Abstract
In recent years there has been increasing evidence suggesting that epilepsy and its treatment can have adverse effects on bone mineralization and calcium metabolism. Many studies have shown a significant reduction in bone mineral density (BMD) and an increased fracture risk in patients treated with enzyme-inducing antiepileptics (phenobarbital, carbamazepine, phenytoin). It is assumed that CYP450-inducing antiepileptic drugs (AEDs) upregulate the enzymes which are responsible for vitamin D metabolism, with the effect of converting 25(OH) vitamin D into inactive metabolites, resulting in reduced calcium absorption with consecutive secondary hyperparathyroidism. Data on bone-specific effects of newer AEDs are limited; nevertheless, alterations of bone metabolism have been reported for oxcarbazepine, gabapentin and, in preclinical studies, for levetiracetam. Prophylactic administration of adequate amounts of calcium and vitamin D is recommended for all patients. For patients with long-term AED exposure, BMD measurement is recommended as part of osteoporosis investigation (especially for patients treated with enzyme-inducing AEDs and where there are major risk factors for fractures). Drug therapy (bisphosphonates) is reserved for the treatment of patients who have a high fracture risk; there are no specific intervention studies available in patients with epilepsy.
Keywords: antiepileptics, epilepsy, fracture, metabolic bone disease, osteoporosis
Introduction
Epilepsy is one of the most common chronic neurological conditions, affecting between 5 and 10 individuals per 1000 population in industrialized nations [Sander, 2003]. Epilepsy occurs predominantly in children and elderly people [Kotsopoulos et al. 2002] and its consequences extend far beyond the occurrence of seizures. In the past, little attention has been paid to metabolic changes associated with long-term intake of antiepileptic drugs (AEDs). One such change affects bone metabolism, with a reduction of bone mineral density (BMD) and an increased risk of fractures. This is particularly unfortunate for people with epilepsy, as they already have an increased propensity to fractures due to other drug side effects (e.g. ataxia), coexisting neurological deficits (e.g. cerebral palsy) and seizure-related falls [Petty et al. 2007].
Osteoporosis-related fractures are frequently observed in postmenopausal women and elderly men. Industrialized nations have a high prevalence of osteoporosis; the average probability of sustaining an osteoporosis-induced fracture in the course of a lifetime (‘lifetime risk’) is about 20–30% for men and 40–56% for women at the age of 50 [Lippuner et al. 2009; Kanis et al. 2000; Jones et al. 1994]. It is difficult to assess the osteoporosis risk if there has been no prior fracture. These days, a case-finding strategy is recommended aimed at identifying persons with a clearly increased fracture risk [Kanis et al. 2008a]. The fracture risk is not only dependent on bone mass, but also on risk factors which contribute to the fracture risk independent of bone density (e.g. age, prior fractures, family history of fracture, lifestyle factors such as nicotine consumption and extraosseous risks such as proneness to falls). Medication history plays a key role in elucidating the causes of secondary osteoporosis. Glucocorticoids, aromatase inhibitors and antiandrogens are frequently associated with osteoporosis. In contrast, the potentially bone-depleting effects of AEDs are less well known. This is illustrated by a survey of 624 neurologists, of whom only 28% were aware that AEDs are associated with reduced bone mass. In this cohort of neurologists, only 9% of paediatric neurologists and 7% of those treating adults prescribed prophylactic calcium and vitamin D supplements for their epilepsy patients [Valmadrid et al. 2001].
In this review we focus on the association between epilepsy and fracture risk, summarize the effects of AEDs on bone metabolism and conclude with practical recommendations for the care of patients with epilepsy.
Epilepsy and fracture risk
People with epilepsy have a 2–6 times greater risk of fractures than the general population [Sheth et al. 2006; Vestergaard, 2005; Mattson and Gidal, 2004], with a particularly increased incidence of fractures of the vertebral bodies and femoral neck [El-Hajj Fuleihan et al. 2008; Souverein et al. 2005; Cummings et al. 1995]. Fractures are not entirely the result of seizures; it is estimated that in both community-dwelling and institutionalized persons, the proportion of fractures directly attributable to seizure activity is about 35% [Vestergaard, 2005]. In addition to this there is an association between fractures and antiepileptic therapy. Importantly, a dose—effect relationship can be observed; the fracture risk increases significantly with the cumulative duration of AED exposure [Souverein et al. 2006]. This is supported by a Danish pharmacoepidemiological study which shows that AED use is associated with an increased fracture risk. The risk was more pronounced in patients using enzyme-inducing AEDs than in those using non-enzyme-inducing AEDs. In this study, the increased fracture risk with AED use was modest: the odds ratio (OR) was 1.18 for carbamazepine (CBZ), 1.79 for phenobarbital, 1.14 for oxcarbazepine (OXC), 1.15 for valproate (VPA) and 1.27 for clonazepam. By contrast, the fracture risk for lamotrigine (LTG), primidone, tiagabine, ethosuximide, topiramate (TOP) and vigabatrin was not significantly increased [Vestergaard et al. 2004]. This adverse effect of enzyme-inducing AEDs was confirmed by some other studies [Tsiropoulos et al. 2008], but not all [Souverein et al. 2006].
Most studies which investigated the influence of AEDs on osteoporosis risk have methodological limitations (e.g. small populations, biased subject selection, lack of adequate control data and failure to take account of confounding factors), which complicates their interpretation [Nakken and Tauboll, 2010]. In summary, the precise risk of fractures in patients with epilepsy is not clearly defined, but it is undoubtedly increased. Both the epilepsy itself and the bone-depleting effect of AEDs contribute to the increased fracture risk, independent of each other.
Epilepsy and low bone mass
The bone mass at a particular age is determined by bone mass formation during adolescence and young adulthood as well as the degree of subsequent bone loss. BMD is measured densitometrically using dual-energy X-ray absorptiometry (DXA); in accordance with the World Health Organization (WHO) definition, differentiation is made between osteopenia (T score −1 to −2.5 SD) and osteoporosis (T score < −2.5 SD), depending on the degree of severity. The term primary osteoporosis covers loss of bone mass in the postmenopausal years as well as reduced bone mass in older men and women. Secondary osteoporosis occurs in association with illnesses or drug effects which result in loss of bone mass.
AED-associated bone loss affects patients of both genders and all age groups. Alteration of bone turnover during the critical period of bone growth and bone mineralization in childhood and adolescence can result in lower peak bone mass in early adulthood. AEDs can also promote age-related bone loss [Coppola et al. 2009]. In an Italian study of 96 children and adolescents with epilepsy (with or without cerebral palsy and/or mental retardation), reduced bone density was found in 58% (n = 56) of patients, with BMD values consistent with osteopenia in 75% and osteoporosis in 25% [Coppola et al. 2009]. In this study, however, cofounding effect due to limited mobility or gait may have contributed to impaired bone mass accrual in children and adolescents. Various studies described low bone density in adults at multiple sites, including the femoral neck and lumbar spine [Pack and Morrell, 2004]. However, most of these studies are cross sectional and often lack control populations, which limits their interpretation.
A longitudinal study revealed that use of AEDs leads to accelerated bone loss at the proximal femur in women with epilepsy aged 65 years and older. The authors concluded that, if unabated, bone loss would be sufficient to increase the risk of hip fracture by 29% over 5 years. According to one estimate, there is a risk of bone density decreasing by 1.8% per year with phenytoin (PHT) treatment [Ensrud et al. 2004].
In their meta-analysis, Vestergaard and colleagues assessed the effects of epilepsy on fracture risk and changes in bone mass in patients with epilepsy. BMD was significantly decreased at the spine and hip. However, the observed increase in relative fracture risk (RR 2.18; 95% confidence interval [CI] 1.94–2.45) was higher than would have been expected based on BMD values (RR 1.2–1.3) [Vestergaard, 2005]. After correction for various covariables, including use of AEDs, the relative risk of sustaining a hip fracture decreased to 1.57 (95% CI 1.30–1.90) [Vestergaard et al. 2004]. This could indicate that although AEDs contribute to reduced bone density, they do not completely account for a higher fracture risk. This means that other factors, such as falls and epileptic seizures, may also contribute to the higher fracture risk. As already mentioned, it has been shown that about 35% of fractures are attributable to seizure episodes [Vestergaard, 2005]. This is further supported by the observation that the type of seizure influences the risk of fractures: patients with tonic—clonic seizures have more fractures than patients with other types of seizure [Persson et al. 2002].
The duration of AED treatment is associated with the rate of drug-induced bone loss [Andress et al. 2002]. Reduced bone mineral content is observed in 20–65% of patients with long-term use of AEDs [Sheth et al. 2008; Stephen et al. 1999].
This poses the question of which AEDs encourage bone loss. Older studies showed that enzyme-inducing AEDs are associated with an increased fracture risk [Valimaki et al. 1994] and that PHT appears to be the drug with the greatest potential to affect bone and mineral metabolism [Pack et al. 2005]. The data on CBZ, also an enzyme inducer, and VPA, an enzyme inhibitor, are somewhat conflicting, but in some studies both drugs have been associated with osteopenia [Ecevit et al. 2004; Sato et al. 2001]. VPA and/or LTG, particularly when used in combination, were associated with reduced bone formation, low BMD and short stature in children [Guo et al. 2001].
The extent to which the newer AEDs influence bone metabolism remains to be determined. Drugs which inhibit carbonic anhydrase (TOP, zonisamide, acetazolamide) can have an unfavourable influence on bone metabolism by causing metabolic acidosis [Nakken et al. 2010]. Recently published data from animal studies show that levetiracetam (LEV) reduces bone strength without altering bone mass [Nissen-Meyer et al. 2007]. Certainly, further studies are needed to investigate the potential effects of newer AEDs on bone quality.
Metabolic consequences of antiepileptic drugs
Cytochrome P450 enzyme-inducing AEDs are most commonly associated with a negative impact on bone. It is assumed that CYP450-inducing AEDs [e.g. phenobarbital, PHT, CBZ, OXC] upregulate the enzymes responsible for the metabolism of vitamin D, resulting in conversion of 25(OH) vitamin D into inactive metabolites (Figure 1). The resulting decrease in 1,25(OH)2 vitamin D leads to reduced calcium absorption, with consecutive secondary hyperparathyroidism, increased bone resorption and accelerated bone loss (Table 1) [Fitzpatrick, 2004].
Figure 1.
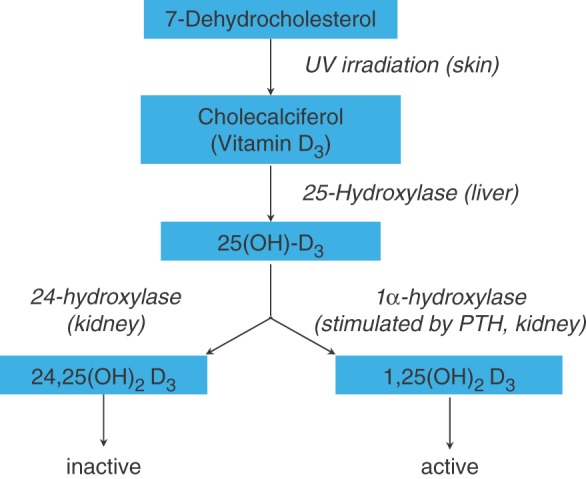
Vitamin D metabolism. 25(OH)-D3, 25-hydroxycholecalciferol; 1,25(OH)2 D3, 1,25-dihydroxycholecalciferol; 24,25(OH)2 D3, 24,25-dihydroxycholecalciferol.
Table 1.
Influence of antiepileptic drugs on bone metabolism (adapted from Verrotti et al. [2010]).
| Drug | BMD | 25(OH)D3 | Ca/P | PTH | Bone turnover marker |
|---|---|---|---|---|---|
| Classic antiepileptics | |||||
| Benzodiazepine | ↓ | ↓ | ↔ | ↔ | ↑bALP, ↑OC, ↑ICTP, ↑NTX |
| Carbamazepine | ↓ | ↓ | ↔ | ↑ | ↑bALP, ↑OC, ↑ICTP, ↑NTX |
| Phenytoin | ↓ | ↓ | ↓ | ↑ | ↑bALP, ↑NTX |
| Phenobarbital | ↓ | ↓ | ↔ | ↑bALP, ↑ICTP | |
| Valproic acid | ↓ | ↔ | ↔ | ↔ | ↑ALP, ↑OC |
| Newer antiepileptics | |||||
| Gabapentin | ↓ | ||||
| Lamotrigine | ↔ | ↔ | ↔ | ? | |
| Levetiracetam | ↔ | ↔ | ↔ | ? | |
| Oxacarbazepine | ↓ | ↓ | ↔ | ↑ | ↑bALP |
Bone formation markers:
BMD, bone mineral density; 25(OH)D3, 25-hydroxycholecalciferol, PTH, parathyroid hormone.
ALP, alkaline phosphatase; bALP, bone-specific alkaline phosphatase; OC, osteocalcin.
Bone resorption markers:
ICTP, C-terminal cross-linked type I collagen telopeptide; NTX, N-terminal cross-linked type I collagen telopeptide.
Bone is a metabolically active tissue that undergoes continuous remodelling by two counteracting processes, namely bone formation and bone resorption. These processes rely on the activity of osteoclasts (resorption), osteoblasts (formation) and osteocytes (maintenance). Under normal conditions, bone resorption and formation are tightly coupled to each other, so that the amount of bone removed is always equal to the amount of newly bone formed. This balance is achieved and regulated through the action of various systemic hormones [e.g. vitamin D, parathyroid hormone (PTH), other steroid hormones] and local mediators (e.g. cytokines, growth factors) [Meier et al. 2008; Seibel, 2000]. As shown in Table 1, most classic AEDs induce accelerated bone turnover as demonstrated by increased serum and urinary levels of biochemical markers of resorption and bone formation. These molecular markers of bone metabolism reflect the dynamics of the metabolic imbalance induced by AEDs and ultimately result in accelerated bone loss. In the most serious form of vitamin D deficiency, osteomalacia, there are extensive mineralization defects of bone (Figure 2).
Figure 2.
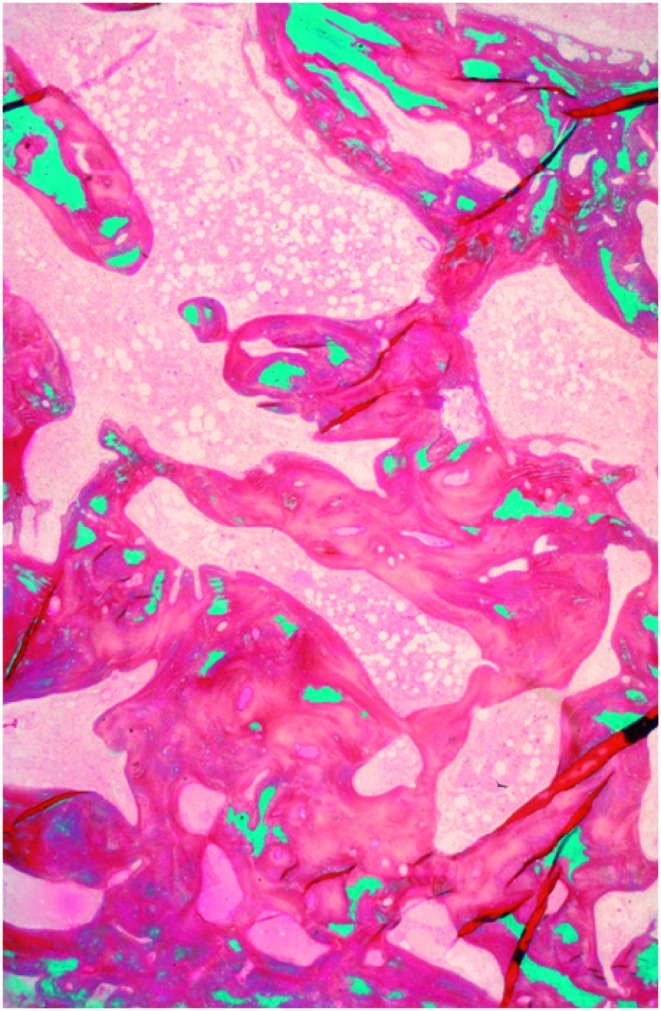
Disturbed mineralization in osteomalacia (osteoidosis): reduced mineralized bone (green), markedly increased unmineralized osteoid (red). Goldner staining.
Several studies have shown that enzyme-inducing AEDs are associated with reduced levels of 25(OH)D [Pack et al. 2008, 2005; Mintzer et al. 2006; Verrotti et al. 2002; Lamberg-Allardt et al. 1990; Hoikka et al. 1984]. In support of this theory, some cross-sectional studies [Sato et al. 2001; Valimaki et al. 1994; Weinstein et al. 1984] have reported elevation of PTH levels in patients treated with enzyme-inducing AEDs as compared with control subjects. Other studies [Verrotti et al. 2002] have observed no significant differences in this respect.
In a longitudinal, controlled investigation to evaluate AED effects on bone density and metabolism, Pack and colleagues followed women on AED monotherapy with PHT, CBZ, LTG or VPA for 1 year. They found significant bone loss at the femoral neck in PHT-treated patients in correlation with reductions in 25(OH)D levels and increases in bone turnover markers. However, patients treated with CBZ or LTG, in whom similar results might be expected, showed no such changes. This study suggests that the individual enzyme-inducing AEDs may differ in their effects on bone metabolism [Mintzer, 2010].
AEDs can also have a negative impact on bone in the absence of vitamin D deficiency. VPA, which does not induce CYP-450 hepatic enzymes, has been shown to compromise skeletal integrity [Farhat et al. 2002; Guo et al. 2001; Sheth et al. 1995]. Possible mechanisms suggested are decreased intestinal absorption of calcium (PHT), resistance to PTH (CBZ), calcitonin deficiency (PHT, primidone), interference with vitamin K metabolism (PHT) and a direct drug effect on bone cell functions (PHT, CBZ, VPA) [Petty et al. 2007]. Other, indirect drug effects, such as hormonal changes (e.g. reduced testosterone levels as a result of increased SHBG levels due to phenobarbital, PHT, CBZ), increase in homocysteine or reduction in IGF-I concentrations can also have an unfavourable effect on bone metabolism [Mintzer, 2010].
Data on newer AEDs is scarce. Gabapentin (GBP) is not metabolized, and it does not induce or inhibit hepatic enzymes. Up till now, no study has examined the relationship between bone metabolism and GBP monotherapy. Nevertheless, several studies in adult epileptic patients treated with various AEDs, including GBP, indicate that long-term GBP therapy can result in bone loss at the hip and lumbar spine [El-Hajj Fuleihan et al. 2008; Andress et al. 2002]. A prospective study by Ensrud and colleagues confirmed that GBP can induce bone loss at the femoral neck in older men [Ensrud et al. 2008]. Only recently was a significant increase in fracture risk found for GBP (adjusted OR 1.49; 95% CI, 1.10–2.02) [Jette et al. 2011]. Although GBP has been shown to induce bone loss it remains unclear, whether GBP has direct deleterious effect on bone or whether increased fracture risk might be attributed to decreased mobility as GBP is frequently used to treat chronic pain syndromes.
There are no data on a direct association between LTG and fractures, but some studies have assessed LTG in relation to its effect on bone density in children [Sheth and Hermann, 2007; Guo et al. 2001] and premenopausal women [Pack, 2008]. Overall, LTG did not induce accelerated bone loss compared with patients treated with other AEDs.
Clinical data on the effects of LEV on skeletal integrity are scarce. A preclinical study in rats observed decreased bone strength at the femoral neck of rats treated with low-dose LEV. In contrast, bone mineral content and bone mass remained unchanged [Nissen-Meyer et al. 2007].
OXC, a weak hepatic enzyme inducer, seems to be associated with reduced 25(OH)D levels and with elevated bone resorption markers which result in increased bone turnover [Cansu et al. 2008; Babayigit et al. 2006; Mintzer et al. 2006].
TOP does not seem to be associated with bone metabolism changes, but there are very few clinical studies available. Patients treated with TOP may develop mild to moderate metabolic acidosis, which can result in kidney stones, osteomalacia and/or osteoporosis [Verrotti et al. 2010].
Practical procedure
A general aspect of everyday clinical practice is to identify persons with an increased risk of fractures, initiate preventive measures and institute therapeutic intervention appropriate to their individual fracture risk. As mentioned it is difficult to assess the fracture risk if no prior fracture has occurred. Nowadays, a case-finding strategy is recommended which is designed to screen for people with a clearly increased risk of fracture [Kanis et al. 2008c]. Further investigation using densitometry is only recommended if an increased fracture risk has been established. Bone density measurement is considered a continuous predictor of fracture risk, but an intervention threshold for treatment cannot be derived from it alone. This is illustrated by the fact that many people sustain a fracture after inadequate trauma without having reached the ‘osteoporosis threshold’ (T score ≤ −2.5 SD). On the other hand, many people with clearly osteoporotic mineral content values have not experienced a fracture. It follows that the fracture risk is not solely dependent on the bone mass, but also on other risk factors, primarily age and body weight, as well as on lifestyle factors and extra-osseous risks (propensity to fall, reaction capacity, muscle mass, eyesight). Some of these factors contribute to the fracture risk independent of bone density.
Evidence-based strategies for investigating and treating osteoporosis in patients with epilepsy and AED-associated osteopathy are limited. Epilepsy and AED therapy in elderly women and men is considered to be a relevant risk factor for fractures of the vertebral bodies and the femoral neck, and BMD measurement is accordingly recommended. This applies in particular to patients with additional risk factors.
In patients receiving long-term therapy with enzyme-inducing AEDs, it is generally recommended that 25(OH) vitamin D levels in the serum should be determined (before treatment, then at 6–12 months). The measurement of biochemical markers of bone turnover in an individual patient on AEDs is of limited diagnostic value and is not recommended to be used in clinical routine [Meier et al. 2009].
All patients with epilepsy and those receiving long-term AED therapy (especially patients taking enzyme-inducing AEDs and VPA) should be advised of their increased risk for fracture and preventive measures should be recommended. General recommendations (Table 2) include adequate supply of calcium and vitamin D, a balanced diet with sufficient protein intake, regular physical activity, strengthening of the neuromuscular function and avoidance of risk factors (nicotine consumption, excessive alcohol intake).
Table 2.
Prophylactic measures against osteoporosis.
| Total daily calcium intake of 1000–1500 mg/day (nutrition and supplements) |
Adequate sunlight exposure, possibly vitamin D3 supplementation
|
| Balanced diet with adequate protein intake |
| Regular physical activity, avoidance of immobility |
Reduction of fall risk by
|
| Possibly hormone replacement therapy, with due consideration of the benefit—risk relationship |
Epileptics have previously been discouraged from participation in physical activity and sports for fear of inducing seizures or increasing seizure frequency [Howard et al. 2004]. Despite a shift in medical recommendations toward encouraging rather than restricting participation, the stigma remains and epileptics continue to be less active than the general population. Hence, education and participation in regular exercise (within the limits of the disease) should be advised.
Oestrogen substitution is to be considered in postmenopausal women, with special consideration of the benefit—risk relationship (breast cancer risk, cardiovascular risk, thrombosis risk). As a rule, time-limited preventive hormone therapy is only prescribed today if there are clear coexisting climacteric complaints and if these require hormone therapy. Even low doses of hormone replacement therapy seem to be effective in preventing postmenopausal bone loss. Finally, consideration must be given to the possibility of epileptic seizures being aggravated by hormone replacement therapy [Harden et al. 2006].
The influence of antiepileptics on vitamin D metabolism requires special consideration. While vitamin D supplementation of 1000–1200 IU/day should be adequate for the daily requirement of patients using non-enzyme-inducing AEDs, a higher daily vitamin D dose (2000–4000 IU) is recommended as preventive therapy in patients on long-term treatment with barbiturates, PHT or CBZ [Bartl, 2007; Drezner, 2004]. Higher doses of vitamin D are required in the case of osteomalacia.
Drug therapy is indicated where there is increased risk of fracture. This applies to patients who have already experienced a fracture, especially a vertebral or hip fracture, or patients with an increased absolute 10-year fracture risk. The individual fracture risk can be evaluated using the ‘WHO Fracture Risk Assessment Tool’ (FRAX©) [Kanis et al. 2009, 2008b]. Antiresorptive preparations, particularly bisphosphonates or selective oestrogen receptor modulators (raloxifene) are primarily used in the therapy of osteoporosis. Treatment should last at least 3–5 years; vitamin D deficiency and osteomalacia must be excluded before drug therapy is initiated. Epilepsy occurs not only in elderly people but also in children. Specifically, the indication and safety of antiresorptive treatment in children and adolescents remains unclear. Further studies in these populations (including women of childbearing age) are needed before bisphosphonate treatment can be recommended.
References
- Andress D.L., Ozuna J., Tirschwell D., Grande L., Johnson M., Jacobson A.F., et al. (2002) Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch Neurol 59: 781–786 [DOI] [PubMed] [Google Scholar]
- Babayigit A., Dirik E., Bober E., Cakmakci H. (2006) Adverse effects of antiepileptic drugs on bone mineral density. Pediatr Neurol 35: 177–181 [DOI] [PubMed] [Google Scholar]
- Bartl R. (2007) [Antiepileptic drug-induced osteopathy. Subtypes, pathogenesis, prevention, early diagnosis and treatment]. Dtsch Med Wochenschr 132: 1475–1479 [DOI] [PubMed] [Google Scholar]
- Cansu A., Yesilkaya E., Serdaroglu A., Hirfanoglu T.L., Camurdan O., Gulbahar O., et al. (2008) Evaluation of bone turnover in epileptic children using oxcarbazepine. Pediatr Neurol 39: 266–271 [DOI] [PubMed] [Google Scholar]
- Coppola G., Fortunato D., Auricchio G., Mainolfi C., Operto F.F., Signoriello G., et al. (2009) Bone mineral density in children, adolescents, and young adults with epilepsy. Epilepsia 50: 2140–2146 [DOI] [PubMed] [Google Scholar]
- Cummings S.R., Nevitt M.C., Browner W.S., Stone K., Fox K.M., Ensrud K.E., et al. (1995) Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 332: 767–773 [DOI] [PubMed] [Google Scholar]
- Drezner M.K. (2004) Treatment of anticonvulsant drug-induced bone disease. Epilepsy Behav 5(Suppl. 2): S41–S47 [DOI] [PubMed] [Google Scholar]
- Ecevit C., Aydogan A., Kavakli T., Altinoz S. (2004) Effect of carbamazepine and valproate on bone mineral density. Pediatr Neurol 31: 279–282 [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G., Dib L., Yamout B., Sawaya R., Mikati M.A. (2008) Predictors of bone density in ambulatory patients on antiepileptic drugs. Bone 43: 149–155 [DOI] [PubMed] [Google Scholar]
- Ensrud K.E., Walczak T.S., Blackwell T., Ensrud E.R., Bowman P.J., Stone K.L. (2004) Antiepileptic drug use increases rates of bone loss in older women: A prospective study. Neurology 62: 2051–2057 [DOI] [PubMed] [Google Scholar]
- Ensrud K.E., Walczak T.S., Blackwell T.L., Ensrud E.R., Barrett-Connor E., Orwoll E.S. (2008) Antiepileptic drug use and rates of hip bone loss in older men: A prospective study. Neurology 71: 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat G., Yamout B., Mikati M.A., Demirjian S., Sawaya R., El-Hajj Fuleihan G. (2002) Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology 58: 1348–1353 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick L.A. (2004) Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav 5(Suppl. 2): S3–S15 [DOI] [PubMed] [Google Scholar]
- Guo C.Y., Ronen G.M., Atkinson S.A. (2001) Long-term valproate and lamotrigine treatment may be a marker for reduced growth and bone mass in children with epilepsy. Epilepsia 42: 1141–1147 [DOI] [PubMed] [Google Scholar]
- Harden C.L., Herzog A.G., Nikolov B.G., Koppel B.S., Christos P.J., Fowler K., et al. (2006) Hormone replacement therapy in women with epilepsy: A randomized, double-blind, placebo-controlled study. Epilepsia 47: 1447–1451 [DOI] [PubMed] [Google Scholar]
- Hoikka V., Alhava E.M., Karjalainen P., Keranen T., Savolainen K.E., Riekkinen P., et al. (1984) Carbamazepine and bone mineral metabolism. Acta Neurol Scand 70: 77–80 [DOI] [PubMed] [Google Scholar]
- Howard G.M., Radloff M., Sevier T.L. (2004) Epilepsy and sports participation. Curr Sports Med Rep 3: 15–19 [DOI] [PubMed] [Google Scholar]
- Jette N., Lix L.M., Metge C.J., Prior H.J., McChesney J., Leslie W.D. (2011) Association of antiepileptic drugs with nontraumatic fractures: A population-based analysis. Arch Neurol 68: 107–112 [DOI] [PubMed] [Google Scholar]
- Jones G., Nguyen T., Sambrook P.N., Kelly P.J., Gilbert C., Eisman J.A. (1994) Symptomatic fracture incidence in elderly men and women: The Dubbo Osteoporosis Epidemiology Study (DOES). Osteoporos Int 4: 277–282 [DOI] [PubMed] [Google Scholar]
- Kanis J.A., Burlet N., Cooper C., Delmas P.D., Reginster J.Y., Borgstrom F., et al. (2008a) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 19: 399–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J.A., Johnell O., Oden A., Johansson H., McCloskey E. (2008b) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19: 385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J.A., Johnell O., Oden A., Sembo I., Redlund-Johnell I., Dawson A., et al. (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11: 669–674 [DOI] [PubMed] [Google Scholar]
- Kanis J.A., McCloskey E.V., Johansson H., Strom O., Borgstrom F., Oden A. (2008c) Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int 19: 1395–1408 [DOI] [PubMed] [Google Scholar]
- Kanis J.A., Oden A., Johansson H., Borgstrom F., Strom O., McCloskey E. (2009) FRAX and its applications to clinical practice. Bone 44: 734–743 [DOI] [PubMed] [Google Scholar]
- Kotsopoulos I.A., van Merode T., Kessels F. G., de Krom M.C., Knottnerus J.A. (2002) Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia 43: 1402–1409 [DOI] [PubMed] [Google Scholar]
- Lamberg-Allardt C., Wilska M., Saraste K.L., Gronlund T. (1990) Vitamin D status of ambulatory and nonambulatory mentally retarded children with and without carbamazepine treatment. Ann Nutr Metab 34: 216–220 [DOI] [PubMed] [Google Scholar]
- Lippuner K., Johansson H., Kanis J.A., Rizzoli R. (2009) Remaining lifetime and absolute 10-year probabilities of osteoporotic fracture in Swiss men and women. Osteoporos Int 20: 1131–1140 [DOI] [PubMed] [Google Scholar]
- Mattson R.H., Gidal B.E. (2004) Fractures, epilepsy, and antiepileptic drugs. Epilepsy Behav 5(Suppl. 2): S36–S40 [DOI] [PubMed] [Google Scholar]
- Meier C., Seibel M.J., Kraenzlin M.E. (2008) Biochemical markers of bone turnover: Clinical aspects, In: Adler R.A. (ed.). Osteoporosis: Pathophysiology and Clinical Management, 2nd ed edn, Humana Press: Totowa, NJ [Google Scholar]
- Meier C., Seibel M.J., Kraenzlin M.E. (2009) Use of bone turnover markers in the real world: Are we there yet? J Bone Miner Res 24: 386–388 [DOI] [PubMed] [Google Scholar]
- Mintzer S. (2010) Metabolic consequences of antiepileptic drugs. Curr Opin Neurol 23: 164–169 [DOI] [PubMed] [Google Scholar]
- Mintzer S., Boppana P., Toguri J., DeSantis A. (2006) Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia 47: 510–515 [DOI] [PubMed] [Google Scholar]
- Nakken K.O., Rytter E.M., Brockmeier F. (2010) [Benzodiazepines in the treatment of epilepsy]. Tidsskr Nor Laegeforen 130: 842–844 [DOI] [PubMed] [Google Scholar]
- Nakken K.O., Tauboll E. (2010) Bone loss associated with use of antiepileptic drugs. Expert Opin Drug Saf 9: 561–571 [DOI] [PubMed] [Google Scholar]
- Nissen-Meyer L.S., Svalheim S., Tauboll E., Reppe S., Lekva T., Solberg L.B., et al. (2007) Levetiracetam, phenytoin, and valproate act differently on rat bone mass, structure, and metabolism. Epilepsia 48: 1850–1860 [DOI] [PubMed] [Google Scholar]
- Pack A. (2008) Bone health in people with epilepsy: Is it impaired and what are the risk factors? Seizure 17: 181–186 [DOI] [PubMed] [Google Scholar]
- Pack A.M., Morrell M.J. (2004) Epilepsy and bone health in adults. Epilepsy Behav 5(Suppl. 2): S24–S29 [DOI] [PubMed] [Google Scholar]
- Pack A.M., Morrell M.J., Marcus R., Holloway L., Flaster E., Done S., et al. (2005) Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol 57: 252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack A.M., Morrell M.J., Randall A., McMahon D.J., Shane E. (2008) Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology 70: 1586–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H.B., Alberts K.A., Farahmand B.Y., Tomson T. (2002) Risk of extremity fractures in adult outpatients with epilepsy. Epilepsia 43: 768–772 [DOI] [PubMed] [Google Scholar]
- Petty S.J., O'Brien T.J., Wark J.D. (2007) Anti-epileptic medication and bone health. Osteoporos Int 18: 129–142 [DOI] [PubMed] [Google Scholar]
- Sander J.W. (2003) The epidemiology of epilepsy revisited. Curr Opin Neurol 16: 165–170 [DOI] [PubMed] [Google Scholar]
- Sato Y., Kondo I., Ishida S., Motooka H., Takayama K., Tomita Y., et al. (2001) Decreased bone mass and increased bone turnover with valproate therapy in adults with epilepsy. Neurology 57: 445–449 [DOI] [PubMed] [Google Scholar]
- Seibel M.J. (2000) Molecular markers of bone turnover: Biochemical, technical and analytical aspects. Osteoporos Int 11(Suppl. 6): S18–S29 [DOI] [PubMed] [Google Scholar]
- Sheth R.D., Binkley N., Hermann B.P. (2008) Progressive bone deficit in epilepsy. Neurology 70: 170–176 [DOI] [PubMed] [Google Scholar]
- Sheth R.D., Gidal B.E., Hermann B.P. (2006) Pathological fractures in epilepsy. Epilepsy Behav 9: 601–605 [DOI] [PubMed] [Google Scholar]
- Sheth R.D., Hermann B.P. (2007) Bone mineral density with lamotrigine monotherapy for epilepsy. Pediatr Neurol 37: 250–254 [DOI] [PubMed] [Google Scholar]
- Sheth R.D., Wesolowski C.A., Jacob J.C., Penney S., Hobbs G.R., Riggs J.E., et al. (1995) Effect of carbamazepine and valproate on bone mineral density. J Pediatr 127: 256–262 [DOI] [PubMed] [Google Scholar]
- Souverein P.C., Webb D.J., Petri H., Weil J., Van Staa T.P., Egberts T. (2005) Incidence of fractures among epilepsy patients: A population-based retrospective cohort study in the General Practice Research Database. Epilepsia 46: 304–310 [DOI] [PubMed] [Google Scholar]
- Souverein P.C., Webb D.J., Weil J.G., Van Staa T.P., Egberts A.C. (2006) Use of antiepileptic drugs and risk of fractures: Case-control study among patients with epilepsy. Neurology 66: 1318–1324 [DOI] [PubMed] [Google Scholar]
- Stephen L.J., McLellan A.R., Harrison J.H., Shapiro D., Dominiczak M.H., Sills G.J., et al. (1999) Bone density and antiepileptic drugs: A case-controlled study. Seizure 8: 339–342 [DOI] [PubMed] [Google Scholar]
- Tsiropoulos I., Andersen M., Nymark T., Lauritsen J., Gaist D., Hallas J. (2008) Exposure to antiepileptic drugs and the risk of hip fracture: A case-control study. Epilepsia 49: 2092–2099 [DOI] [PubMed] [Google Scholar]
- Valimaki M.J., Tiihonen M., Laitinen K., Tahtela R., Karkkainen M., Lamberg-Allardt C., et al. (1994) Bone mineral density measured by dual-energy x-ray absorptiometry and novel markers of bone formation and resorption in patients on antiepileptic drugs. J Bone Miner Res 9: 631–637 [DOI] [PubMed] [Google Scholar]
- Valmadrid C., Voorhees C., Litt B., Schneyer C.R. (2001) Practice patterns of neurologists regarding bone and mineral effects of antiepileptic drug therapy. Arch Neurol 58: 1369–1374 [DOI] [PubMed] [Google Scholar]
- Verrotti A., Coppola G., Parisi P., Mohn A., Chiarelli F. (2010) Bone and calcium metabolism and antiepileptic drugs. Clin Neurol Neurosurg 112: 1–10 [DOI] [PubMed] [Google Scholar]
- Verrotti A., Greco R., Latini G., Morgese G., Chiarelli F. (2002) Increased bone turnover in pre-pubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia 43: 1488–1492 [DOI] [PubMed] [Google Scholar]
- Vestergaard P. (2005) Epilepsy, osteoporosis and fracture risk—a meta-analysis. Acta Neurol Scand 112: 277–286 [DOI] [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L. (2004) Fracture risk associated with use of antiepileptic drugs. Epilepsia 45: 1330–1337 [DOI] [PubMed] [Google Scholar]
- Weinstein R.S., Bryce G.F., Sappington L.J., King D.W., Gallagher B.B. (1984) Decreased serum ionized calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. J Clin Endocrinol Metab 58: 1003–1009 [DOI] [PubMed] [Google Scholar]


