Abstract
Milnacipran, a serotonin and norepinephrfrine reuptake inhibitor with preferential inhibition of norepinephrine reuptake over serotonin, is approved in the United States for the management of fibromyalgia. Owing to its effects on norepinephrine and serotonin, as well as its lack of activity at other receptor systems, it was hypothesized that milnacipran would provide improvements in pain and other fibromyalgia symptoms without some of the unpleasant side effects associated with other medications historically used for treating fibromyalgia. The clinical safety and efficacy of milnacipran 100 and 200 mg/day in individuals with fibromyalgia has been investigated in four large, randomized, double-blind, placebo-controlled studies and three long-term extension studies. The clinical studies used composite responder analyses to identify the proportion of individual patients reporting simultaneous and clinically significant improvements in pain, global status, and physical function, in addition to assessing improvement in various symptom domains such as fatigue and dyscognition. In the clinical studies, patients receiving milnacipran reported significant improvements in pain and other symptoms for up to 15 months of treatment. Most adverse events were mild to moderate in severity and were related to the intrinsic pharmacologic properties of the drug. Long-term exposure to milnacipran did not result in any new safety concerns. As with other serotonin and norepinephrine reuptake inhibitors, increases in heart rate and blood pressure have been observed in some patients with milnacipran treatment.
Keywords: fatigue, fibromyalgia, milnacipran, pain, physical function, serotonin—norepinephrine reuptake inhibitor
Introduction
Fibromyalgia (FM) is a chronic pain disorder that affects approximately 2—4% of the US population [Wolfe et al. 1995]. Although FM is often considered to be a disorder predominantly affecting middle-aged women, it has been observed in men, children, adolescents, and the elderly [Chakrabarty and Zoorob, 2007]. The hallmark symptom of FM is chronic widespread pain, which patients may describe as an overall achiness, deep gnawing or burning pain, or a feeling of swelling in their soft tissues [Bennett, 2009; Arnold et al. 2008; Mease, 2005]. Other commonly reported symptoms include fatigue, stiffness, cognitive dysfunction, disturbed sleep, and psychological distress [Bennett, 2009; Mease et al. 2007].
Although the pathophysiology of FM is not completely understood, a number of genetic, psychosocial, biochemical, and physiologic factors are likely to be involved in the development of this disorder [Bradley, 2009; Mease, 2005]. Increasing evidence suggests, however, that the painful symptoms of FM are attributable to abnormal pain processing in the central nervous system (CNS), including the amplification of pain signals in ascending pain pathways [Staud and Rodriguez, 2006] and the dysregulation of pain signals via descending pain pathways [Bradley, 2009]. The resulting central sensitization can lead to heightened sensitivity to painful stimuli (hyperalgesia) and painful responses to nonpainful stimuli (allodynia) [Staud and Spaeth, 2008]. The transmission of nociceptive information from the periphery to the brain via ascending pain pathways is mediated by various neurotransmitters, including substance P and glutamate [Bradley, 2009]. Neurotransmitters such as serotonin, norepinephrine, and dopamine are involved in the modulation of pain signals in the descending pathways [Dubner and Hargreaves, 1989], and reduced cerebral spinal fluid levels of metabolites of these neurotransmitters have been found in patients with FM compared with healthy controls [Russell et al. 1992]. Thus, medications that increase levels of serotonin, norepinephrine, or dopamine may have clinically beneficial effects on pain in patients with FM. Further, because central sensitization is common in various etiologies of chronic pain, there is currently considerable interest in the possibility that agents inhibiting the reuptake of both serotonin and norepinephrine (serotonin— norepinephrine reuptake inhibitors [SNRIs]) may prove to be valuable in treating a wide variety of chronic pain conditions.
Currently, three drugs are approved by the US Food and Drug Administration (FDA) for the management of FM. Two of these medications, milnacipran and duloxetine, are SNRIs; the third drug, pregabalin, is an alpha-2-delta ligand. A number of other drugs have also been tried in patients with FM, including nonsteroidal anti-inflammatory drugs (NSAIDs), opiates, selective serotonin reuptake inhibitors (SSRIs), and tricyclic antidepressants (TCAs); of these, only the TCAs have demonstrated consistent efficacy in FM clinical studies [Clauw, 2008; Goldenberg et al. 2004]. It has been postulated that similar to the SNRIs, TCAs restore deficits in the descending pain pathways by inhibiting the reuptake of both serotonin and norepinephrine, thereby leading to improvements in pain [Mease, 2009; Clauw, 2008]. However, the efficacy of TCAs is limited by poor tolerability and side effects due to their affinity for histaminergic, cholinergic, and adrenergic receptor systems [Mease, 2005], leading to diminished patient compliance and limited long-term use in patients with FM [Mease, 2009]. In contrast to TCAs, SNRIs such as milnacipran and duloxetine possess no significant affinities for these receptors [Briley et al. 1996; Wong et al. 1993], resulting in a more favorable tolerability profile.
Since 1997, milnacipran has been widely used outside of the United States for the treatment of major depressive disorder, resulting in a large body of safety data from clinical studies [Nakagawa et al. 2009] and from postmarketing surveillance reports. In patients with FM, the safety and efficacy of milnacipran has been investigated in four large, randomized, double-blind, placebo-controlled studies [Branco et al. 2010; Arnold et al. 2009a; Mease et al. 2009b; Clauw et al. 2008a] and in three long-term extension studies [Goldenberg et al. 2010; Branco et al. 2009; Ferrera et al. 2009]. As discussed below, results from these clinical studies indicate that milnacipran significantly improves the pain and other symptoms of FM for up to 15 months of treatment. In addition, findings from key preclinical and clinical pharmacology studies are highlighted below to present a comprehensive review of milnacipran.
Preclinical studies
The inhibitory effects of milnacipran on serotonin and norepinephrine reuptake have been investigated using established physiologic and biochemical experimental methods. In microdialysis studies, milnacipran was found to increase the extracellular levels of both serotonin and nor-epinephrine in the hypothalamus [Moret and Briley, 1997] and prefrontal cortex [Kitaichi et al. 2008; Mochizuki et al. 2002] of rodents. In an in vitro study comparing the effects of various SNRIs on monoamine uptake and transporter binding affinity in human cell lines, milnacipran was found to inhibit norepinephrine reuptake with approximately three-fold greater potency than serotonin reuptake [Vaishnavi et al. 2004]. These in vitro data distinguish milnacipran from other SNRIs (duloxetine and ven-lafaxine), which have been reported to be more potent in inhibiting serotonin reuptake than nor-epinephrine reuptake [Vaishnavi et al. 2004].
There are no well-validated animal models for FM. However, since pain is an integral component of FM, the effects of milnacipran in rodent models of pain are briefly reviewed here. Several studies have shown that this drug reduces hyperalgesic and allodynic behaviors in rodent models of chronic pain. For example, in preclinical studies that use nerve ligation techniques to simulate neuropathic pain, milnacipran diminished hyperalgesic and allodynic behaviors [Ikeda et al. 2009; Takeda et al. 2009; Matsuzawa-Yanagida et al. 2008; King et al. 2006; Obata et al. 2005]. It has been postulated that dysregulation of inhibitory descending pathways contributes to the painful symptoms of FM [Bradley, 2009].
Preclinical results from these nerve ligation studies indicate that that agents acting on both serotonergic and noradrenergic systems are likely to have analgesic effects. For example, in the nerve ligation study that compared paroxetine (an SSRI), maprotiline (a tetracyclic antidepressant with strong noradrenergic reuptake inhibition), and milnacipran (an SNRI), effects on allodynic behavior were only observed with milnacipran [Obata et al. 2005].
Milnacipran has also been found to diminish painful behaviors in a streptozotocin-induced model of diabetic neuropathy [Ikeda et al. 2009], a stress-induced model of muscle hyper-algesia [Suarez-Roca et al. 2006], and in a formalin model of pain [Bardin et al. 2010; Yokogawa et al. 2002]. The formalin model is thought to simulate central sensitization [Bardin et al. 2010], a neurobiological mechanism involving the amplification of nociceptive signals in the CNS that may result in hypersensitivity to pain and other stimuli [Yunus, 2008; Clauw, 2005]. Consistent with findings from the nerve ligation studies, findings from the formalin model indicate that dual reuptake inhibitors such as milnacipran have greater analgesic effects than either SSRIs that act on serotonergic systems or norepinephrine reuptake inhibitors that act solely on noradrenergic systems [Yokogawa et al. 2002]. Therefore, drugs that modulate both noradrener-gic and serotonergic systems involved in the central sensitization process are rational targets for drug development. Although the results of all of these studies do not clarify the exact mechanisms by which milnacipran improves pain in patients with FM, they provide a preclinical basis for the improvements in pain that have been reported in the milnacipran clinical studies.
In addition to its involvement in central pain processing, norepinephrine is thought to play a key regulatory role in cognition via its effects on cellular excitability and synaptic plasticity [Sara, 2009] and via its overlapping activity with dopamine in the prefrontal cortex, the area of the brain that processes higher cognitive functions such as working memory and attentional control [Stahl, 2009; Briand et al. 2007]. Results from several preclinical studies have shown that milnacipran improves cognitive performance in rodents [Moojen et al. 2006; Matsumoto et al. 2005; Rao et al. 2003] and normalizes impaired long-term potentiation or synaptic plasticity in the rat hippocampus [Matsumoto et al. 2005]. It is of interest that results from the milnacipran clinical studies indicate that this drug may improve cognitive deficits in patients with FM.
Pharmacology
Milnacipran is a white crystalline powder that is freely soluble in water. It is available in 12.5, 25, 50, and 100mg tablets [Forest Laboratories, 2009]. The FDA-approved dosage of milnacipran for the management of FM is 100 mg/day (50 mg twice daily), which can be increased to 200 mg/day based on individual patient response. No dose adjustment is necessary based on age or gender. Slow dose escalation may minimize nausea in the early treatment period.
Milnacipran is well absorbed in humans, with the maximal plasma concentration (Cmax) reached within 2—4 hours after oral administration [Forest Laboratories, 2009]. The absolute bio-availability of milnacipran is high (85—90%) [Puozzo et al. 1987], and absorption is not affected by food. The terminal elimination half-life is approximately 6—8 hours [Puozzo and Leonard, 1996; Puozzo et al. 1987] and steady-state concentrations are reached within 36—48 hours. Exposure to milnacipran increases proportionally within the therapeutic dose range and multiple-dose pharmacokinetics are predictable from single-dose data.
Milnacipran and its metabolites are eliminated primarily by renal excretion, with approximately 55% of milnacipran excreted unchanged in urine, 19% as a carbamoyl-O-glucuronide conjugate, 8% as A/-desethyl milnacipran, and the remainder of the administered dose as other minor metabolites, all of which are inactive [Forest Laboratories, 2009; Puozzo and Leonard, 1996].
Consistent with the elimination pathway of milnacipran, subjects with hepatic impairment have not demonstrated clinically relevant changes in milnacipran pharmacokinetic parameters as compared with subjects with normal hepatic function [Puozzo et al. 1998a]. In patients with mild, moderate, and severe renal impairment, however, systemic exposure (AUC0-OO) to milnacipran increased by 16%, 52%, and 199%, respectively, as compared with healthy subjects [Forest Laboratories, 2009; Puozzo et al. 1998b]. Thus, dose adjustment is required in patients in cases of severe renal impairment, and caution is advised in patients with moderate renal impairment or severe hepatic impairment [Forest Laboratories, 2009].
A population pharmacokinetic model was recently developed in order to describe milnacipran pharmacokinetics in patients with FM as compared with healthy subjects [Ghahramani and Periclou, 2009]. Demographic variables and clinical laboratory values were used as covariates to develop the population pharmacokinetic model. The base structural model was a two-compartment model with first-order absorption and elimination and lag time in absorption. Results confirm that creatinine clearance has a significant effect on oral plasma clearance (CL/F) (p> 0.001) with CL/F values decreasing with decreases in renal function, which is consistent with the elimination of milnacipran via the renal pathway. Patients with FM were found to have a significantly lower CL/F than healthy subjects by 12%, but this difference was not considered to be clinically relevant.
The potential for milnacipran to undergo pharmacokinetic drug—drug interactions is limited due to low plasma protein binding (13%) [Puozzo et al. 2002], low hepatic metabolism [Puozzo et al. 1998a], and minimal interactions with the cytochrome P450 system. In vitro study results indicate that even at high concentrations, milnacipran does not induce or inhibit cytochrome P450 enzymes [Paris et al. 2009]. In healthy volunteers, exposure to milnacipran and its metabolites was not affected in either poor or extensive metabolizers of mephenytoin and sparteine/dextromethorphan (metabolized by cytochrome P450 enzymes CYP2C19 and CYP2D6, respectively), indicating that milnacipran oxidative metabolism is not mediated through CYP2C19 or CYP2D6 polymorphic pathways [Puozzo et al. 2005]. Moreover, no significant in vivo interactions were detected between milnacipran and CYP2D6, CYP3A4, CYP2C19, or CYP1A2 isoenzyme activities, indicating flexibility in the therapeutic use of milnacipran. Studies in healthy volunteers have also shown that milnacipran does not undergo pharmacokinetic drug interactions with carbamazepine, digoxin, lithium, or warfarin [Periclou et al. 2009; Puozzo et al. 2002]. In addition, the pharmacokinetics of milnacipran are not affected by switching from clomipramine or fluoxetine to milnacipran without an intervening washout period [Periclou et al. 2009].
Despite the minimal risk of pharmacokinetic interactions, the possibility of pharmacodynamic interactions due to the inherent pharmacology of milnacipran should be considered. Owing to the potential for serotonin syndrome or neurolep-tic malignant syndrome — like reactions that can occur with this class of drugs, concomitant use of milnacipran with serotonergic drugs (e.g. SSRIs, SNRIs, triptans), antipsychotics, dopamine antagonists, CNS-active drugs, certain cardiovascular agents (e.g. digoxin, clonidine), or catecholamines (e.g. epinephrine, norepinephrine) is cautioned; concomitant use with mono-amine oxidase inhibitors is contraindicated [Chwieduk and McCormack, 2010; Forest Laboratories, 2009]. Although activation of mania or hypomania was not observed in FM clinical studies of milnacipran, such events have been reported in patients treated with similar medications for mood disorders; thus, it is advised that milnacipran be used with caution in patients with history of mania. As with other antidepressants, milnacipran may increase suicidality in patients, particularly in children, adolescents, and young adults. Owing to noradrenergic effects, treatment with milnacipran is also cautioned in patients with history of dysuria. Milnacipran can produce mydriasis and is contraindicated in patients with uncontrolled narrow-angle glaucoma; it may be used with caution in patients with controlled narrow-angle glaucoma. In the 2-week discontinuation phase of Study 3, no evidence of discontinuation syndrome was observed in patients who were abruptly switched from milnacipran 100mg/day to placebo [Saxe et al. 2009a]. However, as with other agents in its class, dose tapering and monitoring for withdrawal symptoms are advised in patients discontinuing extended milnacipran treatment [Forest Laboratories, 2009].
In contrast to TCAs, milnacipran lacks clinically meaningful affinity for adrenergic, serotonergic, dopaminergic, histaminergic, muscarinic, or opiate receptors [Assie et al. 1992; Moret et al. 1985]. Because milnacipran increases serotonin and norepinephrine levels by inhibiting reuptake at presynaptic sites rather than through interaction with postsynaptic serotonergic and noradrenergic receptors, this drug is expected to have a side-effect profile consistent with increased peripheral exposure to these neurotransmitters [Spencer and Wilde, 1998].
Efficacy
Clinical study design
The efficacy of milnacipran in FM has been investigated in three randomized, double-blind, placebo-controlled, multicenter, phase 3 studies conducted in the United States (Study 1 [Mease et al. 2009b]; Study 2 [Clauw et al. 2008a]; and Study 3 [Arnold et al. 2009a]) and a phase 3 study in Europe (Study 4 [Branco et al. 2010]). These four studies were designed using relatively similar dosing, inclusion and exclusion criteria, and primary endpoints (Table 1).
Table 1.
Summary of milnacipran phase 3 clinical studies in fibromyalgia.
| Study 1 [Mease et al. 2009b] | Study 2 [Clauw et al. 2008a] | Study 3 [Arnold et al. 2009a] | Study 4 [Branco et al. 2010] | |
|---|---|---|---|---|
| Study Design | ||||
| Study location | US | US | US, Canada | Europe |
| ITT population, N | 888 | 1196 | 1025 | 876 |
| Duration of stable-dose phase | 24 weeks | 12 weeksa | 12 weeks | 12 weeks |
| Study medication dosage(s) | 100 mg/day | 100 mg/day | 100 mg/day | 200 mg/day |
| 200 mg/day | 200 mg/day | |||
| Inclusion Criteria | ||||
| ACR criteria for FM | ✓ | ✓ | ✓ | ✓ |
| Average baseline pain intensity VAS score (range 0–100) | ≥50 | ≥40 | ≥40 and ≤90 | ≥40 and ≤90 |
| FIQ physical function subscale score | NA | ≥4 | ≥ 4 | ≥ 3 |
| Ability and willingness to use electronic PED | ✓ | ✓ | ✓ | ✓ |
| Discontinuation of nonpharmacologic and CNS-active pharmacologic therapies | ✓ | ✓ | ✓ | ✓ |
| Exclusion Criteria | ||||
| Current major depressive episodeb | ✓ | ✓ | ✓ | ✓ |
| BDI score | NA | >25 | > 25 | > 25 |
| Severe psychiatric illness or medical condition | ✓ | ✓ | ✓ | ✓ |
| Primary Endpoints | ||||
| Two-measure composite responderc | ✓ | ✓ | ✓ | ✓ |
| Three-measure composite responderd | ✓ | ✓ | ✓ | NA |
| FIQ total score | NAe | NAe | NAe | ✓ |
Some patients in Study 2 received up to 6 months of treatment; primary efficacy endpoint was at 3 months.
Assessed by Mini-International Neuropsychiatric Interview (MINI).
Defined as an individual patient achieving ≥30% improvement from baseline in PED 24-hour recall pain and Patient Global Impression of Change response of ‘very much improved’ or ‘much improved’.
Defined as an individual patient achieving the criteria for a two-measure composite responder and a ≥6-point improvement from baseline in Short Form-36 Physical Component Summary score.
In Studies 1, 2, and 3, FIQ total score was included as an additional endpoint.
Abbreviations: ✓, applicable; ACR, American College of Rheumatology; BDI, Beck Depression Inventory; CNS, central nervous system; FIQ, Fibromyalgia Impact Questionnaire; FM, fibromyalgia; ITT, intent-to-treat; NA, not applicable; PED, patient experience diary; US, United States; VAS, visual analog scale.
Together, the phase 3 milnacipran clinical studies included approximately 4000 adult patients who met the 1990 American College of Rheumatology (ACR) criteria for FM [Wolfe et al. 1990] (Table 1). In the 3-month studies (Studies 2, 3, and 4), patients were equally randomized to all treatment groups. In the 6-month study (Study 1), patients were randomized at a ratio of 1:1:2 to placebo, milnacipran 100 mg/day, and milnacipran 200 mg/day, respectively, in order to collect more data for the higher dose; this study included 3- and 6-month efficacy endpoints.
In addition to meeting certain criteria for baseline scores on pain and physical function (Table 1), all patients in the milnacipran studies were also required to discontinue nonpharmacologic and CNS-active pharmacologic therapies. Exclusion criteria included current major depressive episode, severe psychiatric illness, and severe medical conditions. Limited rescue medication use was permitted in the US studies for acute exacerbations of FM pain; however, the use of rescue medication during critical efficacy evaluation periods resulted in the invalidation of pain data and/or classification of patients as nonresponders.
Efficacy was assessed daily using an electronic patient experience diary (PED) as well as at clinic visits. A major feature of the efficacy endpoints used in these studies was the inclusion of composite responder classifications. This ensured that any patient who was classified as a responder had demonstrated a clinically significant improvement in each individual component of the composite response definition. Simultaneous evaluation of both pain and patient global responses was part of all of the responder definitions. Included as a primary efficacy end-point in all of the studies was a two-measure composite responder analysis that required patients to meet the following criteria: >30% improvement from baseline in PED visual analog scale (VAS) 24-hour recall pain scores; and a rating of 1 (‘very much improved’) or 2 (‘much improved’) on the Patient Global Impression of Change (PGIC). The three US studies also included a more stringent three-measure composite responder analysis as a primary end-point, which required patients to have a > 6-point improvement from baseline in the Short Form-36 (SF-36) Physical Component Summary (PCS) score in addition to meeting the pain and PGIC criteria described above. In the European study, the Fibromyalgia Impact Questionnaire (FIQ) total score was included as a coprimary endpoint.
As requested by the US FDA, the milnacipran clinical study used a baseline observation carried forward (BOCF) method to impute missing values for the primary efficacy endpoints [USFDA, 2009]. This is a conservative approach that assumes no improvement from baseline occurred in patients with missing data, even if they showed dramatic improvement prior to leaving the study. Sensitivity analyses of the primary endpoints and analyses of secondary endpoints (e.g. improvements in pain, fatigue, global status, and multidimensional functioning) included last observation carried forward (LOCF) and observed cases (OC). The LOCF is a commonly used imputation method that uses the last available data as the endpoint value. The OC approach makes no assumptions about missing values and thus does not account for patients who may have discontinued due to insufficient efficacy. However, the OC approach does provide clinically useful information about patients who can tolerate treatment. In addition to the BOCF, LOCF, and OC methods described above, a mixed-effect model repeated measure (MMRM) method was used for some post hoc analyses of the milnacipran clinical study data. Compared with LOCF, this statistical approach may be more effective in controlling for Type I errors and minimizing bias [Siddiqui et al. 2009].
Baseline characteristics
Patient demographics were generally similar across the four studies [Palmer et al. 2009]. Most patients in these studies were female (>90%) and white (>90%). Mean ages ranged from 48.8 to 50.2 years, and mean duration of FM symptoms was approximately 10 years. The only notable difference between US and European patients was body weight. Mean baseline body mass index (BMI) scores in the US studies (range 30.5—30.9) exceeded the World Health Organization (WHO) criteria for obesity (BMI ≤ 30) [WHO, 2000]. By contrast, the mean baseline BMI in the European study (26.7) fell within the overweight range (BMI 25 to <30). Similarly, proportions of patients who were obese in the US studies (range 47—50%) [Arnold et al. 2009b] were higher than those in the European study (22%) [Branco et al. 2010].
Baseline disease characteristics were also similar among patients in all of the milnacipran studies. In general, patients experienced severe FM-related pain before enrollment in the clinical studies. Mean baseline PED VAS scores were >60 for 24-hour and weekly recall pain scores [Palmer et al. 2009], coinciding with published thresholds for severe pain [Bennett et al. 2005]. Based on severity thresholds established for FIQ total scores [Bennett et al. 2009], patients across the studies also had moderate-to-severe functional impairment at baseline (FIQ total score range, 56.9—64.6) [Palmer et al. 2009]. In addition, mean baseline SF-36 PCS scores indicated that these patients with FM were approximately two standard deviations below US norms in terms of physical functioning [Strand and Singh, 2008; Ware, 2000]. Although patients with severe psychiatric illness or major depressive episodes were excluded from these studies, consistent with clinical experience, a number of patients also exhibited minimal to moderate depressive symptoms at baseline [Palmer et al. 2009], as indicated by mean Beck Depression Inventory (BDI) scores (range 8.9—14.0) [Beck et al. 1988].
Composite responder endpoints
The cardinal symptoms of FM, in addition to chronic widespread pain, include fatigue, sleep disturbances, reduced functioning (physical, mental, and social), and cognitive disturbances [Mease, 2005]. The Outcome Measures in Rheumatology (OMERACT) FM working group has been actively researching the importance of assessing these multiple symptoms of FM in clinical studies. Based on input from patients, clinicians, and researchers, OMERACT has identified the following core domains to be assessed in FM clinical studies: pain, tenderness, fatigue, patient global, multidimensional function, and sleep disturbances [Mease et al. 2009a].
OMERACT has also been instrumental in gaining recognition of the importance of using composite responder indices in clinical studies [Mease et al. 2007]. Such endpoints, which measure clinically meaningful changes across several domains in individual patients, are well suited to capture multidimensional improvements in such complex disorders as FM. Clinical studies for other pain disorders, including rheumatoid arthritis [Schwieterman, 2008] and osteoarthritis [Bingham et al. 2008], have also used composite endpoints to provide information about specific patient responses, as opposed to group mean changes. The two-measure composite responder endpoint used in all four of the milnacipran studies includes two of the core domains identified by OMERACT: pain and patient global. Because these endpoints require patients to meet more than one criterion, they are inherently conservative outcome measures. For example, patients who might have reported dramatic improvements in pain but did not rate their global status as ‘much improved’ or ‘very much improved’ (i.e. PGIC scores of 1 or 2) could not be classified as two-measure composite responders. The three-measure composite responder endpoint used in the three US milnacipran clinical studies represents an even higher hurdle for success by requiring patients to also demonstrate improvements in a physical function component in addition to pain and global status.
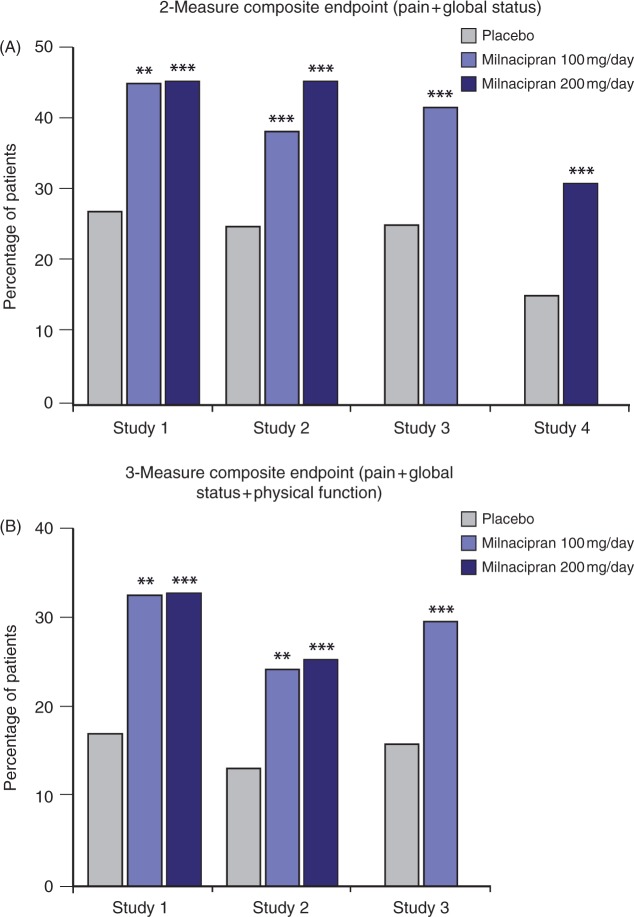
In the phase 3 studies, significantly more milnacipran-treated patients were classified as two-measure and three-measure composite responders as compared with patients receiving placebo. Using the conservative BOCF method to impute missing data, two-measure composite responder rates ranged as follows: 100mg/day, 22.8—28.5%; 200mg/day, 24.8—26.8%; placebo, 16.5—19.3% (all p< 0.05, both doses except for 100mg/day in Study 1). For the three-measure composite endpoint used in the US studies, responder rates ranged as follows: 100mg/day, 14.5—20.0%; 200mg/day, 13.9—19.3%; placebo, 8.7—12.1% (all p< 0.05, both doses; BOCF). Among completers in the US studies, three-measure composite responder rates were approximately twice as high in milnacipran-treated patients as in placebo-treated patients (all p<0.01, both doses versus placebo; OC) (Figure 1).
Figure 1.
Proportion of patients meeting the two-measure and three-measure composite responder criteria at 3 months, observed cases. From Study 1 [Mease et al. 2009b], Study 2 [Clauw ef at. 2008a], Study 3 [Arnold et al. 2009a], and Study 4 [Branco ef at. 2010]. *p < 0.05; **p < 0.01; ***p ≤ 0.001 versus placebo.
In addition to the composite responders, a number of symptom domains were assessed in the milnacipran clinical studies. As discussed below, these include the individual components of the composite responders (i.e. pain, patient global, physical function), as well as fatigue and dyscognition. The potential effects of milnacipran on quality of life, as assessed by measures of multidimensional functioning such as the SF-36 and FIQ, are also discussed.
Pain
Chronic widespread pain is the cardinal symptom of FM and a number of different measures were used to assess pain severity in the milnacipran studies. One innovative aspect of these studies was the use of electronic PEDs in collecting pain data. Patients were prompted several times each day and on a weekly basis to record their recalled pain (24-hour recall, weekly recall) and current (‘real-time’) pain. The advantages of using the PEDs include the minimization of potential recall bias and the ability to capture data in the patients’ home environment [Williams et al. 2008, 2007]. The PED pain data were supplemented by paper-based and computerized tablet-based VAS pain assessments (24-hour and weekly recalled pain) and the Brief Pain Inventory (BPI).
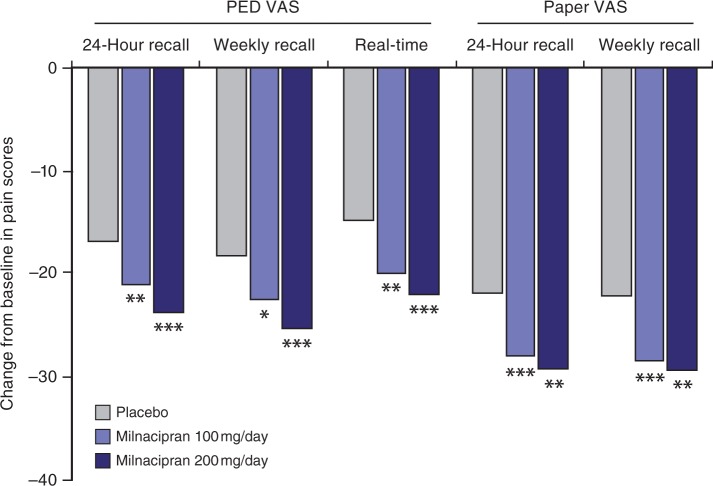
In all of the FM studies, improvements in PED VAS 24-hour recall pain scores were significantly greater with milnacipran than with placebo. In addition, all of the ancillary pain measures described above were consistent with and corroborated these PED data. The significant improvements with milnacipran versus placebo in multiple pain measures are exemplified by the results from Study 2 [Clauw et al. 2008a], which was the only study to include both doses of milnacipran and randomize patients equally to each treatment arm (Figure 2). In all of the studies, significant differences between milnacipran and placebo were reported after Week 1 of treatment, with maximal pain relief observed after 9 weeks of milnacipran treatment. Among study completers, significant improvements in pain with milnacipran treatment were observed at most weeks for up to 6 months of treatment (both doses, p < 0.05; OC) [Mease et al. 2009b]. A pooled analysis of Studies 1 and 2 indicate that consistent pain relief (i.e. ≤30% pain reduction for 80% of days remaining until the 3-month endpoint) was achieved sooner in patients treated with milnacipran as compared with placebo [data on file].
Figure 2.
Least squares mean changes from baseline in pain scores. From Study 2 (placebo, n=401; milnacipran 100 mg/day, n=399; milnacipran 200 mg/day, n=396) [data on file], observed cases. *p<0.05; **p <0.01; ***p<0.001 versus placebo; PED, patient experience diary; VAS, visual analog scale.
The effect of milnacipran on FM pain was also evident in the pain component of the composite responder criteria (i.e. >30% improvement from baseline in PED VAS 24-hour recall pain scores), which reflects clinically meaningful improvements in pain [Dworkin et al. 2008; Farrar et al. 2001]. In all four studies, the proportion of milnacipran-treated patients achieving >30% improvement in pain at 3 months ranged from 46% to 60%, compared with 33% to 39% for placebo (p<0.01, OC) (Table 2). Post hoc analyses of Studies 1 and 2 provide further evidence of the effect of milnacipran on improvements in pain [Mease et al. 2009d]. After 15 weeks of treatment, a significantly higher proportion of milnacipran-treated patients achieved a more conservative definition of pain relief (i.e. ≤50% improvement in pain) compared with patients who received placebo (100mg/day, 27%; 200mg/day, 34%; placebo, 19%; both doses, p < 0.05, OC). Finally, patients treated with milnacipran experienced significantly more days with ≤30% improvements in pain (p< 0.001, OC) and ≤50% improvements in pain (p< 0.001, OC) as compared with patients on placebo. These findings indicate that treatment with milnacipran results in persistent and consistent clinically meaningful improvements in FM pain.
Table 2.
Summary of pain, global, and physical function responder rates at 3 months in milnacipran phase 3 clinical studies in fibromyalgiaa.
| Study 1 [Mease et al. 2009b] | Study 2 [Clauw et al. 2008a] | Study 3 [Arnold et al. 2009a] | Study 4 [Branco et al. 2010] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Milnacipran 100 mg/day | Milnacipran 200 mg/day | Placebo | Milnacipran 100 mg/day | Milnacipran 200 mg/day | Placebo | Milnacipran 100 mg/day | Placebo | Milnacipran 200 mg/day | ||
| Painb | LOCF | 35% | 40% | 42% | 29% | 37%** | 40%*** | 31% | 45%*** | 30% | 39%** |
| OC | 39% | 56%** | 60%*** | 38% | 52%** | 55%*** | 37% | 53%*** | 33% | 46%*** | |
| Globalc | LOCF | 30% | 38% | 39%* | 25% | 35%** | 38%*** | 26% | 42%*** | 21% | 33%*** |
| OC | 37% | 53%** | 55%*** | 32% | 48%*** | 51%*** | 33% | 52%*** | 23% | 41%*** | |
| Physical functiond | LOCF | 34% | 38% | 40% | 25% | 32%* | 28% | 31% | 40%*** | NA | NA |
| OC | 38% | 51%* | 50%* | 30% | 41%** | 35% | 37% | 47%** | NA | NA | |
p<0.05;
p≤0.01;
p≤ 0.001, versus placebo.
From published literature and/or data on file.
Proportion of patients achieving ≥30% improvement from baseline in PED 24-hour recall pain.
Proportion of patients achieving Patient Global Impression of Change response of ‘very much improved’ or ‘much improved’.
Proportion of patients achieving a ≥6-point improvement from baseline in Short Form-36 Physical Component Summary score.
LOCF, last observation carried forward; OC, observed cases; NA, not applicable to Study 4; PED, patient experience diary.
Patient global
Patient global status measures allow patients to integrate into a single evaluation the different aspects of their response to treatment, including pain relief, improvement in multidimensional functioning, and side effects. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has recommended the inclusion of the PGIC as a core outcome measure in chronic pain trials [Dworkin et al. 2005]. Similarly, the OMERACT FM working group has identified ‘patient global’ as an essential domain to measure in FM clinical trials [Mease et al. 2009a]. The PGIC uses a seven-point Likert scale that allows patients to rate their change from ‘very much improved’ to ‘very much worse’ [Guy, 1976]. After 3 months of treatment in the milnacipran clinical studies, 41—55% of patients who received active treatment reported that their FM was ‘very much improved’ or ‘much improved’ since the beginning of the study (p≤0.01, OC) (Table 2). In a post hoc analysis of Studies 1 and 2 that evaluated the impact of FM symptoms on global status, changes in pain scores were found to have the strongest correlation with PGIC scores in patients who reported global improvements [Geisser et al. 2010]. Other independent correlating factors included vitality, sleep, dyscognition, and physical function. Thus, while pain improvement is the fundamental effect of milnacipran treatment in patients with FM, its effects on patient global status also reflect improvement in other important domains.
Physical function
Patients with FM characteristically experience substantial reductions in physical functioning, which can impede the ability to perform daily tasks [Goldenberg, 2009; Culos-Reed and Brawley, 2000]. Clinically meaningful improvement in physical function, defined in the US milnacipran studies as ≤6-point improvement from baseline in SF-36 PCS score, was included as a component of the three-measure composite responder endpoint. In these studies, response rates for SF-36 PCS were significantly higher with milnacipran versus placebo (Table 2). In a pooled analysis of the three US studies, the proportion of patients with clinically meaningful improvements in SF-36 PCS scores at all study visits was significantly higher with milnacipran 100mg/day than with placebo [Saxe et al. 2009b]. In the European study (Study 4), for example, least squares (LS) mean changes from baseline SF-36 PCS scores for milnacipran 200mg/day and placebo were +4.55 and +3.57, respectively, representing a 27% greater improvement with active treatment over placebo (p = 0.025, LOCF) [Branco et al. 2010].
Fatigue
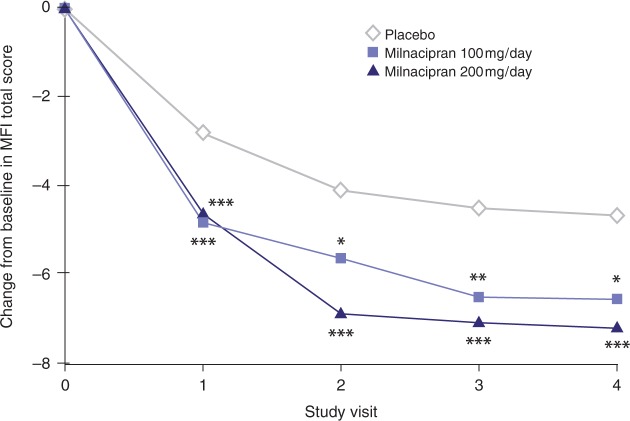
Fatigue, in addition to pain, is one of the most commonly reported and troublesome symptoms of FM [Guymer and Clauw, 2002; Wolfe et al. 1996, 1990], with moderate or severe fatigue occurring in an estimated 75—90% of patients [Yunus, 2005]. In the milnacipran clinical studies, fatigue was measured using the Multidimensional Fatigue Inventory (MFI) [Smets et al. 1995], the fatigue items of the FIQ (‘How tired have you been?’ and ‘How have you felt when you get up in the morning?’) [Burckhardt et al. 1991], and the energy/vitality domain of the SF-36 health survey. A pooled analysis of MFI total scores indicated that milnacipran significantly improved fatigue by Week 3 (i.e. first study visit), with milnacipran-treated patients demonstrating approximately 65—70% improvements over placebo (p< 0.001, MMRM) [Clauw et al. 2008b]. Significant differences between milnacipran and placebo in MFI total scores were sustained over time (Figure 3). Other analyses have shown significant improvements with milnacipran over placebo on the MFI subscale scores (general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity) [Mease et al. 2009c; Thacker et al. 2009], the FIQ fatigue items [Mease et al. 2009c], and the SF-36 energy/vitality domain [Branco et al. 2010; Thacker et al. 2009]
Figure 3.
Least squares mean changes from baseline in Multidimensional Fatigue Inventory total scores. From pooled analysis (N=2084) of Studies 1 and 2 [Clauw et al. 2008b], mixed-effects model repeated measures. Visits correspond to 3 weeks (Visit 1), 7 weeks (Visit 2), 11 weeks (Visit 3), and 15 weeks (Visit 4) of double-blind treatment (including 3 weeks of dose escalation and 12 weeks of stable dose treatment). *p < 0.05; **p ≤ 0.01; ***p < 0.001 versus placebo.
Cognitive dysfunction
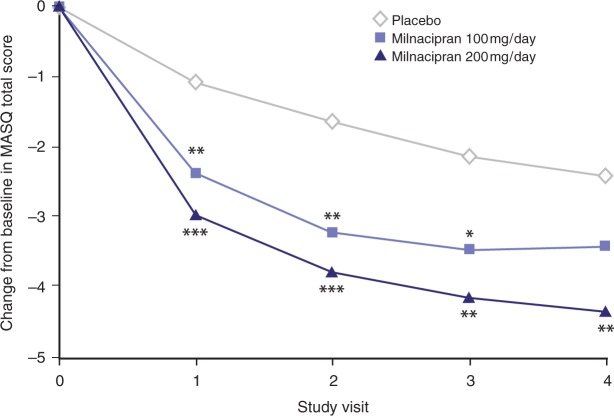
Cognitive dysfunction, sometimes referred to as ‘fibrofog’, is a common complaint among patients with FM [Glass, 2008]. One study reported cognitive impairment in 83% of patients with FM, compared with 30% of patients with other rheumatic disorders [Katz et al. 2004]. This impairment includes increased susceptibility to distraction and loss of memory performance [Glass, 2008; Katz et al. 2004]. In the milnacipran clinical studies, the Multiple Ability Self-Report Questionnaire (MASQ) [Seidenberg et al. 1994] was used to assess improvements in cognitive function. This measure encompasses five different cognitive domains: language ability, visual-perceptual ability, verbal memory, visual memory, and attention. In an analysis of pooled data from Studies 1, 2, and 3, LS mean changes from baseline in MASQ total scores for milnacipran 200mg/day and placebo were —4.3 and —2.4, respectively, indicating significant improvements in cognitive function in patients with FM at 3 months (p = 0.004, MMRM) (Figure 4) [Manevitz et al. 2009]. Significant differences between milnacipran 200 mg/day and placebo were also observed at all study visits for the MASQ attention and verbal memory domains (p < 0.05).
Figure 4.
Least squares mean changes from baseline in Multiple Ability Self-Report Questionnaire total scores. From pooled analysis (A/=3109) of Studies 1, 2, and 3 [Manevitz et al. 2009], mixed-effects model repeated measures. Visits correspond to 0 weeks (Visit 1), 4 weeks (Visit 2), 8 weeks (Visit 3), and 12 weeks (Visit 4) of double-blind stable dose treatment. *p < 0.05; **p≤ 0.01; ***p ≤ 0.001 versus placebo.
Durability of response
The long-term efficacy (up to 15 months) of milnacipran in patients with FM has been investigated in randomized, double-blind, extension studies for Study 1 [Goldenberg et al. 2010], Study 2 [Ferrera et al. 2009], and Study 4 [Branco et al. 2009]. Patients who had received placebo during the lead-in studies were re-randomized to receive milnacipran (100 mg/day, 150 mg/day, or 200 mg/day) during the extension studies. Patients who had received milnacipran 200 mg/day in the lead-in studies were maintained on this dosage during the extension studies. Patients who had received milnacipran 100 mg/day either maintained their dosage or were re-randomized to receive milnacipran 200 mg/day during the extension studies.
Patients receiving ≤1 year continuous milnacipran treatment in the extension studies demonstrated durable improvements in pain. In the extension to Study 1, for example, patients who received milnacipran 200 mg/day for 12 months (n = 209) had similar improvements in pain scores at 6 months (end of placebo-controlled lead-in study) and at 12 months (end of extension study) [Goldenberg et al. 2010]. In these patients, mean changes from baseline pain VAS weekly recall scores were —32.5 and —35.1 at 6 and 12 months, respectively. Similarly, durable results were observed for pain, FIQ, PGIC, and other efficacy measures in all three of the extension studies. Results in patients who were re-randomized from milnacipran 100 to 200 mg/day (n = 92) demonstrated an additional 7.1% improvement in pain after 6 months at the higher dosage [Goldenberg et al. 2010], suggesting that some patients with FM may benefit from receiving milnacipran 200 mg/day.
Cost and quality-of-life considerations
The net effect of the multiple FM symptoms discussed above is that many patients with this disorder report a reduced quality of life, with symptoms such as pain and fatigue interfering with their ability to work, enjoy social activities, and perform regular daily tasks [Hoffman and Dukes, 2008; Mease, 2005]. The quality of life deficit in patients with FM is generally considered to be greater than is observed in many other chronic illnesses, such as rheumatoid arthritis [Hoffman and Dukes, 2008]. In addition to having a negative impact on the lives of individual patients, FM represents a societal burden in terms of healthcare and disability costs.
Recent utilization studies estimate the total healthcare cost per FM patient to be approximately US$10,000 per year [Blum et al. 2009; Silverman et al. 2009; White et al. 2008]. Moreover, it has been estimated that patients with FM miss 29.8 working days per year, which is significantly more days than found in matched controls (10.4 days, p < 0.0001) or patients with osteoarthritis (25.7 days, p < 0.0001) [White et al. 2008]. The impact of FM on healthcare and disability costs was confirmed by a recent analysis of claims from patients in a primary care setting [Sicras-Mainar et al. 2009]. An important finding of this study was that poorer patient-reported health status and quality of life, as measured by the FIQ and European quality of life (EQ-5D), correlated significantly with higher costs (FIQ, p< 0.001; EQ-5D, p<0.05). These results suggest that medications which improve the multiple symptom domains of FM might also improve quality of life for these patients and reduce the cost burden.
Although the actual cost benefits of milnacipran have not yet been evaluated, significant improvements with milnacipran versus placebo have been observed in several quality of life measures, including the FIQ and SF-36. The FIQ measures the overall impact of FM on many symptoms, including pain, fatigue, physical function, sleep disturbance, and psychological distress [Burckhardt et al. 1991]. In the European clinical study (Study 4), which investigated the efficacy of milnacipran 200mg/day versus placebo and included FIQ total score as a coprimary end-point, the LS mean changes from baseline FIQ total score for milnacipran 200mg/day and placebo were —14.18 and —11.18, respectively, representing significant improvements with active treatment over placebo (p = 0.015, LOCF) [Branco et al. 2010]. Significant differences between milnacipran and placebo in FIQ total score were also found in Studies 2 and 3 [Arnold et al. 2009a; Mease et al. 2009b; Clauw et al. 2008a]. These SF-36 and FIQ results indicate that milnacipran improves physical, mental, and social function, potentially leading to an improved quality of life.
The SF-36 measures 8 domains from which the PCS score (described above) and Mental Component Summary (MCS) score are derived [Ware, 2000]. In all US studies, significant improvements in SF-36 MCS scores were observed with milnacipran 100 or 200mg/day versus placebo [Branco et al. 2010; Arnold et al. 2009a; Clauw et al. 2008a] (all p < 0.05, LOCF). Significant improvements over placebo were also observed for milnacipran 100 and 200 mg/day in all eight domains of the SF-36 [Branco et al. 2010] and [data on file].
Safety
Milnacipran was generally well tolerated at daily doses of 100 and 200 mg in clinical studies of patients with FM [Branco et al. 2010; Arnold et al. 2009a; Mease et al. 2009b; Clauw et al. 2008a]. In a pooled safety analysis that included 2209 patients (milnacipran, w=1557; placebo, n = 652) from Studies 1 and 2 [Mease et al. 2009b; Clauw et al. 2008a] and the milnacipran phase 2 study in patients with FM [Gendreau et al. 2005], discontinuation due to adverse events (AEs) occurred in 23% and 26% of patients receiving milnacipran 100 and 200 mg/day, respectively, compared with 12% of patients receiving placebo [Gendreau et al. 2008]. The only AEs that led to the premature discontinuation of therapy in ≤2% of milnacipran recipients and at a higher incidence then placebo were nausea (100 mg/day, 4%; 200 mg/day, 7%; placebo, 0.6%), palpitations (100 mg/day, 3%; 200 mg/day, 3%; placebo, 0.6%), 450and headache (100 mg/day, 2%; 200mg/day, 2%; placebo, 0.2%). Rates of discontinuation due to AEs were comparatively lower in Study 3 (100 mg/day, 18%; placebo, 14%). The lower placebo-corrected rates of discontinuation in Study 3 (4% versus 11—14% in previous studies) may be related to the slower, more flexible dose escalation period used in this study compared with the other milnacipran studies [Arnolds al. 2009a]. The lower rate of discontinuation due to AEs observed in Study 3 is consistent with anecdotal evidence from the clinic that more gradual and flexible dose escalation of milnacipran may improve tolerability.
The most common treatment emergent AE (TEAE) in all treatment groups was nausea (pooled safety analysis, placebo-corrected rates: 100 mg/day, 15%; 200 mg/day, 20%), which tended to be mild to moderate in severity [Gendreau et al. 2008]. Although nausea incidence rates for placebo and milnacipran treatment groups tended to vary by study, placebo-corrected rates are similar across studies. In all of the studies, patients were advised to take the medication with food, a recommendation that tends to lessen nausea associated with use of milnacipran. Pooled analysis of Studies 1, 2, and 3 demonstrates that approximately 70% of nausea episodes resolved within 3 weeks after onset [data on file]. Additional TEAEs occurring in ≤5% of milnacipran-treated patients and at a rate twice that of placebo include constipation, hot flush, hyperhidrosis, palpitations, vomiting, heart rate increased, dry mouth, and hypertension [Gendreau et al. 2008]. The profiles of newly emergent AEs (NEAEs) in long-term extension studies were similar to the TEAEs observed in the lead-in studies (i.e. Studies 1 and 2); most TEAEs and NEAEs were mild to moderate in severity [Goldenberg et al. 2010; Ferrera et al. 2009; Gendreau et al. 2008] and [data on file]. In the pooled safety analysis, the incidence of serious AEs did not differ among treatment groups (100 mg/day, 2%; 200 mg/day, 2%; placebo, 3%) [Gendreau et al. 2008], and prolonged exposure to milnacipran (i.e. up to 15 months) did not result in any new safety concerns [Goldenberg et al. 2010; Ferrera et al. 2009].
As has been reported for other medications commonly used to treat FM, including TCAs [Glassman, 1984] and SNRIs [Stahl et al. 2005], changes in heart rate and blood pressure have been observed in patients treated with milnacipran [Gendreau et al. 2008]. In the pooled safety analysis, mean increases in systolic blood pressure (SBP) and diastolic blood pressure (DBP) (up to 3.1mmHg) and heart rate (approximately 7—8 beats per minute [bpm]) were observed after 3 months of treatment with milnacipran [Forest Laboratories, 2009; Gendreau et al. 2008]. Similar increases in blood pressure and heart rate were observed in Studies 3 [Arnold et al. 2009a] and 4 [Branco et al. 2010]. Among FM patients who were nonhypertensive at baseline, approximately twice as many milnacipran-treated patients became hypertensive at the end of the study (SBP ≤140mmHg or DBP ≤90mmHg) compared with patients receiving placebo (100 mg/day, 20%; 200 mg/day, 17%; placebo, 7%) [Forest Laboratories, 2009]. Among patients who met SBP criteria for prehypertension at baseline (SBP of 120 to 139 mmHg), more patients in the milnacipran treatment groups became hypertensive at the end of the study as compared with placebo (lOOmg/day, 14%; 200mg/day, 14%; placebo, 9%).
Changes of clinical interest in blood pressure (i.e. SBP >140mmHg, SBP > 15 mmHg from baseline, DBP <90mmHg, or DBP ≤ 10 mmHg from baseline) occurred in 7% to 15% of patients receiving milnacipran 100 mg/day or 200 mg/day, compared with 3—8% of placebo-treated patients [data on file]. Changes of clinical interest in heart rate (i.e. <100bpm or ≤20bpm increase from baseline) occurred in 9—10% of milnacipran-treated patients, compared with 0.5% in placebo-treated patients [data on file]. Sustained increases in SBP (≤ 15 mmHg increase from baseline for three consecutive visits) and DBP (≤ 10 mmHg increase from baseline for 3 consecutive visits) occurred more frequently with milnacipran versus placebo (SBP: 100 mg/day, 9%; 200 mg/day, 6%; placebo, 2%; DBP: 100mg/day, 13%; 200 mg/day, 10%; placebo, 4%) [Forest Laboratories, 2009]. Sustained increases in heart rate (≤100bpm or ≤20bpm increase from baseline for three consecutive visits) were also more common with milnacipran versus placebo (100 mg/day, 8%; 200 mg/day, 10%; placebo, 0.2%) [data on file]. Potentially clinically significant changes in vital signs (i.e. increases in supine SBP to ≤ 180 mmHg with a ≤ 20 mmHg increase from baseline, increases in DBP to ≤ 110 mmHg with a ≤ 15 mmHg increase from baseline, and increases in heart rate t+o >120bpm with a >20bpm increase from baseline) were uncommon, with each occurring in less than 1% of milnacipran-treated patients [Gendreau et al. 2008].
In a study that monitored 24-hour ambulatory blood pressure and heart rate, normotensive and hypertensive patients with FM received placebo (w=lll) or milnacipran (w = 210) titrated to 100 mg/day (for 3 weeks) and then to 200 mg/day (for 2 weeks) over a 7-week double-blind period [ClinicalTrials.gov, 2009]. At the end of the double-blind period, mean ambulatory vital sign changes with milnacipran were slightly higher than observed in the efficacy studies (where measurements were made at rest), but outlier analyses showed that categorical shifts in hypertensive status were similar to those observed in the milnacipran FM clinical studies [data on file]. A thorough QT/QTc study, conducted to investigate the effect of milnacipran at a dosage of 600 mg/day (i.e. 3—6 times the recommended milnacipran dosage) on cardiac repolarization, indicated that milnacipran would not significantly affect cardiac repolarization at clinically relevant therapeutic and supratherapeu-tic concentrations [Periclou et al. 2010].
In the milnacipran studies, there were few changes in clinical laboratory parameters of potential clinical concern. Some patients had mild elevations (i.e. 1—3 times the upper limit of normal [ULN]) in alanine transaminase (ALT) levels (100 mg/day, 6%; 200 mg/day, 7%; placebo, 3%) and aspartate transaminase (AST) levels (100 mg/day, 3%; 200 mg/day, 5%; placebo, 2%) [Forest Laboratories, 2009]. However, no clinically significant increases in bilirubin were observed, and no patients were found to have ALT >3 times the ULN associated with bilirubin ≤2 times the ULN. Thus, no apparent clinically relevant differences among treatment groups in hematology, urinalysis, or other clinical laboratory parameters were detected [Gendreau et al. 2008].
In both the US and European milnacipran studies, most patients (>75% and 58%, respectively) were overweight or obese at baseline [Arnold et al. 2009b] and [data on file], an observation that is consistent with reports that more patients with FM are overweight or obese compared with the general population [Bennett et al. 2007]. In each of the US studies, milnacipran-treated patients lost approximately 1kg (—0.8 to —1.1kg; —1.8 to —2.41b) compared with approximately 0 kg (—0.3 to 0.4 kg; —0.6 to 0.9 lb) in placebo-treated patients (p< 0.05) [Arnold et al. 2009b]. Similar results were demonstrated in milnacipran-treated patients who were overweight or obese at baseline (p < 0.05, OC) as well as in patients exposed to milnacipran for up to 15 months [Goldenberg et al. 2010] and [data on file]. The incidence of nausea in milnacipran-treated patients who lost weight was not significantly different from those who did not lose weight (p < 0.05) [Arnold et al. 2009b], suggesting that nausea is unlikely to account for the weight loss. Because many patients with FM are overweight or obese, such concurrent comorbidities should be considered in the selection of FM pharmacotherapies, and the negligible effects of milnacipran on body weight may positively influence compliance in the FM patient population.
Conclusions
Milnacipran is an SNRI, with a preferential effect on norepinephrine over serotonin reuptake. It was approved in the United States for the management of FM in 2009. Milnacipran is rapidly absorbed in humans, undergoes minimal hepatic metabolism, has little propensity for pharmacokinetic drug interactions, and is excreted in the urine, mainly unchanged or as the glucuronide conjugate, with a plasma half-life of 6—8 hours.
Milnacipran has been studied in four phase 3 clinical studies with remarkably consistent results. In all four studies, milnacipran resulted in statistically significant increases in the proportions of composite responders when compared with placebo. Composite responders were defined as patients simultaneously meeting the criteria for clinically significant improvements in pain (≤30% improvement in VAS 24-hour recall pain) and patient global status (‘much improved’ or ‘very much improved’), combined into a single endpoint. A second primary endpoint added the requirement for a simultaneous clinically significant change in function—an important consequence of impairment in the various domains affected in FM. While changes in these other domains are reflected, presumably, in the overall global evaluation of the patient, additional analyses confirmed that other important domains, such as fatigue and cognition, are also improved with milnacipran.
Composite responder analyses, which were the primary endpoints in the milnacipran clinical studies of FM, are important for evaluating syndromes with multiple integral symptom domains. They have found utility in evaluating diseases such as rheumatoid arthritis, and now FM. Responder analyses ensure that statistical differences among treatment groups in a clinical study represent a clinically meaningful response for the individual responders, and that the actual responder rates simply reflect the extent of the drug effect in a population rather than the clinical significance of the response. Post hoc analyses of the milnacipran clinical study data confirm the clinical impression that some patients have rather dramatic improvement, while others have lesser effects.
The inclusion of secondary efficacy analyses has also confirmed beneficial effects of milnacipran on the FM-associated domains of fatigue and cognition. Instruments used to assess these domains (i.e. MFI and MASQ) are less well established, and quantitative changes in response to milnacipran were less consistent from study to study; nevertheless, pooled analyses clearly demonstrated an independent effect of milnacipran on these domains. Similarly, milnacipran resulted in improvements in mood, although path analyses have confirmed that the effects of milnacipran on other symptoms of FM, such as pain, are not a result of changes in mood.
The safety profile of milnacipran has been well established by clinical studies in FM and by post-marketing experience with this drug for the treatment of major depression outside of the United States. The side effects associated with milnacipran use are generally mild and not serious. Overdose experience has been relatively benign, possibly due to the emetogenic effect of large doses. The most troublesome side effect is nausea, which occurs in 10—20% of patients treated with milnacipran (corrected for placebo rates); clinical experience suggests that slow uptitration, guided by clinical response, is most likely to result in patients continuing on treatment. Other side effects commonly seen include constipation, hot flush, hyperhidrosis, palpitations, vomiting, heart rate increased, dry mouth, and hypertension. In total, side effects resulted in the discontinuation of approximately 6% of patients (corrected for placebo discontinuations) from FM clinical studies.
From a clinical perspective, the most important side effect is the increase in blood pressure and heart rate seen in some but not all patients. This usually occurs early during treatment, but can occur later; moreover, these changes can be marked, requiring prompt medical attention. However, changes in vital signs are not necessarily consistent or predictable. Therefore, monitoring of patients receiving milnacipran is mandatory prior to and throughout treatment, and increases should be treated appropriately. Although QT prolongation may occur, the increase is not considered clinically relevant. Mild elevations of liver enzymes can occur but have not been associated with clinically important liver injury in the clinical studies. In addition, suicidal ideation, which is a concern for this class of drugs, has been infrequent (based primarily on experience in treating major depression). Of course, other known and unknown possible side effects, as described in the Prescribing Information, should be considered when prescribing milnacipran [Forest Laboratories, 2009].
Milnacipran represents a new addition to the group of drugs shown to be useful in the management of FM. There are, as yet, no controlled clinical studies comparing the safety and efficacy of milnacipran with that of other drugs used in FM, and studies looking at adding milnacipran to patients already receiving a drug with a different mechanism of action should be of interest.
Acknowledgements
The authors would like to thank Prescott Medical Communications Group (Chicago, IL) for providing medical writing assistance. This work was supported by Forest Laboratories, Inc., New York, NY, USA.
Footnotes
Dr Robert Palmer is a Senior Medical Director at Forest Research Institute, a subsidiary of Forest Laboratories, Inc., and a shareholder in Forest Laboratories, which is one of the companies involved in the development of milnacipran for the management of fibromyalgia. Dr Antonia Periclou and Dr Pradeep Banerjee are employees in the Clinical Pharmacology and Drug Dynamics and Pharmacology departments, respectively, at Forest Research Institute and also hold stock Forest Laboratories, Inc.
References
- Arnold L.M., Crofford L.J., Mease P.J., Burgess S.M., Palmer S.C., Abetz L., et al. (2008) Patient perspectives on the impact of fibromyalgia. Patient Educ Couns 73: 114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L.M., Gendreau R.M., Spera A., Gendreau J., Wang Y. (2009a) Milnacipran 100 mg/day in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial [poster]. In: Proceedings of the American College of Rheumatology 73rd Annual Scientific Meeting, Vol. 60, 16–21 October 2009, Philadelphia, PA. [Google Scholar]
- Arnold L.M., Palmer R.H., Hufford M.R., Chen W. (2009b) Milnacipran's effect on body weight by baseline BMI: results across 3 randomized, double-blind, placebo-controlled fibromyalgia trials [abstract]. Arthritis Rheum 60(10 Suppl): S532 [Google Scholar]
- Assie M.B., Charveron M., Palmier C, Puozzo C, Moret C., Briley M. (1992) Effects of prolonged administration of milnacipran, a new antidepressant, on receptors and monoamine uptake in the brain of the rat. Neuropharmacology 31: 149–155 [DOI] [PubMed] [Google Scholar]
- Bardin L., Gregoire S., Aliaga M., Malfetes N., Vitton O., Ladure P., et al. (2010) Comparison of milnacipran, duloxetine and pregabalin in the formalin pain test and in a model of stress-induced ultrasonic vocalizations in rats. Neurosci Res 66: 135–140 [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Garbin M.G. (1988) Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 8: 77–100 [Google Scholar]
- Bennett R.M. (2009) Clinical manifestations and diagnosis of fibromyalgia. Rheum Dis Clin North Am 35: 215–232 [DOI] [PubMed] [Google Scholar]
- Bennett R.M., Bushmakin A.G., Cappelleri J.C., Zlateva G., Sadosky A.B. (2009) Minimal clinically important difference in the fibromyalgia impact questionnaire. Rheumatol 36: 1304–1311 [DOI] [PubMed] [Google Scholar]
- Bennett R.M., Jones J., Turk D.C., Russell I.J., Matallana L. (2007) An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R.M., Schein J., Kosinski M.R., Hewitt D.J., Jordan D.M., Rosenthal N.R. (2005) Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis Rheum 53: 519–527 [DOI] [PubMed] [Google Scholar]
- Bingham C.O., III, Bird S.R., Smugar S.S., Xu X., Tershakovec A.M. (2008) Responder analysis and correlation of outcome measures: pooled results from two identical studies comparing etoricoxib, celecoxib, and placebo in osteoarthritis. Osteoarthritis Cartilage 16: 1289–1293 [DOI] [PubMed] [Google Scholar]
- Blum S.I., Juday T., Erder M.H. (2009) Economic burden of fibromyalgia compared to other chronic conditions [abstract]. Value Health 12: A135 [Google Scholar]
- Bradley L.A. (2009) Pathophysiology of fibromyalgia. Am J Med 122(12 Suppl): S22—S30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco J.C., Cherin P., Spath M., Mainguy Y. (2009) Long-term therapeutic response to milnacipran treatment for fibromyalgia. A European 1-year extension study following a 3-month study [abstract]. Arthritis Rheum 60(10 Suppl): S529. [DOI] [PubMed] [Google Scholar]
- Branco J.C., Zachrisson O., Perrot S., Mainguy Y. (2010) A European, multicenter, randomized, double-blind, placebo-controlled monotherapy clinical trial of milnacipran in the treatment of fibromyalgia. J Rheumatol 37: 851–859 [DOI] [PubMed] [Google Scholar]
- Briand L.A., Gritton H., Howe W.M., Young D.A., Sarter M. (2007) Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol 83: 69–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley M., Prost J.F., Moret C. (1996) Preclinical pharmacology of milnacipran. Int Clin Psychopharmacol 11(Suppl 4): 9–14 [DOI] [PubMed] [Google Scholar]
- Burckhardt C.S., Clark S.R., Bennett R.M. (1991) The Fibromyalgia Impact Questionnaire: development and validation. J Rheumatol 18: 728–733 [PubMed] [Google Scholar]
- Chakrabarty S., Zoorob R. (2007) Fibromyalgia., Am Fam Physician 76: 247–254 [PubMed] [Google Scholar]
- Chwieduk CM, McCormack P.L. (2010) Milnacipran: in fibromyalgia. Drugs 70: 99–108 [DOI] [PubMed] [Google Scholar]
- Clauw D.J. (2005) The taxonomy of chronic pain: moving toward more mechanistic classifications, In: Wallace D.J., Clauw D.J. (eds), Fibromyalgia and Other Central Pain Syndromes, Lippincott Williams & Williams: Philadelphia, PA, pp. 9–16 [Google Scholar]
- Clauw D.J. (2008) Pharmacotherapy for patients with fibromyalgia. J Clin Psychiatry 69(Suppl 2): 25–29 [PubMed] [Google Scholar]
- Clauw D.J., Mease P., Palmer R.H., Gendreau R.M., Wang Y. (2008a) Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial (published correction appears in Clin Ther 31: 446). Clin Ther 30: 1988–2004 [DOI] [PubMed] [Google Scholar]
- Clauw D.J., Palmer R.H., Hufford M.R., Zablocki R., Wang Y. (2008b) Milnacipran improves fatigue in patients with fibromyalgia: results from two randomized, double-blind, placebo-controlled trials [poster]. In: Proceedings of the American College of Rheumatology 72nd Annual Scientific Meeting, Vol. 58, 24–29 October 2008, San Francisco, CA. [Google Scholar]
- ClinicalTrials.gov (2009) A study of milnacipran in patients with fibromyalgia: effects on 24 hour ambulatory blood pressure monitoring [clinical trial]. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00618956 (accessed 11 February 2010).
- Culos-Reed S.N., Brawley L.R. (2000) Fibromyalgia, physical activity, and daily functioning: the importance of efficacy and health-related quality of life. Arthritis Care Res 13: 343–351 [DOI] [PubMed] [Google Scholar]
- Dubner R., Hargreaves K.M. (1989) The neurobiology of pain and its modulation. Clin J Pain 5(Suppl 2): S1—S4, discussion S4–S6. [DOI] [PubMed] [Google Scholar]
- Dworkin R.H., Turk D.C., Farrar J.T., Haythornthwaite J.A., Jensen M.P., Katz N.P., et al. (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113: 9–19 [DOI] [PubMed] [Google Scholar]
- Dworkin R.H., Turk D.C., Wyrwich K.W, Beaton D., Cleeland C.S., Farrar J.T, et al. (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9: 105–121 [DOI] [PubMed] [Google Scholar]
- Farrar J.T, Young J.P., Jr, LaMoreaux L., Werth J.L., Poole R.M. (2001) Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94: 149–158 [DOI] [PubMed] [Google Scholar]
- Ferrera R., Palmer R., Chen W, Gendreau R. (2009) Improvements in fibromyalgia symptoms are sustained for 1 year with milnacipran treatment: results from 2 double-blind, dose-controlled extension studies [abstract]. J Pain 10(4 Suppl 1): S60 [Google Scholar]
- Forest Laboratories Inc. (2009) Savella prescribing information, St. Louis, Missouri. Available at: http://www.frx.com/pi/Savella_pi.pdf (accessed 11 February 2010).
- Geisser M.E., Clauw D.J., Strand V., Gendreau R.M., Palmer R.H., Williams D.A. (2010) Contributions of change in clinical status parameters to Patient Global Impression of Change (PGIC) scores among persons with fibromyalgia treated with milnacipran. Pain 149: 373–378 [DOI] [PubMed] [Google Scholar]
- Gendreau J., Palmer R.H., Thacker K. (2008) Milnacipran is safe and well tolerated in the treatment of fibromyalgia syndrome [poster]. In: Proceedings of the 28th Annual Scientific Meeting of the American Pain Society, 8–10 May 2008, Tampa, FL.
- Gendreau R.M., Thorn M.D., Gendreau J.F., Kranzler J.D., Ribeiro S., Gracely R.H., et al. (2005) Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol 32: 1975–1985 [PubMed] [Google Scholar]
- Ghahramani P., Periclou A. (2009) Population pharmacokinetic analysis of milnacipran in fibromyalgia patients and healthy volunteers [poster]. In: Proceedings of the American Conference on Pharmacometrics, 4–7 October 2009, Mashantucket, CT.
- Glass J.M. (2008) Fibromyalgia and cognition. J Clin Psychiatry 69(Suppl 2): 20–24 [PubMed] [Google Scholar]
- Glassman A.H. (1984) Cardiovascular effects of tricyclic antidepressants. Annu Rev Med 35: 503–511 [DOI] [PubMed] [Google Scholar]
- Goldenberg D.L. (2009) Diagnosis and differential diagnosis of fibromyalgia. Am J Med 122(12 Suppl): S14—S21 [DOI] [PubMed] [Google Scholar]
- Goldenberg D.L., Burckhardt C., Crofford L. (2004) Management of fibromyalgia syndrome. JAMA 292: 2388–2395 [DOI] [PubMed] [Google Scholar]
- Goldenberg D.L., Clauw D.J., Palmer R.H., Mease P., Chen W, Gendreau R.M. (2010) Durability of therapeutic response to milnacipran treatment for fibromyalgia. Results of a randomized, double-blind, monotherapy 6-month extension study. Pain Med 11: 180–194 [DOI] [PubMed] [Google Scholar]
- Guy W. (1976) ECDEU assessment manual for psychopharmacology (DHEW Publication No. ADM 76–338). Washington, DC: US Government Printing Office [Google Scholar]
- Guymer E.K., Clauw D.J. (2002) Treatment of fatigue in fibromyalgia. Rheum Dis Clin North Am 28: 367–378 [DOI] [PubMed] [Google Scholar]
- Hoffman D.L., Dukes E.M. (2008) The health status burden of people with fibromyalgia: a review of studies that assessed health status with the SF-36 or the SF-12. Int J Clin Pract 62: 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Ishida Y, Naono R., Takeda R., Abe H., Nakamura T., et al. (2009) Effects of intrathecal administration of newer antidepressants on mechanical allodynia in rat models of neuropathic pain. Neurosci Res 63: 42–46 [DOI] [PubMed] [Google Scholar]
- Katz R.S., Heard A.R., Mills M., Leavitt F. (2004) The prevalence and clinical impact of reported cognitive difficulties (fibrofog) in patients with rheumatic disease with and without fibromyalgia. J Clin Rheumatol 10: 53–58 [DOI] [PubMed] [Google Scholar]
- King T., Rao S., Vanderah T., Chen Q., Vardanyan A., Porreca F. (2006) Differential blockade of nerve injury-induced shift in weight bearing and thermal and tactile ypersensitivity by milnacipran. J Pain 7: 513–520 [DOI] [PubMed] [Google Scholar]
- Kitaichi Y., Inoue T., Izumi T., Nakagawa S., Tanaka T., Masui T., et al. (2008) Effect of co-administration of a serotonin-noradrenaline reuptake inhibitor and a dopamine agonist on extracellular monoamine concentrations in rats. Eur J Pharmacol 584: 285–290 [DOI] [PubMed] [Google Scholar]
- Manevitz A., Palmer R.H., Chen W., Gendreau R.M. (2009) The effects of milnacipran on self-reported complaints of decreased cognitive functioning in fibromyalgia patients [poster]. In: Proceedings of the American College of Rheumatology 73rd Annual Scientific Meeting, Vol. 60, 16–21 October 2009, Philadelphia, PA. [Google Scholar]
- Matsumoto M., Tachibana K., Togashi H., Tahara K., Kojima T., Yamaguchi T., et al. (2005) Chronic treatment with milnacipran reverses the impairment of synaptic plasticity induced by conditioned fear stress. Psychopharmacology (Ber) 179: 606–612 [DOI] [PubMed] [Google Scholar]
- Matsuzawa-Yanagida K., Narita M., Nakajima M., Kuzumaki N., Niikura K., Nozaki H., et al. (2008) Usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology 33: 1952–1965 [DOI] [PubMed] [Google Scholar]
- Mease P. (2005) Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol 32(Suppl 75): 6–21 [PubMed] [Google Scholar]
- Mease P. (2009) Further strategies for treating fibromyalgia: the role of serotonin and norepinephrine reuptake inhibitors. Am J Med 122(12 Suppl): S44—S55 [DOI] [PubMed] [Google Scholar]
- Mease P., Arnold L., Bennett R., Boonen A., Buskila D., Carville S., et al. (2007) Fibromyalgia syndrome. J Rheumatol 34: 1415–1425 [PubMed] [Google Scholar]
- Mease P., Arnold L., Choy E., Clauw D., Crofford L., Glass J., et al. (2009a) Fibromyalgia syndrome module at OMERACT 9: domain construct. J Rheumatol 36: 2318–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P., Clauw D., Gendreau R., Rao S., Kranzler J., Chen W., et al. (2009b) The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial (published correction appears in J Rheumatol 36: 661). J Rheumatol 36: 398–409 [DOI] [PubMed] [Google Scholar]
- Mease P., Palmer R., Wang Y., Gendreau R. (2009c) Milnacipran improves fatigue in fibromyalgia: pooled analyses from 3 randomized, placebo-controlled clinical trials [abstract]. Arthritis Rheum 60(10 Suppl): S532—S533 [Google Scholar]
- Mease P., Palmer R., Wang Y., Hufford M. (2009d) A day-to-day analysis of the analgesic efficacy of milnacipran in the treatment of fibromyalgia [abstract]. J Pain 10(4 Suppl): S60 [Google Scholar]
- Mochizuki D., Tsujita R., Yamada S., Kawasaki K., Otsuka Y, Hashimoto S., et al. (2002) Neurochemical and behavioural characterization of milnacipran, a serotonin and noradrenaline reuptake inhibitor in rats. Psychopharmacology (Berl) 162: 323–332 [DOI] [PubMed] [Google Scholar]
- Moojen V.K., Martins M.R., Reinke A., Feier G., Agostinho F.R., Cechin E.M., et al. (2006) Effects of milnacipran in animal models of anxiety and memory. Neurochem Res 31: 571–577 [DOI] [PubMed] [Google Scholar]
- Moret C., Briley M. (1997) Effects of milnacipran and pindolol on extracellular noradrenaline and serotonin levels in guinea pig hypothalamus. J Neurochem 69: 815–822 [DOI] [PubMed] [Google Scholar]
- Moret C., Charveron M., Finberg J.P., Couzinier J.P., Briley M. (1985) Biochemical profile of midalcipran (F 2207), 1-phenyl-l-diethyl-aminocarbonyl-2-aminomethyl-cyclopropane (Z) hydrochloride, a potential fourth generation antidepressant drug. Neuropharmacology 24: 1211–1219 [DOI] [PubMed] [Google Scholar]
- Nakagawa A., Watanabe N., Omori I.M., Barbui C, Cipriani A., McGuire H., et al. (2009) Milnacipran versus other antidepressive agents for depression. Cochrane Database SystRev 3: CD006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H., Saito S., Koizuka S., Nishikawa K., Goto F. (2005) The monoamine-mediated antiallodynic effects of intrathecally administered milnacipran, a serotonin noradrenaline reuptake inhibitor, in a rat model of neuropathic pain. Anesth Analg 100: 1406–1410 [DOI] [PubMed] [Google Scholar]
- Palmer R.H., Clauw D.J., Mainguy Y, Wang Y, Gendreau R.M. (2009) Baseline characteristics of fibromyalgia patients in 4 clinical trials of milnacipran [abstract]. Arthritis Rheum 60(10 Suppl): S527 [Google Scholar]
- Paris B.L., Ogilvie B.W, Scheinkoenig J.A., Ndikum-Moffor F., Gibson R., Parkinson A. (2009) In vitro inhibition and induction of human liver cytochrome p450 enzymes by milnacipran. Drug Metab Dispos 37: 2045–2054 [DOI] [PubMed] [Google Scholar]
- Periclou A., Coutts S.M., Rao S.R., Palmer R.H., Thacker K., Trugman J. (2009) Milnacipran has low potential for drug-drug interactions [abstract]. Neurology 72(Suppl 3): A212 [Google Scholar]
- Periclou A., Palmer R.H., Zheng H., Lindamood C. (2010) Effects of milnacipran on cardiac repolarization in healthy participants. J Clin Pharmacol 50: 422–433 [DOI] [PubMed] [Google Scholar]
- Puozzo C.j, Albin H., Vincon G., Deprez D., Raymond J.M., Amouretti M. (1998a) Pharmacokinetics of milnacipran in liver impairment. Eur J Drug Metab Pharmacokinet 23: 273–279 [DOI] [PubMed] [Google Scholar]
- Puozzo C.j, Lens S., Reh C, Michaelis K., Rosillon D., Deroubaix X., et al. (2005) Lack of interaction of milnacipran with the cytochrome p450 isoenzymes frequently involved in the metabolism of antidepressants. Clin Pharmacokinet 44: 977–988 [DOI] [PubMed] [Google Scholar]
- Puozzo C., Leonard B.E. (1996) Pharmacokinetics of milnacipran in comparison with other antidepressants. Int Clin Psychopharmacol 11(Suppl 4): 15–27 [DOI] [PubMed] [Google Scholar]
- Puozzo C., Panconi E., Deprez D. (2002) Pharmacology and pharmacokinetics of milnacipran. Int Clin Psychopharmacol 17(Suppl 1): S25—S35 [DOI] [PubMed] [Google Scholar]
- Puozzo C., Pozet N., Deprez D., Baille P., Ung H.L., Zech P. (1998b) Pharmacokinetics of milnacipran in renal impairment. Eur J Drug Metab Pharmacokinet 23: 280–286 [DOI] [PubMed] [Google Scholar]
- Puozzo C., Rostin M., Montastruc J.L., Houin G. (1987) Absolute bioavailability study of midalcipran (F 2207) in volunteers [paper]. In: Proceedings of the Third European Congress of Biopharmaceutics and Pharmacokinetics, Freiburg, West Germany.
- Rao S.G., Trzaska Z.J., Kranzler J.D., Bilsky E.J. (2003) Milnacipran enhances performance in the morris water maze in BALB/C mice, an inbred mouse model of anxiety [abstract]. Biol Psychiatry 53(8 Suppl 1): S80 [Google Scholar]
- Russell I.J., Vaeroy H., Javors M., Nyberg F. (1992) Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum 35: 550–556 [DOI] [PubMed] [Google Scholar]
- Sara S.J. (2009) The locus coeruleus and noradren-ergic modulation of cognition. Nat Rev Neurosci 10: 211–223 [DOI] [PubMed] [Google Scholar]
- Saxe PA, Arnold L.A., Gendreau R.M., Spera A., Gendreau J., Wang Y. (2009a) A randomized, double-blind, placebo-controlled clinical trial of milnacipran 100mg/day for the management of fibromyalgia: results from a 2-week discontinuation phase. Arthritis Rheum 60(10 Suppl): S532 [Google Scholar]
- Saxe PA, Palmer R.H., Wang Y., Gendreau R.M. (2009b) Milnacipran improves physical function in patients with fibromyalgia: pooled results from 3 phase III trials [poster]. In: Proceedings of the American College of Rheumatology 73rd Annual Scientific Meeting, Vol. 60, 16–21 October 2009, Philadelphia, PA. [Google Scholar]
- Schwieterman WD. (2008) Issues in the design of new clinical trials for rheumatoid arthritis therapeutics. Nat Clin Pract Rheumatol 4: 641–648 [DOI] [PubMed] [Google Scholar]
- Seidenberg M., Haltiner A., Taylor M.A., Hermann B.B., Wyler A. (1994) Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol 16: 93–104 [DOI] [PubMed] [Google Scholar]
- Sicras-Mainar A., Rejas J., Navarro R., Blanca M., Morcillo A., Larios R., et al. (2009) Treating patients with fibromyalgia in primary care settings under routine medical practice: a claim database cost and burden of illness study. Arthritis Res Ther 11: R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui O., Hung H.M., O'Neill R. (2009) MMRM vs. LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. J Biopharm Stat 19: 227–246 [DOI] [PubMed] [Google Scholar]
- Silverman S., Dukes E.M., Johnston S.S., Brandenburg N.A., Sadosky A., Huse D.M. (2009) The economic burden of fibromyalgia: comparative analysis with rheumatoid arthritis. Curr Med Res Opin 25: 829–840 [DOI] [PubMed] [Google Scholar]
- Smets E.M., Garssen B., Bonke B., De Haes J.C. (1995) The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 39: 315–325 [DOI] [PubMed] [Google Scholar]
- Spencer CM, Wilde M.I. (1998) Milnacipran. A review of its use in depression. Drugs 56: 405–427 [DOI] [PubMed] [Google Scholar]
- Stahl S.M. (2009) Fibromyalgia—pathways and neurotransmitters. Hum Psychopharmacol 24(Suppl 1): S11—S17 [DOI] [PubMed] [Google Scholar]
- Stahl S.M., Grady M.M., Moret C., Briley M. (2005) SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr 10: 732–747 [DOI] [PubMed] [Google Scholar]
- Staud R., Rodriguez M.E. (2006) Mechanisms of disease: pain in fibromyalgia syndrome. Nat Clin Pract Rheumatol 2: 90–98 [DOI] [PubMed] [Google Scholar]
- Staud R., Spaeth M. (2008) Psychophysical and neurochemical abnormalities of pain processing in fibromyalgia. CNS Spectr 13(3 Suppl 5): 12–17 [DOI] [PubMed] [Google Scholar]
- Strand V., Singh JA. (2008) Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am f Manag Care 14: 234–254 [PubMed] [Google Scholar]
- Suarez-Roca H., Quintero L., Arcaya J.L., Maixner W, Rao S.G. (2006) Stress-induced muscle and cutaneous hyperalgesia: differential effect of milnacipran. Physiol Behav 88: 82–87 [DOI] [PubMed] [Google Scholar]
- Takeda R., Watanabe Y, Ikeda T, Abe H., Ebihara K., Matsuo H., et al. (2009) Analgesic effect of milnacipran is associated with c-Fos expression in the anterior cingulate cortex in the rat neuropathic pain model. Neurosci Res 64: 380–384 [DOI] [PubMed] [Google Scholar]
- Thacker K., Trugman J., Rao S., Wang Y. (2009) The effect of milnacipran on cognitive function and fatigue in fibromyalgia patients [abstract]. Neurology 72(Suppl 3): A213 [Google Scholar]
- USFDA (2009) U.S. Drug approval package, Savella (milnacipran HCl tablets). Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022256s000TOC.cfm2010).
- Vaishnavi S.N., Nemeroff C.B., Plott S.J., Rao S.G., Kranzler J., Owens M.J. (2004) Milnacipran: a comparative analysis of human monoamine uptake and transporter binding affinity. Biol Psychiatry 55: 320–322 [DOI] [PubMed] [Google Scholar]
- Ware J.E., Jr (2000) SF-36 health survey update. Spine 25: 3130–3139 [DOI] [PubMed] [Google Scholar]
- White L.A., Birnbaum H.G., Kaltenboeck A., Tang J., Mallett D., Robinson R.L. (2008) Employees with fibromyalgia: medical comorbidity, healthcare costs, and work loss. J Occup Environ Med 50: 13–24 [DOI] [PubMed] [Google Scholar]
- WHO (2000) Obesity: Preventing and Managing the Global Epidemic. Report of a WHO consultation (technical report). Geneva, Switzerland: World Health Organization; Available at: http://whqlibdoc.who.int/trs/WHO_TRS_894.pdf (accessed 10 February 2010). [PubMed] [Google Scholar]
- Williams D.A., Gendreau R.M., Clauw D.J. (2007) Electronic diaries have superior discrimination compared to paper-based assessment in individuals with fibromyalgia [abstract]. Arthritis Rheum 56(9 Suppl): S607 [Google Scholar]
- Williams D.A., Gendreau R.M., Clauw D.J.(2008) A comparison between electronic diaries and paper-based pain assessment in individuals with fibromyalgia (FM) [abstract]. J Pain 9(4 Suppl 2): 16 [Google Scholar]
- Wolfe F., Hawley D.J., Wilson K. (1996) The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol 23: 1407–1417 [PubMed] [Google Scholar]
- Wolfe F., Ross K., Anderson J., Russell I.J., Hebert L. (1995) The prevalence and characteristics of fibromyalgia in the general population. Arthritis Bombardier, C., Goldenberg, D.L. et al. (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33: 160–1722306288 [Google Scholar]
- Wong D.T., Bymaster F.P., Mayle D.A., Reid L.R., Krushinski J.H., Robertson D.W. (1993) LY248686, a new inhibitor of serotonin and norepinephrine uptake. Neuropsychopharmacology 8: 23–33 [DOI] [PubMed] [Google Scholar]
- Yokogawa F., Kiuchi Y, Ishikawa Y, Otsuka N., Masuda Y, Oguchi K., et al. (2002) An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Anesth Analg 95: 163–168 [DOI] [PubMed] [Google Scholar]
- Yunus M.B. (2005) Symptoms and signs of fibromyalgia syndrome: an overview. In: Wallace D.J., Clauw D.J. (eds), Fibromyalgia and Other Central Pain Syndromes. Philadelphia, PA: Lippincott Williams & Williams, pp. 125–132 [Google Scholar]
- Yunus M.B. (2008) Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 37: 339–352 [DOI] [PubMed] [Google Scholar]