Abstract
Objectives:
We tested the ability of the VASFIQ, a seven-item scale composed of Fibromyalgia Impact Questionnaire (FIQ) visual analog scales (VASs), to quantify fibromyalgia global disease severity and identify fibromyalgia patients with significant symptoms of fatigue, poor sleep, depression or anxiety.
Methods:
Spearman rank correlations were used to compare global VASFIQ, FIQ and Patient Global Impression of Change (PGIC) scores and individual FIQ VAS scores with full-length, validated questionnaire scores for fatigue (Multidimensional Assessment of Fatigue—Global Fatigue Index [MAF-GFI]), poor sleep (Medical Outcomes Study Sleep Problems Index [SPI]) and depression and anxiety (Hospital Anxiety and Depression Scale [HADS]). Patient scores used in the analyses were derived from 2229 patients enrolled in three pregabalin fibromyalgia trials. Receiver operating characteristic analyses determined VASFIQ cutoff scores identifying patients with clinically significant symptom levels using full-length, validated symptom questionnaires to define cases.
Results:
Global VASFIQ and FIQ scores correlated highly at baseline and study endpoints (ρ = 0.94 and 0.97, respectively; both p<0.0001). Change in global VASFIQ and FIQ scores correlated similarly to PGIC scores at study endpoints (ρ = 0.58 and 0.61, respectively; both p<0.0001). Individual FIQ VAS scores correlated with corresponding full-length symptom questionnaire scores at baseline and study endpoints (VASfatigue with MAF-GFI, ρ = 0.64 and 0.76; VASsleep with SPI, ρ = 0.50 and 0.67; VASdepression with HADS-D, ρ = 0.43 and 0.62; VASanxiety with HADS-A, ρ = 0.47 and 0.67, respectively; p <0.0001 for all). Patients with significant symptoms of fatigue were identified by VASfatigue >7.5, poor sleep by VASsleep >7.9, depression by VASdepression >5.8 and anxiety by VASanxiety >6.0. VASFIQ global scores ≥31.4 and ≥45.0 identified patients with moderate and severe global fibromyalgia symptoms, respectively.
Conclusions:
The VASFIQ scale accurately quantifies global fibromyalgia severity and identifies patients with significant symptoms of fatigue, poor sleep, depression or anxiety with brevity, enabling rapid patient assessment and informing treatment decisions in busy clinics.
Keywords: anxiety, depression, fatigue, fibromyalgia, pain, poor sleep, screening, symptoms
Introduction
Fibromyalgia (FM) is a complex disorder of chronic widespread pain and tenderness associated with numerous other symptoms including fatigue, poor sleep quality, depression and anxiety [Wolfe, 1990]. The complexity of FM can make patients challenging to treat. While all patients with FM experience pain, individual patients often vary widely in the severity of associated FM symptoms from which they suffer. Quantification of the severity of associated FM symptoms is required for diagnosis under new preliminary diagnostic criteria for FM recommended by the American College of Rheumatology (ACR) [Wolfe et al. 2010], and management of all clinically significant symptoms is recommended for effective FM management [Carville et al. 2008]. However, assessment is often hampered by the inability of many patients to effectively articulate their symptom burden and the inability of clinicians to interpret the patients' complaints into a coherent intellectual framework from which to make diagnostic and treatment decisions. While self-report questionnaires to quantify symptom severity exist, including the Multidimensional Assessment of Fatigue (MAF) for fatigue [Belza, 1995], the Medical Outcomes Study—Sleep Scale (MOS—Sleep) for sleep quality [Hays and Stewart, 1992] and the Hospital Anxiety and Depression Scale (HADS) for anxiety and depression [Zigmond and Snaith, 1983], their length and complexity typically preclude use in busy clinical practices.
The Fibromyalgia Impact Questionnaire (FIQ) is the standard patient self-report assessment for quantifying the global severity of FM symptoms in research studies [Burckhardt et al. 1991]. The FIQ is composed of 20 items that assess disease severity over the past week with 11 questions to assess physical functioning, two ‘day-of-the-week’ items to quantify the number of days patients ‘felt good’ or ‘missed work’, and seven visual analog scales (VASs) to assess symptoms of fatigue, sleep quality, depression, anxiety, stiffness, pain and work disability. However, clinical utility of the FIQ is limited by its length, the scoring complexity of physical functioning and ‘day-of-the-week’ items, and the lack of functionality to identify patients with clinically significant symptom levels that require treatment. While clinical utility of the entire FIQ is limited, the FIQ VASs have properties that make them ideal for clinical use. VASs are simple to score and have been shown to perform as well as longer scales in respect to sensitivity to change and correlation with clinical variables [Wolfe, 2004]. In addition, the FIQ VASs evaluate domains that have been identified as important in assessing patients with FM [Mease et al. 2009]. Finally, prior research in a small FM cohort showed the FIQ VASs performed as well as the full FIQ in assessing global disease severity, and cutoff scores on individual FIQ VASs could be established to identify patients with significant symptoms of fatigue, poor sleep and depression [Boomershine et al. 2008].
In this work, we challenged the hypothesis that a scale composed of the seven FIQ VASs (the VASFIQ, Figure 1) could provide a brief, clinically useful FM assessment measure, with VAS scores summed to provide a measure of global disease severity and cutoff scores developed for individual VASs that identify patients with clinically significant symptoms of fatigue, poor sleep quality, depression and anxiety.
Figure 1.
The VASFIQ Questionnaire. FIQ, Fibromyalgia Impact Questionnaire; VAS, Visual Analogue Scale. FIQ VASs reproduced with permission from R.M. Bennett and figure reproduced with permission from The Journal of Rheumatology© 1991 [Burckhardt et al. 1991].
Patients and methods
Combined data from 2229 patients enrolled in three pregabalin (Lyrica®) FM treatment trials (A0081056 [ClinicalTrials.gov identifier: NCT00645398], A0081077 [ClinicalTrials.gov identifier: NCT00230776] and A0081100 [ClinicalTrials.gov identifier: NCT00333866]) [Arnold et al. 2008; Mease et al. 2008b; Pauer et al. 2008] were analyzed. All trials had similar inclusion and exclusion criteria. Patients were included if they were ≥18 years of age, had a baseline pain score of at least 40 mm on a 100-mm pain VAS and fulfilled ACR classification criteria for FM (i.e. widespread pain present for ≥3 months and pain on palpation at ≥11 of 18 specific tender point sites) [Wolfe et al., 1990]. Exclusion criteria included evidence of inflammatory rheumatic disease, severe painful disorders other than FM, clinically significant or unstable medical or psychiatric disorders, history of illicit drug or alcohol abuse within the past 2 years, previous pregabalin treatment and receiving or applying for disability benefits. Patients were also required to discontinue use of other concomitant medications taken for FM as well as agents used to treat pain and insomnia prior to entering the trials. Each trial complied with Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice Guidelines, was approved by local research ethics committees, and written informed consent was obtained from all patients.
Questionnaire data analyzed from the trials included baseline and endpoint scores for the FIQ, HADS, MOS-Sleep, MAF and Patient Global Impression of Change (PGIC). Endpoints’ scores were determined using last observation carried forward. The MAF measures fatigue and the effect of fatigue on activities using 16 items resulting in a Global Fatigue Index (MAF-GFI) that can range from 1 to 50, with higher scores indicating greater fatigue severity [Belza, 1995]. The MAF-GFI has been shown a valid fatigue measure in a variety of patient populations with chronic conditions [Neuberger, 2003]. Since there are no set cut-off scores on the MAF to define clinically significant fatigue, a MAF-GFI score of ≥30 was used to define clinically significant fatigue since this score is one standard deviation (SD) above the mean seen in healthy controls [Belza, 1995]. The MOS-Sleep comprises 12 items that measure sleep parameters across six domains yielding a composite Sleep Problems Index II (SPI) score that can range from 0 to 100, with higher scores indicating worse sleep problems [Hays and Stewart, 1992]. The MOS-Sleep has proven relevance for the impact of FM on sleep quality in FM patients [Martin et al. 2009] and is considered the best questionnaire for assessing sleep disturbance in patients with pain [Cole et al. 2007]. The MOS-Sleep has traditionally utilized a recall period of 4 weeks, as utilized in trial A0081056 [Arnold et al. 2008]. However, the US Food and Drug Administration (FDA) has recommended against use of patient-reported outcomes with long recall periods [US Department of Health and Human Services, 2009], and a 1-week recall period has been shown reliable for use of the MOS-Sleep in studies evaluating sleep disturbance in patients with FM [Sadosky et al. 2009], so a 1-week recall period was used in studies A0081077 and A0081100 [Mease et al. 2008b; Pauer et al. 2008]. The MOS-Sleep has been validated for use in patients with FM and has good reliability over time using a test—retest model [Cappelleri et al. 2009; Sadosky et al. 2009]. Since there are no set cutoff scores on the MOS-sleep to define clinically significant sleep problems, an SPI score of ≥50 was used to define clinically significant sleep problems since this score is one SD above the mean seen in healthy controls [Hays and Stewart, 1992] and identifies patients with moderately severe sleep interference [Viala-Danten et al. 2008]. The HADS is one of the most frequently used scales to assess anxiety and depression symptoms in somatically ill patients since its items exclude somatic symptoms of psychologic distress [Zigmond and Snaith, 1983]. The HADS has been validated in numerous patient populations including patients with musculoskeletal disorders and found to be internally consistent for assessing the severity of anxiety and depression symptoms [Pallant and Bailey, 2005]. The HADS consists of two, seven-item subscales to assess anxiety (HADS-A) and depression (HADS-D) symptoms with scores for each that can range from 0 to 21, with higher scores indicating higher levels of each symptom. HADS-A and HADS-D scores ≥11 were used to define clinically significant anxiety and depression levels, respectively, since these are the most widely accepted cutoff scores with a sensitivity and specificity for identifying patients of approximately 80% [Bjelland et al. 2002].
The FIQ is the accepted standard for quantifying the global severity of FM symptoms [Bennett et al. 2009]. The FIQ is composed of 20 items that assess disease severity over the past week with 11 questions to assess physical functioning, two ‘day-of-the-week’ items to quantify the number of days patients ‘felt good’ or ‘missed work’, and seven VASs to assess symptoms of fatigue, sleep quality, depression, anxiety, stiffness, pain and work disability. The 11 FIQ physical functioning items evaluate the ability of patients to perform large muscle activities and each were scored on a 0-to-3 Likert scale, with 0 indicating ‘always’ and 3 indicating ‘never’ [Bennett et al. 2009]. A composite physical functioning score was derived by summing the physical functioning items, dividing by the number of questions answered, and multiplying by 3.33 to yield a 0–10 score. The ‘felt good’ ‘day-of-the-week’ score was derived by reverse scoring (to obtain the number of days patients felt bad) and multiplying the result by 1.43 to yield a 0–10 score. The ‘missed work’ ‘day-of-the-week’ score was derived by multiplying the number of days by 1.43 to yield a 0–10 score. VAS scores were derived by measuring the distance from the origin to the patient mark in centimeters to yield a 0–10 score for each symptom. FIQ global scores were derived by summing the composite physical functioning, ‘day-of-the-week’ and VAS scores, with higher scores indicating more severe FM. To maintain a maximum possible score of 100, an ‘equalization calculation’ was used if patients did not answer all 10 sections by multiplying the global score by 10 and dividing by the number of sections answered. VASFIQ global scores were derived by summing scores from the seven FIQ VASs to yield a 0–70 score, with higher scores indicating more severe FM. Owing to its brevity, VASFIQ global scores were only calculated for patients who answered all VASs.
Statistical analysis
All analyses were conducted on an intention-to-treat population (i.e. randomized patients that took a dose and contributed efficacy data). Summary statistics were described for the demographics data, such as sex, race and age. Baseline efficacy characteristics were descriptively summarized for the FIQ global, VASFIQ global, HADS-D, HADS-A, SPI and MAF-GFI. Spearman rank correlations compared FIQ global scores with VASFIQ global scores at baseline and study endpoints, change in FIQ and VASFIQ global scores from baseline to study endpoints, and individual FIQ VAS scores with corresponding full-length symptom questionnaires at baseline and study endpoints. Spearman rank correlations also were used to compare PGIC scores at study endpoints with change in global FIQ and VASFIQ scores. Percentage of patients with minimal (floor) and maximal (ceiling) values also were determined for individual FIQ VASs and the corresponding full-length symptom scales. Receiver operating characteristic (ROC) [Harris, 2010; Zweig and Campbell, 1993; Hanley and McNeil, 1982; Metz, 1978] analyses of baseline patient data identified cutoff scores on individual FIQ VASs that classified patients with clinically significant symptom levels using corresponding validated questionnaires to define cases (clinically significant cases were defined for anxiety if HADS-A scores were ≥11, depression if HADS-D scores were ≥11, fatigue if MAF-GFI scores were ≥30, and poor sleep if SPI scores were ≥50). We also calculated the sensitivity and specificity of the tests using the identified cutoffs. The intersection of the sensitivity and specificity curves established the cutoff scores on individual FIQ VASs, according to methods previously described [Harris, 2010]. Scatterplot and regression analyses were used to determine corresponding values between global FIQ and VASFIQ scores.
Results
While questionnaire data were derived primarily from white, middle-aged females (Table 1), the cohort included a number of patients from other groups including Hispanics, Blacks, and men. Owing to its brevity, VASFIQ global scores were only calculated for patients who answered all VASs, resulting in the exclusion of only 18 of 2225 patients with FIQ global scores (Table 2). The patients typically had moderate-to-severe FM as indicated by an average FIQ global score >60 (Table 2) [Burckhardt et al. 1991]. Patients also typically had clinically significant symptoms of fatigue and poor sleep quality as evidenced by average MAF-GFI scores > 30 and average SPI scores > 50 (Table 2). Median scores in the HADS-A (score = 9) and HADS-D (score = 7) indicate that our FM cohort had levels of anxiety and depression consistent with those previously recorded in patients with chronic musculoskeletal pain (i.e. higher than in patients with other chronic medical conditions but lower than in outpatients with psychiatric conditions) [Pallant and Bailey, 2005; Zigmond and Snaith, 1983]. Consistent with the full-length questionnaire scores, VAS scores for fatigue and sleep quality tended to be in the severe range (averaging near 8 for both; Table 2), while anxiety and depression VAS scores indicated more moderate symptoms (averaging near 5 for both; Table 2).
Table 1.
Summary of FM patient characteristics.
| Characteristic | All patients (n = 2229) |
|---|---|
| Mean age (SD), years | 49.12 (11.18) |
| Range, years | 18–82 |
| Race, n (%) | |
| White | 1912 (85.8) |
| Black | 69 (3.1) |
| Hispanic | 149 (6.7) |
| Other | 99 (4.4) |
| Gender, n (%) | |
| Women | 2083 (93.4) |
| Men | 146 (6.6) |
| Mean FM duration, months (SD) | 111.28 (95.42) |
| Median | 85 |
| Range | 1–656 |
FM, fibromyalgia; SD, standard deviation.
Table 2.
Summary of fibromyalgia patient baseline questionnaire scores.
| Questionnaire | N | Mean (SD) | Median | Range |
|---|---|---|---|---|
| FIQ global | 2225 | 61.72 (14.73) | 61.9 | 8.8–99.1 |
| VASFIQ global | 2207 | 46.90 (10.63) | 47 | 5.2–70 |
| VASfatigue | 2216 | 7.86 (1.85) | 8.3 | 0.1–10 |
| VASsleep | 2215 | 7.81 (1.94) | 8.3 | 0.2–10 |
| VASanxiety | 2217 | 4.99 (2.94) | 5.2 | 0–10 |
| VASdepression | 2214 | 4.59 (2.98) | 4.7 | 0–10 |
| HADS-A | 2215 | 9.02 (4.42) | 9 | 0–21 |
| HADS-D | 2215 | 7.63 (4.20) | 7 | 0–21 |
| SPI | 2197 | 60.54 (17.75) | 61.4 | 4.5–100 |
| MAF-GFI | 2193 | 36.39 (7.95) | 37.8 | 7.5–50 |
FIQ, Fibromyalgia Impact Questionnaire; FM, fibromyalgia; HADS-A, Hospital Anxiety and Depression Scale—Anxiety sub-scale; HADS-D, Hospital Anxiety and Depression Scale—Depression subscale; MAF-GFI, Multidimensional Assessment of Fatigue — Global Fatigue Index; SD, standard deviation; SPI, Medical Outcomes Study Sleep Problems Index II; VAS, visual analog scale.
To compare global scores on the brief questionnaire VASFIQ with its full-length counterpart FIQ, Spearman correlations for baseline, endpoint and change scores were performed (Table 3). VASFIQ global scores were highly correlated with FIQ global scores at baseline (ρ = 0.94, p<0.0001) and at study endpoints (ρ = 0.97, p<0.0001). Change in VASFIQ and FIQ global scores at study endpoints were also highly correlated with one another (ρ = 0.97, p<0.0001) and moderately correlated with PGIC scores (ρ = 0.61, p<0.0001 for FIQ global and ρ = 0.58, p<0.0001 for VASFIQ global). Individual FIQ VASs scores correlated well with corresponding full-length questionnaire scores at baseline (VASfatigue with MAF-GFI, ρ = 0.67; VASanxiety with HADS-A, ρ = 0.64; VASdepression with HADS-D, ρ = 0.57; VASsleep with SPI, ρ = 0.50; p<0.0001 for all) and at study endpoints (VASfatigue with MAF-GFI, ρ = 0.76; VASanxiety with HADS-A, ρ = 0.67; VASdepression with HADS-D, ρ = 0.62; VASsleep with SPI, ρ = 0.67; p<0.0001 for all). Similarly, change in individual FIQ VAS scores were also correlated with change in the corresponding full-length questionnaire scores at study endpoints (VASfatigue with MAF-GFI, ρ = 0.64; VASanxiety with HADS-A, ρ = 0.47; VASdepression with HADS-D, ρ = 0.43; VASsleep with SPI, ρ = 0.57; p<0.0001 for all; Table 3).
Table 3.
Summary of correlations between questionnaire scores.
| Questionnaire comparison | Baseline | Endpoint | Change |
|---|---|---|---|
| FIQ global versus VASFIQ, ρ | 0.94 | 0.97 | 0.97 |
| PGIC versus FIQ global, ρ | n/a | n/a | 0.61 |
| PGIC versus VASFIQ, ρ | n/a | n/a | 0.58 |
| MAF-GFI versus VASfatigue, ρ | 0.67 | 0.76 | 0.64 |
| HADS-A versus VASanxiety, ρ | 0.64 | 0.67 | 0.47 |
| HADS-D versus VASdepression, ρ | 0.57 | 0.62 | 0.43 |
| SPI versus VASsleep, ρ | 0.50 | 0.67 | 0.57 |
FIQ, Fibromyalgia Impact Questionnaire; HADS-A, Hospital Anxiety and Depression Scale — Anxiety subscale; HADS-D, Hospital Anxiety and Depression Scale — Depression subscale; MAF-GFI, Multidimensional Assessment of Fatigue — Global Fatigue Index; n/a, not applicable; PGIC, Patient Global Impression of Change; SPI, Medical Outcomes Study Sleep Problems Index II; VAS, visual analog scale.
Overlapping scoring density plots of baseline scores were used to compare the distribution of VAS symptom scores to their full-length counterparts (Figure 2). Depiction of scores in the density plots were made comparable by subtracting the mean from each score and dividing by the SD. There was considerable overlap of the scoring distributions between the VASs and the full-length questionnaires. Distributions of full-length questionnaires were normally distributed, with HADS-A and HADS-D scores shifted towards lower scores (Figure 2(a) and (b), respectively), whereas the MAF-GFI and SPI were shifted towards higher scores (Figure 2(c) and (d), respectively). The distribution of VASanxiety and VASdepression scores showed a bimodal pattern centered on the mean (Figure 2(a) and (b), respectively). In contrast, the VASfatigue and VASsleep scoring distributions were unimodal (Figure 2(c) and (d), respectively). VASFIQ global, FIQ global, individual FIQ VAS and full-length symptom questionnaire baseline scores at various patient percentile levels were calculated to compare floor and ceiling effects. No floor effects were seen for either the global VASFIQ or FIQ scales, and ceiling effects were low with 0% for FIQ global and 0.3% for VASFIQ global scores. Floor and ceiling effects were also low for all full-length symptom questionnaires at 1% or less. Floor effects for the VASanxiety and VASdepression scores were 2.2% and 2.7%, respectively, and floor effects for the VASfatigue and VASsleep scores were zero. Ceiling effects for the VASfatigue and VASsleep scores were 6.8% and 7.1%, respectively, while VASanxiety and VASdepression ceiling effects were lower at 1.6% and 1.4%, respectively.
Figure 2.
Overlapping scoring density plots of baseline VAS and full-length questionnaire scores. HADS-A, Hospital Anxiety and Depression Scale — Anxiety subscale; HADS-D, Hospital Anxiety and Depression Scale — Depression subscale; MAF-GFI, Multidimensional Assessment of Fatigue — Global Fatigue Index; SPI, Medical Outcomes Study Sleep Problems Index II; VAS, visual analog scale.
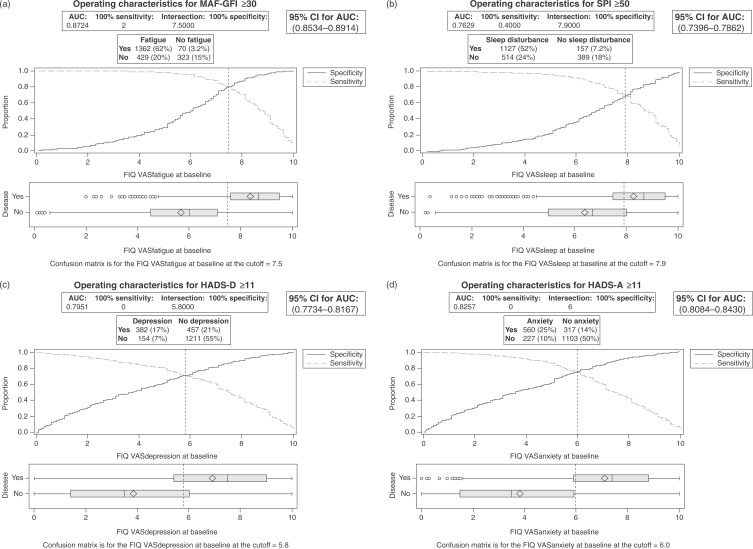
ROC analyses were performed to determine VAS cutoff scores that identified FM patients with clinically significant symptom levels with maximum sensitivity and specificity using the corresponding full-length questionnaires as reference standards. AVASfatigue score of >7.5 was 76.0% sensitive and 82.2% specific for clinically significant fatigue (ROC area under the curve [AUC] = 0.87, 95% confidence interval [CI] = 0.85–0.90; Figure 3(a)). A VASsleep score of > 7.9 was 68.7% sensitive and 71.2% specific for significant sleep disturbance (ROC AUC = 0.76, 95% CI = 0.74–0.79; Figure 3(b)). A VASdepression score of > 5.8 was 71.3% sensitive and 72.6% specific for significant depressive symptoms (ROC AUC = 0.80, 95% CI = 0.77–0.82; Figure 3(c)), and a VASanxiety score of > 6.0 was 71.2% sensitive and 77.7% specific for clinically significant anxiety (ROC AUC = 0.83, 95% CI = 0.81–0.84; Figure 3(d)).
Figure 3.
Receiver operating characteristic analyses for (a) VASfatigue, (b) VASsleep, (c) VASdepression and (d) VASanxiety baseline scores. AUC, area under the curve; CI, confidence interval; FIQ, Fibromyalgia Impact Questionnaire; HADS-A, Hospital Anxiety and Depression Scale — Anxiety subscale; HADS-D, Hospital Anxiety and Depression Scale — Depression subscale; MAF-GFI, Multidimensional Assessment of Fatigue — Global Fatigue Index; ROC, receiver operating characteristic; SPI, Medical Outcomes Study Sleep Problems Index II; VAS, visual analog scale.
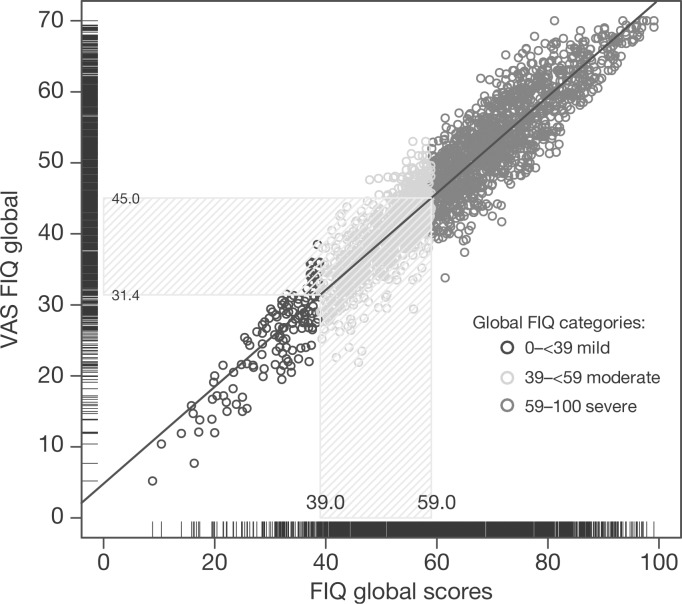
Correspondence between the global VASFIQ and FIQ baseline scores were demonstrated in a scatterplot (Figure 4). FIQ global scores that define moderate (≥39) and severe (≥59) symptoms [Bennett et al. 2010] corresponded to VASFIQ global scores of ≥31.4 and ≥45.0, respectively (Figure 4).
Figure 4.
Correspondence between baseline VASFIQ and FIQ global scores. FIQ, Fibromyalgia Impact Questionnaire; VAS, visual analog scale.
Discussion
The complexity and heterogeneity of symptoms experienced by patients with FM can make them very challenging to manage. To improve management, evidence-based FM guidelines recommend development of an individualized treatment plan tailored according to pain intensity, function and associated features such as depression, fatigue and sleep disturbance; this should be based on a comprehensive assessment of pain, function and psychosocial context [Carville et al. 2008]. In addition, new preliminary diagnostic criteria recommended by the ACR for FM require physicians to quantify symptom severity [Wolfe et al. 2010]. However, comprehensive assessment of FM symptoms can be difficult to perform within the time available in a busy clinical practice. While the FIQ VASs have previously been recommended as a rapid FM assessment [Boomershine and Crofford, 2009], this advice was based on research conducted in a single rheumatology practice on a small number of patients [Boomershine et al. 2008].
The work outlined herein provides support for use of the VASFIQ in FM symptom assessment. Baseline, endpoint and change global scores on the VASFIQ correlated with corresponding FIQ global scores (Table 3 and Figure 4) [Bennett et al. 2010]. This, taken together with the finding that global VASFIQ and FIQ change scores had similar correlations with PGIC endpoint scores (Table 3) and similarly low floor and ceiling effects, suggests that the seven FIQ VAS items can be summed to provide global FM disease severity information. In addition to providing global disease severity information in aggregate, correlations between baseline, followup and change scores of individual VASs and corresponding full-length symptom questionnaires (Table 3) indicate VASFIQ items can quantify the severity of symptoms of fatigue, anxiety, depression and sleep quality. ROC analyses (Figure 3) also demonstrate that cutoff scores on the FIQ VASs can be identified that characterize FM patients with clinically significant symptoms of fatigue (>7.5), poor sleep (>7.9), anxiety (>6.0) and depression (>5.8).
The symptoms measured by the VASFIQ are widely regarded as clinically relevant to patients with FM. A Delphi exercise studying the impact of FM symptoms on patients’ lives demonstrated that pain, fatigue and sleep disturbance are among the most detrimental [Mease et al. 2008a]; this led researchers to classify these symptoms among a core set of domains that are essential to measure in all clinical FM trials [Mease et al. 2009]. Depression was classified among domains to be assessed at some point in a clinical development program, whereas anxiety and stiffness were considered domains of research interest. In addition, a cluster analysis performed to identify clinical features of FM that patients would most like to see improved found that clusters of pain and fatigue symptoms were selected most frequently at 90% and 89%, respectively [Bennett et al. 2010]. Disturbed sleep was included as a clinical feature in the pain cluster and was identified as the second most important individual symptom behind ‘pain or discomfort’. The affective cluster, which included feelings of anxiety or depression, was selected fifth overall at 21%.
VASs have been used since the 1920s to measure subjective phenomena owing to their brevity, universality, ease of use and scoring [Wewers and Lowe, 1990]. These properties, along with research showing the performance of VASs rival longer scales with respect to correlation with clinical variables and sensitivity to change [Wolfe, 2004], have led to widespread use of VASs in both clinical medicine and research. VASs have shown great utility in measuring numerous symptoms relevant to FM including mood, sleep quality, functional ability and pain [Wewers and Lowe, 1990]. VASs measuring fatigue also have been shown to be highly reproducible and sensitive for quantifying symptom severity and treatment response in multiple patient populations [Wolfe, 2004; Grant et al. 1999]. Similar to the VASFIQ, VAS questionnaire sets have been used previously in other populations with complex symptom burdens such as cancer patients being treated with chemotherapy [Padilla et al. 1983; Presant et al. 1981]. As with the VASFIQ, these scales used individual VAS scores to quantify specific symptoms and combined VAS scores to measure overall quality of life.
The present work has several limitations. Numerous limitations were introduced by the types of patients included in the treatment trials from which the data were collected [Arnold et al. 2008; Mease et al. 2008b; Pauer et al. 2008]. Study participants consisted primarily of white, middle-aged females (Table 1), which may limit applicability of these data to other populations. All patients included in the trials were required to meet ACR classification criteria for FM and have baseline pain scores in the moderate-to-severe range. These inclusion criteria are likely to have skewed the study population toward patients with more severe symptoms than the average patient with FM typically seen in clinical practice, as patients who fulfill the ACR criteria are known to have more severe symptom levels [Katz et al. 2006]. Conversely, patients were excluded from the trials if they had severe depression or unstable psychiatric disorders, and this may have falsely reduced scores on psychiatric assessments compared with those seen in routine clinical practice. However, the proportions of patients with significant depressive or anxiety symptoms as determined by the HADS (approximately 25% for depression and 40% for anxiety) are similar to those previously reported for patients in the community with FM [Herrmann, 1997].
The study also was limited by the full-length questionnaires available for use as reference standards to characterize patients with clinically significant symptom levels. The present analyses were performed on data from pre-existing pregabalin FM clinical trials [Arnold et al. 2008; Mease et al. 2008b; Pauer et al. 2008], and so we were dependent on the questionnaires that were utilized in these trials. The HADS is the best available self-report questionnaire for assessing the severity of anxiety and depression symptoms in patients with FM since it was specifically designed to exclude the effects of complicating physical and emotional symptoms of medical illness [Zigmond and Snaith, 1983] and its structure has proven validity in numerous populations including patients with chronic musculoskeletal pain [Pallant and Bailey, 2005]. While the HADS has a sensitivity and specificity for identifying patients with clinically significant depression and anxiety symptoms of approximately 80% [Bjelland et al. 2002], it is not a diagnostic instrument. Accurate diagnosis of depressive and anxiety disorders requires a structured diagnostic interview; an interview such as the MiniInternational Neuropsychiatric Interview (MINI) would be preferred to categorize patients with clinically significant symptoms [Sheehan et al. 1998]. Similarly, while the MOS-Sleep has demonstrated validity and reliability in quantifying the severity of sleep symptoms in FM patients [Cappelleri et al. 2009; Sadosky et al. 2009], it is not a diagnostic instrument. A structured clinical interview or a questionnaire such as the Athens Insomnia Scale based on International Classification of Diseases, Tenth Revision criteria for insomnia diagnosis with predetermined cutoff scores would have been preferable for identifying patients with clinically significant sleep disturbance [Soldatos et al. 2000]. Future studies using gold-standard assessments to diagnose FM patients with mood disorders and insomnia are needed to assess the accuracy of FIQ VAS cut points identified in the current study.
The VASFIQ has some inherent limitations that could impact its clinical utility [Wewers and Lowe, 1990]. Patients sometimes have difficulty understanding the VAS concept, and initial training is required to ensure accurate completion. VASFIQ completion requires self-administration of a paper questionnaire and VAS scoring requires distance measurement with a ruler, both of which can be cumbersome to perform in the clinic setting. Oral administration of the VASFIQ using a 0–10 numeric rating scale has been proposed as a way to circumvent these issues [Boomershine, 2010; Boomershine and Crofford, 2009]. However, since oral administration would require changes to the VASFIQ, including addition of a small introductory explanation and third-person wording of the items, validation of an interviewer-administered version is needed before it can be recommended. While the VASFIQ assesses many symptoms of clinical importance in FM [Mease et al. 2009], it fails to evaluate the severity of cognitive dysfunction necessary for ACR FM diagnostic criteria [Wolfe et al. 2010].
Two revisions of the FIQ VASs have sought to address these limitations [Boomershine, 2010; Bennett et al. 2009]. A revision of the FIQ (FIQR) has been developed recently that provides a rapid alternative to the full-length FIQ [Bennett et al. 2009]. This patient questionnaire is longer than the VASFIQ, uses a different format for answering questions, and includes a subscale for patient function. Although the FIQR shows promise as a screening tool, it has only been tested in two studies: an online and focus group study [Bennett et al. 2009], and an interventional pilot study [Carson et al. 2010]. Further validation in the clinical setting is necessary before it can be recommended. While VASs perform well in quantifying baseline symptom severity and gauging symptom improvement at follow up, they often perform poorly in assessing symptom worsening over time [Farrar et al. 2001]. VASs perform poorly at follow up when baseline scores are at or near the upper limit, as seen with the VASfatigue and VASsleep items, because ceiling effects do not allow patients to indicate symptom worsening. This is an important limitation of using VASs in FM management, because medications that improve some FM symptoms often worsen other symptoms and many patients with FM have high baseline VAS scores. A revision of the VASFIQ that utilizes more extreme wording for the upper anchors to decrease ceiling effects and a patient impression of change format at follow up has been proposed to improve assessment of symptom worsening in patients with FM [Boomershine, 2010], but this revision needs to be validated.
Finally, we want to stress that the VASFIQ should not be used in isolation to make treatment decisions. The VASFIQ is a rapid screen that can quantify symptom severity, but it is not intended for use as a diagnostic instrument. A careful clinical evaluation is required to identify the underlying cause of symptoms and determine the most appropriate course of therapy. The VASFIQ can improve FM management by providing a mechanism for patients to effectively articulate their symptom burden and enhance the ability of clinicians to interpret the patient's complaints into a coherent intellectual framework from which to make diagnostic and treatment decisions.
Acknowledgements
Editorial support was provided by Patricia McChesney, PhD, of UBC Scientific Solutions and was funded by Pfizer Inc.
Footnotes
This study was funded by Pfizer Inc. Chad S. Boomershine is supported by the NIH (award number K08DK080219).
Chad S. Boomershine has previously received research support from Pfizer Inc. for developing the VASFIQ tool. Dr. Boomershine also serves as a consultant for Pfizer Inc., Eli Lilly and Company and Forest Pharmaceuticals Inc. Birol Emir and Gergana Zlateva are full-time employees of Pfizer Inc. Yi Wang performed a paid internship at Pfizer Inc.
References
- Arnold L.M., Russell I.J., Diri E.W., Duan W.R., Young J.P., Jr., Sharma U., et al. (2008) A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain 9: 792–805 [DOI] [PubMed] [Google Scholar]
- Belza B.L. (1995) Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol 22: 639–643 [PubMed] [Google Scholar]
- Bennett R.M., Friend R., Jones K.D., Ward R., Han B.K., Ross R.L. (2009) The Revised Fibromyalgia Impact Questionnaire (FIQR): Validation and psychometric properties. Arthritis Res Ther 11: R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R.M., Russell J., Cappelleri J.C., Bushmakin A.G., Zlateva G., Sadosky A. (2010) Identification of symptom and functional domains that fibromyalgia patients would like to see improved: A cluster analysis. BMC Musculoskelet Disord 11: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. (2002) The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52: 69–77 [DOI] [PubMed] [Google Scholar]
- Boomershine C.S. (2010) The FIBRO System: A rapid strategy for assessment and management of fibromyalgia syndrome. Therapeut Adv Musculoskel Dis 2: 187–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomershine C.S., Crofford L.J. (2009) A symptom-based approach to pharmacologic management of fibromyalgia. Nat Rev Rheumatol 5: 191–199 [DOI] [PubMed] [Google Scholar]
- Boomershine C.S., Titova D., Chung C.P., Oeser A., Stein M. (2008) Five visual analogue scales quantify global disease severity and identify clinically significant symptoms in fibromyalgia syndrome. Arthritis Rheum 58: S686–S687 [Google Scholar]
- Burckhardt C.S., Clark S.R., Bennett R.M. (1991) The fibromyalgia impact questionnaire: Development and validation. J Rheumatol 18: 728–733 [PubMed] [Google Scholar]
- Cappelleri J.C., Bushmakin A.G., McDermott A.M., Dukes E., Sadosky A., Petrie C.D., et al. (2009) Measurement properties of the Medical Outcomes Study Sleep Scale in patients with fibromyalgia. Sleep Med 10: 766–770 [DOI] [PubMed] [Google Scholar]
- Carson J.W., Carson K.M., Jones K.D., Bennett R.M., Wright C.L., Mist S.D. (2010) A pilot randomized controlled trial of the Yoga of Awareness program in the management of fibromyalgia. Pain 151: 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carville S.F., Arendt-Nielsen S., Bliddal H., Blotman F., Branco J.C., Buskila D., et al. (2008) EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 67: 536–541 [DOI] [PubMed] [Google Scholar]
- Cole J.C., Dubois D., Kosinski M. (2007) Use of patient-reported sleep measures in clinical trials of pain treatment: A literature review and synthesis of current sleep measures and a conceptual model of sleep disturbance in pain. Clin Ther 29(Suppl): 2580–2588 [DOI] [PubMed] [Google Scholar]
- Farrar J.T., Young J.P., Jr., LaMoreaux L., Werth J.L., Poole R.M. (2001) Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94: 149–158 [DOI] [PubMed] [Google Scholar]
- Grant S., Aitchison T., Henderson E., Christie J., Zare S., McMurray J., et al. (1999) A comparison of the reproducibility and the sensitivity to change of visual analogue scales, Borg scales, and Likert scales in normal subjects during submaximal exercise. Chest 116: 1208–1217 [DOI] [PubMed] [Google Scholar]
- Hanley J.A., McNeil B.J. (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143: 29–36 [DOI] [PubMed] [Google Scholar]
- Harris K. (2010) ROC hard? No ROC made easy! In: SAS Global Forum 2010, Seattle, WA, available at: http://support.sas.com/resources/papers/proceedings10/222-2010.pdf
- Hays R.D., Stewart A.L. (1992) Sleep measures, In: Stewart A.L., Ware J.E. (eds). Measuring Functioning and Well-being: The Medical Outcomes Study Approach, Duke University Press: Durham, NC, pp. 235–259 [Google Scholar]
- Herrmann C. (1997) International experiences with the Hospital Anxiety and Depression Scale—a review of validation data and clinical results. J Psychosom Res 42: 17–41 [DOI] [PubMed] [Google Scholar]
- Katz R.S., Wolfe F., Michaud K. (2006) Fibromyalgia diagnosis: A comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum 54: 169–176 [DOI] [PubMed] [Google Scholar]
- Martin S., Chandran A., Zografos L., Zlateva G. (2009) Evaluation of the impact of fibromyalgia on patients’ sleep and the content validity of two sleep scales. Health Qual Life Outcomes 7: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P.J., Arnold L.M., Choy E.H., Clauw D.J., Crofford L.J., Glass J.M., et al. (2009) Fibromyalgia syndrome module at OMERACT 9: Domain construct. J Rheumatol 36: 2318–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P.J., Arnold L.M., Crofford L.J., Williams D.A., Russell I.J., Humphrey L., et al. (2008a) Identifying the clinical domains of fibromyalgia: Contributions from clinician and patient Delphi exercises. Arthritis Rheum 59: 952–960 [DOI] [PubMed] [Google Scholar]
- Mease P.J., Russell I.J., Arnold L.M., Florian H., Young J.P., Jr., Martin S.A., et al. (2008b) A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol 35: 502–514 [PubMed] [Google Scholar]
- Metz C.E. (1978) Basic principles of ROC analysis. Semin Nucl Med 8: 283–298 [DOI] [PubMed] [Google Scholar]
- Neuberger G.B. (2003) Measures of Fatigue, The Fatigue Questionnaire, Fatigue Severity Scale, Multidimensional Assessment of Fatigue Scale, and Short Form-36 Vitality (Energy/Fatigue) Subscale of the Short Form Health Survey. Arthritis Rheum 49: S175–S183 [Google Scholar]
- Padilla G.V., Presant C., Grant M.M., Metter G., Lipsett J., Heide F. (1983) Quality of life index for patients with cancer. Res Nurs Health 6: 117–126 [DOI] [PubMed] [Google Scholar]
- Pallant J.F., Bailey C.M. (2005) Assessment of the structure of the Hospital Anxiety and Depression Scale in musculoskeletal patients. Health Qual Life Outcomes 3: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauer L., Danneskiold-Samsoe B., Jespersen A., Atkinson G., Whelan L., Leon T., et al. (2008) Pregabalin for management of fibromyalgia (FM): A 14-week, randomized, double-blind, placebo-controlled, monotherapy trial (study A0081100). Ann Rheum Dis 67(Suppl. II): 25617604285 [Google Scholar]
- Presant C.A., Klahr C., Hogan L. (1981) Evaluating quality-of-life in oncology patients: Pilot observations. Oncol Nurs Forum 8: 26–30 [PubMed] [Google Scholar]
- Sadosky A., Dukes E., Evans C. (2009) Reliability of a 1-week recall period for the Medical Outcomes Study Sleep Scale (MOS-SS) in patients with fibromyalgia. Health Qual Life Outcomes 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl. 20): 22–33, quiz 34–57. [PubMed] [Google Scholar]
- Soldatos C.R., Dikeos D.G., Paparrigopoulos T.J. (2000) Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J Psychosom Res 48: 555–560 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (2009) Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims, available at: http://www.fda.gov/downloads/Drugs/GuideanceComplianceRegulatoryInformation/GuidancesAUCM193282.pdf [DOI] [PMC free article] [PubMed]
- Viala-Danten M., Martin S., Guillemin I., Hays R.D. (2008) Evaluation of the reliability and validity of the Medical Outcomes Study sleep scale in patients with painful diabetic peripheral neuropathy during an international clinical trial. Health Qual Life Outcomes 6: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers M.E., Lowe N.K. (1990) A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 13: 227–236 [DOI] [PubMed] [Google Scholar]
- Wolfe F. (1990) Fibromyalgia. Rheum Dis Clin North Am 16: 681–698 [PubMed] [Google Scholar]
- Wolfe F. (2004) Fatigue assessments in rheumatoid arthritis: Comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J Rheumatol 31: 1896–1902 [PubMed] [Google Scholar]
- Wolfe F., Clauw D.J., Fitzcharles M.A., Goldenberg D.L., Katz R.S., Mease P., et al. (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 62: 600–610 [DOI] [PubMed] [Google Scholar]
- Wolfe F., Smythe H.A., Yunus B., Bennett R.M., Bombardier C., Goldenberg D.C., et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum 33: 160–172 [DOI] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361–370 [DOI] [PubMed] [Google Scholar]
- Zweig M.H., Campbell G. (1993) Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 39: 561–577 [PubMed] [Google Scholar]