a potent activator of TGF-β. However, the role of this mechanism in fibrosis is still unclear [Riessen et al. 1999].
The other proposed targets in the treatment of fibrosis are the genes encoding collagens. Those genes that encode collagens type I and III have attracted much attention. This is because tissue fibrosis in scleroderma, the main clinical manifestation of the disease, is caused by the accumulation of these two types of collagen and other connective tissue components in the extracellular space. In the study of Rosenbloom and colleagues, GGTI-296, a specific inhibitor of geranylgeranyl transferase, inhibited type I gene expression in normal and systemic sclerosis fibroblasts [Rosenbloom et al. 2000]. This observation was further confirmed by a study with simvastatin, where a marked reduction of gene expression in cultures of normal and systemic sclerosis dermal fibroblasts caused a substantial reduction of collagen synthesis [Louneva et al. 2006].
Conclusions
At present it is not clear whether statin therapy will be important in the treatment of systemic sclerosis. Results from animal and in vitro models should be interpreted with caution and perhaps cannot be translated directly into the human clinic. Taking into account accelerated macrovascular disease among patients with systemic sclerosis, statins should be the first choice. However, additional immunosuppressive and antifibrotic properties should be proven in well-designed clinical trials.
The promising results of statins from various models of human auto-immunity and connective tissue diseases confirm the potential of HMG-CoA reductase inhibitors in the treatment of diseases. We are steadily accumulating research in this field. Unfortunately, most studies utilize animal and in vitro models, where very high doses of statins are used, and promising results cannot be found in the majority of connective tissue disease presentation in humans. However, treatment with statins may give us a unique opportunity to stop the burden of accelerated atherosclerosis and perhaps modulate immune response and fibrotic processes. It is clear that statins will not be substitutes for steroids and other immunosuppressants but may lead to a reduction in doses/amounts of classic drugs.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
None declared.
References
- Abud-Mendoza C, de la Fuente H., Cuevas-Orta E., Baranda L., Cruz-Rizo J., Gonzalez-Amaro R. (2003) Therapy with statins in patients with refractory rheumatic diseases: A preliminary study. Lupus 12: 607–611 [DOI] [PubMed] [Google Scholar]
- Aktas O., Waiczies S., Smorodchenko A., Dorr J., Seeger B., Prozorovski T., et al. (2003) Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med 197: 725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki I., Aoki N., Kawano K., Shimoyama K., Maki A., Homori M., et al. (1997) Platelet-dependent thrombin generation in patients with hyperlipidemia. J Am Coll Cardiol 30: 91–96 [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown J.A., Freeman G.J., Hafler D.A. (2001) CD4+CD25 high regulatory cells in human peripheral blood. J Immunol 167: 1245–1253 [DOI] [PubMed] [Google Scholar]
- Barandier C, Ming X.F., Yang Z. (2003) Small G proteins as novel therapeutic targets in cardiovascular medicine. News Physiol Sci 18: 18–22 [DOI] [PubMed] [Google Scholar]
- Botazzo G.F., Pujol-Borrel R., Hanafusa T., Feldmann M. (1983) Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet 2: 1115–1119 [DOI] [PubMed] [Google Scholar]
- Braga V.M.M. (2002) Cell-cell adhesion and signaling. Curr Opin Cell Biol 14: 546–556 [DOI] [PubMed] [Google Scholar]
- Bulkley B.H., Roberts W.C. (1975) The heart in systemic lupus erythematosus and the changes induced in it by the corticosteroid therapy. A study of 36 necropsy patients. Am J Med 58: 243–264 [DOI] [PubMed] [Google Scholar]
- Camera M., Toschi V., Comparato C, Baetta R., Rossi F.j, Fuortes M., et al. (2002) Cholesterol-induced thrombogenicity of the vessel wall: Inhibitory effect of fluvastatin. Thromb Haemost 87: 748–755 [PubMed] [Google Scholar]
- Cipollone F., Fazia M., Iezzi A., Zucchelli M., Pini B., De Cesare D., et al. (2003) Suppression of the functionally coupled cyclooxygenase-2/prostaglandin E synthase as a basis of simvastatin dependent plaque stabilization in humans. Circulation 107: 1479–1485 [DOI] [PubMed] [Google Scholar]
- Clark D.A., Coker R. (1998) Transforming growth facto-beta (TGF-P). Int Biochem Cell Biol 30: 293–298 [DOI] [PubMed] [Google Scholar]
- Cohen L.H., Pieterman E., van Leeuwen R.E., Overhand M., Burm B.E., van der Marel G.A., et al. (2000) Inhibitors of prenylation of Ras and other G-proteins and their application as therapeutics. Biochem Pharmacol 60: 1061–1068 [DOI] [PubMed] [Google Scholar]
- Colicelli J. (2004) Human RAS superfamily proteins and related GTPases. Sci STKE 250: RE13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colli S., Eligini S., Lalli M., Camera M., Paoletti R., Tremoli E. (1997) Vastatins inhibit tissue factor in cultured human macrophages: A novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol 17: 265–272 [DOI] [PubMed] [Google Scholar]
- Comparato C, Altana C, Bellosta S., Baetta R., Paoletti R., Corsini A. (2001) Clinically relevant pleiotropic effects of statins: Drug properties or effects of profound cholesterol reduction? Nutr Metab Cardiovasc Dis 11: 328–343 [PubMed] [Google Scholar]
- Corsini A., Mazzotti M., Raiteri M., Soma M.R., Gabbiani G., Fumagalli R., et al. (1993) Relationship between mevalonate pathway and arterial myocyte proliferation: In vitro studies with inhibitors of HMG-CoA reductase. Atherosclerosis 1001: 117–125 [DOI] [PubMed] [Google Scholar]
- Corsini A., Pazzucconi F., Arnaboldi L., Pfister P., Fumagalli R., Paoletti R., et al. (1998) Direct effects of statins on the vascular wall. J Cardiovasc Pharmacol 31: 773–778 [DOI] [PubMed] [Google Scholar]
- Costenbader K.H., Liang M.H., Chibnik L.B., Aizer J., Kwon H., Gall V., et al. (2007) A pravastatin dose-escalation study in systemic lupus erythematosus. Rheumatol Int 27: 1071–1077 [DOI] [PubMed] [Google Scholar]
- Cristea A., Rus H., Niculescu F., Bedeleanu D., Vlaicu R. (1986) Characterization of circulating immune complexes in heart disease. Immunol Lett 13: 45–49 [DOI] [PubMed] [Google Scholar]
- Dahlback B. (2000) Blood coagulation. Lancet 355: 1627–1632 [DOI] [PubMed] [Google Scholar]
- Daniel J.L., Dangelmaier C, Jin J., Kim Y.B., Kunapuli S.P. (1999) Role of intracellular signaling events in ADP-induced platelet aggregation. Thromb Haemost 82: 1322–1326 [PubMed] [Google Scholar]
- de Kruif M.D., Limper M., Hansen H. R., de Ruiter J., Spek C.A., van Gorp E.C. (2009) Effects of a 3-month course of rosuvastatin in patients with systemic lupus erythematosus. Ann Rheum Dis 68: 1654. [DOI] [PubMed] [Google Scholar]
- Esdaile J.M., Abrahamowicz M., Grodzicky T, Li Y., Panaritis C., du Berger R., et al. (2001) Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 44: 2331–2337 [DOI] [PubMed] [Google Scholar]
- Esteve E., Castro A., Lopez-Bermejo A., Endrell J., Ricart W., Fernandez-Real J.M. (2007) Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care 30: 939–945 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. (2002) Rho GTPases in cell biology. Nature 420: 629–635 [DOI] [PubMed] [Google Scholar]
- Eto M., Barandier C, Rathgeb L., Kozai T, Joch H, Yang Z., et al. (2001) Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin converting enzyme-1 expression by distinct pathways: Role of Rho/ROCK and mitogen-activated protein kinase. Circ Res 89: 583–590 [DOI] [PubMed] [Google Scholar]
- Eto M., Kozai T, Cosentino F., Joch H., Lilscher T.F. (2002) Statins prevent tissue factor expression in human endothelial cells: Role of Rho/Rho-kinase and Akt pathways. Circulation 87: 748–755 [DOI] [PubMed] [Google Scholar]
- Ferreira G.A., Navarro T.P., Telles R.W., Andrade L.E., Sato E.I. (2007) Atorvastatin therapy improves endothelial-dependent vasodilation in patients with systemic lupus erythematosus: An 8 week controlled trial. Rheumatology (Oxford) 46: 1560–1565 [DOI] [PubMed] [Google Scholar]
- Ferro D., Basili S., Alessandri C, Cara D., Violi F. (2000) Inhibition of tissue-factor-mediated thrombin generation by simvastatin. Atherosclerosis 149: 111–116 [DOI] [PubMed] [Google Scholar]
- Frazie K, Williams S., Kothapalli D., Klapser H., Grotendorst G.R. (1996) Stimulation of fibro-blast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol 107: 404–411 [DOI] [PubMed] [Google Scholar]
- Fuster V., Badimon L., Badimon J.J., Chesebro J.H. (1992) The pathogenesis of coronary artery disease and acute coronary syndromes 2. N Engl J Med 326: 310–318 [DOI] [PubMed] [Google Scholar]
- George J., Afek A., Keren P., Herz I., Goldberg I., Haklai R., et al. (2002) Functional inhibition of Ras by s-trans, trans-farnesyl thiosalicylic acid attenuates atherosclerosis in apolipoprotein E knockout mice. Circulation 105: 2416–2422 [DOI] [PubMed] [Google Scholar]
- Glomset J.A., Farnsworth C.C. (1994) Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu Rev Cell Biol 10: 181–205 [DOI] [PubMed] [Google Scholar]
- Hakamada-Taguchi R., Uehara Y., Kuribayashi K., Numabe A., Saito K., Negoro H., et al. (2003) Inhibition of hydroxymethylglutaryl coenzyme A reductase reduces Thl development and promotes Th2 development. Circ Res 93: 948–956 [DOI] [PubMed] [Google Scholar]
- Han K.H., Ryu J., Hong K.H., Ko J., Pak Y.K., Kim J.B., et al. (2005) HMG-CoA reductase inhibition reduces monocyte CC chemokine receptor 2 expression and monocyte chemoattractant protein-1 mediated monocyte recruitment in vivo. Circulation 111: 1439–1447 [DOI] [PubMed] [Google Scholar]
- Hanson J., Bossingham D. (1998) Lupus-like syndrome associated with simvastatin. Lancet 352: 1070. [DOI] [PubMed] [Google Scholar]
- Heinrich J., Assmann G. (1995) Fibrinogen and cardiovascular risk. J Cardiovasc Risk 2: 197–205 [PubMed] [Google Scholar]
- Henney A.M., Wakeley P.R., Davies M.J., Foster K., Hembry R., Murphy G., et al. (1991) Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA 88: 8154–8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson H., Nived O., Sturfelt G. (1989) Outcome in systemic lupus erythematosus: A prospective study of patients from a defined population. Medicine (Baltimore) 68: 141–150 [PubMed] [Google Scholar]
- Kanda H., Hamasaki K., Kubo K., Tateishi S., Yonezumi A., Kanda Y., et al. (2002) Antiniflammatory effect of simvastatin in patients with rheumatoid arthritis. J Rheumatol 29: 2024–2026 [PubMed] [Google Scholar]
- Kaneider N.C., Egger P., Dunzendorfer S., Wiedermann C.J. (2002) Rho-GTPase-dependent platelet-neutrophil interaction affected by HMG-CoA reductase inhibition with altered adenosine nucleotide release and function. Arterioscler Thromb Vasc Biol 22: 1029–1035 [DOI] [PubMed] [Google Scholar]
- Kim Y.C., Kim K.K., Shevach E.M. (2010) Simvastatin induces Foxp3 T regulatory cells by modulation of transforming growth factor-beta signal transduction. Immunology 130: 484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotyla P.J. (2009) Pravastatin, a 3-hydroxy-3-methyl-glutharyl coenzyme A inhibitor does not show pleiotropic effects in patients with systemic lupus erythematosus. Rheumatol Int 29: 353–354 [DOI] [PubMed] [Google Scholar]
- Kotyla P.J., Sliwinska-Kotyla B., Kucharz E.J. (2006) TNF alpha as a potential target in the treatment of SLE: A Role for the HMG-CoA reductase inhibitor simvastatin. J Rheumatol 33: 2361. [PubMed] [Google Scholar]
- Krysiak R., Okopien B., Herman Z.S. (2003) Effects of HMG-CoA reductase inhibitors on coagulation and fibrinolysis processes. Drugs 63: 1821–1854 [DOI] [PubMed] [Google Scholar]
- Kwak B., Mulhaupt F., Myit S., Mach F. (2000) Statins as newly recognized type of immunomodulator. Nat Med 6: 1399–1402 [DOI] [PubMed] [Google Scholar]
- Kwak B., Mulhaupt F., Veillard N., Pelli G., Mach F. (2001) The HMG-CoA reductase inhibitor simvastatin inhibits IFN-gamma induced MHC class II expression in human vascular endothelial cells. Swiss Med Wkly 131: 41–46 [DOI] [PubMed] [Google Scholar]
- Lawman S., Mauri C, Jury E.C., Cook H.T., Ehrenstein M.R. (2004) Atorvastatin inhibits auto-reactive B cell activation and delays lupus development in New Zeland Black/White F1 mice. J Immunol 173: 7641–7646 [DOI] [PubMed] [Google Scholar]
- Leung B.P., Sattar N., Crilly A., Prach M., McCarey D.W., Payne H., et al. (2003) A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol 170: 1524–1530 [DOI] [PubMed] [Google Scholar]
- Li C., Lim S.W., Choi B.S., Lee S.H., Cha J.H., Kim I.S., et al. (2005) Inhibitory effect of pravastatin on transforming growth factor pi-inducible gene h3 expression in a rat model of chronic cyclosporine nephropathy. Am J Nephrol 25: 611–620 [DOI] [PubMed] [Google Scholar]
- Liao J.K. (2002) Isoprenoids as mediators of the biological effect of statins. J Clin Invest 110: 285–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ridker P.M., Maseri A. (2002) Inflammation and atherosclerosis. Circulation 105: 1135–1143 [DOI] [PubMed] [Google Scholar]
- Lin H., Xiao Y, Chen G., Fu D., Ye Y, Liang L., et al. (2010) HMG-CoA reductase inhibitor simvastatin suppresses Toll-like receptor 2 ligand-induced activation of nuclear factor kappa B by preventing RhoA activation in monocytes from rheumatoid arthritis patients. Rheumatol Int, [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Liu M.F., Wang C.R., Fung L.L., Wu C.R. (2004) Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol 59: 198–202 [DOI] [PubMed] [Google Scholar]
- Liuzzo G., Kopecky S.L., Frye R.L., O'Fallon W.M., Maseri A., Goronzy J.J., et al. (1999) Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 100: 2135–2139 [DOI] [PubMed] [Google Scholar]
- Louneva N., Huaman G., Fertala J., Jimenez S.A. (2006) Inhibition of systemic sclerosis dermal fibroblast type I collagen production and gene expression by simvastatin. Arthritis Rheum 54: 1298–1308 [DOI] [PubMed] [Google Scholar]
- Mackay D.J., Hall A. (1998) Rho GTPases. J Biol Chem 273: 20685–20688 [DOI] [PubMed] [Google Scholar]
- Mahmud A., Feely J. (2005) Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 46: 1118–1122 [DOI] [PubMed] [Google Scholar]
- Manzi S., Meilahn E.N., Rairie J.E., Conte C.G., Medsger T.A., Jr, Jansen-McWilliams L., et al. (1997) Age specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the Framingham Study. Am J Epidemiol 145: 408–415 [DOI] [PubMed] [Google Scholar]
- Maradit-Kremers H., Crownson C.S., Nicola P.J., Ballman K.V., Roger V.L., Jacobsen S.J., et al. (2005) Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum 52: 402–411 [DOI] [PubMed] [Google Scholar]
- McCarey D.W., Mclnnes LB, Madhok R., Hampson R., Scherbakov O., Ford I., et al. (2004) Trial of atorvastatin in rheumatoid arthritis (TARA): Double blind, randomized placebo controlled trial. Lancet 363: 2015–2021 [DOI] [PubMed] [Google Scholar]
- Meroni P.L., Tremoli E. (2003) Modulation of adhesion molecule expression on endothelial cells: To be or not to be? J Thromb Haemost 1: 2280–2282 [DOI] [PubMed] [Google Scholar]
- Mezzetti A. (2005) Pharmacological modulation of plaque instability. Lupus 14: 769–772 [DOI] [PubMed] [Google Scholar]
- Musial J., Undas A., Undas R., Brozek J., Szczeklik A. (2001) Treatment with simvastatin and low-dose aspirin depresses thrombin generation in patients with coronary heart disease and borderline-high cholesterol levels. Thromb Haemost 85: 221–225 [PubMed] [Google Scholar]
- Mustafa A., Nityanand S., Berglund L., Lithell H., Lefvert A.K. (2000) Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation 102: 2576–2581 [DOI] [PubMed] [Google Scholar]
- Navab M., Berliner J.A., Watson A.D., Hama S.Y., Territo M.C., Lusis A.J., et al. (1996) The yin and yang of oxidation in the development of fatty streak: A review based on 1994 George Lymann Duff Memorial Lecture. Arterioscler Thromb Vasc Biol 16: 831–842 [DOI] [PubMed] [Google Scholar]
- Petri M. (2000) Detection of coronary artery disease and the role of traditional risk factors in the Hopkins lupus cohort. Lupus 9: 170–175 [DOI] [PubMed] [Google Scholar]
- Pierre-Paul D., Gahtan V. (2003) Noncholesterol-lowering effects of statins. Vasc Endovascular Surg 37: 301–313 [DOI] [PubMed] [Google Scholar]
- Porreca E., Di F.C., Baccante G., Di N.M., Cuccurullo F. (2002) Increased transforming growth factor-beta (1) circulating levels and production in human monocytes after 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase inhibition with pravastatin. J Am Coll Cardiol 39: 1752–1757 [DOI] [PubMed] [Google Scholar]
- Postlethwaite A.E. (1995) Role of T cells and cyto-kines in effecting fibrosis. Int Rev Immunol 12: 247–258 [DOI] [PubMed] [Google Scholar]
- Riessen R., Axel D.I., Fenchel M., Herzog U.U., Rossmann H., Karsch K.R. (1999) Effect of HMG-CoA reductase inhibitors on extracellular matrix expression in human vascular smooth cells. Basic Res Cardiol 94: 322–332 [DOI] [PubMed] [Google Scholar]
- Roman M.J., Salmon J.E., Sobel R., Lockshin M.D., Sammaritano L., Schwartz J.E., et al. (2001) Prevalance and relation to risk factors of carotid atherosclerosis and left ventricular hypertrophy in systemic lupus erythematosus and antiphospholipid antibody syndrome. Am J Cardiol 87: 663–666 [DOI] [PubMed] [Google Scholar]
- Romano M., Diomede L., Sironi M., Massimiliano L., Sottocorno M., Polentarutti N., et al. (2000a) Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab Invest 80: 1095–1100 [DOI] [PubMed] [Google Scholar]
- Romano M., Mezzetti A., Marulli C, Ciabattoni G., Febo F., Di Ienno S., et al. (2000b) Fluvastatin reduces soluble P-selectin and ICAM-1 levels in hypercholesterolemic patients: Role of nitric oxide. J Investig Med 48: 183–189 [PubMed] [Google Scholar]
- Rosenbloom J., Saitta B., Gaidarova S., Sandorfi N.j, Rosenbloom J.C., Abrams W.R., et al. (2000) Inhibition of type I gene expression in normal and systemic sclerosis fibroblasts by a specific inhibitor of geranylgeranyl transferase I. Arthritis Rheum 43: 1624–1632 [DOI] [PubMed] [Google Scholar]
- Rosenson R.S., Tangney C.C. (1998) Antiatherothrombotic properties of statins: Implications for cardiovascular event reduction. JAMA 279: 1643–1650 [DOI] [PubMed] [Google Scholar]
- Rosenson R.S., Tangney C.C., Schaefer E.J. (2001) Comparative study of HMG-CoA reductse inhibitors on fibrinogen. Atherosclerosis 155: 463–466 [DOI] [PubMed] [Google Scholar]
- Ross R. (1999) Atherosclerosis an inflammatory disease. N Eng J Med 340: 115–126 [DOI] [PubMed] [Google Scholar]
- Ryan S.T., Koteliansky V.E., Gotwals P.J., Lindner V. (2003) Transforming growth factor-beta-dependent events in vascular remodeling following arterial injury. J Vasc Res 40: 37–46 [DOI] [PubMed] [Google Scholar]
- Sadeghi M.M., Tiglio A., Sadigh K., O'Donnell L., Collinge M., Pardi R., et al. (2001) Inhibition of interferon-gamma-mediated microvascular endothelial cell major histocompati-bility complex class II gene activation by HMG-CoA reductase inhibitors. Transplantation 71: 1262–1268 [DOI] [PubMed] [Google Scholar]
- Schror K. (1995) Antiplatelet drugs. A comparative review. Drugs 50: 7–28 [DOI] [PubMed] [Google Scholar]
- Somlyo A.P., Somlyo A.V. (2000) Signal trans-duction by G-proteins, Rho-kinase and protein phos-phatase to smooth muscle and non-muscle myosin II. J Physiol 522: 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.C., White CM. (2001) Do HMG-CoA reductase inhibitors affect fibrinogen. Ann Pharmacother 35: 236–241 [DOI] [PubMed] [Google Scholar]
- Szczeklik A., Musial J., Undas A., Gajewski P., Gora P., Swadzba J. (1999) Inhibition of thrombin generation by simvastatin and lack of additive effects of aspirin in patients with marked hypercholesterolemia. J Am Coll Cardiol 33: 1286–1293 [DOI] [PubMed] [Google Scholar]
- Takai Y., Sasaki T., Matozataki T. (2001) Small GTP-binging proteins. Physiol Rev 81: 153–208 [DOI] [PubMed] [Google Scholar]
- Takemoto M., Liao J.K. (2001) Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol 11: 328–343 [DOI] [PubMed] [Google Scholar]
- Tan A., Levrey H., Dahm C, Polunovsky V.A., Rubins J., Bitterman P.B., et al. (1999) Lovastatin induces fibroblast apoptosis in vitro and in vivo: A possible therapy for fibroproliferative disorders. Am J Respir Crit Care Med 159: 220–227 [DOI] [PubMed] [Google Scholar]
- Urowitz M.B., Bookman A.A., Koehler B.E., Gordon D.A., Smythe H.A., Ogryzlo MA. (1976) The bimodal mortality pattern of systemic lupus erythematosus. Am J Med 60: 221–225 [DOI] [PubMed] [Google Scholar]
- Valencia X., Yarboro C, Illei G., Lipsky P.E. (2007) Deficient CD4+CD25 high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 178: 2579–2588 [DOI] [PubMed] [Google Scholar]
- van Haelst P.L., van Doormaal J.J., May J.F., Gans R.O., Crijns H.J., Cohen-Tervaert J.W. (2001) Secondary prevention with fluvastatin decreases levels of adhesion molecules, neopterin and C reactive protein. Eur J Intern Med 12: 503–509 [DOI] [PubMed] [Google Scholar]
- Varga J., Rosenbloom J., Jimenez S.A. (1987) Transforming growth factor beta (TGF-beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J 247: 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan C.J., Gotto A.M. (2004) Update on statins: 2003. Circulation 110: 886–892 [DOI] [PubMed] [Google Scholar]
- Vaughan C.J., Gotto A.M., Basson C.T. (2000) The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol 35: 1–10 [DOI] [PubMed] [Google Scholar]
- Warkentin T.E. (1995) Hemostasis and atherosclerosis. Can J Cardiol ll(Suppl C): 29–34 [PubMed] [Google Scholar]
- Watts G.M., Beurskens F.J., Martin-Padura I., Ballantyne C.M., Klickstein L.B., Brenner M.B., et al. (2005) Manifestations of inflammatory arthritis are critically dependent on LFA-1. J Immunol 174: 3668–3675 [DOI] [PubMed] [Google Scholar]
- Watts K.L., Cottrell E., Hoban P.R., Spiteri M.A. (2006) RhoA signaling modulates cyclin Dl expression in human lung fibroblasts: Implication for idiopathic pulmonary fibrosis. Respir Res 7: 88–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts K.L., Spiteri M. (2004) Connective tissue growth factor expression and induction by transforming growth factor is abrogated by simvastatin via Rho signaling mechanism. Am J Physiol Lung Cell Mol Physiol 287: L1323–L1332 [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G., Welzenbach K., Brinkmann V, Kamata T, Kallen J., Bruns C., et al. (2001) Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med 7: 687–692 [DOI] [PubMed] [Google Scholar]
- Yokota K., Miyazaki T, Hirano M., Akiyama Y, Mimura T. (2006) Simvastatin inhibits production of interleukin 6 (IL-6) and IL-8 and cell proliferation induced by tumor necrosing factor-a in fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Rheumatol 33: 463–471 [PubMed] [Google Scholar]
- Yokota K., Miyoshi F., Miyazaki T, Sato K., Yoshida Y., Asanuma Y. (2008) High concentration simvastatin induces apoptosis in fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Rheumatol 35: 193–200 [PubMed] [Google Scholar]
- Youssef S., Stüve O., Patarroyo J.C., Ruiz P.J., Radosevich J.L., Hur E.M., et al. (2002) The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420: 78–84 [DOI] [PubMed] [Google Scholar]
- Zhang F.L., Casey P.J. (1996) Protein prenylation: Molecular mechanism and functional consequences. Annu Rev Biochem 65: 241–269 [DOI] [PubMed] [Google Scholar]