Abstract
Cutaneous adverse reactions are reported for many therapeutic agents and, in general, are observed in between 0% and 8% of treated patients depending on the drug. Antiosteoporotic agents are considered to be safe in terms of cutaneous effects, however there have been a number of case reports of cutaneous adverse reactions which warrant consideration. This was the subject of a working group meeting of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis in April 2009, which focused on the impact of cutaneous adverse reactions and drug-induced hypersensitivity in the management of postmenopausal osteoporosis. This position paper was drafted following these discussions and includes a flowchart for their recognition. Cutaneous adverse reactions observed with antiosteoporotic agents were reviewed and included information from case reports, regulatory documents and pharmacovigilance. These reactions ranged from benign effects including exanthematous or maculopapular eruption (drug rash), photosensitivity and urticaria, to the severe and potentially life-threatening reactions of angioedema, drug rash with eosinophilia and systemic symptoms (DRESS), Stevens Johnson syndrome and toxic epidermal necrolysis. A review of the available evidence demonstrates that cutaneous adverse reactions occur with all commonly used antiosteoporotic treatments. Notably, there are reports of Stevens Johnson syndrome and toxic epidermal necrolysis for bisphosphonates, and of DRESS and toxic epidermal necrolysis for strontium ranelate. These severe reactions remain very rare (<1 in 10,000 cases). In general, with proper management and early recognition, including immediate and permanent withdrawal of the culprit agent, accompanied by hospitalization, rehydration and systemic corticosteroids if necessary, the prognosis is positive.
Keywords: antiosteoporosis treatments, cutaneous adverse reactions, hypersensitivity reactions, osteoporosis
Introduction
The general definition of an adverse drug reaction is any reaction resulting from an intervention related to the use of a medicinal product [Edwards and Aronson, 2000]. Although such reactions are frequent and can reach many organs, the skin is a common site for adverse drug reactions, and current estimates are that 2.2% of all hospitalized patients have cutaneous adverse reactions to their treatment [Hunziker et al. 1997]. Cutaneous adverse reactions are reported for a wide range of therapeutic agents and are observed in between 0% and 8% of patients for most drugs, with the highest rates reported for antibiotics (1—8%) and nonsteroidal anti-inflammatory drugs (NSAIDs) (0.3—0.7%) [Bigby, 2001; Hunziker et al. 1997]. Most are due to drug-induced hypersensitivity, that is, activation of an unexpected and exaggerated immune response, and clinically resemble an allergic reaction or viral disease.
Although drugs used to treat osteoporosis are considered safe in terms of cutaneous effects, reported cases have appeared in the literature of a variety of cutaneous adverse reactions associated with antiosteoporotic therapy. Owing to the rarity of these events, the circumstances for cutaneous adverse reactions associated with anti-osteoporotic agents had never been reviewed. Therefore, a working group organized by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis in April 2009 focused on the impact of cutaneous adverse reactions and drug-induced hypersensitivity in managing postmenopausal osteoporosis. This review was drafted following these discussions and aims to describe the recognition and management of cutaneous adverse reactions and drug-induced hypersensitivity, in addition to reviewing current knowledge on antiosteoporotic agents in clinical use. This should help facilitate the early recognition and appropriate management of any such cases occurring with antiosteoporotic agents.
Methods
Relevant articles, authorative reviews and case reports were identified through a PubMed/MEDLINE search of English-language articles published between 1996 and February 2009. The search strategy included the terms osteoporosis, pharmacovigilance, dermatology, cutaneous adverse reaction, hypersensitivity, rash, eruption, urticaria, photosensitivity, drug rash with eosinophilia and systemic symptoms (DRESS), Stevens Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), bisphospho-nate, alendronate, risedronate, ibandronate, zole-dronic acid, raloxifene, strontium ranelate, teriparatide, and parathyroid hormone. Separate subsearches were also performed using the above terms and a filter of case reports, as well as a cross search using combinations of the above terms. Overall, 646 articles were detected, 17 of which described case reports of cutaneous adverse reactions to antiosteoporotic agents which were selected by the authors for inclusion in this review. Event rates were determined from the current European or American regulatory files, as well as postmarketing data collected from the Pharmapendium website for the more severe events [US Food and Drug Administration (FDA), 2009]. The Pharmapendium database is principally supplied with data from the FDA Approval Package Database and Adverse Event Reporting System (ARES; last updated January 2009), but also includes information from other sources, notably the European Medicines Agency (EMA) European Public Assessment Report database.
Recognizing drug-induced cutaneous adverse reactions
Cutaneous adverse reactions are reported for many therapeutic agents and there are a number of factors that may predict an adverse reaction or drug-induced hypersensitivity. In relation to drug-related aspects, high molecular weight (>1000 Da), cytotoxicity and direct binding to immune receptors, such as T-cell receptors, and major histocompatibility complex, may all increase the risk for an immunogenic or allergenic response [Gomes and Demoly, 2005]. Other risk factors are related to patient profiles, for example, adverse drug reactions appear to be more common in women than in men, and in certain ethnic groups. Genetic predisposition to severe drug-induced hypersensitivity to allopuri-nol or carbamazepine has been described in Han Chinese carrying certain genetic markers for human leukocyte antigen (HLA) [Hung et al. 2005; Chung et al. 2004], suggesting a possible route to testing for such reactions. In this context, a randomized trial has indicated that screening patients with human immunodeficiency virus (HIV) for the presence of an HLA allele known to be associated with hypersensitivity to abacavir can reduce the risk of an adverse reaction [Mallal et al. 2008].
Infections, particularly viral infections, also considerably increase the risk for an allergic response to drugs. Pathogenic links between drug-induced hypersensitivity and a range of viruses have been postulated, including the herpes simplex virus HHV-6, the Epstein—Barr virus, cytomegalovirus, HIV, influenza and viral hepatitis [Eshki et al. 2009; Mockenhaupt, 2007; Gomes and Demoly, 2005]. The presence of connective tissue disease or autoimmune disease has also been suggested to increase risk.
Owing to the rarity of these reactions and the overlap between syndromes, diagnosis outside the dermatology clinic is difficult. Beyond the clinical presentation, better knowledge of culprit agents and of the delay to onset after treatment initiation can help differential diagnosis. Selected cutaneous adverse reactions are therefore summarized in Table 1 [Valeyrie-Allanore et al. 2007; Roujeau, 2005]. The cutaneous and systemic symptoms, the delay to onset, and the results of laboratory tests (for hypereosinophilia, renal or hepatic disorders for example) help determine causality. The list includes the benign reactions of exanthematous or maculopapular eruptions (drug rash), photosensitivity and urticaria, in addition to the more severe cutaneous adverse reactions of angioedema, DRESS, SJS, and TEN, otherwise known as Lyell syndrome, all of which are potentially life threatening (Figure 1). This review is limited to cutaneous adverse reactions reported for antiosteoporotic agents and therefore does not cover reactions such as acute generalized exanthematous pustulosis, anaphylactic shock or fixed drug eruptions. For information on those reactions, the reader is referred to a number of exhaustive reviews on the topic [Aberer and Kranke, 2008; Roujeau, 2005].
Table 1.
Identification of cutaneous adverse reactions.
| Cutaneous adverse reaction | Delay to reaction onset | Clinical signs and symptoms | Common culprit drugs | Incidence of cutaneous adverse reaction |
|---|---|---|---|---|
| Photosensitivity | Hours or days after sunlight exposure | Erythema linked to ultraviolet UVA radiation exposure | Fluoroquinolone antibiotics, NSAIDs, amiodarone, tricyclic antidepressants, thiazide diuretics, quinidine | Photoallergy: 1—10 cases per 10,000 patients |
| Exanthematous or maculopapular eruptions | 4—15 days | Erythematous macules or papules, beginning onthe trunk and upper extremities, mucosal involvement rare Low-grade fever | Allopurinol, antiepileptic agents, antibacterial sulfonamides, aminopenicillins, and cephalosporins | > 3% of patients |
| Urticaria | < 24 hours | Transient eruptionof erythematousand oedematous papules and laques, associated pruritus Associated with angioedema in 50% of cases | Antibiotics, aspirin, general anaesthetics, NSAIDs, angiotensinconverting enzyme inhibitors | 1—10 cases per 10,000 patients |
| Drug rash with eosinophiliaand systemic symptoms | 2—6 weeks | Severe generalized eruption, facial oedema, macularor papular, hemorrhagic orbullous exanthemaand erythematous centrofacialswelling Fever, general malaise, lymphadenopathy, eosinophilia, nephritis, interstitial pneumonia and hepatitis | Sulfonamides, aromaticantiepileptic agents, allopurinol, NSAIDs, captopril, lamotrigine, antibiotics, tuberculostatic drugs, neuroleptics, calcium channel blockers | 1 case per 10,000 patients (data for antiepileptics and ulfonamides) [Wolf et al. 2005] |
| Stevens Johnson syndrome | 5—10 days | Blisters on purple macula ortarget-like lesions, widespread but predominanton trunk, epidermal detachment on 10% of bodysurface area, evere erosion of mucous membrane Fever | Antibacterial sulfonamides, anticonvulsants, allopurinol, sulfasalazine, nevirapine | 1—6 cases per million per year [Borchers et al., 2008] |
| Toxic epidermal necrolysis (Lyell syndrome) | 5—10 days | Blisters on purple macula ortarget-like lesions, widespread but predominanton trunk, epidermal detachment on 30% of bodysurface area, severe erosion of mucous membrane Fever | Antibacterial sulfonamides, anticonvulsants, allopurinol, sulfasalazine, nevirapine | 1—2 cases per million per year [Borchers et al., 2008] |
NSAIDs, nonsteroidal anti-inflammatory drugs.
Figure 1.
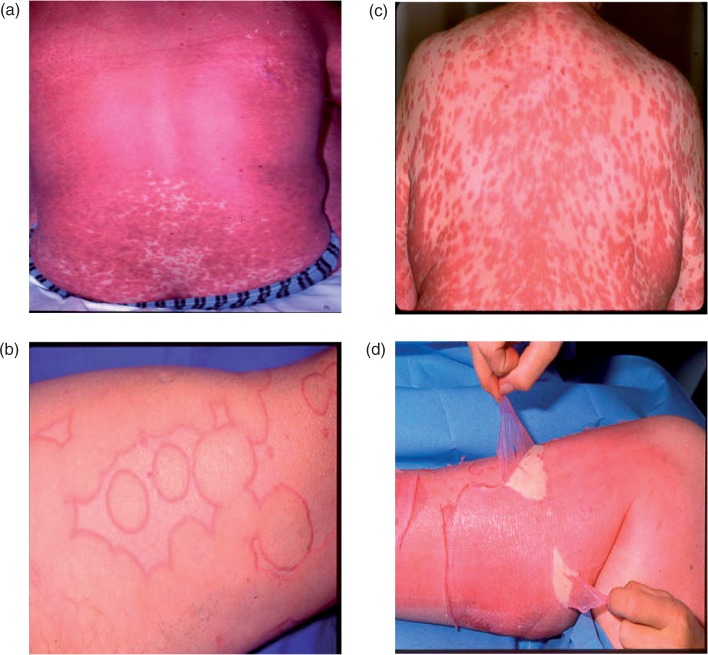
Severe cutaneous adverse reactions. (a) Exanthematous or maculopapular eruption, (b) urticaria, (c) drug rash with eosinophilia and systemic symptoms, (d) Stevens Johnson syndrome/toxic epidermal necrolysis. Reproduced with permission from the Department of Dermatology, Rouen University Hospital, Rouen, France.
Photosensitivity
Drug-induced photosensitivity is the occurrence of an erythema, or an exaggerated sunburn, a few hours or days after exposure to sunlight in a treated patient [Valeyrie-Allanore et al. 2007; Morison, 2004; Svensson et al. 2001]. Individual response is determined by dose and variations in absorption and metabolism, but also by skin phenotype, with fair-skinned people being the most susceptible. Photosensitivity reactions have been reported for a large number of agents, including fluoroquinolone antibiotics, NSAIDs, amiodarone, tricyclic antidepressants, thiazide diuretics and quinidine. Most drugs associated with photosensitivity reactions absorb in the ultraviolet UVA region.
Photosensitivity reactions are further divided into phototoxic and photoallergic reactions [Valeyrie-Allanore et al. 2007; Morison, 2004; Svensson et al. 2001]. A phototoxic reaction may occur in any individual receiving the culprit agent and may be reduced by distancing intake from exposure to sunlight, for example, by dosing in the evening, or by reducing sun exposure during treatment. Photoallergic reactions correspond to drug-induced hypersensitivity, and are more typical of delayed-type immune-mediated reactions. The incidence of photoallergy is generally rare (1—10 cases per 10,000). It is more likely to be eczematous and pruritic in nature and is usually transient, although it may persist in rare cases for months or years.
Exanthematous or maculopapular eruption
Exanthematous or maculopapular eruptions are the most common cutaneous adverse reactions and are often more simply described as ‘drug rash’ or a ‘drug eruption’. Clinically, these begin as erythematous macules or papules on the trunk and upper extremities (Figure 1(a)), which progressively become confluent and spread symmetrically downward. Typically, the eruption is polymorphous, although it may be associated with morbilliform, urticarial or purpuric lesions. Mucosal involvement is rare. The eruption may be accompanied by a low-grade fever.
Exanthematous or maculopapular eruptions have been reported for most drugs with a rate of about 1% of users [Hunziker et al. 1997]. Higher rates (>3% of users) have been reported for the anti-urolithic agent, allopurinol, antiepileptic agents and antibacterial sulfonamides, aminopenicillins and cephalosporins. This benign rash accounts for over 90% of all cutaneous adverse reactions [Hunziker et al. 1997] and generally requires little more than drug withdrawal and symptomatic treatment. The delay to onset of reaction is between 4 and 15 days after treatment initiation, although it may appear 2 days after treatment cessation (the so-called ‘9th day’ eruption).
Urticaria and angioedema
Urticaria consists of a transient eruption of itchy erythematous and oedematous papules and plaques (Figure 1(b)), often associated with pruritus [Valeyrie-Allanore et al. 2007; Roujeau, 2005]. The reaction is termed angioedema when it involves dermal and subcutaneous tissues. In about 50% of cases, urticaria is associated with angioedema. More severe cases may include angioedema of the buccal mucosa, tongue, larynx and pharynx, and possibly other systems, leading to anaphylactic shock. The eruption may occur anywhere on the body and fever may occur in cases with extensive facial angioedema.
Urticaria is a common reaction to many drugs and is associated, for example, with antibiotics, aspirin and general anaesthetics. NSAIDs and angiotensin-converting enzyme (ACE) inhibitors are known to produce combined urticaria and angioedema. The incidence of these reactions remains low, at around 1 case per 10,000, with slightly higher rates for ACE inhibitors (2—10 cases per 10,000) [Roujeau, 2005].
Drug-induced urticaria and angioedema usually appear within 24 h of intake. One strong feature of these reactions is the expanding and fading over a day — the reaction may last for a few hours and then disappear within 24 h without leaving any scarring [Svensson et al. 2001]. These effects generally resolve spontaneously and completely on withdrawal of the culprit drug.
Drug rash with eosinophilia and systemic symptoms
The DRESS syndrome, also identified as ‘drug hypersensitivity syndrome’ or ‘drug-induced hypersensitivity syndrome’, was first described in 1939, a year after the introduction of phenytoin [Wolf et al. 2005]. The DRESS syndrome includes a severe cutaneous eruption (Figure 1(c)), as well as lymph node enlargement, fever, and systemic involvement such as hepatitis, interstitial nephritis, interstitial pneumonia and hae-matological abnormalities [Shiohara et al. 2007; Valeyrie-Allanore et al. 2007; Wolf et al. 2005; Bocquet et al. 1996]. The multisystem involvement, including visceral tissue, clinically differentiates DRESS from a simple drug rash.
The cutaneous reaction begins as a macular erythema, progressing into asymmetrical, red, pruritic, confluent, papular eruption, possibly with pustules. Mucosal involvement may be present and facial oedema is frequent. The eruption begins on the upper trunk and face and descends to the lower extremities. Clinically, the patient presents with general malaise and a high and spiking fever (>38.5°C) [Wolf et al. 2005], although tissue culture is negative for underlying infection. In 70% of cases, the predominant biological finding is hypereosinophilia. In terms of visceral tissue, the kidney and liver are involved, with varying degrees of hepatitis in 60% of cases. Lymphadenopathy is also frequent because of lymphoid hyperplasia. More rarely, the brain, heart, lungs and thyroid may be implicated.
A distinctive element of the DRESS syndrome is the delay to onset, which occurs between 2 and 6 weeks after treatment initiation. The rate of mortality is generally reported to be between 8% and 10% [Roujeau, 2005]. Rapid drug withdrawal and appropriate well-informed management, including rapid initiation of systemic corticosteroids, can improve the prognosis.
Agents known to provoke a DRESS syndrome include sulfonamides, aromatic antiepileptic agents (e.g. phenytoin and phenobarbital), allopurinol, NSAIDs, captopril, lamotrigine, antibiotics, tuberculostatic drugs, neuroleptics and calcium channel blockers. The incidence of DRESS is estimated at between 1 and 10 cases per 10,000 for antiepileptic agents and sulfonamides [Valeyrie-Allanore et al. 2007; Wolf et al. 2005]. The factors implicated in the severity of this syndrome remain unclear, although the presence of HHV-6 infection may exacerbate the prognosis in relation to visceral involvement [Eshki et al. 2009].
Stevens Johnson syndrome and toxic epidermal necrolysis
Of the cutaneous adverse reactions, SJS and TEN are the most severe [Valeyrie-Allanore et al. 2007; Roujeau, 2005; Wolf et al. 2005]. The eruption begins as small blisters on purple maculae and target-like lesions predominantly on the trunk, and rapidly extends to the rest of the body, including severe erosion of the mucous membrane. Confluence of the necrotic lesions leads to extensive erythema and epidermal detachment (Figure 1(d)). The actual diagnosis is determined by the extent of the epidermal detachment, with SJS demonstrating epidermal detachment on less than 10% of the body surface. TEN is more severe than SJS, with the same lesions, but leading to epidermal detachment on over 30% of the body surface (intermediate cases of 10—30% are defined as SJS—TEN overlap) [Borchers et al. 2008; Roujeau, 2005]. Fever precedes the cutaneous and mucosal eruption by 24—48 h. Systemic involvement includes mild elevation of liver enzymes, as well as pulmonary and intestinal manifestations, with detachment of epithelia similar to the cutaneous effects, leading to respiratory distress and diarrhoea [Roujeau, 2005]. In the case of pulmonary involvement, the prognosis may be very poor.
The delay to onset of SJS and TEN is generally 5—10 days after treatment initiation. The rate of mortality depends on the severity of the lesions and is currently reported to be about 10% for SJS and over 30% for TEN [Valeyrie-Allanore et al. 2007], mainly due to respiratory failure or sepsis. Mortality can be reduced by rapid recognition and an effective management strategy. SJS and TEN are extremely rare, with an estimated incidence of 1—6 cases per million per year, about 70% of which are linked to adverse drug reactions. Drugs associated with a higher risk of SJS and TEN [Mockenhaupt et al. 2008] include antibacterial sulfonamides, antiepileptic agents, allopurinol, sulfasalazine, and the antiretroviral agent nevirapine.
Management of cutaneous adverse reactions
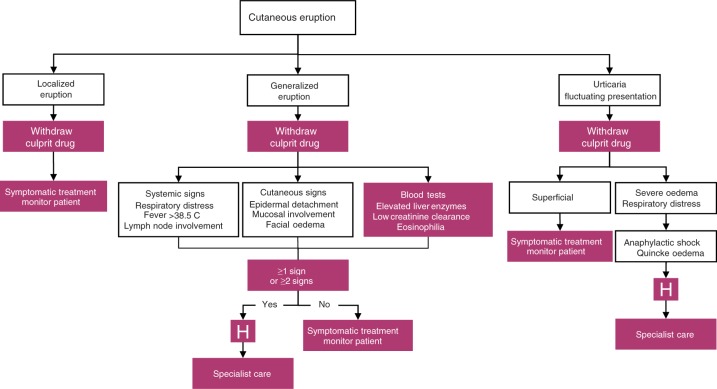
Management strategies depend on the type and severity of the reaction [Aberer and Kranke, 2008], but should always remain on the side of caution. In Figure 2, a decision flowchart is presented for the physician faced with a cutaneous eruption in a patient receiving a new treatment. The most important action is to immediately withdraw the culprit agent, and refer for specialist care if necessary.
Figure 2.
Decision flowchart in the event of cutaneous eruptions in patients receiving newly prescribed treatments.
Symptomatic treatment (emollients) and monitoring may be the only intervention necessary in the management of the more benign cutaneous adverse reactions of exanthematous or maculopapular eruption (drug rash), photosensitivity, urticaria and angioedema. More severe forms of these benign eruptions may require systemic anti-histamines and topical corticosteroids, and intravenous adrenaline in the most severe cases of angioedema bordering on anaphylactic shock. Rechallenge should be avoided.
In cases of severe cutaneous adverse reaction, immediate and permanent withdrawal of the culprit agent is essential. The DRESS syndrome requires systemic corticosteroid therapy (0.5—1mg/kg) in cases of visceral involvement [Shiohara et al. 2007; Wolf et al. 2005; Ghislain and Roujeau, 2002]. Regular monitoring is particularly important, and appropriate management should be instigated in cases of liver, pulmonary or kidney failure. TEN and SJS require specialist care because of the epidermal detachment, with procedures paralleling those applied in the burns unit [Wolf et al. 2005; Ghislain and Roujeau, 2002]. Systemic cortico-steroids and intravenous immunoglobulins have been tried in SJS, but their use in TEN remains controversial and is not recommended in the absence of randomized trial evidence [Allanore and Roujeau, 2007].
The potential involvement of intravenous immunoglobulins and other experimental treatments in the management of severe cutaneous adverse reactions is currently under investigation. On the basis of a hypothesis of an intimate relationship between reactivation of herpes virus and the onset of a hypersensitivity syndrome, patients with DRESS have been successfully treated with pulsed intravenous immunoglobulin G (IgG) in a small-scale study [Shiohara et al. 2006]. Some authors have also found promising results with intravenous IgG in patients with TEN [Viard et al. 1998], but other series were inconclusive. There is insufficient evidence to support the use of IgG as part of the management strategy for these patients [Allanore and Roujeau, 2007; Faye and Roujeau, 2005].
Cutaneous adverse reactions to antiosteoporotic agents
Although rare, recent case reports of cutaneous adverse reactions to antiosteoporotic agents underline the importance of their recognition by practitioners treating postmenopausal osteoporosis. Table 2 summarizes cutaneous adverse reactions reported or associated with currently available antiosteoporotic agents.
Table 2.
Cutaneous adverse reactions linked to antiosteoporosis treatments.
| Treatment | Reported cutaneous adverse reactions | Frequency |
|---|---|---|
| Alendronate [EMA, 2008a] | Rash, pruritus, erythema | Uncommon (1—10 per 1000) |
| Urticaria, angioedema, rash with photosensitivity | Rare (1—10 per 10,000) | |
| SJS, TEN | Very rare (<1 per 10,000) | |
| Ibandronate [EMA, 2007a] | Skin reactions at the injection site (irritation, pain and swelling) | Uncommon (1—10 per 1000) |
| Angioedema, facial oedema, urticaria | Rare (1—10 per 10,000) | |
| Hypersensitivity reactions | Rare (1—10 per 10,000) | |
| Parathyroid hormone and derivatives [EMA, 2008e, 2007b] | Sweating, erythema at injection site | Common (1—10 per 100) |
| Rash | Rare (1—10 per 10,000) | |
| Raloxifene [EMA, 2008d] | Rash | Very rare (<1 per 10,000) |
| Risedronate [Vidal, 2009; Barrera et al. 2005; FDA, 2002] | Rash | Uncommon (1—10 per 1000) |
| Pruritus | Rare (1—10 per 10,000) | |
| Urticaria, angioedema, bullous reactions, photosensitivity | Very rare (<1 per 10,000) | |
| SJS | Very rare (<1 per 10,000) | |
| Strontium ranelate [EMA, 2008c] | Dermatitis, eczema | Common (1—10 per 100) |
| Hypersensitivity reactions (angioedema, pruritus, urticaria) | Very rare (<1 per 10,000) | |
| DRESS, SJS, TEN | Very rare (<1 per 10,000) | |
| Zoledronic acid [EMA, 2008b] | Rash | Uncommon (1—10 per 1000) |
| Redness, swelling and/or pain at infusion site | Rare (1—10 per 10,000) |
DRESS, drug rash with eosinophilia and systemic symptoms; SJS, Stevens Johnson syndrome; TEN, toxic epidermal necrolysis.
Bisphosphonates as a class are associated with a range of benign and severe cutaneous adverse reactions, whatever their mode of administration. European regulatory documents for oral alendronate cite rates of about 0.1—1% for drug rash, pruritus and erythema, and between 0.01% and 0.1% for urticaria, photosensitivity and angioedema [EMA, 2008a]. This corresponds with case reports of urticaria [Kimura et al. 2003; Kontoleon et al. 2000], erythema multiforme and angioedema [Biswas et al. 2003], superficial gyrate erythema [High et al. 2003] and maculopapular eruptions [Brinkmeier et al. 2007], which have appeared since alendronate became available in the mid 1990s. There is also one report of hypertrophic lichen planus [Lazarov et al. 2002] which included an itching rash on the trunk and extremities, combined with livid flat papules and hypertrophic prurigo-like papules, but no mucosal involvement. Alendronate is also associated with more severe cutaneous adverse reactions with cases of SJS and TEN, although these remain very rare (<1 case per 10,000 users) [EMA, 2008a]. Alendronate has been in clinical use since 1993, and since that time there have been 19 cases of SJS and 15 cases of TEN reported to the FDA ARES database [FDA, 2009].
Risedronate, the other commonly used oral bisphosphonate, has been in clinical use since 1997. A similar range of cutaneous adverse reactions to alendronate have been reported: rash in 3—4% of cases and pruritus in 2% [Vidal, 2009; FDA, 2002]. This was confirmed in a pharmacovigilance survey of more than 13,000 patients in England, which also reported cases of urticaria and photosensitivity [Barrera et al. 2005]. A case report of drug eruption on the lower limbs 3 weeks after initiation of treatment, including multiple infiltrated purpuric plaques, was diagnosed as cutaneous vasculitis [Belhadjali et al. 2008]. This case resolved completely upon withdrawal of risedronate, although it did reappear on rechallenge. In relation to severe cutaneous adverse reactions, the English pharmacovigilance study cited one case of SJS, while in the FDA ARES database there are five reported cases of SJS with risedronate, as well as two cases of TEN [FDA, 2009].
Ibandronate and zoledronic acid, the intravenous bisphosphonates, are relative newcomers to the class and have been in clinical use since 2005—2007. Irritation at injection site, including pain and swelling, are provoked by both agents. The regulatory files state that these bisphosphonates are also associated with angioedema, facial swelling or oedema, and urticaria for ibandronate (1 case per 10,000) [EMA, 2007a], and rash, erythema and pruritus for zoledronic acid (1—10 cases per 1000) [EMA, 2008b]. In addition, there is one case report of a pruritic maculopapular rash on the lower extremities with fever (39°C), which appeared 10 days after administration [Rizos et al. 2006]. The patient was treated with intravenous corticosteroids and oral antihis-tamines and the rash and other symptoms subsided within 48 h.
It may be too early in the lifecycle of these two agents to determine the incidence of severe drug hypersensitivity, although this is mentioned in the European regulatory file for ibandronate [EMA, 2007a]. The FDA ARES cites one case of SJS with ibandronate, and four cases of SJS and four cases of TEN with zoledronic acid [FDA, 2009].
The European regulatory documents for strontium ranelate cite dermatitis and eczema as common (rates 2.3% and 1.8% versus 2.0% and 1.4% for placebo), and rash, pruritus, urticaria and angioedema as very rare (<1 case per 10,000) [EMA, 2008c]. There is one case report of an erythematous rash with violaceous patches and plaques which was diagnosed as an interstitial granulomatous reaction [Groves, 2008]. Another case of a generalized cutaneous eruption [Boada et al. 2009] with no fever resolved completely after withdrawal of treatment. Hypersensitivity syndromes, such as DRESS [Jonville-Bera et al. 2009; Pernicova et al. 2008] and TEN [Lee et al. 2009], have also been reported. However, they are very rare (<1 case per 10,000) and with early recognition and appropriate management, the prognosis can be improved [EMA, 2008c; Grosso et al. 2008]. Because strontium ranelate is not currently available in the USA, the FDA ARES database does not include cases of hypersensitivity reactions, although it does mention two cases of SJS from the European files [FDA, 2009].
The remaining antiosteoporotic treatments are associated with low rates of cutaneous adverse reactions. Treatment with the selective oestrogen receptor modulator raloxifene is associated with rash, flushing and sweating [EMA, 2008d; Layton et al. 2005], in line with its mode of action on oestrogen receptors. To the best of the authors' knowledge, there are no case reports of other cutaneous adverse reactions with raloxifene. The use of parathyroid hormone has been linked to erythema at the injection site, which is reported in 0.1—1% of patients [EMA, 2008e, 2007b]. The anabolic agent teriparatide is associated with rash and increased sweating [EMA, 2008e] at a slightly higher frequency (1—10%). There is no evidence of hypersensitivity reactions with either of these agents. Finally, phase II trials of the human monoclonal antibody denosumab have reported moderately increased rates of rash (2—11%, according to dosage versus 0% in placebo groups) [Burnett-Bowie, 2008; McClung et al. 2006]. Whether these effects are serious remains to be seen when the results of ongoing phase III trials are available.
When there are severe cutaneous side effects with one particular therapeutic class, it is recommended that the prescription of an antiosteoporotic in the same class is avoided, even with a different administration, in preference of another therapeutic class.
Discussion and conclusion
A review of the available limited evidence available from regulatory documents and case reports demonstrates that all commonly used antiosteoporotic agents are associated with cutaneous adverse reactions. These reactions are usually benign, because in their most severe forms they are extremely rare, occurring in less than 1 in 10,000 cases. These rates are on a par with drugs such as antibiotics and antiepileptics. These cutaneous adverse reactions occur at lower rates and with lesser severity than other agents used in rheumatology, for example, allopurinol, sulfasalazine (for which, notably, there are 27 cases of SJS and 14 cases TEN cited in the FDA ARES database [FDA, 2009]) and NSAIDs. The incidence of cutaneous adverse reactions with antiosteoporotic agents should be regarded as a reason for vigilance and action when any unusual cutaneous effects occur. Overall, it is important to weigh the risks involved against the benefits of treatment in terms of prevention of osteoporotic fracture, particularly hip fracture, which carries a high risk of complications such as impaired function, loss of independence, need for nursing home care, financial cost and mortality [Bliuc et al. 2009; Autier et al. 2000; Center et al. 1999; Chrischilles et al. 1994; Cooper et al. 1993].
Perusal of the case reports indicates more cutaneous adverse reactions with agents that have been in clinical use the longest (e.g. alendronate) and fewer for more recent agents (e.g. zoledronic acid and ibandronate). This may be a simple consequence of the rarity of the events which mount up as the number of treated patients increase. This illustrates the difficulty of comparing the different treatments, even among those from the same class.
The pathogenesis of these cutaneous adverse reactions remains unclear. There has been speculation that these reactions result from the offending drug acting as a hapten or prohapten, or because of some pharmacological interaction with the immune system [Schnyder and Pichler, 2009]. Whether this also applies to antiosteoporotic agents remains to be elucidated by further research, although this may be compromised by the low incidence of these events. In general, however, with proper management and early recognition, including immediate and permanent withdrawal of all culprit agents, accompanied by hospitalization, rehydration and systemic corticosteroids if necessary, the prognosis is positive.
Footnotes
Professor P. Musette is a consultant/advisor for Servier. Dr J-M. Kaufman reports no conflict of interest for this manuscript. Professor R. Rizzoli has disclosed that he is a consultant/advisor to Merck, Roche, Servier, Danone, Nycomed, Eli Lilly and Amgen; and that he is on the speakers' bureau for Roche, Novartis, Servier and Amgen. Professor P. Cacoub has disclosed that he has been the recipient of research grants from Schering Plough, Roche, Servier and Gilead; he has received honoraria from Roche, Servier, Bristol Myers Squibb, Sanofi Aventis, Gilead and Schering Plough; and he is a consultant/advisor to Roche, Servier, Bristol Myers Squibb, Sanofi Aventis and Gilead Schering Plough. Professor M-L. Brandi reports no conflict of interest for this manuscript. Professor J-Y. Reginster has disclosed that he has been the recipient of research grants from Bristol Myers Squibb, Merck Sharp & Dohme, Rottapharm, Teva, Lilly, Novartis, Roche, GlaxoSmithKline, Amgen and Servier; he is a consultant/advisor to Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed, NPS, Theramex and UCB; and he has been the recipient of lecture fees for speaking at meetings on behalf of Merck Sharp and Dohme, Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GlaxoSmithKline, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Theramex, Nycomed and NovoNordisk.
References
- Aberer W., Kranke B. (2008) Clinical manifestations and mechanisms of skin reactions after systemic drug administration. Drug Discovery Today 5: e237–e247 [Google Scholar]
- Allanore L., Roujeau J.C. (2007) Clinic and pathogenesis of severe bullous skin reactions: Stevens–Johnson syndrome, toxic epidermal necrolysis. In: Pichler W.J. (ed.) Drug Hypersensitivity. Basel: Karger, pp. 267–277 [Google Scholar]
- Autier P., Haentjens P., Bentin J., Baillon J.M., Grivegnee A.R., Closon M.C., et al. (2000) Costs induced by hip fractures: A prospective controlled study in Belgium. Belgian Hip Fracture Study Group. Osteoporos Int 11: 373–380 [DOI] [PubMed] [Google Scholar]
- Barrera B.A., Wilton L., Harris S., Shakir S.A. (2005) Prescription-event monitoring study on 13,164 patients prescribed risedronate in primary care in England. Osteoporos Int 16: 1989–1998 [DOI] [PubMed] [Google Scholar]
- Belhadjali H., Slim R., Aouam K., Youssef M., Zili J. (2008) Cutaneous vasculitis induced by risedronate. Allergy 63: 1405. [DOI] [PubMed] [Google Scholar]
- Bigby M. (2001) Rates of cutaneous reactions to drugs. Arch Dermatol 137: 765–770 [PubMed] [Google Scholar]
- Biswas P.N., Wilton L.V., Shakir S.A. (2003) Pharmacovigilance study of alendronate in England. Osteoporos Int 14: 507–514 [DOI] [PubMed] [Google Scholar]
- Bliuc D., Nguyen N.D., Milch V.E., Nguyen T.V., Eisman J.A., Center J.R. (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301: 513–521 [DOI] [PubMed] [Google Scholar]
- Boada A., Carrascosa J.M., Leal L., Ferrandiz C. (2009) Generalized cutaneous drug eruption due to strontium ranelate. J Eur Acad Dermatol Venereol 23: 321–322 [DOI] [PubMed] [Google Scholar]
- Bocquet H., Bagot M., Roujeau J.C. (1996) Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin Cutan Med Surg 15: 250–257 [DOI] [PubMed] [Google Scholar]
- Borchers A.T., Lee J.L., Naguwa S.M., Cheema G.S., Gershwin M.E. (2008) Stevens–Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev 7: 598–605 [DOI] [PubMed] [Google Scholar]
- Brinkmeier T., Kugler K., Lepoittevin J.P., Frosch P.J. (2007) Adverse cutaneous drug reaction to alendronate. Contact Dermatitis 57: 123–125 [DOI] [PubMed] [Google Scholar]
- Burnett-Bowie S.A. (2008) Is twice-yearly denosu-mab beneficial in postmenopausal women with osteo-penia but no history of fracture? Nat Clin Pract Endocrinol Metab 4: 660–661 [DOI] [PubMed] [Google Scholar]
- Center J.R., Nguyen T.V., Schneider D., Sambrook P.N., Eisman J.A. (1999) Mortality after all major types of osteoporotic fracture in men and women: An observational study. Lancet 353: 878–882 [DOI] [PubMed] [Google Scholar]
- Chrischilles E., Shireman T., Wallace R. (1994) Costs and health effects of osteoporotic fractures. Bone 15: 377–386 [DOI] [PubMed] [Google Scholar]
- Chung W.H., Hung S.I., Hong H.S., Hsih M.S., Yang L.C., Ho H.C., et al. (2004) Medical genetics: A marker for Stevens–Johnson syndrome. Nature 428: 486. [DOI] [PubMed] [Google Scholar]
- Cooper C., Atkinson E.J., Jacobsen S.J., O'Fallon W.M., Melton L.J., 3rd (1993) Population-based study of survival after osteoporotic fractures. Am J Epidemiol 137: 1001–1005 [DOI] [PubMed] [Google Scholar]
- Edwards I.R., Aronson J.K. (2000) Adverse drug reactions: Definitions, diagnosis, and management. Lancet 356: 1255–1259 [DOI] [PubMed] [Google Scholar]
- Eshki M., Allanore L., Musette P., Milpied B., Grange A., Guillaume J.C., et al. (2009) Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: A cause of unpredictable multiorgan failure. Arch Dermatol 145: 67–72 [DOI] [PubMed] [Google Scholar]
- EMA (2007a) Ibandronate. Summary of Product Characteristics. European Medicines Agency; Available at: http://www.ema.europa.eu (accessed 11 February 2009). [Google Scholar]
- EMA (2007b) Parathyroid Hormone. Summary of Product Characteristics. European Medicines Agency; Available at: http://www.ema.europa.eu (accessed 11 February 2009). [Google Scholar]
- EMA (2008a) Alendronate. Summary of Product Characteristics. European Medicines Agency; Available at: http://www.ema.europa.eu (accessed 11 February 2009). [Google Scholar]
- EMA (2008b) Zoledronic Acid. Summary of Product Characteristics. European Medicines Agency; Available at: http://www.ema.europa.eu (accessed 11 February 2009). [Google Scholar]
- EMA (2008c) Strontium Ranelate. Summary of Product Characteristics. European Medicines Agency; Available at: http://www.ema.europa.eu (accessed 11 February 2009). [Google Scholar]
- EMA (2008d) Raloxifene. Summary of Product Characteristics. European Medicines Agency; Available at: http://www.ema.europa.eu (Accessed 11 February 2009). [Google Scholar]
- EMA (2008e) Teriparatide. Summary of Product Characteristics. European Medicines Agency; Available at: http://www.ema.europa.eu (Accessed 11 February 2009). [Google Scholar]
- Faye O., Roujeau J.C. (2005) Treatment of epidermal necrolysis with high-dose intravenous immunoglobulins (IV Ig): Clinical experience to date. Drugs 65: 2085–2090 [DOI] [PubMed] [Google Scholar]
- FDA (2002) Risedronate. Description. US Food and Drug Administration; Available at: http://www.fda.gov (accessed 11 February 2009). [Google Scholar]
- FDA (2009) Pharmapendium. US Food and Drug Administration; Available at: www.pharmapendium.com (accessed 23 February 2009). [Google Scholar]
- Ghislain P.D., Roujeau J.C. (2002) Treatment of severe drug reactions: Stevens–Johnson syndrome, toxic epidermal necrolysis and hypersensitivity syndrome. Dermatol Online J 8: 5. [PubMed] [Google Scholar]
- Gomes E.R., Demoly P. (2005) Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol 5: 309–316 [DOI] [PubMed] [Google Scholar]
- Grosso A., Douglas I., Hingorani A., MacAllister R., Smeeth L. (2008) Post-marketing assessment of the safety of strontium ranelate: A novel case-only approach to the early detection of adverse drug reactions. Br J Clin Pharmacol 66: 689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves C. (2008) Interstitial granulomatous reaction to strontium ranelate. Arch Dermatol 144: 268–269 [DOI] [PubMed] [Google Scholar]
- High W.A., Cohen J.B., Wetherington W, Cockerell C.J. (2003) Superficial gyrate erythema as a cutaneous reaction to alendronate for osteoporosis. J Am Acad Dermatol 48: 945–946 [DOI] [PubMed] [Google Scholar]
- Hung S.I., Chung W.H., Liou L.B., Chu C.C., Lin M.j, Huang H.P., et al. (2005) HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA 102: 4134–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker T., Kunzi U.P., Braunschweig S., Zehnder D., Hoigne R.(1997) Comprehensive hospital drug monitoring (CHDM): Adverse skin reactions, a 20-year survey. Allergy 52: 388–393 [DOI] [PubMed] [Google Scholar]
- Jonville-Bera A.P., Crickx B., Aaron L., Hartingh I., Autret-Leca E. (2009) Strontium ranelate-induced DRESS syndrome: First two case reports. Allergy 64: 658–659 [DOI] [PubMed] [Google Scholar]
- Kimura M., Kawada A., Murayama Y., Murayama M. (2003) Drug eruption due to alendronate sodium hydrate. Contact Dermatitis 48: 116. [DOI] [PubMed] [Google Scholar]
- Kontoleon P., Ilias I., Stavropoulos P.G., Papapetrou P.D. (2000) Urticaria after administration of alendronate. Ada Derm Venereol 80: 398. [PubMed] [Google Scholar]
- Layton D., Clarke A., Wilton L.V., Shakir S.A. (2005) Safety profile of raloxifene as used in general practice in England: Results of a prescription-event monitoring study. Osteoporos Int 16: 490–500 [DOI] [PubMed] [Google Scholar]
- Lazarov A., Moss K., Plosk N., Cordoba M., Baitelman L. (2002) Alendronate-induced lichen planus. Isr Med Assoc J 4: 389–390 [PubMed] [Google Scholar]
- Lee H.Y., Lie D., Lim K.S., Thirumoorthy T., Pang S.M. (2009) Strontium ranelate-induced toxic epidermal necrolysis in a patient with post-menopausal osteoporosis. Osteoporos Int 20: 161–162 [DOI] [PubMed] [Google Scholar]
- Mallal S., Phillips E., Carosi G., Molina J.M., Workman C., Tomazic J., et al. (2008) HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 358: 568–579 [DOI] [PubMed] [Google Scholar]
- McClung M.R., Lewiecki E.M., Cohen S.B., Bolognese M.A., Woodson G.C., Moffett A.H., et al. (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354: 821–831 [DOI] [PubMed] [Google Scholar]
- Mockenhaupt M. (2007) Epidemiology and causes of severe cutaneous adverse reactions to drugs, In: Pichler W.J. (ed.). Drug Hypersensitivity, Karger: Basel, pp. 18–31 [Google Scholar]
- Mockenhaupt M., Viboud C., Dunant A., Naldi L., Halevy S., Bouwes-Bavinck J.N., et al. (2008) Stevens–Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 128: 35–44 [DOI] [PubMed] [Google Scholar]
- Morison W.L. (2004) Clinical practice. Photosensitivity. N Engl J Med 350: 1111–1117 [DOI] [PubMed] [Google Scholar]
- Pernicova I., Middleton E.T., Aye M. (2008) Rash, strontium ranelate and DRESS syndrome put into perspective. European Medicine Agency on the alert. Osteoporos Int 19: 1811–1812 [DOI] [PubMed] [Google Scholar]
- Rizos E.C., Milionis H.J., Elisaf M.S. (2006) Fever with rash following zolendronic acid administration. Clin Exp Rheumatol 24: 455. [PubMed] [Google Scholar]
- Roujeau J.C. (2005) Clinical heterogeneity of drug hypersensitivity. Toxicology 209: 123–129 [DOI] [PubMed] [Google Scholar]
- Schnyder B., Pichler W.J. (2009) Mechanisms of drug-induced allergy. Mayo Clin Proc 84: 268–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohara T., Inaoka M., Kano Y. (2006) Drug-induced hypersensitivity syndrome (DIHS): A reaction induced by a complex interplay among herpes viruses and antiviral and antidrug immune responses. Allergol Int 55: 1–8 [DOI] [PubMed] [Google Scholar]
- Shiohara T., Takahashi R., Kano Y. (2007) Drug-induced hypersensitivity syndrome and viral reactivation, In: Pichler W.J. (ed.). Drug Hypersensitivity, Karger: Basel, pp. 251–266 [Google Scholar]
- Svensson C.K., Cowen E.W, Gaspari A.A. (2001) Cutaneous drug reactions. Pharmacol Rev 53: 357–379 [PubMed] [Google Scholar]
- Valeyrie-Allanore L., Sassolas B., Roujeau J.C. (2007) Drug-induced skin, nail and hair disorders. Drug Saf 30: 1011–1030 [DOI] [PubMed] [Google Scholar]
- Viard I., Wehrli P., Bullani R., Schneider P., Holler N., Salomon D., et al. (1998) Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 282: 490–493 [DOI] [PubMed] [Google Scholar]
- Vidal L. (2009) Le Dictionnaire, 85th edn, Issy-les-Moulineaux, France: Vidal [Google Scholar]
- Wolf R., Orion E., Marcos B., Matz H. (2005) Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol 23: 171–181 [DOI] [PubMed] [Google Scholar]