Abstract
Background/objectives: This postmarketing surveillance study assessed the preference, satisfaction, usability, and tolerability of subcutaneous self-administration of a high-concentration (50 mg/ml) ready-to-use formulation of methotrexate (MTX) in patients with rheumatoid arthritis or psoriatic arthritis.
Methods: The study enrolled 403 patients with rheumatoid or psoriatic arthritis. The first injection was administered by the attending physician or nurse, followed by five self-administered injections at weekly intervals. The high-concentration formulation consisted of a prefilled syringe with MTX 50 mg/ml solution and a pre-attached needle. Questionnaires were used to document outcomes.
Results: The overall assessment was ‘very good’ and ‘good’ in 87.6% of the patients and in 92.8% of the physicians/study nurses. Availability and use of a pre-attached needle was considered as very advantageous and advantageous by 91.8% of the patients and 88.8% of the physicians/study nurses. A total of 96% of the patients described the feeling of the injection as comfortable or tolerable. Patients reported that self-administration led to a feeling of more independence (89.1%) and an improved quality of life (83.6%). A total of 109 patients reported previous self-administration of low-concentration MTX formulations; 94.5% of them stated that they would prefer the high-concentration MTX formulation in the future. The formulation was generally well tolerated. Physicians’ expectations concerning the benefit of switching to MTX self-administration was met in 92.8% of the patients. A total of 96.3% of the patients were considered suitable for subcutaneous self-administration of the MTX formulation.
Conclusions: The 50 mg/ml prefilled syringe appears to be a valuable treatment option for patients with rheumatoid and psoriatic arthritis in need of MTX. This is supported by the strong appreciation of the patients as well as their attending healthcare professionals for its convenience and tolerability. The results confirm the findings and experience from a clinical study performed in Germany in 2009, which showed that 93% of the patients prefer the 50 mg/ml prefilled syringe with a pre-attached needle.
Keywords: methotrexate, parenteral, postmarketing surveillance study, pre-attached needle, prefilled syringe, psoriatic arthritis, rheumatoid arthritis, self-administration, subcutaneous injection
Introduction
Methotrexate (MTX) has been established as a gold standard in the first-line medical treatment of rheumatoid arthritis (RA) [Ahern and Chandran, 1995; Sieper and Braun, 1996; Ward and Fries, 1998]. MTX is a disease-modifying antirheumatic drug that in the past has been mainly administered orally at doses between 7.5 and 30 mg once a week. Clinical data support the use of subcutaneously (SC) administered MTX that is well absorbed, well tolerated, and appears to solve some of the issues encountered with oral administration, e.g. variable absorption and saturation of the absorption mechanism with increasing doses [Balis et al. 1988]. A 24-week SC administration of MTX at a dose of 15 mg/week was significantly more effective than oral administration [Braun et al. 2008]. In comparison to intramuscular injection, SC administration has been shown to be better tolerated with improved usability [Brooks et al. 1990]. In addition, SC administration of MTX was associated with a significant reduction in gastrointestinal side effects compared with oral administration of the same MTX dose [Rutkowska-Sak et al. 2009].
In a 6-month, multicenter, prospective, randomized, double-blind, two-arm phase IV trial, SC and oral MTX administrations were compared in 384 patients with RA [Braun et al. 2008]. MTX doses were 15 mg/week either orally (two 7.5 mg tablets) or SC (prefilled syringe containing a medium-concentrated formulation of 10 mg/ml). Tolerability between treatments was similar. However, significantly more patients receiving SC MTX than with oral MTX showed ACR20 (78% versus 70%) and ACR70 (41% versus 33%) responses. Patients with a disease duration ≥12 months had even higher ACR20 response rates (89% for SC administration and 63% for oral).
In a pharmacokinetic study in 12 healthy male subjects, 15 mg of MTX was administered SC at concentrations of either 50 mg/ml or 10 mg/ml solution. Both concentrations were shown to be bioequivalent with regard to area under the curve (AUC; medac, data on file). However, the rate of absorption (C max) was higher after administration of the higher concentrated solution. Rate and extent of absorption after SC administration with the two solutions was similar for the metabolite 7hydroxy-MTX. Since both concentrations were bioequivalent, no difference in the efficacy and the safety of the two formulations was expected.
Two MTX formulations for SC use were tested in an open-label, comparative, within-patient controlled, multicenter study in 132 patients with RA [Müller-Ladner et al. 2010]. MTX treatment consisted of 20 mg/week administered as a medium-concentration formulation (2.0 ml of 10 mg/ml solution in prefilled syringe; separate needle) which was compared to a novel high-concentration formulation (0.4 ml of 50 mg/ml in prefilled syringe; pre-attached needle). Each treatment was given for 3 weeks. Questionnaires and visual analog scales were used to measure outcomes. The total smaller volume of administered drug and the improved usability of a pre-attached needle in combination with a smaller prefilled syringe resulted in preference of the patients for the high-concentration formulation. In addition, local tolerability was slightly better compared with the medium-concentration formulation. These assessments were confirmed by the attending healthcare professionals.
This postmarketing surveillance study assessed preference, satisfaction, and usability of SC self-administration of a high-concentration (50 mg/ml) ready-to-use MTX formulation (Metex®/Metoject® 50 mg/ml; medac GmbH, Wedel, Germany) in patients with RA or psoriatic arthritis.
Patients and methods
This postmarketing surveillance study (Anwen-dungsbeobachtung) was conducted between June 2009 and May 2010 in 52 outpatient rheumatology practices in Germany and enrolled patients with a diagnosis of RA or psoriatic arthritis. Decisions about medical treatment were exclusively made by the treating physicians. The physicians selected appropriate patients, i.e. patients suffering from RA or psoriatic arthritis, for whom MTX therapy (Metex®/Metoject® 50 mg/ml; medac GmbH, Wedel, Germany) was indicated. MTX was contained in a prefilled syringe that included a pre-attached needle. Physicians recorded patient history, previous and concomitant MTX therapy, and the Metex®/Metoject® 50 mg/ml dose administered. They also recorded physicians’/patients’ assessments of usability and preference as well as assessments of local tolerability by the patient at the respective visits. Adverse drug reactions were to be reported.
Table 1 summarizes questions and answers concerning patient-, physician-, and study-nurse-reported outcomes.
Table 1.
Preference, usability, and tolerability outcomes*.
| Patient-, physician-, and study-nurse-reported outcomes |
| Visibility of information ‘Dose strength of ready-to-use syringe’ printed on outer package Four categories were suggested: ‘very good’, ‘good’, ‘mediocre’, and ‘poor’. |
| Visibility of information ‘Application once a week’ printed on outer package Four categories were suggested: ‘very good’, ‘good’, ‘mediocre’, and ‘poor’. |
| Usability concerning (1) removal of ready-to-use syringe from package; (2) removal of the rubber stopper; (3) handling of syringe at time of prick; (4) handling of syringe during injection; and (5) easiness of depressing the syringe plunger Three categories were suggested: ‘very satisfactory’, ‘satisfactory’, ‘less satisfactory’. |
| Patient’s tolerability of the subcutaneous injection
Three categories were suggested: ‘comfortable’, ‘tolerable’, ‘intolerable’. |
| Occurrence of pain at injection site ‘Yes/no’; if ‘yes’: Three categories were suggested: ‘slight’, ‘moderate’, ‘severe’. |
| Overall assessment of therapy with MTX ready-to-use syringe at the end of a 5-week treatment Five categories were suggested: ‘very poor’, ‘poor’, ‘satisfactory’, ‘good’ and ‘very good’. |
| Syringe with or without pre-attached needle in patients with previous use of the low-concentration (10 mg/ml) MTX ready-to-use syringe
‘How do you like the pre-attached needle (small syringe) in comparison to the one which still has to be attached (large syringe)?’
Five categories were suggested: ‘great disadvantage’, ‘disadvantage’, ‘no difference’, ‘advantage’, and ‘great advantage’. |
| Physician- and study-nurse-reported outcomes |
| Patient’s suitability for subcutaneous self-injection with MTX ready-to-use syringe Three categories were suggested: ‘very suitable’, ‘suitable’, ‘less suitable’. |
| Expectations met for switch to self-administration of MTX ready-to-use syringe Five categories were suggested: ‘fully met’, ‘largely met’, ‘partly met’, ‘less met’ and ‘not met’. |
| Improved patient compliance expected with smaller injection volume of MTX ready-to-use syringe Two categories were suggested: ‘yes’, ‘no’. |
| Continuation of treatment with MTX ready-to-use syringe Two categories were suggested: ‘yes’, ‘no’. |
| Patient-reported outcomes |
| Effort needed for self-injection Three categories were suggested: ‘little’, ‘moderate’, ‘great’. |
| More patient independence through self-administration Three categories were suggested: ‘yes’, ‘no’, ‘not yet assessable at this time’. |
| Improved patient quality of life through self-administration Three categories were suggested: ‘yes’, ‘no’, ‘not yet assessable at this time’. |
| Patient-reported outcomes in patients with previous use of the low-concentration (10 mg/ml) MTX ready-to-use syringe |
| Smaller injection volume Five categories were suggested: ‘very unpleasant’, ‘unpleasant’, ‘no difference’, ‘pleasant’ and ‘very pleasant’. |
| Preference of smaller, high-concentration (50 mg/ml) versus larger, low-concentration (10 mg/ml) MTX ready-to-use syringe Two categories were suggested: ‘smaller syringe’, ‘larger syringe’. |
Original in German
Treatment duration was 5 weeks, during which patients received a total of six MTX injections administered subcutaneously. At baseline, the first MTX treatment was administered by the physician/nurse to instruct the patient. One week later (week 1), the patients self-injected the second MTX dose under the supervision of the physician/nurse. The next 3 MTX injections at week 2, 3, and 4 were performed by the patients at their homes. The sixth and last MTX injection was self-administered by the patient at the physician’s office (see Table 2). No diagnostic and therapeutic measures were requested for the conduct of the study. This postmarketing surveillance study did not require any additional diagnostic or therapeutic measures beyond usual standard medical procedures.
Table 2.
Study procedures.
| Start of therapy | Week 1 | Weeks 2, 3 and 4 | Week 5 |
|---|---|---|---|
| • Medical history | • Subcutaneous self-administration under supervision of physician/study nurse | • Subcutaneous self-administration at home | • Subcutaneous self-administration under supervision of physician/study nurse |
| • Instructions on subcutaneous self-administration | |||
| • Injection #1 | • Injection #2 | • Injections #3, #4 and #5 | • Injection #6 |
| • Documentation | • Documentation | • Documentation |
Since no statistical hypotheses were prespecified only descriptive statistical methods were employed and no confirmatory analyses of the data were performed. In accordance with confidentiality regulations, no data were recorded which could be directly assigned to patients.
The study was conducted in compliance with German Drug Law regulations (§ 67 Section 6) applicable to postmarketing surveillance studies.
Results
Demographics and baseline characteristics
A total of 71 physicians enrolled 403 patients into the study: 122 (30.3%) were men, 275 (68.2%) were women; information on gender was missing in 6 (1.5%) patients. Mean weight was 76.5 kg, median height 168.4 cm, and mean body mass index 27.8 kg/m2. A total of 310 (76.9%) patients had RA, 59 (14.6%) psoriatic arthritis, 28 (6.9%) other rheumatic diseases/arthritis, and no information was available for 6 (1.5%) patients. Of these, 221 (54.8%) patients had previously received MTX treatment at dosages ranging between 7.5 and 25 mg/week and a treatment duration for up to 23 years, and 92 (41.6%) of these patients had received MTX as subcutaneous injections. The most common reasons for a change to MTX self-administration were improved efficacy due to better bioavailability (43.0%) compared with previous treatments, improved usability (25.3%) and dislike to MTX tablets (13.6%). Table 3 summarizes demographics and baseline characteristics.
Table 3.
Demographics and baseline characteristics (n = 403).
| Gender, n (%) | |
| Men | 122 (30.3) |
| Woman | 275 (68.2) |
| Information missing | 6 (1.5) |
| Weight, mean ± SD (kg) | 76.5 ± 16.6 |
| Height, mean ± SD (cm) | 168.4 ± 11.1 |
| Body mass index, mean ± SD (kg/m2) | 27.8 ± 16.6 |
| Diagnosis of rheumatic disease, n (%) | |
| Rheumatoid arthritis | 310 (76.9) |
| Psoriatic arthritis | 59 (14.7) |
| Others | 28 (6.9) |
| Information missing | 6 (1.5) |
| Pretreatment with MTX, n (%) | |
| Yes | 221 (54.8) |
| No | 180 (44.7) |
| Information missing | 2 (0.5) |
| Previous MTX dose, n (%) | |
| 7.5 mg | 10 (4.5) |
| 10 mg | 53 (24.0) |
| 15 mg | 112 (50.7) |
| 20 mg | 34 (15.4) |
| 25 mg | 6 (2.7) |
| Information missing | 6 (2.7) |
| Mean dose | 14.5 ± 3.9 mg |
| Median dose (range) | 15 mg (7.5–25 mg) |
| Previous mode of MTX administration, n (%) | |
| Oral | 106 (48.0) |
| Parenteral | 115 (52.0) |
| Start of MTX treatment, n (%) | |
| >20 years ago | 3 (1.3) |
| >10 to 20 years ago | 22 (9.9) |
| >5 to 10 years ago | 62 (28.2) |
| 0 to 5 years ago | 132 (59.7) |
| Information missing | 2 (0.9) |
| Reasons for change to MTX self-administration, n (%) | |
| Improved bioavailability | 95 (43.0) |
| Improved usability | 56 (25.3) |
| Low bioavailability of previous MTX treatment | 32 (14.5) |
| Dislike to MTX tablets | 30 (13.6) |
| Adverse events of previous MTX treatment | 23 (10.4) |
| Others | 25 (11.3) |
MTX, methotrexate; SD, standard deviation
Patient-, physician-, and study-nurse-reported outcomes
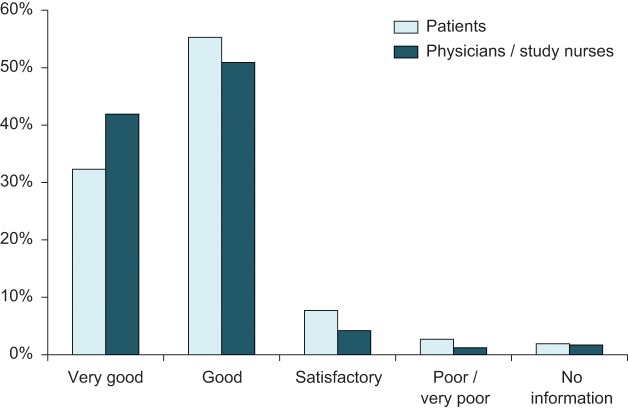
At the end of the study after a 5-week self-administered treatment with MTX ready-to-use syringe (50 mg/ml), the overall assessment was ‘very good’ and ‘good’ in 87.6% of the patients compared with 2.7% with a ‘poor’ and ‘very poor’ assessment. The corresponding assessments by physicians/study nurses were 92.8% (‘very good’ and ‘good’) and 1.2% (‘poor’ and ‘very poor’) (see Figure 1).
Figure 1.
Overall assessment of MTX prefilled syringe 50 mg/ml (n = 403).
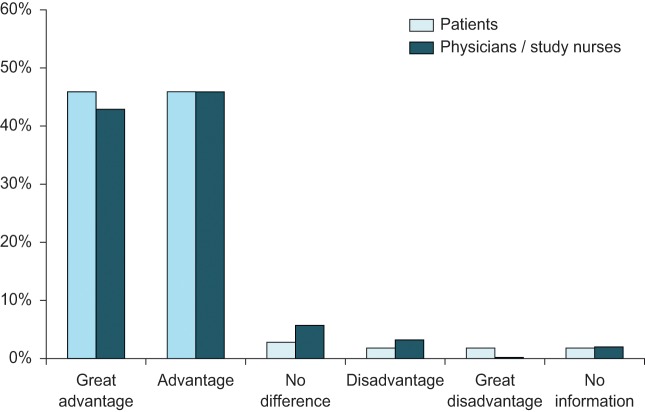
Availability and use of a pre-attached needle was considered as advantageous by 91.8% of the patients and physicians/study nurses (see Figure 2).
Figure 2.
Overall assessment of pre-attached needle in patients with previous experience of low-concentration MTX 10 mg/ml (n = 109).
Figure 3.
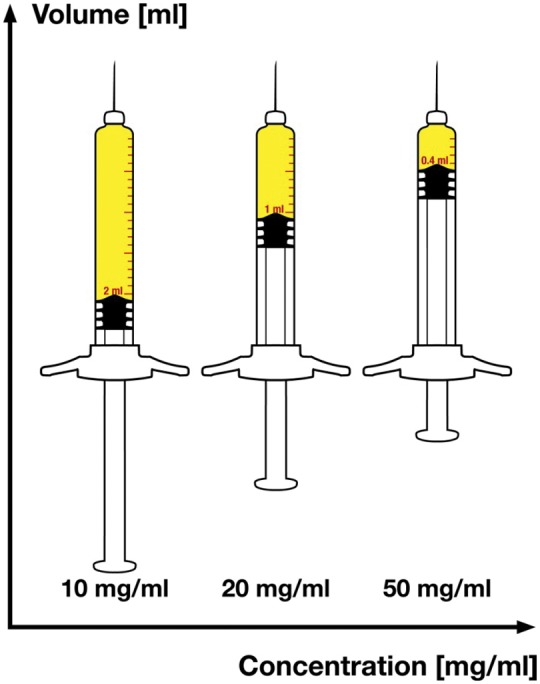
Different volumes of a 20 mg dose comparing the concentrations 10 mg/ml (2 ml to inject), 20 mg/ml (1 ml to inject) or 50 mg/ml (0.4 ml to inject).
A total of 96% of the patients described the feeling of the injection as comfortable or tolerable between the first and sixth injection; severe pain occurred only once.
Patient-reported outcomes
Most patients got used to the effort in applying self-injection of MTX. Twenty (5.0%) patients reported administration errors with regard to administration sites and disinfection. A total of 89.6% of the patients evaluated the labeling of the dosage as ‘very good’ and ‘good’, which confirms the usefulness of the color-coded backstop matching to the carton. Patients reported that self-administration led to a feeling of more independence (89.1%) and an improved quality of life (83.6%). A total of 109 patients reported previous self-administration of medium-concentration MTX formulations; 94.5% of them stated that they would prefer the high-concentration MTX formulation with 50 mg/ml in the future. This was mostly due to a better tolerated injection with reduced volume (93.6%) and the pre-attached needle (91.8%).
Physician- and study-nurse-reported outcomes
Physicians’ expectations concerning the benefit of switching to MTX self-administration were met in 92.8% of the patients and 96.3% of the patients were considered suitable for subcutaneous self-administration of the MTX formulation.
Safety
No serious adverse drug reactions were reported.
Discussion
This was a postmarketing surveillance study that evaluated preference, satisfaction, and usability of subcutaneous self-administration of a high-concentration (50 mg/ml) ready-to-use formulation of MTX in 403 patients with RA or psoriatic arthritis. The results show a high acceptance by patients (87.6%) and healthcare professionals (92.8%) of the MTX prefilled syringe with a pre-attached needle. In particular, 91.8% of the patients and physicians/study nurses valued the availability and use of a pre-attached needle as high. The formulation was generally well tolerated. In addition, self-administration of the MTX formulation was associated with an improved quality of life in 84% of the patients. Physicians considered 96.3% of the patients suitable for SC self-administration of the MTX formulation.
A total of 109 patients had previous experience in using medium-concentration MTX formulations. Of these, 94.5% preferred to use the new high-concentration MTX formulation in the future. These results are similar to those reported previously in a study comparing a medium-concentration with a high-concentration MTX formulation for SC self-administration [Müller-Ladner et al. 2010]. The smaller injection volume may improve the comfort of injection and the pre-attached needle makes the handling of the syringe safer, both contributing to the patients’ preference for this MTX formulation [Müller-Ladner et al. 2010]. In addition, other studies have confirmed the improved convenience and tolerability of subcutaneous administration of MTX also in comparison with intramuscular injection [Brooks et al. 1990; Sander et al. 1996; Zackheim, 1992].
This postmarketing surveillance study focused on subjective assessments of preference, satisfaction, and usability of the high-concentration MTX formulation and does not address safety and efficacy. However, with regard to efficacy, a recently performed 6 month, multicenter, randomized, double-blind, controlled trial compared oral MTX in 384 MTX-naïve patients with active RA to subcutaneously administered MTX [Braun et al. 2008]; the latter formulation showed superior efficacy over the oral MTX formulation and thus similar clinical results may be expected for the high-concentration MTX formulation. In addition, current practice guidelines support the use of parenteral MTX treatment in patients with poor compliance, inadequate effectiveness, or gastrointestinal side effects [Pavy et al. 2006; Tarner et al. 2009].
Conclusions
The 50 mg/ml prefilled syringe appears to be a valuable treatment option for patients with RA and psoriatic arthritis in need of MTX. This is supported by the strong appreciation of the patients as well as their attending healthcare professionals for its convenience and tolerability. The results confirm the findings and experience from a previously performed clinical study.
Acknowledgments
The authors wish to thank all participating centers who enrolled at least one patient:
H. Schmidt, Berlin; C. Busch-Mauz and T. Busch, Ilvesheim; I. Dahmann, Göttingen; R. Dockhorn, Weener; C. Eisterhues, Braunschweig; J. Frey, Neuburg a. d. Donau; A. Göbel, Lippstadt; W. Harmuth, Marktredwitz; H. Heintz, Hamburg; M. Hesse, Bad Kreuznach; T. Karger, Köln; M. Leidert, Lüneburg; A. Liebhaber, Halle; T. Marycz, Amberg; A. Melzer, Sessen; A. Reck, Mittelherwigsdorf; S. Remstedt, Berlin; U. Schoo, Rheine; H. Schulte and Partner, Hamburg; F. Schumann, Reken; M. Schürmann, Mülheim; F. Striesow, Bonn; W. Thies, Herrsching; C. Weimann, Magdeburg; S. Worsch, Mühlhausen; W.-D. Wörth, Wiesbaden; P. Veress, Mönchengladbach; K. Babinsky, Halle; C. Stille, Hannover; C. Baumann, Plauen; S. Bödeker, Marl; A. Rittstieg, Gelsenkirchen; E. Bräuning, Kahla; H.-E. Langer, Düsseldorf; B. Proba, Lüdenscheid; B. Heilig, Heidelberg; U. Kaeding, Parchim; V. Kubitza, Aschaffenburg; D. Lassak-Siedl, Heidelberg; D. Nottarp, Hanau; G. Hübner, Rheine; M. Linke, Stadtbergern; M. Reichardt, Berlin; J.-C. Nolde, Hannover; R. Haux, Berlin; S. Kupka, Altenburg; H.-J. Menne, Dortmund; B. Pech, Eberswalde; A. Stein, München; G. Donath, Freystadt; and N. Rinaldi, Ulm..
Martin Bornemann MD provided medical writing support on behalf of medac GmbH, Germany.
Footnotes
This postmarketing surveillance study was sponsored by medac Gesellschaft für klinische Spezialpräparate mbH.
FS has no conflicts of interest to declare. AB is an employee of medac GmbH.
References
- Ahern M.J., Chandran G. (1995) Category III symptom-modifying antirheumatic drugs. Clin Immunother 3: 196–217 [Google Scholar]
- Balis F.M., Mirro J., Jr, Reaman G.H., Evans W.E., McCully C., Doherty K.M., et al. (1988) Pharmacokinetics of subcutaneous methotrexate. J Clin Oncol 6: 1882–1886 [DOI] [PubMed] [Google Scholar]
- Braun J., Kaestner P., Flaxenberg P., Wahrisch J., Hanke P., Demary W., et al. (2008) Comparison of the clinical efficacy and safety of subcutaneous versus oral administration of methotrexate in patients with active rheumatoid arthritis: results of a six-month, multicenter, randomized, double-blind, controlled, phase IV trial. Arthritis Rheum 58: 73–81 [DOI] [PubMed] [Google Scholar]
- Brooks P.J., Spruill W.J., Parish R.C., Birchmore D.A. (1990) Pharmacokinetics of methotrexate administered by intramuscular and subcutaneous injections in patients with rheumatoid arthritis. Arthritis Rheum 33: 91–94 [DOI] [PubMed] [Google Scholar]
- Müller-Ladner U., Rockwitz K., Brandt-Jürgens J., Haux R., Kastner P., Braun J., et al. (2010) Tolerability and patient/physician satisfaction with subcutaneously administered methotrexate provided in two formulations of different drug concentrations in patients with rheumatoid arthritis. Open Rheumatol J 4: 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavy S., Constantin A., Pham T., Gossec L., Maillefert J.F., Cantagrel A., et al. (2006) Methotrexate therapy for rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. Joint Bone Spine 73: 388–395 [DOI] [PubMed] [Google Scholar]
- Rutkowska-Sak L., Rell-Bakalarska M., Lisowska B. (2009) Oral vs. subcutaneous low-dose methotrexate treatment in reducing gastrointestinal side effects. Reumatologia 47: 207–211 [Google Scholar]
- Sander O., Huebner G., Rau R. (1996) [Subcutaneous MTX - a reasonable addition of established modes of administration)]. Z Rheumatol 55(Suppl. 1): 111 [Google Scholar]
- Sieper J., Braun J. (1996) [Therapy of rheumatoid arthritis]. Deut Med Wochenschr 121: 563–567 [DOI] [PubMed] [Google Scholar]
- Tarner I.H., Manger B., Fleck M. (2009) [Evidence-based recommendations of a national group of experts on the use of methotrexate in inflammatory rheumatic diseases]. Akt Rheumatol 34: 59–66 [Google Scholar]
- Ward M.M., Fries J.F. (1998) Trends in antirheumatic medication use among patients with rheumatoid arthritis, 1981–1996. J Rheumatol 25: 408–416 [PubMed] [Google Scholar]
- Zackheim H.S. (1992) Subcutaneous administration of methotrexate. J Am Acad Dermatol 26: 1008. [DOI] [PubMed] [Google Scholar]